Glycyrrhizic Acid Prevents Paclitaxel-Induced Neuropathy via Inhibition of OATP-Mediated Neuronal Uptake
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Experiments
2.1.1. Cell Lines
2.1.2. Paclitaxel Accumulation
2.1.3. Assessment of Different Uptake Mechanisms
2.1.4. Overexpression of Drug Transporters
2.1.5. Knock-Out of Drug Transporters
2.2. MTT Assay
2.3. RT-qPCR
2.4. LC-MS/MS
2.5. Isolation and Culture of Murine Primary Sensory Neurons
2.6. Immunofluorescence and Microscopy
2.6.1. Immunofluorescence Staining of Cells
2.6.2. Quantitative Microscopy
2.7. Animals
2.7.1. Drug Administration
2.7.2. Nerve Conduction Studies
2.8. Statistical Analysis
3. Results
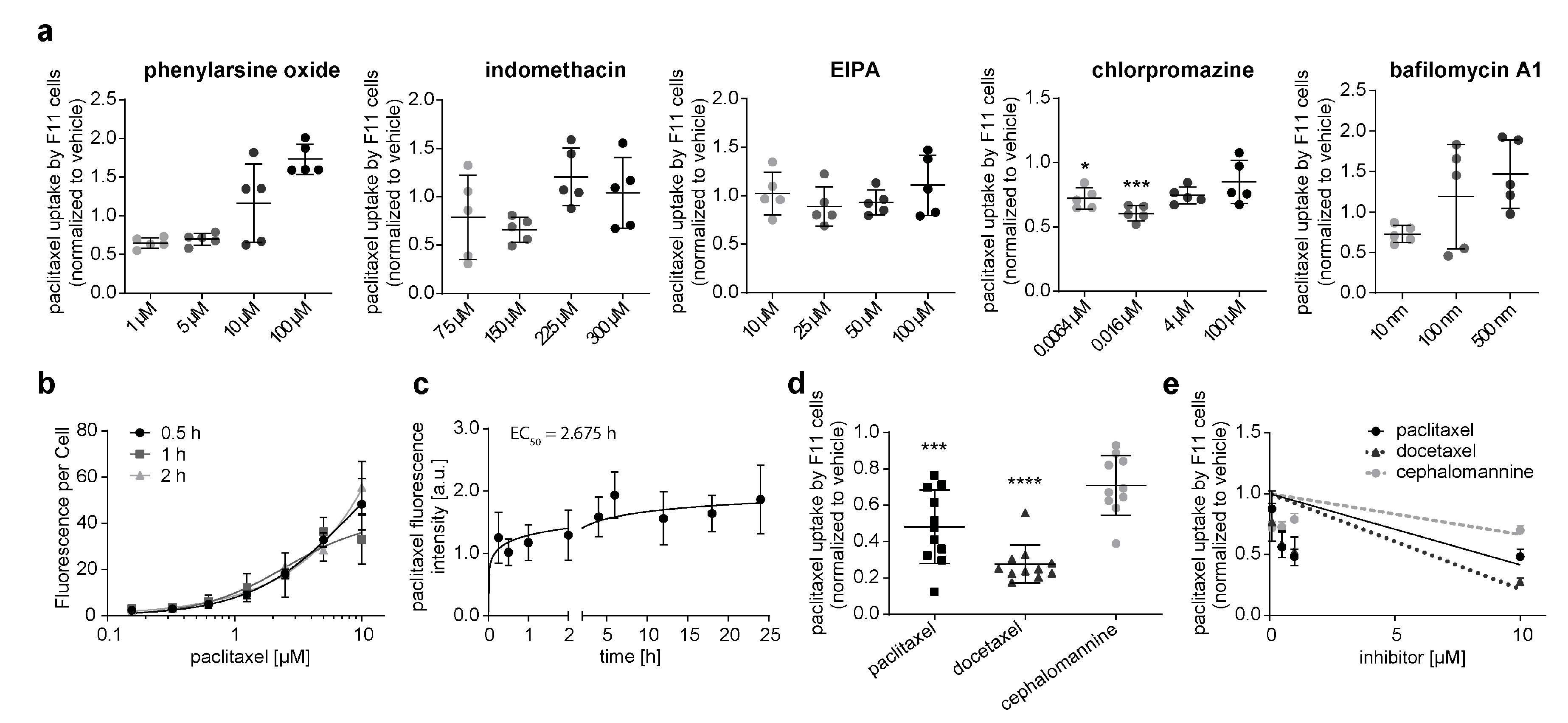
3.1. Neuronal Cell Line Rapidly Takes up Paclitaxel via an Active Transport Mechanism
3.2. Paclitaxel Is Actively Transported by OATP1A1, OATP1B2, and OATP2A1 in a Neuronal Cell Line
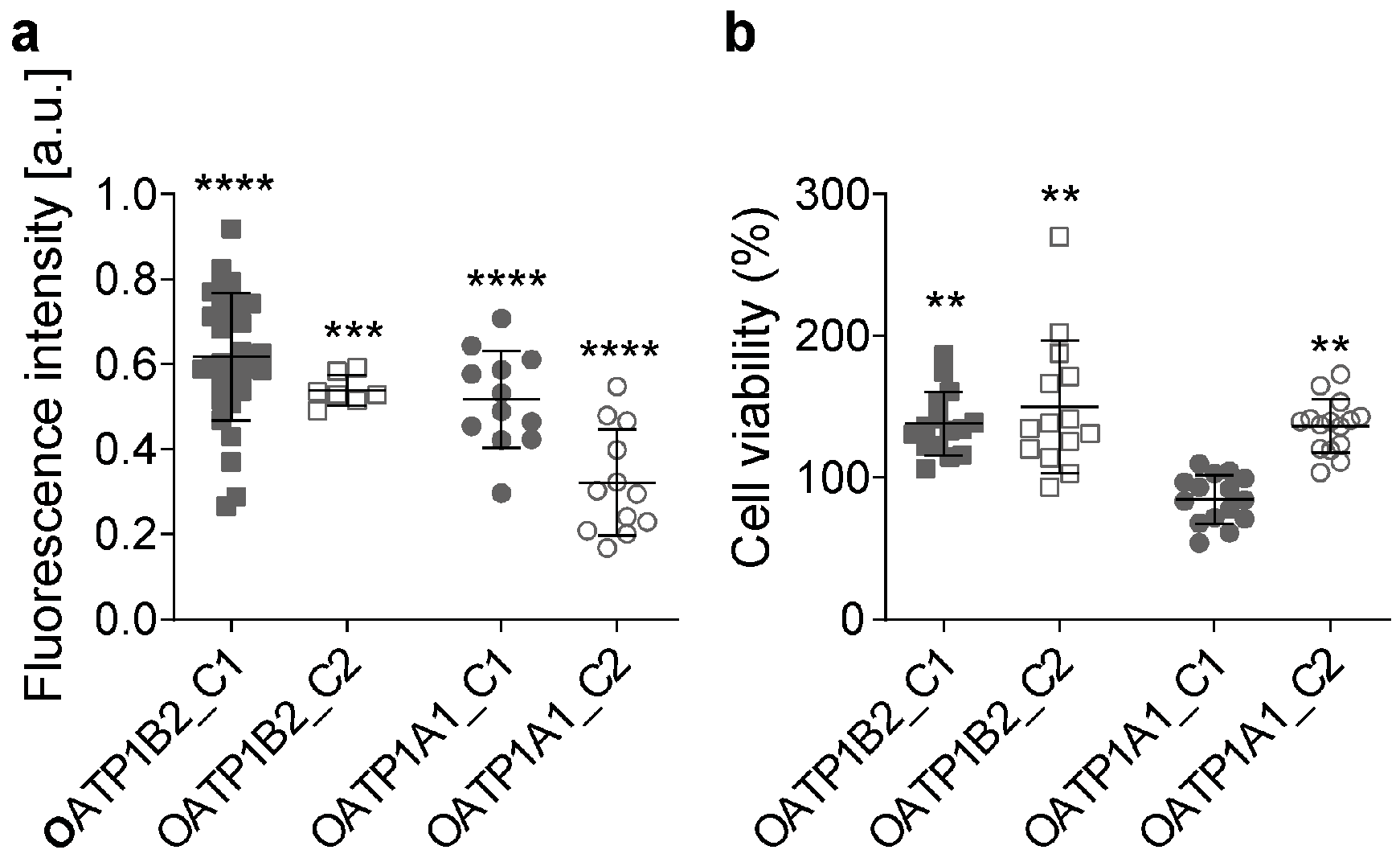
3.3. Overexpression of OATPs Increases Paclitaxel Uptake
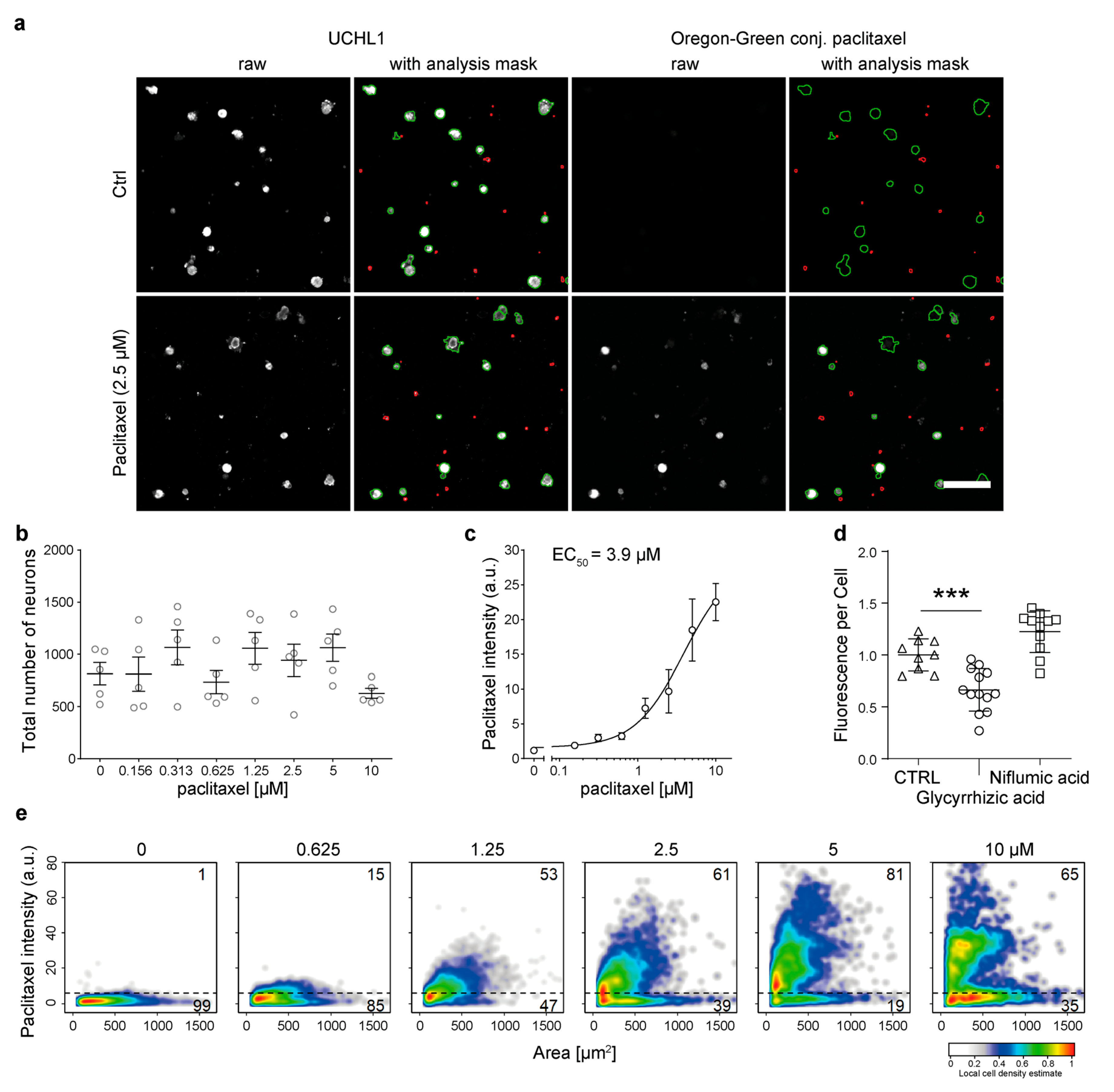
3.4. Primary Murine DRG Neurons Actively Take up Paclitaxel
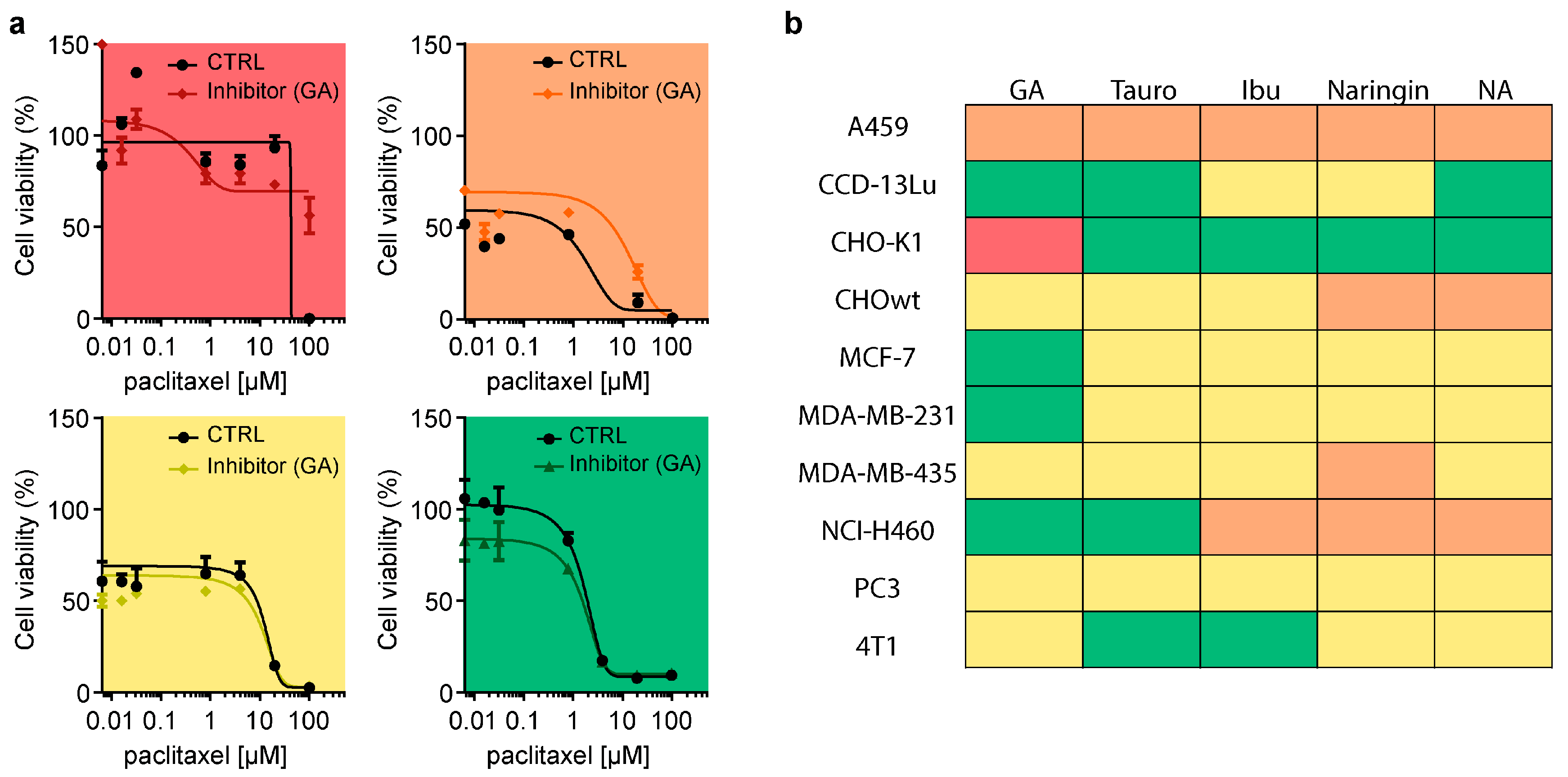
3.5. Glycyrrhizic Acid Inhibits OATP1A1- and OATP1B2-Mediated Paclitaxel Transport
3.6. OATP Inhibition Protects against Paclitaxel-Induced Neurotoxicity In Vitro
3.7. Inhibition of OATP Does Not Attenuate Paclitaxel Cancer Cell Toxicity
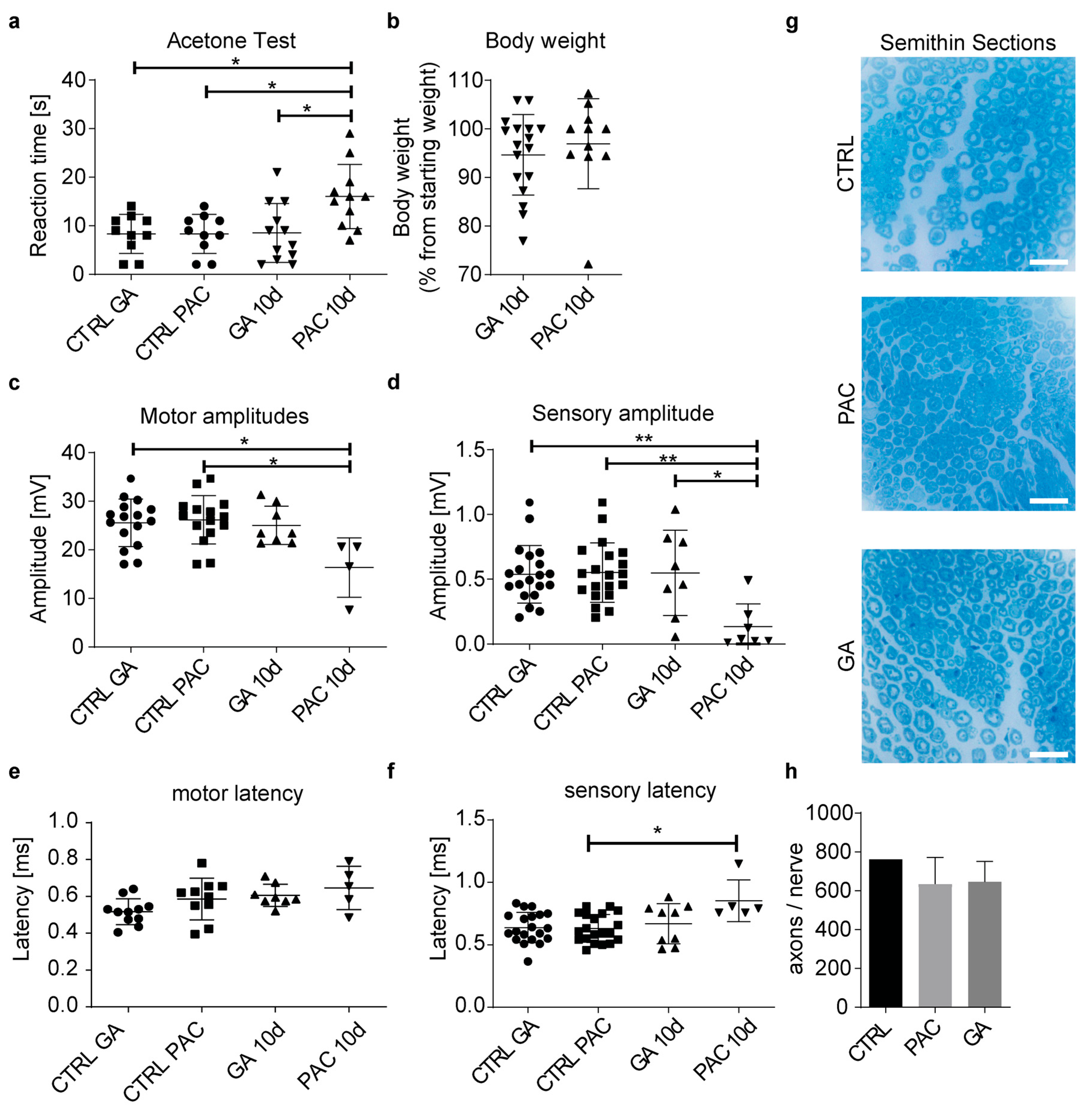
3.8. Glycyrrhizic Acid Prevents Paclitaxel Neurotoxicity In Vivo
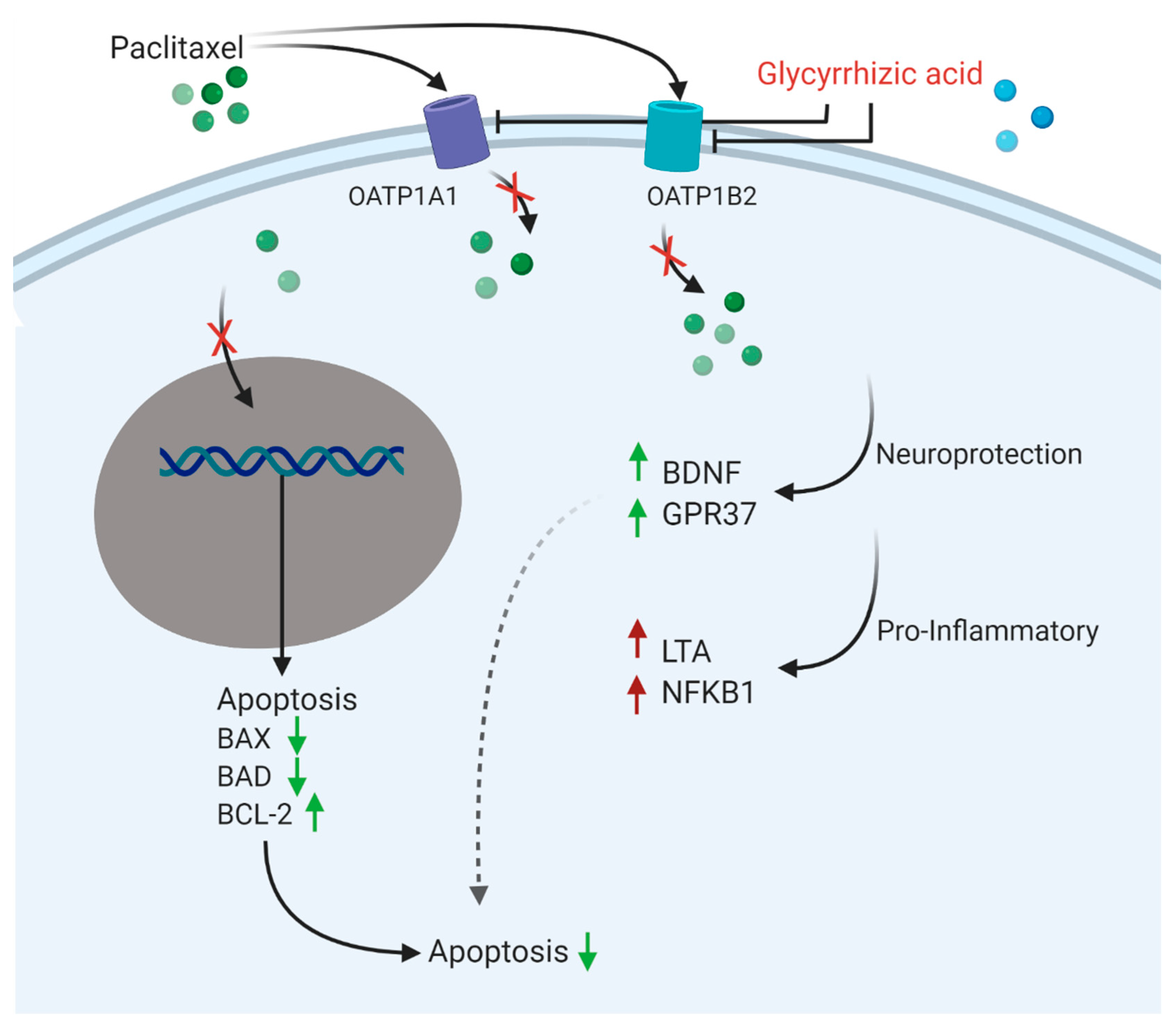
4. Discussion
- Inhibition of endocytosis, autophagy, macropinocytosis, and clathrin- or caveolae-mediated transport did not attenuate paclitaxel translocation into neurons. An exception was increased paclitaxel uptake at high inhibitor concentrations, which was likely caused by increased inhibitor cytotoxicity at escalating concentrations. If cells undergo apoptosis and become porous, this could increase paclitaxel influx or the accumulation of fluorescence in the fluorescent paclitaxel that binds to cell structures.
- Our kinetic experiments demonstrate that peak paclitaxel concentrations in F11 were measured not before 5 h after incubation, which was much longer than one would expect in the case of uptake into the sensory neuron via diffusion. Diffusion is a highly efficient process, and equilibrium is reached after seconds or minutes.
- Pre-exposure of F11 cells with paclitaxel and its structurally closely related derivative docetaxel prevented paclitaxel uptake, which strongly indicates a saturable, specific transport mechanism. Cephalomannine, on the other hand, was not able to alter paclitaxel influx, probably because of the different hydroxylation sites in the molecular structure, and hence, its induced metabolic differences [28,29]. Fluorescent paclitaxel uptake is inversely correlated with the concentration of docetaxel and paclitaxel used. This indicates the competitive inhibition of fluorescent paclitaxel uptake. Notably, paclitaxel has been suggested to be a drug-transport inhibitor itself, inhibiting OATP1B2 and OATP2B1 [30,31]. This could further alter experiment outcomes with predispositions to paclitaxel and its derivatives.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, S.B.; Kwok, J.B.; Loy, C.T.; Friedlander, M.L.; Lin, C.S.-Y.; Krishnan, A.V.; Lewis, C.R.; Kiernan, M.C. Paclitaxel-induced neuropathy: Potential association of MAPT and GSK3B genotypes. BMC Cancer 2014, 14, 993. [Google Scholar] [CrossRef] [PubMed]
- Scripture, C.D.; Figg, W.; Sparreboom, A. Peripheral Neuropathy Induced by Paclitaxel: Recent Insights and Future Perspectives. Curr. Neuropharmacol. 2006, 4, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ferlini, C.; Gallo, D.; Scambia, G. New taxanes in development. Expert Opin. Investig. Drugs 2008, 17, 335–347. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Schiff, P.B.; Fant, J.; Horwitz, S.B. Promotion of microtubule assembly in vitro by taxol. Nature 1979, 277, 665–667. [Google Scholar] [CrossRef]
- Peters, C.M.; Jimenez-Andrade, J.M.; Jonas, B.M.; Sevcik, M.A.; Koewler, N.J.; Ghilardi, J.R.; Wong, G.Y.; Mantyh, P.W. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp. Neurol. 2007, 203, 42–54. [Google Scholar] [CrossRef]
- Cavaletti, G.; Cavalletti, E.; Oggioni, N.; Sottani, C.; Minoia, C.; D’Incalci, M.; Zucchetti, M.; Marmiroli, P.; Tredici, G. Distribution of Paclitaxel Within The Nervous System Of The Rat After Repeated Intravenous Administration. J. Peripher. Nerv. Syst. 2000, 6, 61. [Google Scholar] [CrossRef]
- Chaudhry, V.; Rowinsky, E.K.; Rn, S.E.S.; Donehower, R.C.; Cornblath, D.R. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: Clinical and electrophysiological studies. Ann. Neurol. 1994, 35, 304–311. [Google Scholar] [CrossRef]
- Lipton, R.B.; Apfel, S.C.; Dutcher, J.P.; Rosenberg, R.; Kaplan, J.; Berger, A.; Einzig, A.I.; Wiernik, P.; Schaumburg, H.H. Taxol produces a predominantly sensory neuropathy. Neurology 1989, 39, 368. [Google Scholar] [CrossRef]
- Dougherty, P.M.; Cata, J.P.; Cordella, J.V.; Burton, A.; Weng, H.-R. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain 2004, 109, 132–142. [Google Scholar] [CrossRef]
- Sisignano, M.; Angioni, C.; Park, C.-K.; Dos Santos, S.M.; Jordan, H.; Kuzikov, M.; Liu, D.; Zinn, S.; Hohman, S.W.; Schreiber, Y.; et al. Targeting CYP2J to reduce paclitaxel-induced peripheral neuropathic pain. Proc. Natl. Acad. Sci. USA 2016, 113, 12544–12549. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.; Lehmann, H.C. Pathomechanisms of Paclitaxel-Induced Peripheral Neuropathy. Toxics 2021, 9, 229. [Google Scholar] [CrossRef]
- Bobylev, I.; Joshi, A.R.; Barham, M.; Ritter, C.; Neiss, W.F.; Höke, A.; Lehmann, H.C. Paclitaxel inhibits mRNA transport in axons. Neurobiol. Dis. 2015, 82, 321–331. [Google Scholar] [CrossRef]
- Areti, A.; Yerra, V.G.; Naidu, V.; Kumar, A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014, 2, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, Z.; Chen, X. Mechanisms underlying paclitaxel-induced neuropathic pain: Channels, inflammation and immune regulations. Eur. J. Pharmacol. 2022, 933, 175288. [Google Scholar] [CrossRef]
- Nishida, K.; Kuchiiwa, S.; Oiso, S.; Futagawa, T.; Masuda, S.; Takeda, Y.; Yamada, K. Up-regulation of matrix metalloproteinase-3 in the dorsal root ganglion of rats with paclitaxel-induced neuropathy. Cancer Sci. 2008, 99, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.; Wiesen, M.H.J.; Albert, V.; Bobylev, I.; Joshi, A.R.; Müller, C.; Lehmann, H.C. Impact of drug formulations on kinetics and toxicity in a preclinical model of paclitaxel-induced neuropathy. J. Peripher. Nerv. Syst. 2021, 26, 216–226. [Google Scholar] [CrossRef]
- Staff, N.P.; Fehrenbacher, J.C.; Caillaud, M.; Damaj, M.I.; Segal, R.A.; Rieger, S. Pathogenesis of paclitaxel-induced peripheral neuropathy: A current review of in vitro and in vivo findings using rodent and human model systems. Exp. Neurol. 2020, 324, 113121. [Google Scholar] [CrossRef]
- Smith, N.F.; Acharya, M.R.; Desai, N.; Figg, I.W.D.; Sparreboom, A. Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol. Ther. 2005, 4, 815–818. [Google Scholar] [CrossRef]
- Smith, N.F.; Marsh, S.; Scott-Horton, T.J.; Hamada, A.; Mielke, S.; Mross, K.; Figg, W.D.; Verweij, J.; McLeod, H.L.; Sparreboom, A. Variants in the SLCO1B3 Gene: Interethnic Distribution and Association with Paclitaxel Pharmacokinetics. Clin. Pharmacol. Ther. 2007, 81, 76–82. [Google Scholar] [CrossRef]
- Nieuweboer, A.J.; Hu, S.; Gui, C.; Hagenbuch, B.; Moghaddam-Helmantel, I.M.G.; Gibson, A.A.; de Bruijn, P.; Mathijssen, R.H.; Sparreboom, A. Influence of Drug Formulation on OATP1B-Mediated Transport of Paclitaxel. Cancer Res 2014, 74, 3137–3145. [Google Scholar] [CrossRef]
- Leblanc, A.F.; Sprowl, J.; Alberti, P.; Chiorazzi, A.; Arnold, W.D.; Gibson, A.A.; Hong, K.W.; Pioso, M.S.; Chen, M.; Huang, K.M.; et al. OATP1B2 deficiency protects against paclitaxel-induced neurotoxicity. J. Clin. Investig. 2018, 128, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Wlcek, K.; Taferner, B.; Hering, S.; Stieger, B.; Tong, D.; Zeillinger, R.; Thalhammer, T.; Jäger, W. Expression of organic anion-transporting polypeptides 1B1 and 1B3 in ovarian cancer cells: Relevance for paclitaxel transport. Biomed. Pharmacother. 2011, 65, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Unno, M.; Onogawa, T.; Tokui, T.; Kondo, T.N.; Nakagomi, R.; Adachi, H.; Fujiwara, K.; Okabe, M.; Suzuki, T.; et al. LST-2, A human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology 2001, 120, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.L.; Kwon, E.M.; Ostrander, E.A.; Montgomery, R.B.; Lin, D.W.; Vessella, R.; Stanford, J.L.; Mostaghel, E.A. Expression of SLCO Transport Genes in Castration-Resistant Prostate Cancer and Impact of Genetic Variation in SLCO1B3 and SLCO2B1 on Prostate Cancer Outcomes. Cancer Epidemiology Biomarkers Prev. 2011, 20, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Isensee, J.; Kaufholz, M.; Knape, M.J.; Hasenauer, J.; Hammerich, H.; Gonczarowska-Jorge, H.; Zahedi, R.P.; Schwede, F.; Herberg, F.W.; Hucho, T. PKA-RII subunit phosphorylation precedes activation by cAMP and regulates activity termination. J. Cell Biol. 2018, 217, 2167–2184. [Google Scholar] [CrossRef] [PubMed]
- Isensee, J.; Krahé, L.; Moeller, K.; Pereira, V.; Sexton, J.E.; Sun, X.; Emery, E.; Wood, J.N.; Hucho, T. Synergistic regulation of serotonin and opioid signaling contributes to pain insensitivity in Na v 1.7 knockout mice. Sci. Signal. 2017, 10, eaah4874. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Ge, G.-B.; Liu, Y.; Wang, L.-M.; Liu, X.-B.; Zhang, Y.-Y.; Li, W.; He, Y.-Q.; Wang, Z.-T.; Sun, J.; et al. Taxane’s Substituents at C3′ Affect Its Regioselective Metabolism: Different in Vitro Metabolism of Cephalomannine and Paclitaxel. Drug Metab. Dispos. 2007, 36, 418–426. [Google Scholar] [CrossRef]
- Jelínek, M.; Balušíková, K.; Daniel, P.; Němcová-Fürstová, V.; Kirubakaran, P.; Jaček, M.; Wei, L.; Wang, X.; Vondrášek, J.; Ojima, I.; et al. Substituents at the C3′ and C3′N positions are critical for taxanes to overcome acquired resistance of cancer cells to paclitaxel. Toxicol. Appl. Pharmacol. 2018, 347, 79–91. [Google Scholar] [CrossRef]
- Gui, C.; Miao, Y.; Thompson, L.; Wahlgren, B.; Mock, M.; Stieger, B.; Hagenbuch, B. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur. J. Pharmacol. 2008, 584, 57–65. [Google Scholar] [CrossRef]
- Letschert, K.; Faulstich, H.; Keller, D.; Keppler, D. Molecular Characterization and Inhibition of Amanitin Uptake into Human Hepatocytes. Toxicol. Sci. 2006, 91, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Garrison, D.A.; Talebi, Z.; Eisenmann, E.D.; Sparreboom, A.; Baker, S.D. Role of OATP1B1 and OATP1B3 in Drug-Drug Interactions Mediated by Tyrosine Kinase Inhibitors. Pharmaceutics 2020, 12, 856. [Google Scholar] [CrossRef]
- van de Steeg, E.; van Esch, A.; Wagenaar, E.; Kenworthy, K.E.; Schinkel, A.H. Influence of Human OATP1B1, OATP1B3, and OATP1A2 on the Pharmacokinetics of Methotrexate and Paclitaxel in Humanized Transgenic Mice. Clin. Cancer Res. 2013, 19, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Bruna, J.; Alberti, P.; Calls-Cobos, A.; Caillaud, M.; Damaj, M.I.; Navarro, X. Methods for in vivo studies in rodents of chemotherapy induced peripheral neuropathy. Exp. Neurol. 2020, 325, 113154. [Google Scholar] [CrossRef] [PubMed]
- Openshaw, H.; Beamon, K.; Synold, T.W.; Longmate, J.; Slatkin, N.E.; Doroshow, J.H.; Forman, S.; Margolin, K.; Morgan, R.; Shibata, S.; et al. Neurophysiological Study of Peripheral Neuropathy after High-Dose Paclitaxel. Clin. Cancer Res. 2004, 10, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Carozzi, V.A.; Canta, A.R.; Oggioni, N.; Sala, B.; Chiorazzi, A.; Meregalli, C.; Bossi, M.; Marmiroli, P.; Cavaletti, G. Neurophysiological and neuropathological characterization of new murine models of chemotherapy-induced chronic peripheral neuropathies. Exp. Neurol. 2010, 226, 301–309. [Google Scholar] [CrossRef]
- Shibata, S. A Drug over the Millennia: Pharmacognosy, Chemistry, and Pharmacology of Licorice. ChemInform 2000, 120, 849–862. [Google Scholar] [CrossRef]
- Fiore, C.; Eisenhut, M.; Ragazzi, E.; Zanchin, G.; Armanini, D. A history of the therapeutic use of liquorice in Europe. J. Ethnopharmacol. 2005, 99, 317–324. [Google Scholar] [CrossRef]
- Yoshida, T.; Abe, K.; Ikeda, T.; Matsushita, T.; Wake, K.; Sato, T.; Sato, T.; Inoue, H. Inhibitory effect of glycyrrhizin on lipopolysaccharide and d-galactosamine-induced mouse liver injury. Eur. J. Pharmacol. 2007, 576, 136–142. [Google Scholar] [CrossRef]
- Ram, A.; Mabalirajan, U.; Das, M.; Bhattacharya, I.; Dinda, A.K.; Gangal, S.V.; Ghosh, B. Glycyrrhizin alleviates experimental allergic asthma in mice. Int. Immunopharmacol. 2006, 6, 1468–1477. [Google Scholar] [CrossRef]
- Hoever, G.; Baltina, L.; Michaelis, M.; Kondratenko, R.; Baltina, L.; Tolstikov, G.A.; Doerr, H.W.; Cinatl, J. Antiviral Activity of Glycyrrhizic Acid Derivatives against SARS−Coronavirus. J. Med. Chem. 2005, 48, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Arase, Y.; Ikeda, K.; Murashima, N.; Chayama, K.; Tsubota, A.; Koida, I.; Suzuki, Y.; Saitoh, S.; Kobayashi, L.; Kumada, H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer 1997, 79, 1494–1500. [Google Scholar] [CrossRef]
- Selyutina, O.; Polyakov, N.E.; Korneev, D.; Zaitsev, B. Influence of glycyrrhizin on permeability and elasticity of cell membrane: Perspectives for drugs delivery. Drug Deliv. 2016, 23, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-H.; Zhang, Q.; Liang, Q.-Y.; Wang, S.-Q.; Zhao, B.-X.; Wang, Y.-T.; Cai, Y.; Li, G.-F. Bioavailability Enhancement of Paclitaxel via a Novel Oral Drug Delivery System: Paclitaxel-Loaded Glycyrrhizic Acid Micelles. Molecules 2015, 20, 4337–4356. [Google Scholar] [CrossRef]
- Shi, L.; Tang, C.; Yin, C. Glycyrrhizin-modified O-carboxymethyl chitosan nanoparticles as drug vehicles targeting hepatocellular carcinoma. Biomaterials 2012, 33, 7594–7604. [Google Scholar] [CrossRef] [PubMed]




| Cell Line | Medium | FBS Concentration |
|---|---|---|
| F11; A549; CHO-K1; CHOwt; PC-3 | DMEM | 10% |
| AR42J | F12-K | 20% |
| 4T1; NCI-H460 | RPMI-1640 | 10% |
| CCD-13Lu; MCF-7 | EMEM | 10% |
| MDA-MB-231; MDA-MB-435 | L15 | 10% |
| Uptake Mechanism | Inhibitory Drug |
|---|---|
| Clathrin-mediated endocytosis | Chlorpromazine |
| Caveolae-mediated endocytosis | Indomethacin |
| Macropinocytosis | Ethyl isopropyl amiloride (EIPA) |
| Autophagy | Bafilomycin A1 |
| Phagocytosis | Phenylarsine oxide |
| Uptake mechanism | Inhibitory drug |
| Inhibitor | Transporter | Solvent |
|---|---|---|
| Ibuprofen | OATP1A1 | DMSO |
| OATP2B1 | ||
| Glycyrrhizic acid | OATP1A1 | H2O |
| OATP1B2 | ||
| Taurocholate | OATP1A4 | H2O |
| OATP1B2 | ||
| Naringin | OATP1A2 | DMSO |
| OATP1A5 | ||
| OATP2B1 | ||
| Niflumic acid | OATP2A1 | DMSO |
| Ibuprofen | OATP1A1 | DMSO |
| OATP2B1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, I.; Isensee, J.; Wiesen, M.H.J.; Imhof, T.; Wassermann, M.K.; Müller, C.; Hucho, T.; Koch, M.; Lehmann, H.C. Glycyrrhizic Acid Prevents Paclitaxel-Induced Neuropathy via Inhibition of OATP-Mediated Neuronal Uptake. Cells 2023, 12, 1249. https://doi.org/10.3390/cells12091249
Klein I, Isensee J, Wiesen MHJ, Imhof T, Wassermann MK, Müller C, Hucho T, Koch M, Lehmann HC. Glycyrrhizic Acid Prevents Paclitaxel-Induced Neuropathy via Inhibition of OATP-Mediated Neuronal Uptake. Cells. 2023; 12(9):1249. https://doi.org/10.3390/cells12091249
Chicago/Turabian StyleKlein, Ines, Jörg Isensee, Martin H. J. Wiesen, Thomas Imhof, Meike K. Wassermann, Carsten Müller, Tim Hucho, Manuel Koch, and Helmar C. Lehmann. 2023. "Glycyrrhizic Acid Prevents Paclitaxel-Induced Neuropathy via Inhibition of OATP-Mediated Neuronal Uptake" Cells 12, no. 9: 1249. https://doi.org/10.3390/cells12091249
APA StyleKlein, I., Isensee, J., Wiesen, M. H. J., Imhof, T., Wassermann, M. K., Müller, C., Hucho, T., Koch, M., & Lehmann, H. C. (2023). Glycyrrhizic Acid Prevents Paclitaxel-Induced Neuropathy via Inhibition of OATP-Mediated Neuronal Uptake. Cells, 12(9), 1249. https://doi.org/10.3390/cells12091249







