SARS-CoV-2 Enters Human Leydig Cells and Affects Testosterone Production In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. hiPSC
2.2. Cell Lines
2.3. Plasmids
2.4. hLLC Induction
2.5. SARS-CoV-2 Spike-Pseudotyped Viral Vector VSVΔG and Bald Vector Production
2.6. Animals
2.7. Histology
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Protein Quantification
2.10. Immunoblotting
2.11. Fluorescent Immunocytochemistry
2.12. Cell Binding Assay
2.13. SARS-CoV-2 Spike Pseudovector System and Pseudovector-Based Inhibition Assay
2.14. MTT Cell Proliferation and Cell Viability Assay
2.15. Fluorescent-Activated Cell Sorting (FACS)
2.16. ELISA
2.17. Statistical Analysis
3. Results
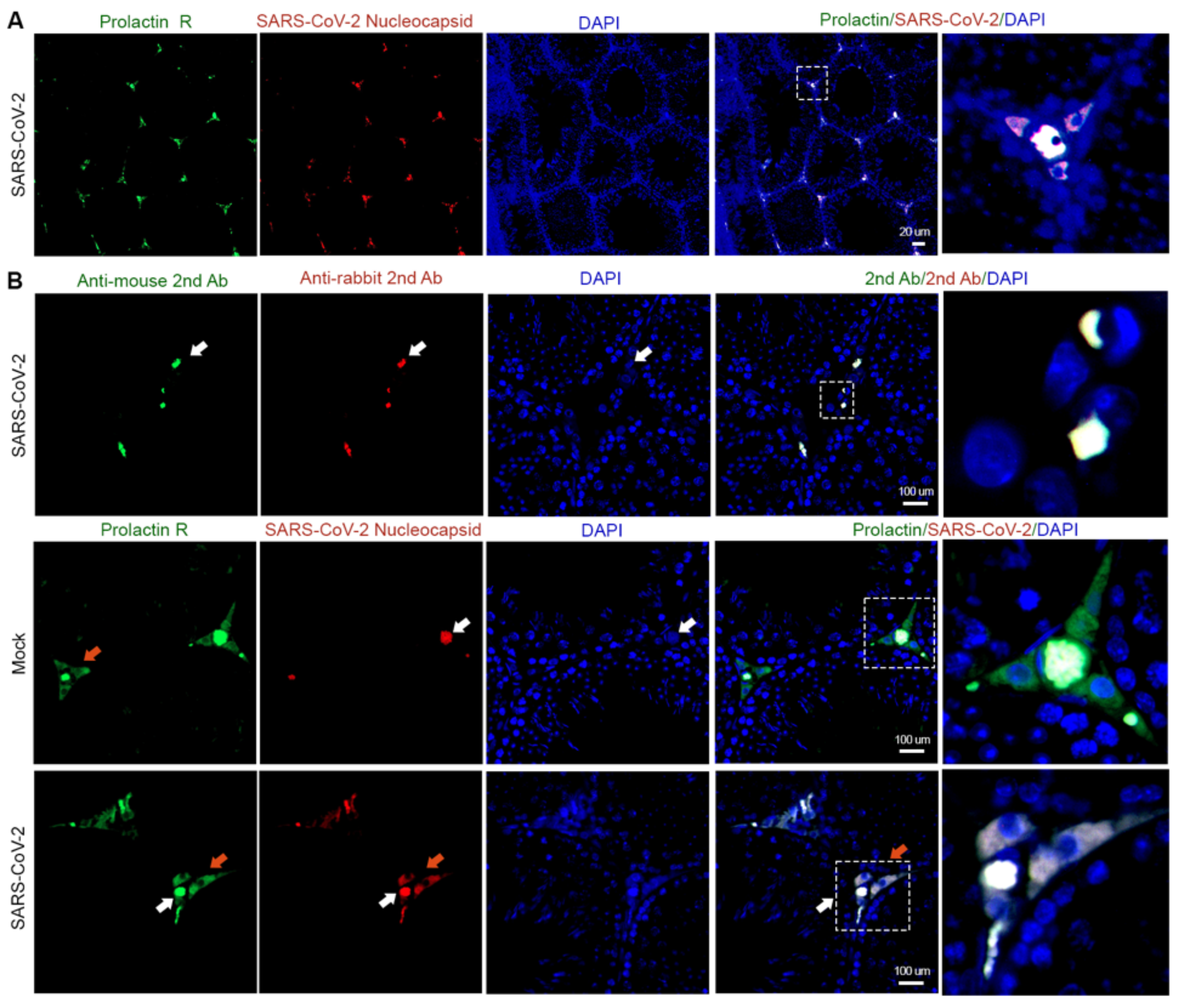
3.1. Interstitial Cells Are the Main Targets of SARS-CoV-2 in the Testis
3.2. SARS-CoV-2 Spike Protein Subunit 1 (S1) Can Bind to hLLCs and Alter Their Function
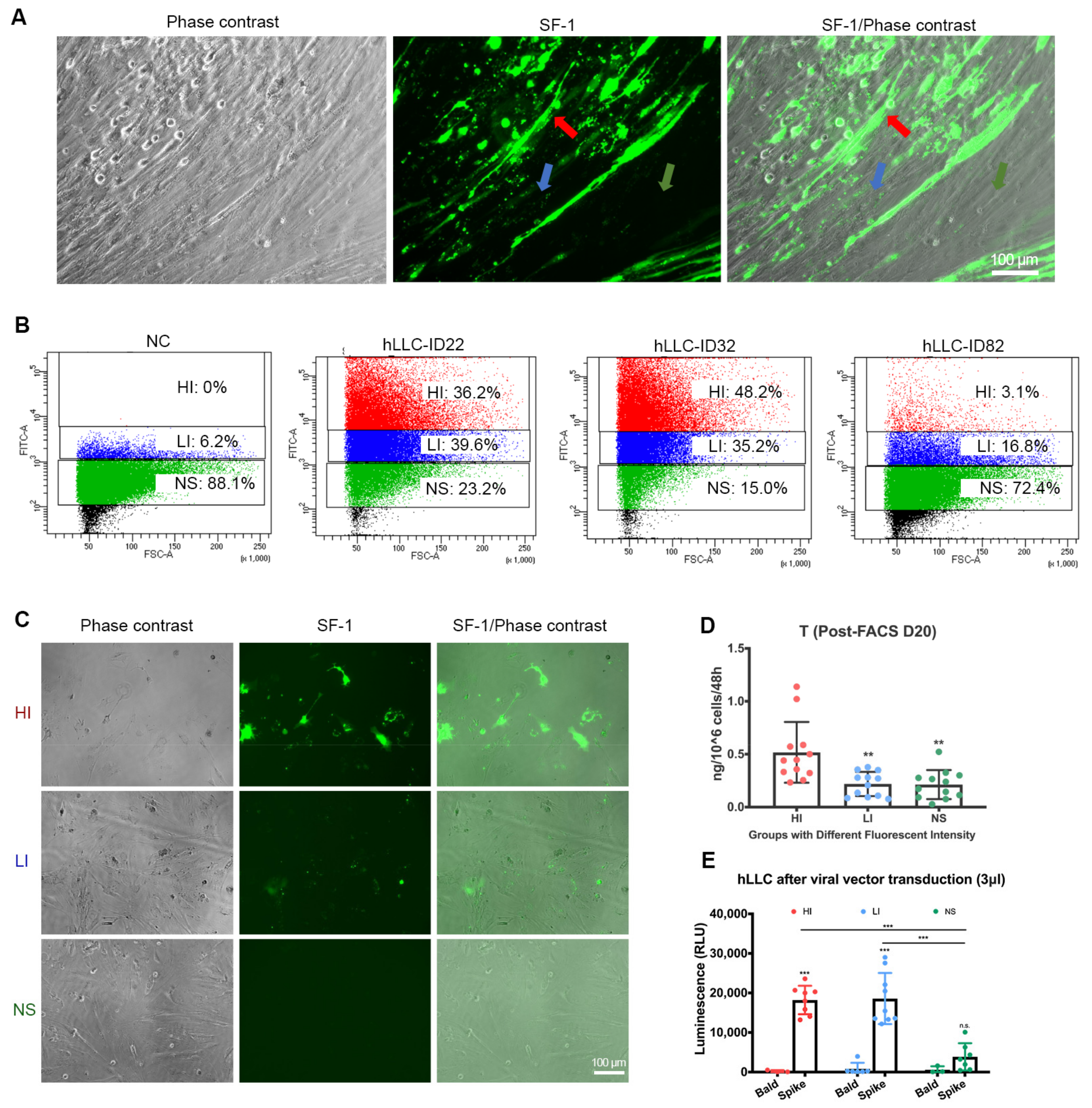
3.3. SARS-CoV-2 Spike Pseudovector Can Enter hLLCs
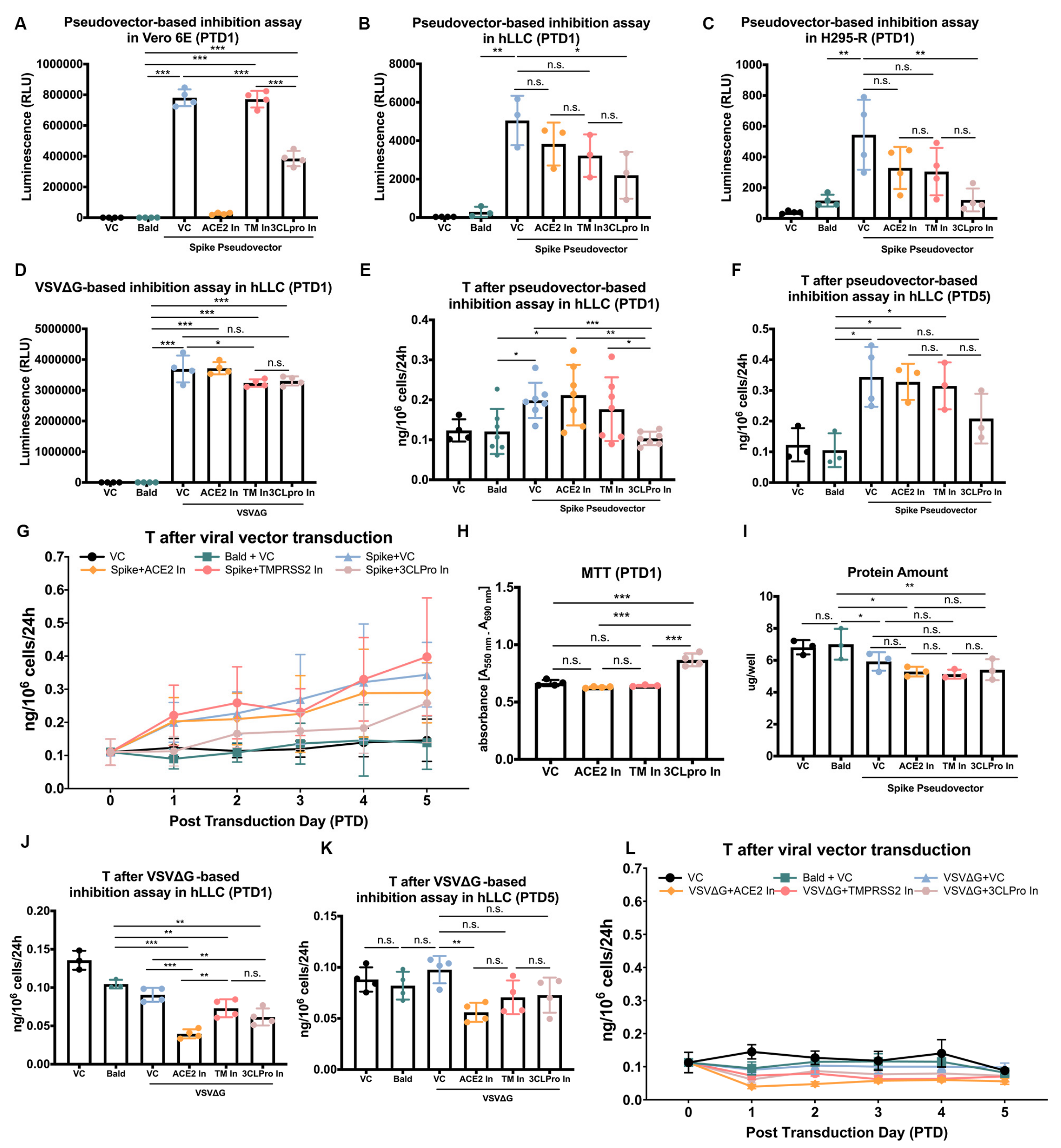
3.4. SARS-CoV-2 Spike Pseudovectors Enter hLLCs through a Pathway Different from Those Present in Vero E6 Cells
3.5. SARS-CoV-2 Spike Pseudovector Entry Dysregulates T Biosynthesis of hLLCs
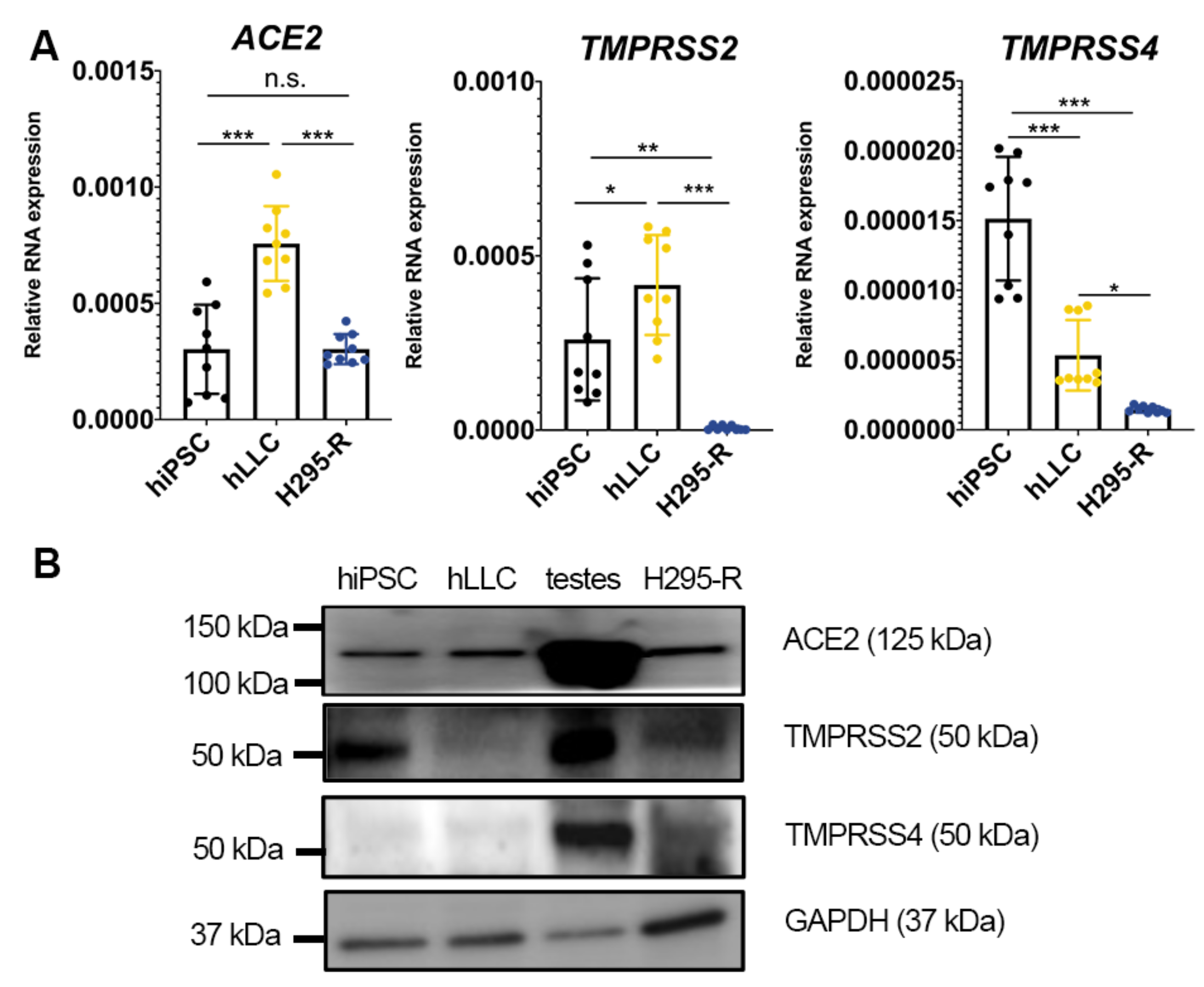
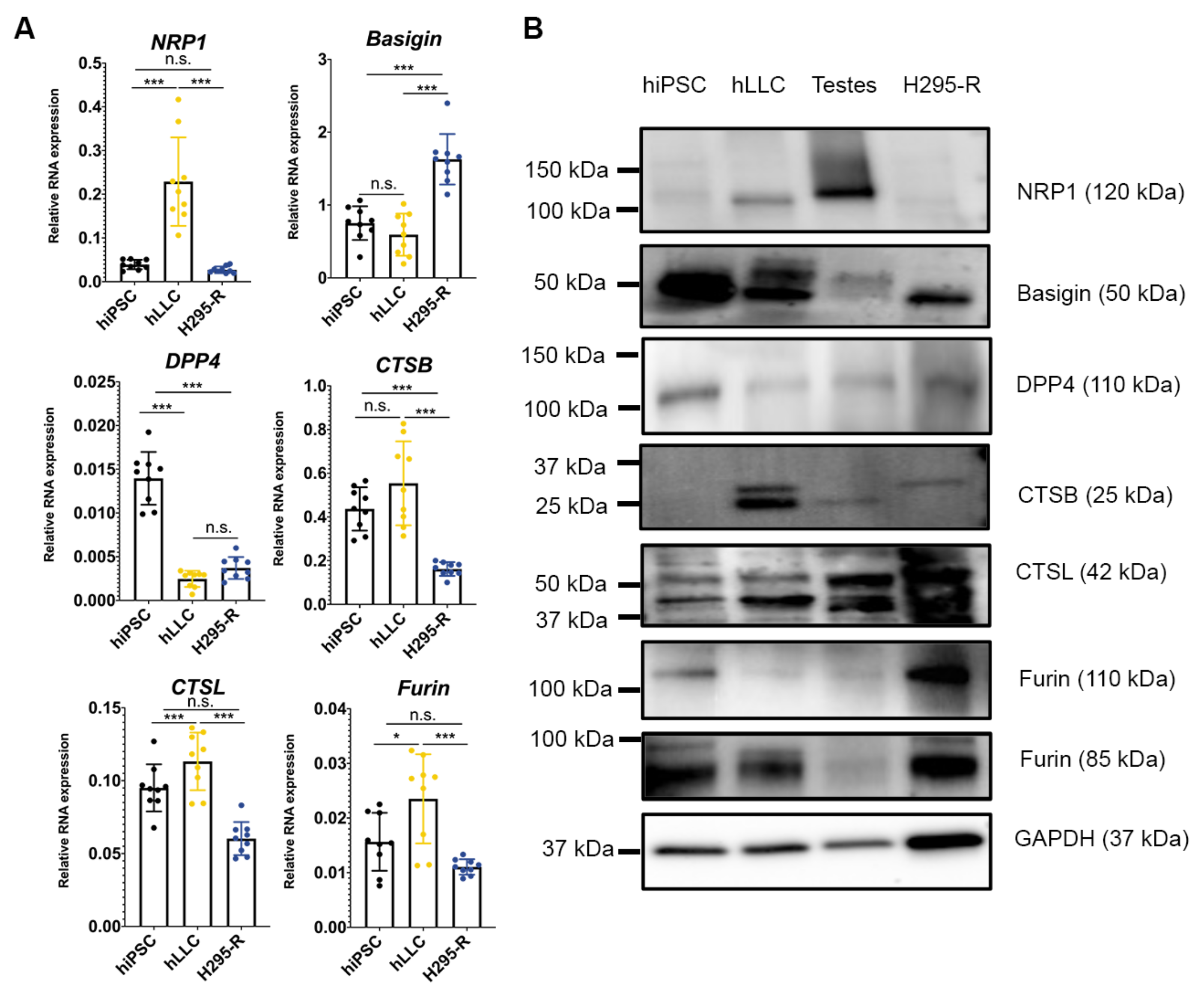
3.6. Human Testis and Leydig-like Cells Present Other Receptors or Proteases for SARS-CoV-2 Entry
4. Discussion
4.1. T Production and SARS-CoV-2
4.2. SARS-CoV-2 Entry into Leydig Cells
4.3. Future Work on SARS-CoV-2 Infection and the Testis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gralinski, L.E.; Menachery, V.D. Return of the Coronavirus: 2019-nCoV. Viruses 2020, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Hikmet, F.; Mear, L.; Edvinsson, A.; Micke, P.; Uhlen, M.; Lindskog, C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020, 16, e9610. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Neto, A.N.; Teixeira, T.A.; Caldini, E.G.; Kanamura, C.T.; Gomes-Gouvea, M.S.; Dos Santos, A.B.G.; Monteiro, R.A.A.; Pinho, J.R.R.; Mauad, T.; da Silva, L.F.F.; et al. Testicular pathology in fatal COVID-19: A descriptive autopsy study. Andrology 2022, 10, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Achua, J.K.; Chu, K.Y.; Ibrahim, E.; Khodamoradi, K.; Delma, K.S.; Iakymenko, O.A.; Kryvenko, O.N.; Arora, H.; Ramasamy, R. Histopathology and Ultrastructural Findings of Fatal COVID-19 Infections on Testis. World J. Mens Health 2021, 39, 65–74. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X. scRNA-seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, A Target for SARS-CoV-2 Infection in Spermatogonia, Leydig and Sertoli Cells. Cells 2020, 9, 920. [Google Scholar] [CrossRef]
- Salonia, A.; Pontillo, M.; Capogrosso, P.; Gregori, S.; Tassara, M.; Boeri, L.; Carenzi, C.; Abbate, C.; Cignoli, D.; Ferrara, A.M.; et al. Severely low testosterone in males with COVID-19: A case-control study. Andrology 2021, 9, 1043–1052. [Google Scholar] [CrossRef]
- Apaydin, T.; Sahin, B.; Dashdamirova, S.; Dincer Yazan, C.; Elbasan, O.; Ilgin, C.; Bilgin, H.; Cam, H.K.; Bahramzada, G.; Kucuk, A.; et al. The association of free testosterone levels with coronavirus disease 2019. Andrology 2022, 10, 1038–1046. [Google Scholar] [CrossRef]
- Salonia, A.; Pontillo, M.; Capogrosso, P.; Gregori, S.; Carenzi, C.; Ferrara, A.M.; Rowe, I.; Boeri, L.; Larcher, A.; Ramirez, G.A.; et al. Testosterone in males with COVID-19: A 7-month cohort study. Andrology 2022, 10, 34–41. [Google Scholar] [CrossRef]
- Cinislioglu, A.E.; Cinislioglu, N.; Demirdogen, S.O.; Sam, E.; Akkas, F.; Altay, M.S.; Utlu, M.; Sen, I.A.; Yildirim, F.; Kartal, S.; et al. The relationship of serum testosterone levels with the clinical course and prognosis of COVID-19 disease in male patients: A prospective study. Andrology 2022, 10, 24–33. [Google Scholar] [CrossRef]
- Campos, R.K.; Camargos, V.N.; Azar, S.R.; Haines, C.A.; Eyzaguirre, E.J.; Rossi, S.L. SARS-CoV-2 Infects Hamster Testes. Microorganisms 2021, 9, 1318. [Google Scholar] [CrossRef]
- Li, C.; Ye, Z.; Zhang, A.J.X.; Chan, J.F.W.; Song, W.; Liu, F.; Chen, Y.; Kwan, M.Y.W.; Lee, A.C.Y.; Zhao, Y. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection by Intranasal or Intratesticular Route Induces Testicular Damage. Clin. Infect. Dis. 2022, 75, e974–e990. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Sottas, C.; Culty, M.; Fan, J.; Hu, Y.; Cheung, G.; Chemes, H.E.; Papadopoulos, V. Directing differentiation of human induced pluripotent stem cells toward androgen-producing Leydig cells rather than adrenal cells. Proc. Natl. Acad. Sci. USA 2019, 116, 23274–23283. [Google Scholar] [CrossRef]
- Kreitzer, F.R.; Salomonis, N.; Sheehan, A.; Huang, M.; Park, J.S.; Spindler, M.J.; Lizarraga, P.; Weiss, W.A.; So, P.L.; Conklin, B.R. A robust method to derive functional neural crest cells from human pluripotent stem cells. Am. J. Stem Cells 2013, 2, 119–131. [Google Scholar]
- Chen, H.Y.; Huang, C.; Tian, L.; Huang, X.; Zhang, C.; Llewellyn, G.N.; Rogers, G.L.; Andresen, K.; O’Gorman, M.R.G.; Chen, Y.W.; et al. Cytoplasmic Tail Truncation of SARS-CoV-2 Spike Protein Enhances Titer of Pseudotyped Vectors but Masks the Effect of the D614G Mutation. J. Virol. 2021, 95, e0096621. [Google Scholar] [CrossRef]
- Whitt, M.A. Generation of VSV pseudotypes using recombinant DeltaG-VSV for studies on virus entry, identification of entry inhibitors, and immune responses to vaccines. J. Virol. Methods 2010, 169, 365–374. [Google Scholar] [CrossRef]
- Ichim, C.V.; Wells, R.A. Generation of high-titer viral preparations by concentration using successive rounds of ultracentrifugation. J. Transl. Med. 2011, 9, 137. [Google Scholar] [CrossRef]
- Mulka, K.R.; Beck, S.E.; Solis, C.V.; Johanson, A.L.; Queen, S.E.; McCarron, M.E.; Richardson, M.R.; Zhou, R.; Marinho, P.; Jedlicka, A.; et al. Progression and Resolution of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Golden Syrian Hamsters. Am. J. Pathol. 2022, 192, 195–207. [Google Scholar] [CrossRef]
- Council, N.R. Guide for the Care and Use of Laboratory Animals; The National Academies Press: Washinton, DC, USA, 2010. [Google Scholar]
- Matzkin, M.E.; Ambao, V.; Carino, M.H.; Rossi, S.P.; Gonzalez, L.; Turyn, D.; Campo, S.; Calandra, R.S.; Frungieri, M.B. Prolactin (PRL) induction of cyclooxygenase 2 (COX2) expression and prostaglandin (PG) production in hamster Leydig cells. Mol. Cell. Endocrinol. 2012, 348, 33–46. [Google Scholar] [CrossRef]
- Botteri Principato, N.L.; Suarez, J.D.; Laws, S.C.; Klinefelter, G.R. The use of purified rat Leydig cells complements the H295R screen to detect chemical-induced alterations in testosterone production. Biol. Reprod. 2018, 98, 239–249. [Google Scholar] [CrossRef]
- Zang, R.; Gomez Castro, M.F.; McCune, B.T.; Zeng, Q.; Rothlauf, P.W.; Sonnek, N.M.; Liu, Z.; Brulois, K.F.; Wang, X.; Greenberg, H.B.; et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020, 5, eabc3582. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Essaidi-Laziosi, M.; Perez Rodriguez, F.J.; Hulo, N.; Jacquerioz, F.; Kaiser, L.; Eckerle, I. Estimating clinical SARS-CoV-2 infectiousness in Vero E6 and primary airway epithelial cells. Lancet Microbe 2021, 2, e571. [Google Scholar] [CrossRef] [PubMed]
- Terali, K.; Baddal, B.; Gulcan, H.O. Prioritizing potential ACE2 inhibitors in the COVID-19 pandemic: Insights from a molecular mechanics-assisted structure-based virtual screening experiment. J. Mol. Graph. Model. 2020, 100, 107697. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hofmann-Winkler, H.; Smith, J.C.; Kruger, N.; Arora, P.; Sorensen, L.K.; Sogaard, O.S.; Hasselstrom, J.B.; Winkler, M.; Hempel, T.; et al. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine 2021, 65, 103255. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.X.; Gong, L.; et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef]
- Valencia, I.; Peiro, C.; Lorenzo, O.; Sanchez-Ferrer, C.F.; Eckel, J.; Romacho, T. DPP4 and ACE2 in Diabetes and COVID-19: Therapeutic Targets for Cardiovascular Complications? Front. Pharmacol. 2020, 11, 1161. [Google Scholar] [CrossRef]
- Hashimoto, R.; Sakamoto, A.; Deguchi, S.; Yi, R.; Sano, E.; Hotta, A.; Takahashi, K.; Yamanaka, S.; Takayama, K. Dual inhibition of TMPRSS2 and Cathepsin Bprevents SARS-CoV-2 infection in iPS cells. Mol. Ther. Nucleic Acids 2021, 26, 1107–1114. [Google Scholar] [CrossRef]
- Zhao, M.M.; Yang, W.L.; Yang, F.Y.; Zhang, L.; Huang, W.J.; Hou, W.; Fan, C.F.; Jin, R.H.; Feng, Y.M.; Wang, Y.C.; et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Target. Ther. 2021, 6, 134. [Google Scholar] [CrossRef]
- Peacock, T.P.; Goldhill, D.H.; Zhou, J.; Baillon, L.; Frise, R.; Swann, O.C.; Kugathasan, R.; Penn, R.; Brown, J.C.; Sanchez-David, R.Y.; et al. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nat. Microbiol. 2021, 6, 899–909. [Google Scholar] [CrossRef]
- Wang, D. Discrepancy between mRNA and protein abundance: Insight from information retrieval process in computers. Comput. Biol. Chem. 2008, 32, 462–468. [Google Scholar] [CrossRef]
- Rambhatla, A.; Bronkema, C.J.; Corsi, N.; Keeley, J.; Sood, A.; Affas, Z.; Dabaja, A.A.; Rogers, C.G.; Liroff, S.A.; Abdollah, F. COVID-19 Infection in Men on Testosterone Replacement Therapy. J. Sex. Med. 2021, 18, 215–218. [Google Scholar] [CrossRef]
- Li, L.; Papadopoulos, V. Advances in stem cell research for the treatment of primary hypogonadism. Nat. Rev. Urol. 2021, 18, 487–507. [Google Scholar] [CrossRef]
- Lanser, L.; Burkert, F.R.; Thommes, L.; Egger, A.; Hoermann, G.; Kaser, S.; Pinggera, G.M.; Anliker, M.; Griesmacher, A.; Weiss, G.; et al. Testosterone Deficiency Is a Risk Factor for Severe COVID-19. Front. Endocrinol. 2021, 12, 694083. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Punjani, N.; Flannigan, R. Androgens and COVID-19: Exploring the role of testosterone replacement therapy. Int. J. Impot. Res. 2022, 34, 649–651. [Google Scholar] [CrossRef]
- Leisegang, K.; Henkel, R. The in vitro modulation of steroidogenesis by inflammatory cytokines and insulin in TM3 Leydig cells. Reprod. Biol. Endocrinol. 2018, 16, 26. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Li, L.; Samplaski, M. Why does COVID-19 kill more elderly men than women? Is there a role for testosterone? Andrology 2021, 9, 65–72. [Google Scholar] [CrossRef]
- Ahmad, I.; Pawara, R.; Surana, S.; Patel, H. The Repurposed ACE2 Inhibitors: SARS-CoV-2 Entry Blockers of Covid-19. Top. Curr. Chem. 2021, 379, 40. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Guan, C.; Chen, R.; Wang, Y.; Feng, S.; Wang, R.; Qu, G.; Zhao, S.; Wang, F.; Wang, X.; et al. Pathological and molecular examinations of postmortem testis biopsies reveal SARS-CoV-2 infection in the testis and spermatogenesis damage in COVID-19 patients. Cell. Mol. Immunol. 2021, 18, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z.; Shinn, P.; Itkin, Z.; Eastman, R.T.; Bostwick, R.; Rasmussen, L.; Huang, R.; Shen, M.; Hu, X.; Wilson, K.M.; et al. Drug Repurposing Screen for Compounds Inhibiting the Cytopathic Effect of SARS-CoV-2. Front. Pharmacol. 2020, 11, 592737. [Google Scholar] [CrossRef] [PubMed]
- Murgolo, N.; Therien, A.G.; Howell, B.; Klein, D.; Koeplinger, K.; Lieberman, L.A.; Adam, G.C.; Flynn, J.; McKenna, P.; Swaminathan, G.; et al. SARS-CoV-2 tropism, entry, replication, and propagation: Considerations for drug discovery and development. PLoS Pathog. 2021, 17, e1009225. [Google Scholar] [CrossRef]
- Bergman, J.; Botling, J.; Fagerberg, L.; Hallstrom, B.M.; Djureinovic, D.; Uhlen, M.; Ponten, F. The Human Adrenal Gland Proteome Defined by Transcriptomics and Antibody-Based Profiling. Endocrinology 2017, 158, 239–251. [Google Scholar] [CrossRef]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Agolli, A.; Yukselen, Z.; Agolli, O.; Patel, M.H.; Bhatt, K.P.; Concepcion, L.; Halpern, J.; Alvi, S.; Abreu, R. SARS-CoV-2 effect on male infertility and its possible pathophysiological mechanisms. Discoveries 2021, 9, e131. [Google Scholar] [CrossRef]
- Alwani, M.; Yassin, A.; Al-Zoubi, R.M.; Aboumarzouk, O.M.; Nettleship, J.; Kelly, D.; Al-Qudimat, A.R.; Shabsigh, R. Sex-based differences in severity and mortality in COVID-19. Rev. Med. Virol. 2021, 31, e2223. [Google Scholar] [CrossRef]
- Zou, P.; Heath, A.; Sewell, C.; Lu, Y.; Tran, D.; Seo, S.K. EXOGENOUS Sex Hormones and Sex Hormone Receptor Modulators in COVID-19: Rationale and Clinical Pharmacology Considerations. Clin. Pharmacol. Ther. 2022, 111, 559–571. [Google Scholar] [CrossRef]
- Iketani, S.; Forouhar, F.; Liu, H.; Hong, S.J.; Lin, F.Y.; Nair, M.S.; Zask, A.; Huang, Y.; Xing, L.; Stockwell, B.R.; et al. Lead compounds for the development of SARS-CoV-2 3CL protease inhibitors. Nat. Commun. 2021, 12, 2016. [Google Scholar] [CrossRef]
- Yang, H.; Yang, M.; Ding, Y.; Liu, Y.; Lou, Z.; Zhou, Z.; Sun, L.; Mo, L.; Ye, S.; Pang, H.; et al. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc. Natl. Acad. Sci. USA 2003, 100, 13190–13195. [Google Scholar] [CrossRef]
- Thiel, V.; Herold, J.; Schelle, B.; Siddell, S.G. Viral replicase gene products suffice for coronavirus discontinuous transcription. J. Virol. 2001, 75, 6676–6681. [Google Scholar] [CrossRef]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.E.; Williamson, M.K.; Anton-Plagaro, C.; Shoemark, D.K.; Simon-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef]
- Perez-Miller, S.; Patek, M.; Moutal, A.; Cabel, C.R.; Thorne, C.A.; Campos, S.K.; Khanna, R. In silico identification and validation of inhibitors of the interaction between neuropilin receptor 1 and SARS-CoV-2 Spike protein. bioRxiv 2020. [Google Scholar] [CrossRef]
- Moutal, A.; Martin, L.F.; Boinon, L.; Gomez, K.; Ran, D.; Zhou, Y.; Stratton, H.J.; Cai, S.; Luo, S.; Gonzalez, K.B.; et al. SARS-CoV-2 spike protein co-opts VEGF-A/neuropilin-1 receptor signaling to induce analgesia. Pain 2021, 162, 243–252. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Dixit, N.M. Evidence of increased Cathepsin B/L and decreased TMPRSS2 usage for cell entry by the SARS-CoV-2 Omicron variant. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hook, G.; Jacobsen, J.S.; Grabstein, K.; Kindy, M.; Hook, V. Cathepsin B is a New Drug Target for Traumatic Brain Injury Therapeutics: Evidence for E64d as a Promising Lead Drug Candidate. Front. Neurol. 2015, 6, 178. [Google Scholar] [CrossRef]
- Simmons, G.; Gosalia, D.N.; Rennekamp, A.J.; Reeves, J.D.; Diamond, S.L.; Bates, P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 2005, 102, 11876–11881. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef]
- Millet, J.K.; Jaimes, J.A.; Whittaker, G.R. Molecular diversity of coronavirus host cell entry receptors. FEMS Microbiol. Rev. 2021, 45, fuaa057. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Frere, J.J.; Serafini, R.A.; Pryce, K.D.; Zazhytska, M.; Oishi, K.; Golynker, I.; Panis, M.; Zimering, J.; Horiuchi, S.; Hoagland, D.A. SARS-CoV-2 infection in hamsters and humans results in lasting and unique systemic perturbations post recovery. Sci. Transl. Med. 2022, 14, eabq3059. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer FW | Primer RW |
|---|---|---|
| ACE2 | ACAGTCCACACTTGCCCAAAT | TGAGAGCACTGAAGACCCATT |
| TMPRSS2 | GTCCCCACTGTCTACGAGGT | CAGACGACGGGGTTGGAAG |
| TMPRSS4 | GACAAACAGCACGTCTGTGGA | GCCTGAGAAAGTGAGTGGGAA |
| NRP1 | ACGTGGAAGTCTTCGATGGAG | CACCATGTGTTTCGTAGTCAGA |
| Basigin | GAAGTCGTCAGAACACATCAACG | TTCCGGCGCTTCTCGTAGA |
| DPP4 | GGGTCACATGGTCACCAGTG | TCTGTGTCGTTAAATTGGGCATA |
| CTSB | AGAGTTATGTTTACCGAGGACCT | GATGCAGATCCGGTCAGAGA |
| CTSL | AGGGTCAGTGTGGTTCTTGTTG | TGAGATAAGCCTCCCAGTTTTC |
| Furin | CCTGGTTGCTATGGGTGGTAG | AAGTGGTAATAGTCCCCGAAGA |
| S18 | ATTAAGGGTGTGGGCCGAAG | TGGCTAGGACCTGGCTGTAT |
| Antibody | Host | Company | Catalogue No. | Dilution | Application |
|---|---|---|---|---|---|
| ACE2 | Rabbit | Abcam | ab108252 | 1:1000 | WB |
| TMPRSS2 | Rabbit | Abcam | ab109131 | 1:1000 | WB |
| TMPRSS4 | Rabbit | ThermoFisher Scientific | 11283-1-AP | 1:500 | WB |
| NRP1 | Rabbit | Abcam | ab81321 | 1:1000 | WB |
| Basigin | Rabbit | Cell Signaling | 13287S | 1:1000 | WB |
| DPP4 | Mouse | ThermoFisher Scientific | TA500733 | 1:500 | WB |
| CTSB | Rabbit | Cell Signaling | 31718T | 1:1000 | WB |
| CTSL | Mouse | ThermoFisher Scientific | BMS1032 | 1:1000 | WB |
| Furin | Mouse | R&D | MAB15032-SP | 1:500 | WB |
| GAPDH | Rabbit | Cell Signaling | 2118L | 1:1000 | WB |
| SARS-CoV-2 Nucleocapsid | Rabbit | ThermoFisher Scientific | MA5-29982 | 1:50 | IFC |
| Prolactin R | Mouse | Novus Bio | NB300-561 | 1:200 | IFC |
| Goat Anti-Mouse IgG | Goat | LI-COR Biosciences | 926-80010 | 1:4000 | WB |
| Goat Anti-Rabbit IgG | Goat | LI-COR Biosciences | 926-80011 | 1:4000 | WB |
| Goat Anti-Rabbit IgG | Goat | ThermoFisher Scientific | A21244 | 1:300 | IFC |
| Name | Functions | Concentrations | Company | Catalogue No. |
|---|---|---|---|---|
| MLN-4760 | ACE2 inhibitor | 37 nM (16 ng/mL) | Millipore Sigma | 530616001 |
| Camostat mesylate | TMPRSS2 inhibitor | 50 µM (24 µg/mL) | Millipore Sigma | SML0057-10MG |
| Cinanserin hydrochloride | 3CL proteases inhibitor | 40 µM (15 µg/mL) | Tocris | 0460 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Sottas, C.M.; Chen, H.-Y.; Li, Y.; Cui, H.; Villano, J.S.; Mankowski, J.L.; Cannon, P.M.; Papadopoulos, V. SARS-CoV-2 Enters Human Leydig Cells and Affects Testosterone Production In Vitro. Cells 2023, 12, 1198. https://doi.org/10.3390/cells12081198
Li L, Sottas CM, Chen H-Y, Li Y, Cui H, Villano JS, Mankowski JL, Cannon PM, Papadopoulos V. SARS-CoV-2 Enters Human Leydig Cells and Affects Testosterone Production In Vitro. Cells. 2023; 12(8):1198. https://doi.org/10.3390/cells12081198
Chicago/Turabian StyleLi, Lu, Chantal M. Sottas, Hsu-Yu Chen, Yuchang Li, Haoyi Cui, Jason S. Villano, Joseph L. Mankowski, Paula M. Cannon, and Vassilios Papadopoulos. 2023. "SARS-CoV-2 Enters Human Leydig Cells and Affects Testosterone Production In Vitro" Cells 12, no. 8: 1198. https://doi.org/10.3390/cells12081198






