Progerinin, an Inhibitor of Progerin, Alleviates Cardiac Abnormalities in a Model Mouse of Hutchinson–Gilford Progeria Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Progerinin (SLC-D011) Synthesis and Charaterization Data
2.3. Chemical Pharmacokinetic (PK) Analysis
2.4. Preparation of Feed Pellets
2.5. Progerinin Treatment
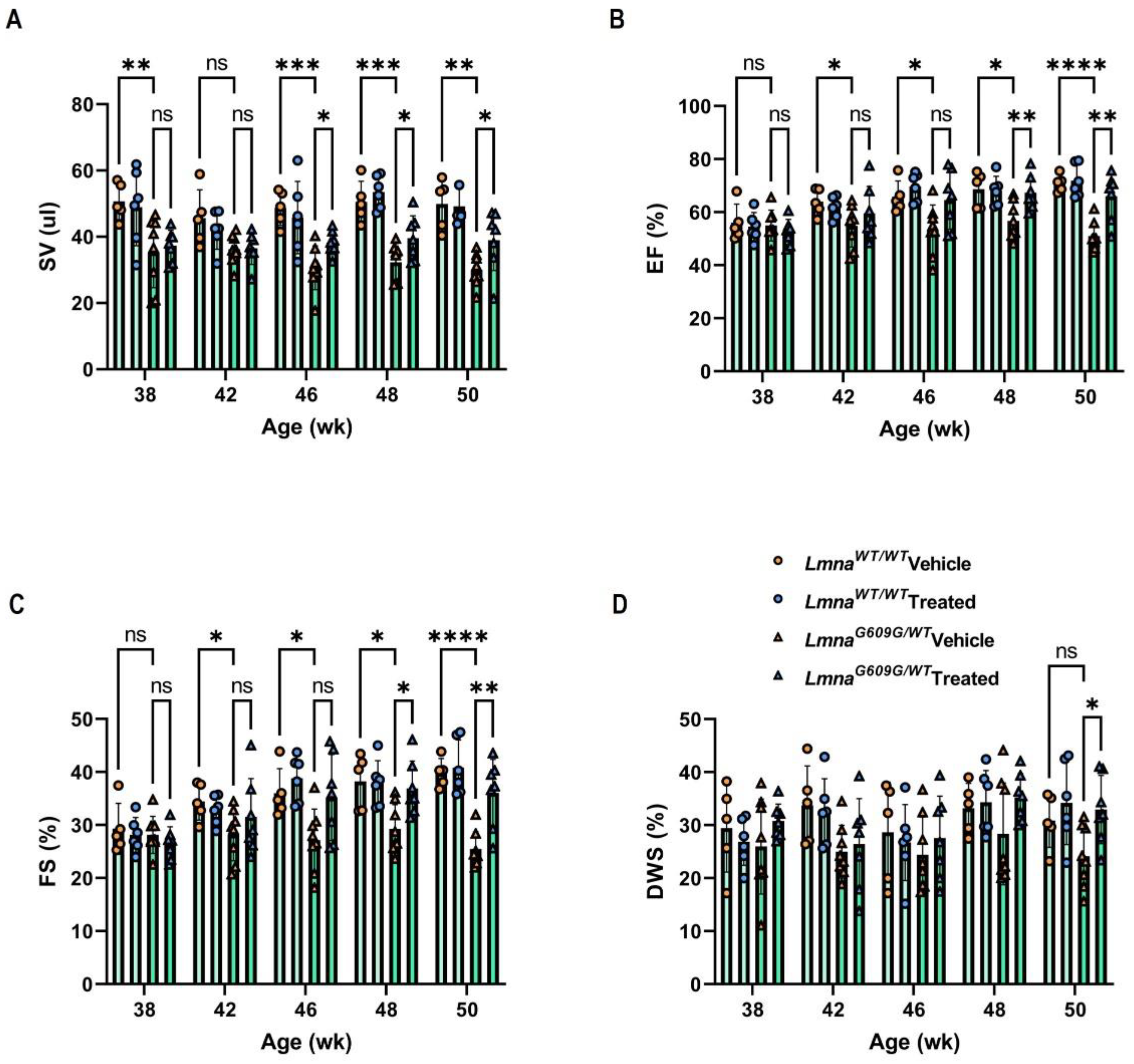
2.6. Echocardiography and Cardiac Function Analysis
2.7. Western Blot for Tissue Samples
2.8. Histology Analysis
2.9. Mouse Cytokine Array
2.10. Statistical Analysis
3. Results
3.1. Progerinin Improves Cardiac Dysfunction in LmnaG609G/WT Mice
3.2. Progerinin Reduces Histological Aortic Defects in LmnaG609G/WT Mice
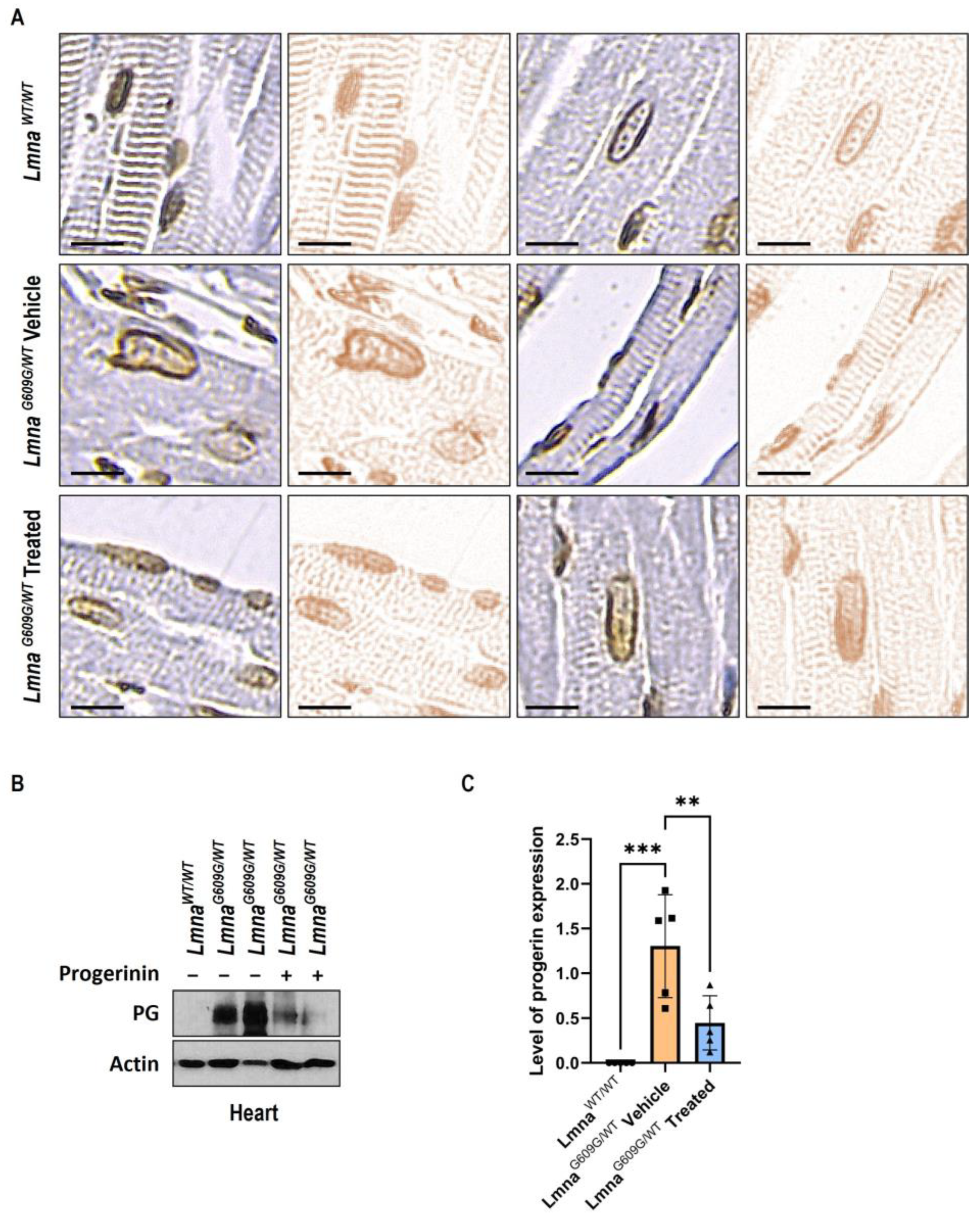
3.3. Progerinin Reduces Nuclear Deformation and Progerin Expression in Heart Tissue of LmnaG609G/WT Mice
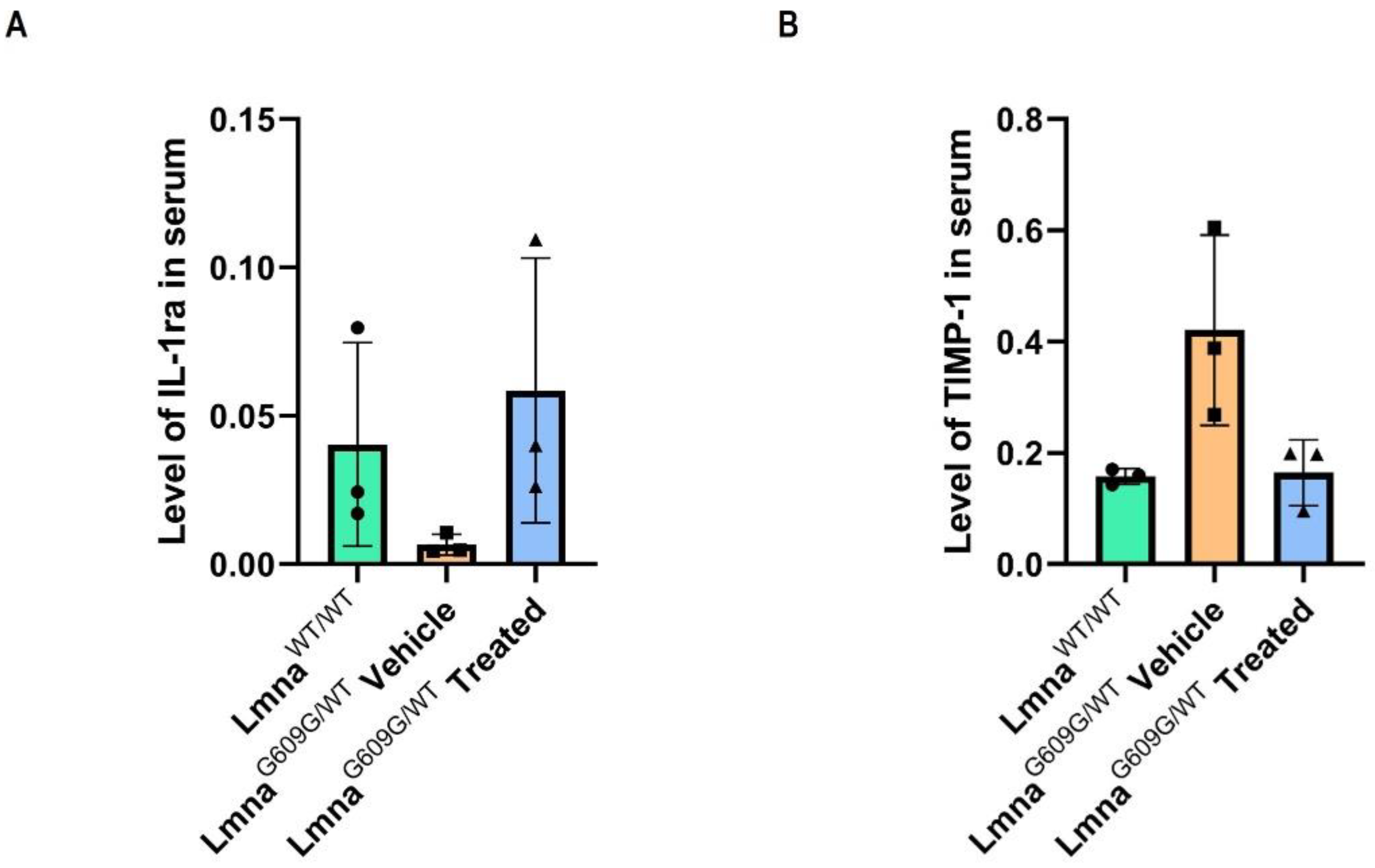
3.4. Progerinin Reverses the Levels of Cytokines in Serum of LmnaG609G/WT Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalo, S.; Kreienkamp, R.; Askjaer, P. Hutchinson-Gilford Progeria Syndrome: A premature aging disease caused by LMNA gene mutations. Ageing Res. Res. Rev. 2017, 33, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Lamis, A.; Siddiqui, S.W.; Ashok, T.; Patni, N.; Fatima, M.; Aneef, A.N. Hutchinson-Gilford Progeria Syndrome: A Literature Review. Cureus 2022, 14, e28629. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.B.; Rothman, F.G.; López-Otín, C.; Misteli, T. Progeria: A paradigm for translational medicine. Cell 2014, 156, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Ikram, S.; Bibi, N.; Mir, A. Hutchinson–Gilford progeria syndrome: A premature aging disease. Mol. Neurobiol. 2018, 55, 4417–4427. [Google Scholar] [CrossRef] [PubMed]
- Hennekam, R.C. Hutchinson–Gilford progeria syndrome: Review of the phenotype. Am. J. Med. Genet. Part A 2006, 140, 2603–2624. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Yoon, M.; Park, B. Laminopathies; Mutations on single gene and various human genetic diseases. BMB Rep. 2018, 51, 327. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ferdous, K.S.; Ahmed, M.; Islam, M.T.; Khan, M.; Perveen, A.; Ashraf, G.M.; Uddin, M. Hutchinson-Gilford progeria syndrome: An overview of the molecular mechanism, pathophysiology and therapeutic approach. Curr. Gene Ther. 2021, 21, 216–229. [Google Scholar]
- Osorio, F.G.; Navarro, C.L.; Cadiñanos, J.; López-Mejía, I.C.; Quirós, P.M.; Bartoli, C.; Rivera, J.; Tazi, J.; Guzmán, G.; Varela, I. Splicing-directed therapy in a new mouse model of human accelerated aging. Sci. Transl. Med. 2011, 3, 106ra107. [Google Scholar] [CrossRef]
- Zaghini, A.; Sarli, G.; Barboni, C.; Sanapo, M.; Pellegrino, V.; Diana, A.; Linta, N.; Rambaldi, J.; D’Apice, M.R.; Murdocca, M. Long term breeding of the Lmna G609G progeric mouse: Characterization of homozygous and heterozygous models. Exp. Gerontol. 2020, 130, 110784. [Google Scholar] [CrossRef]
- Lee, S.; Jung, Y.; Yoon, M.; Kang, S.; Oh, A.; Lee, J.; Jun, S.; Woo, T.; Chun, H.; Kim, S.K. Interruption of progerin–lamin A/C binding ameliorates Hutchinson-Gilford progeria syndrome phenotype. J. Clin. Invest 2016, 126, 3879–3893. [Google Scholar] [CrossRef]
- Kang, S.; Yoon, M.; Ahn, J.; Kim, J.; Kim, S.Y.; Kang, S.Y.; Joo, J.; Park, S.; Cho, J.; Woo, T. Progerinin, an optimized progerin-lamin A binding inhibitor, ameliorates premature senescence phenotypes of Hutchinson-Gilford progeria syndrome. Commun. Biol. 2021, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, P.; Misteli, T. Lamin A-dependent nuclear defects in human aging. Science 2006, 312, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Hah, J.; Kim, D. Deciphering nuclear mechanobiology in laminopathy. Cells 2019, 8, 231. [Google Scholar] [CrossRef]
- Bujak, M.; Frangogiannis, N.G. The role of IL-1 in the pathogenesis of heart disease. Arch Immunol. Ther. Exp. 2009, 57, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Pfeiler, S.; Winkels, H.; Kelm, M.; Gerdes, N. IL-1 family cytokines in cardiovascular disease. Cytokine 2019, 122, 154215. [Google Scholar] [CrossRef]
- Böni-Schnetzler, M.; Méreau, H.; Rachid, L.; Wiedemann, S.J.; Schulze, F.; Trimigliozzi, K.; Meier, D.T.; Donath, M.Y. IL-1beta promotes the age-associated decline of beta cell function. Iscience 2021, 24, 103250. [Google Scholar] [CrossRef]
- Sundström, J.; Evans, J.C.; Benjamin, E.J.; Levy, D.; Larson, M.G.; Sawyer, D.B.; Siwik, D.A.; Colucci, W.S.; Wilson, P.W.; Vasan, R.S. Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: The Framingham heart study. Eur. Heart J. 2004, 25, 1509–1516. [Google Scholar] [CrossRef]
- Lindsay, M.M.; Maxwell, P.; Dunn, F.G. TIMP-1: A marker of left ventricular diastolic dysfunction and fibrosis in hypertension. Hypertension 2002, 40, 136–141. [Google Scholar] [CrossRef]
- Xu, S.; Jin, Z. Hutchinson–Gilford progeria syndrome: Cardiovascular pathologies and potential therapies. Trends Biochem. Sci. 2019, 44, 561–564. [Google Scholar] [CrossRef]
- Hamczyk, M.R.; Villa-Bellosta, R.; Gonzalo, P.; Andrés-Manzano, M.J.; Nogales, P.; Bentzon, J.F.; López-Otín, C.; Andrés, V. Vascular smooth muscle–specific progerin expression accelerates atherosclerosis and death in a mouse model of Hutchinson-Gilford progeria syndrome. Circulation 2018, 138, 266–282. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.; Smoot, L.B.; Wake, N.; Kieran, M.W.; Kleinman, M.E.; Miller, D.T.; Schwartzman, A.; Giobbie-Hurder, A.; Neuberg, D.; Gordon, L.B. Mechanisms of premature vascular aging in children with Hutchinson-Gilford progeria syndrome. Hypertension 2012, 59, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Hamczyk, M.R.; del Campo, L.; Andrés, V. Aging in the cardiovascular system: Lessons from Hutchinson-Gilford progeria syndrome. Annu. Rev. Physiol. 2018, 80, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Hamczyk, M.R.; Nevado, R.M. Vascular smooth muscle cell aging: Insights from Hutchinson-Gilford progeria syndrome. Clínica E Investig. En Arterioscler. 2023, 35, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Olive, M.; Harten, I.; Mitchell, R.; Beers, J.K.; Djabali, K.; Cao, K.; Erdos, M.R.; Blair, C.; Funke, B.; Smoot, L. Cardiovascular pathology in Hutchinson-Gilford progeria: Correlation with the vascular pathology of aging. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Gordon, L.B.; Kleinman, M.E.; Gurary, E.B.; Massaro, J.; D’Agostino, R.; Kieran, M.W.; Gerhard-Herman, M.; Smoot, L. Cardiac abnormalities in patients with Hutchinson-Gilford progeria syndrome. JAMA Cardiol. 2018, 3, 326–334. [Google Scholar] [CrossRef]
- Benedicto, I.; Dorado, B.; Andrés, V. Molecular and Cellular Mechanisms Driving Cardiovascular Disease in Hutchinson-Gilford Progeria Syndrome: Lessons Learned from Animal Models. Cells 2021, 10, 1157. [Google Scholar] [CrossRef]
- Harsha, D.W.; Bray, G.A. Weight loss and blood pressure control (Pro). Hypertension 2008, 51, 1420–1425. [Google Scholar] [CrossRef]
- Karason, K.; Wallentin, I.; Larsson, B.; Sjöströmt, L. Effects of obesity and weight loss on cardiac function and valvular performance. Obes. Res. 1998, 6, 422–429. [Google Scholar] [CrossRef]
- Constable, P.; III, W.M.; Sisson, D. Clinical assessment of left ventricular relaxation. J. Vet. Intern. Med. 1999, 13, 5–13. [Google Scholar] [CrossRef]
- Chengode, S. Left ventricular global systolic function assessment by echocardiography. Ann. Card. Anaesth. 2016, 19, S26. [Google Scholar] [CrossRef]
- Wessels, A.; Sedmera, D. Developmental anatomy of the heart: A tale of mice and man. Physiol. Genom. 2003, 15, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, D.; Xanthos, T.; Dontas, I.; Lelovas, P.; Perrea, D. The use of mice and rats as animal models for cardiopulmonary resuscitation research. Lab. Anim. 2008, 42, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Nikolov, A.; Popovski, N. Extracellular matrix in heart disease: Focus on circulating collagen type I and III derived peptides as biomarkers of myocardial fibrosis and their potential in the prognosis of heart failure: A concise review. Metabolites 2022, 12, 297. [Google Scholar] [CrossRef]
- Liu, T.; Song, D.; Dong, J.; Zhu, P.; Liu, J.; Liu, W.; Ma, X.; Zhao, L.; Ling, S. Current understanding of the pathophysiology of myocardial fibrosis and its quantitative assessment in heart failure. Front. Physiol. 2017, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A. Elastic fibers during aging and disease. Ageing Res. Rev. 2021, 66, 101255. [Google Scholar] [CrossRef]
- North, B.J.; Sinclair, D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Riches-Suman, K.; Hussain, A. Identifying and targeting the molecular signature of smooth muscle cells undergoing early vascular ageing. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2022, 1868, 166403. [Google Scholar] [CrossRef]
- Harhouri, K.; Frankel, D.; Bartoli, C.; Roll, P.; De Sandre-Giovannoli, A.; Lévy, N. An overview of treatment strategies for Hutchinson-Gilford Progeria syndrome. Nucleus 2018, 9, 265–276. [Google Scholar] [CrossRef]
- Harhouri, K.; Cau, P.; Casey, F.; Guedenon, K.M.; Doubaj, Y.; Van Maldergem, L.; Mejia-Baltodano, G.; Bartoli, C.; De Sandre-Giovannoli, A.; Lévy, N. MG132 Induces Progerin Clearance and Improves Disease Phenotypes in HGPS-like Patients’ Cells. Cells 2022, 11, 610. [Google Scholar] [CrossRef]
- Macicior, J.; Marcos-Ramiro, B.; Ortega-Gutiérrez, S. Small-molecule therapeutic perspectives for the treatment of progeria. Int. J. Mol. Sci. 2021, 22, 7190. [Google Scholar] [CrossRef]
- Mojiri, A.; Walther, B.K.; Jiang, C.; Matrone, G.; Holgate, R.; Xu, Q.; Morales, E.; Wang, G.; Gu, J.; Wang, R. Telomerase therapy reverses vascular senescence and extends lifespan in progeria mice. Eur. Heart J. 2021, 42, 4352–4369. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Brehm, M.; Fassbach, M.; Pelzer, B.; Scheuring, S.; Küry, P.; Strauer, B.E.; Schwartzkopff, B. Increased cardiac mRNA expression of matrix metalloproteinase-1 (MMP–1) and its inhibitor (TIMP–1) in DCM patients. Clin. Res. Cardiol. 2006, 95, 261–269. [Google Scholar] [CrossRef]
- Takawale, A.; Zhang, P.; Patel, V.B.; Wang, X.; Oudit, G.; Kassiri, Z. Tissue inhibitor of matrix metalloproteinase-1 promotes myocardial fibrosis by mediating CD63–integrin β1 interaction. Hypertension 2017, 69, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Santiago, C.; Quevedo-Abeledo, J.C.; Hernández-Hernández, V.; de Vera-González, A.; Gonzalez-Delgado, A.; González-Gay, M.Á.; Ferraz-Amaro, I. Interleukin 1 receptor antagonist relation to cardiovascular disease risk in patients with rheumatoid arthritis. Sci. Rep. 2022, 12, 13698. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; De Las Heras Gala, T.; Carstensen-Kirberg, M.; Huth, C.; Zierer, A.; Wahl, S.; Sudduth-Klinger, J.; Kuulasmaa, K.; Peretz, D.; Ligthart, S. Circulating levels of interleukin 1-receptor antagonist and risk of cardiovascular disease: Meta-analysis of six population-based cohorts. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1222–1227. [Google Scholar] [CrossRef]
- Herder, C.; Donath, M.Y. Interleukin-1 receptor antagonist: Friend or foe to the heart? Lancet Diabetes Endocrinol. 2015, 3, 228–229. [Google Scholar] [CrossRef]
- Chen, X.; Yao, H.; Kashif, M.; Revêchon, G.; Eriksson, M.; Hu, J.; Wang, T.; Liu, Y.; Tüksammel, E.; Strömblad, S. A small-molecule ICMT inhibitor delays senescence of Hutchinson-Gilford progeria syndrome cells. Elife 2021, 10, e63284. [Google Scholar] [CrossRef]
- Koblan, L.W.; Erdos, M.R.; Wilson, C.; Cabral, W.A.; Levy, J.M.; Xiong, Z.; Tavarez, U.L.; Davison, L.M.; Gete, Y.G.; Mao, X. In vivo base editing rescues Hutchinson–Gilford progeria syndrome in mice. Nature 2021, 589, 608–614. [Google Scholar] [CrossRef]
- Puttaraju, M.; Jackson, M.; Klein, S.; Shilo, A.; Bennett, C.F.; Gordon, L.; Rigo, F.; Misteli, T. Systematic screening identifies therapeutic antisense oligonucleotides for Hutchinson–Gilford progeria syndrome. Nat. Med. 2021, 27, 526–535. [Google Scholar] [CrossRef]
- Marcos-Ramiro, B.; Gil-Ordóñez, A.; Marín-Ramos, N.I.; Ortega-Nogales, F.J.; Balabasquer, M.; Gonzalo, P.; Khiar-Fernández, N.; Rolas, L.; Barkaway, A.; Nourshargh, S. Isoprenylcysteine carboxylmethyltransferase-based therapy for Hutchinson–Gilford progeria syndrome. ACS Cent. Sci. 2021, 7, 1300–1310. [Google Scholar] [CrossRef]
- Murtada, S.; Mikush, N.; Wang, M.; Ren, P.; Kawamura, Y.; Ramachandra, A.B.; Li, D.S.; Braddock, D.T.; Tellides, G.; Gordon, L.B. Lonafarnib improves cardiovascular function and survival in a mouse model of Hutchinson-Gilford Progeria Syndrome. eLife 2023, 12, e82728. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-m.; Seo, S.; Song, E.J.; Kweon, O.; Jo, A.-h.; Park, S.; Woo, T.-G.; Kim, B.-H.; Oh, G.T.; Park, B.-J. Progerinin, an Inhibitor of Progerin, Alleviates Cardiac Abnormalities in a Model Mouse of Hutchinson–Gilford Progeria Syndrome. Cells 2023, 12, 1232. https://doi.org/10.3390/cells12091232
Kang S-m, Seo S, Song EJ, Kweon O, Jo A-h, Park S, Woo T-G, Kim B-H, Oh GT, Park B-J. Progerinin, an Inhibitor of Progerin, Alleviates Cardiac Abnormalities in a Model Mouse of Hutchinson–Gilford Progeria Syndrome. Cells. 2023; 12(9):1232. https://doi.org/10.3390/cells12091232
Chicago/Turabian StyleKang, So-mi, Seungwoon Seo, Eun Ju Song, Okhee Kweon, Ah-hyeon Jo, Soyoung Park, Tae-Gyun Woo, Bae-Hoon Kim, Goo Taeg Oh, and Bum-Joon Park. 2023. "Progerinin, an Inhibitor of Progerin, Alleviates Cardiac Abnormalities in a Model Mouse of Hutchinson–Gilford Progeria Syndrome" Cells 12, no. 9: 1232. https://doi.org/10.3390/cells12091232




