Zbtb40 Deficiency Leads to Morphological and Phenotypic Abnormalities of Spermatocytes and Spermatozoa and Causes Male Infertility
Abstract
:1. Introduction
2. Results
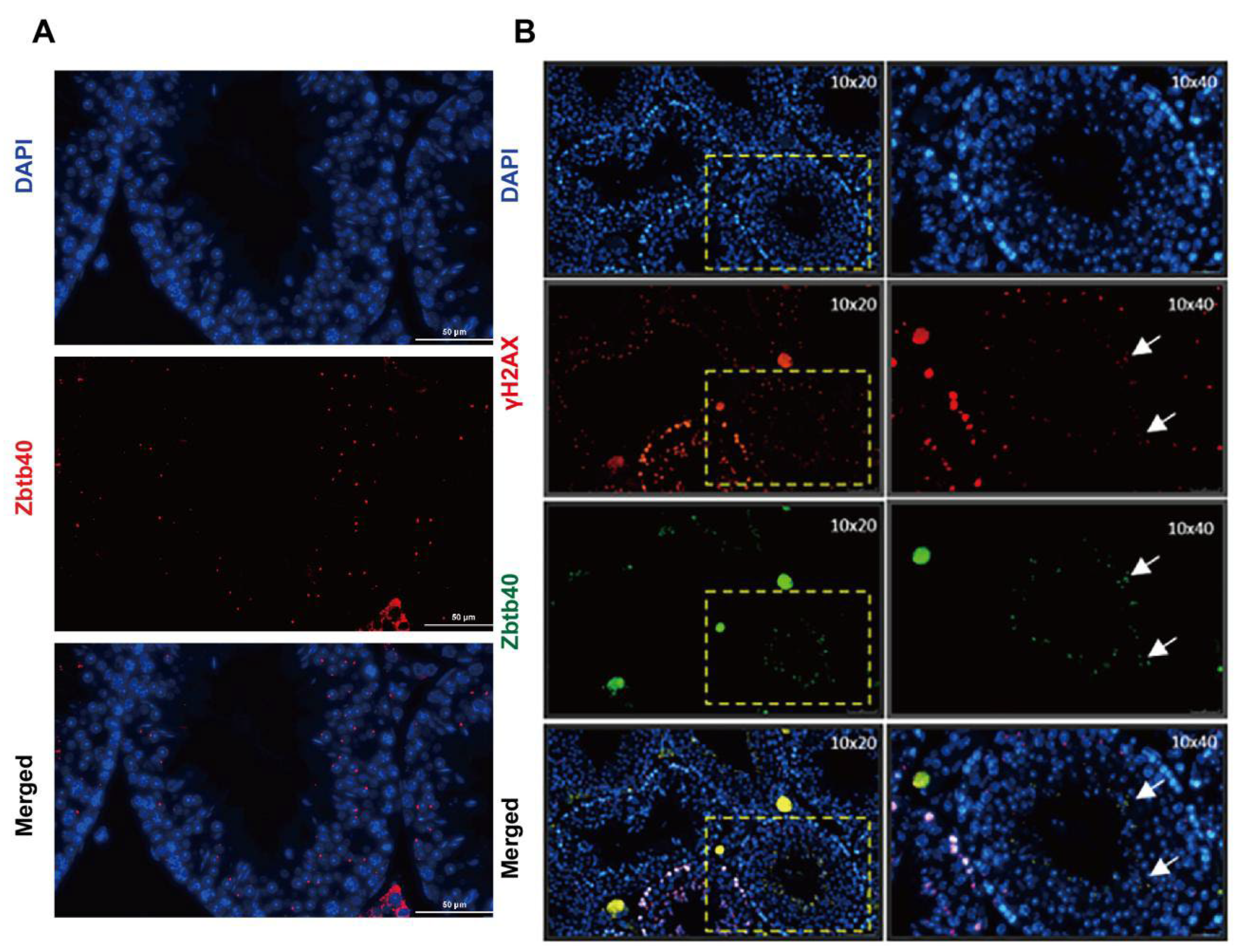
2.1. ZBTB40 Is Expressed in Mouse Spermatocytes
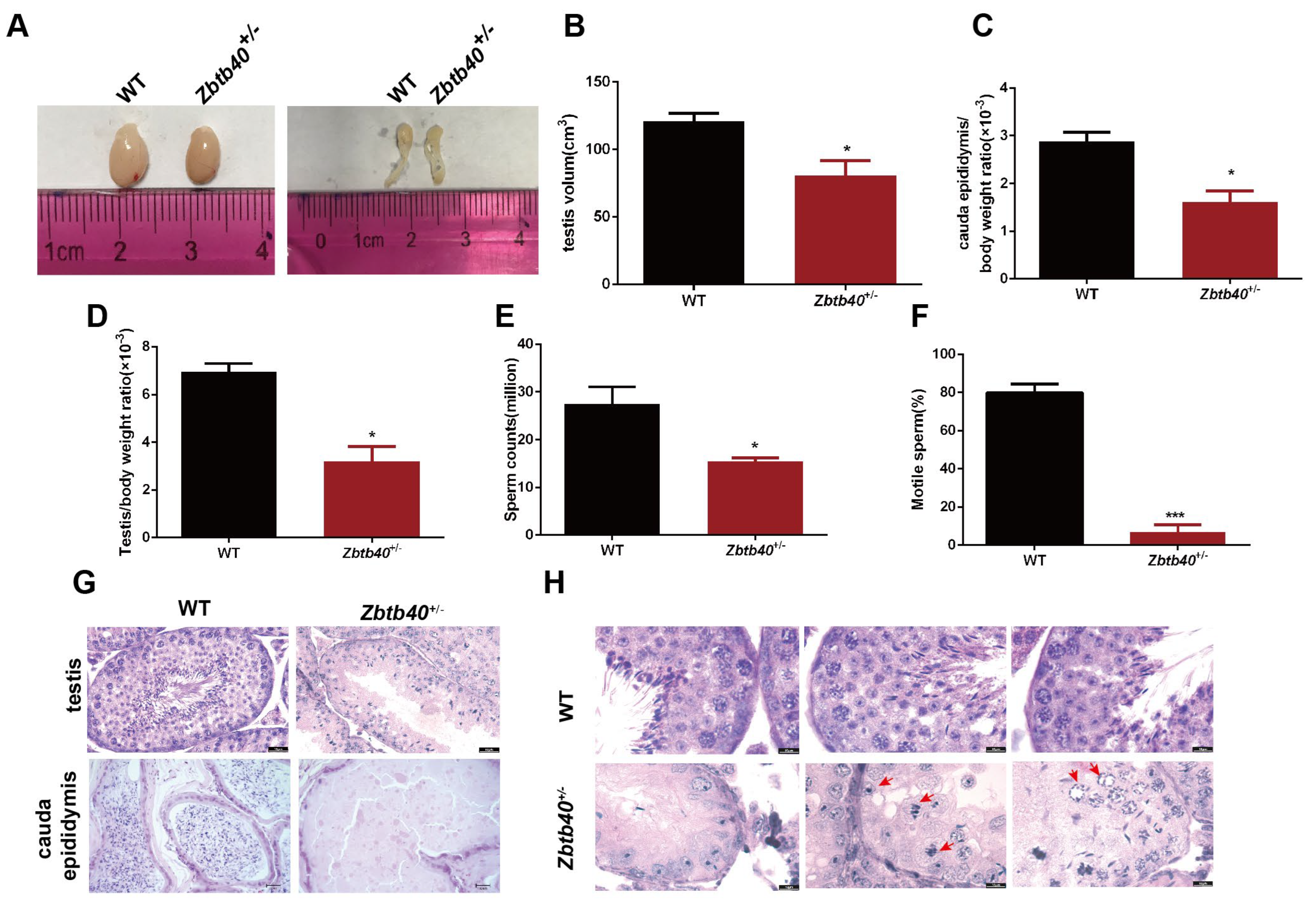
2.2. Zbtb40+/− Male Mice Exhibit Infertility
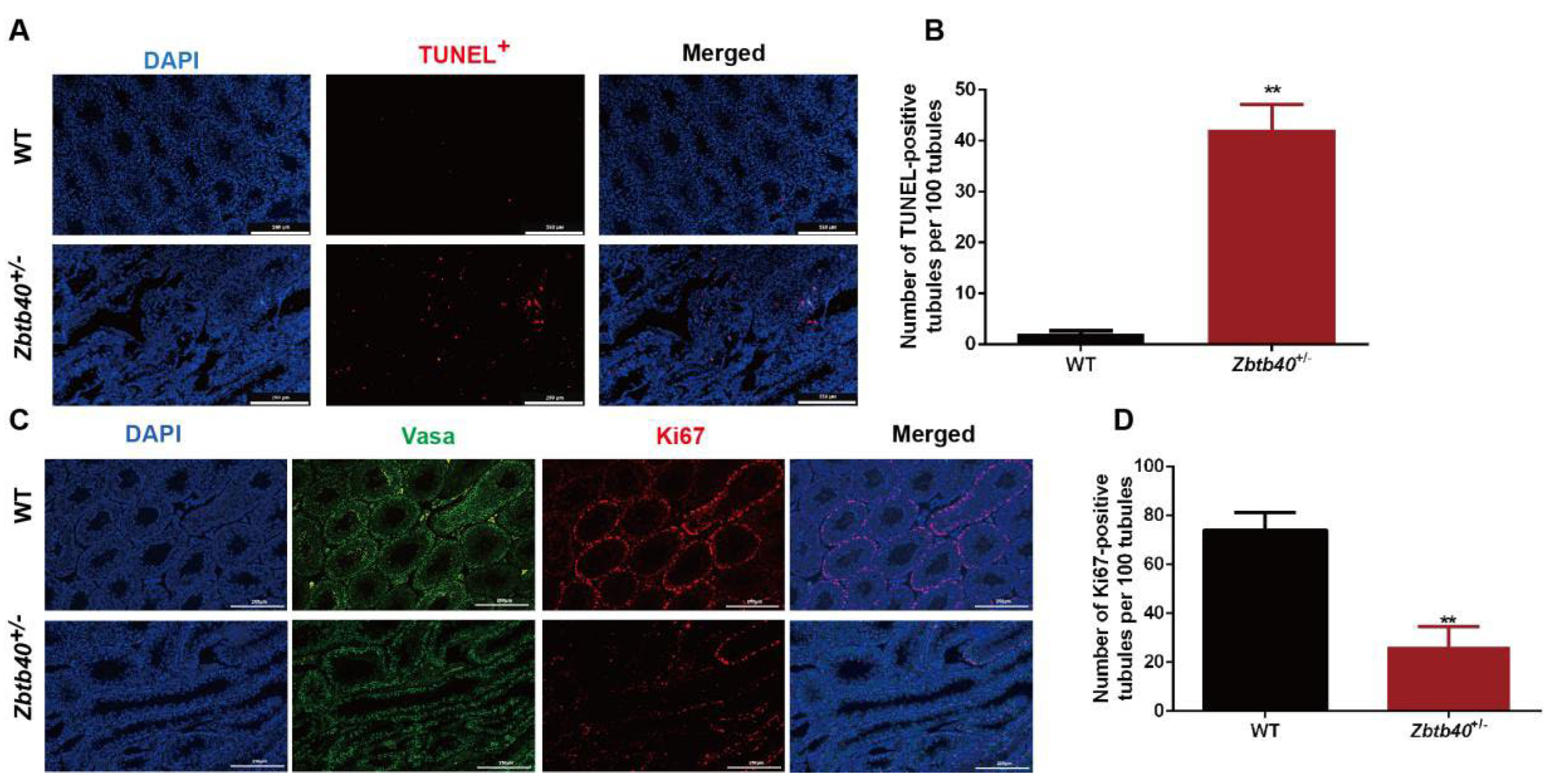
2.3. Zbtb40 KO Causes Apoptosis and Inhibits the Proliferation of Male Germ Cells
2.4. Zbtb40+/− Affects Spermatocyte Recombination and Pairing of the Sex Chromosomes
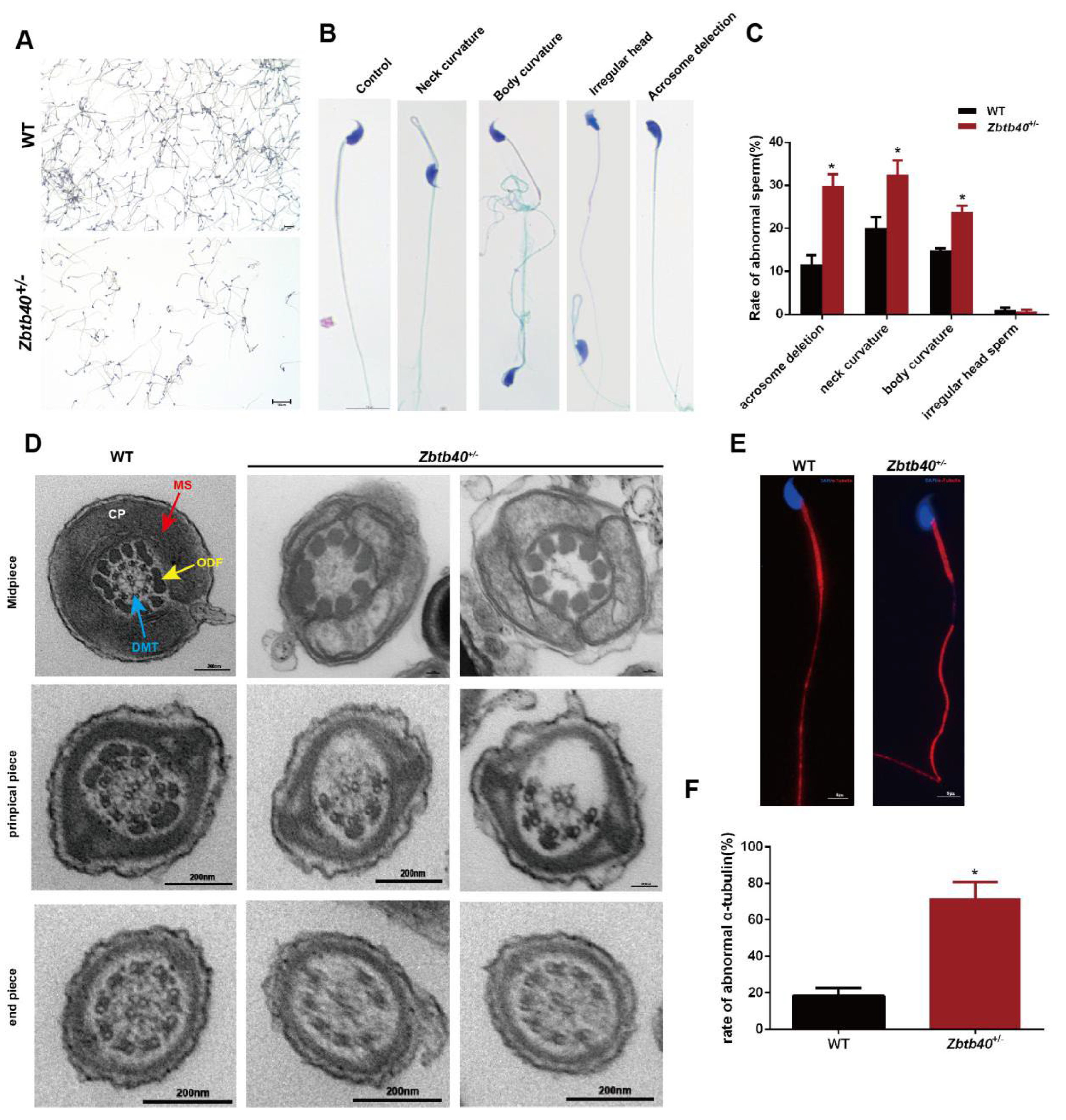
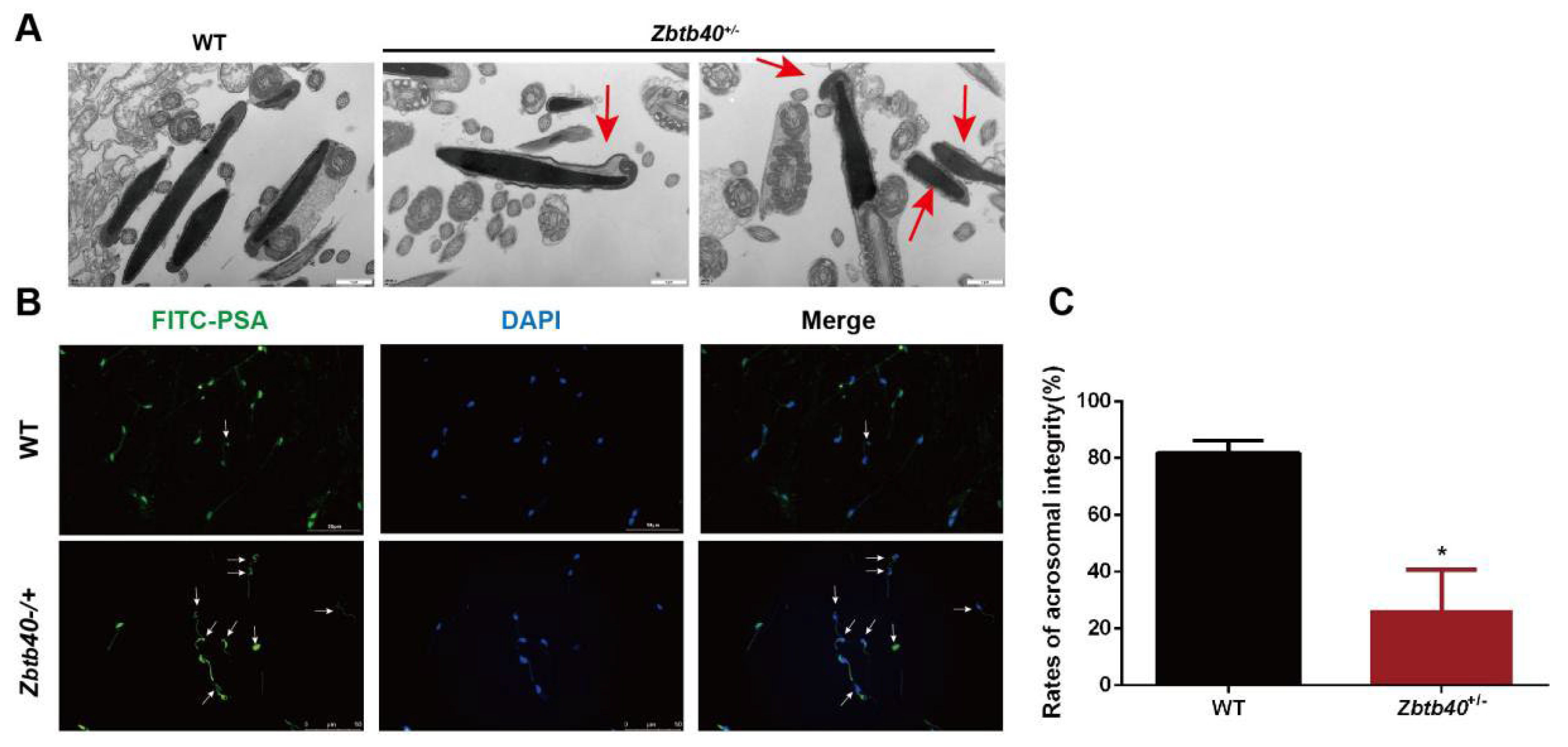
2.5. The Spermatozoa of Zbtb40+/− Male Mice Showed Flagellum Deformities and Abnormal Acrosome Biogenesis
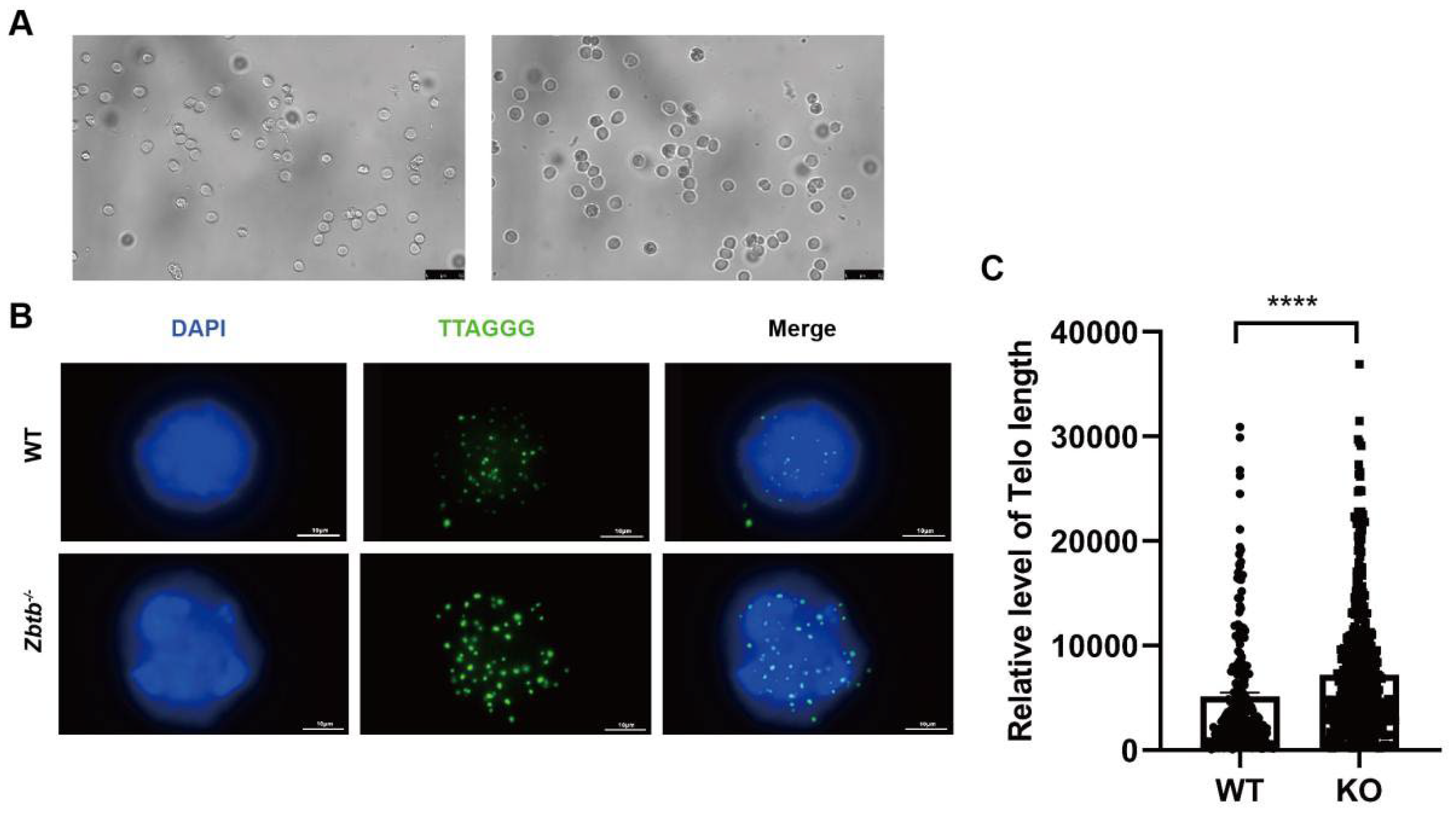
2.6. Zbtb40+/− Mice Have Longer Telomeres in Spermatocytes
2.7. ZBTB 40 Mutation Is Associated with Non-Obstructive Azoospermia (NOA)
3. Discussion
4. Material and Methods
4.1. Zbtb40 Knockout Mice
4.2. Western Blots
4.3. Fluorescence In Situ Hybridization (FISH)
4.4. Hematoxylin and Eosin (H&E) Staining
4.5. Immunohistochemistry
4.6. TUNEL Assay
4.7. Isolation of Mouse Male Germ Cells
4.8. STA-PUT Separation of Mouse Spermatocytes
4.9. Spermatocyte Spreading and Immunofluorescence Staining
4.10. Sperm Counting
4.11. Transmission Electron Microscope (TEM)
4.12. Pap Staining
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Rooij, D.G. Proliferation and differentiation of spermatogonial stem cells. Reproduction 2001, 121, 347–354. [Google Scholar] [CrossRef]
- O’Donnell, L.; Nicholls, P.K.; O’Bryan, M.K.; McLachlan, R.I.; Stanton, P.G. Spermiation: The process of sperm release. Spermatogenesis 2011, 1, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Tu, C.; Cong, J.; Zhang, Q.; He, X.; Zheng, R.; Yang, X.; Gao, Y.; Wu, H.; Lv, M.; Gu, Y.; et al. Bi-allelic mutations of DNAH10 cause primary male infertility with asthenoteratozoospermia in humans and mice. Am. J. Hum. Genet. 2021, 108, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ma, H.; Khan, T.; Ma, A.; Li, T.; Zhang, H.; Gao, J.; Zhou, J.; Li, Y.; Yu, C.; et al. A DNAH17 missense variant causes flagella destabilization and asthenozoospermia. J. Exp. Med. 2020, 217, e20182365. [Google Scholar] [CrossRef]
- Guo, T.; Tu, C.F.; Yang, D.H.; Ding, S.Z.; Lei, C.; Wang, R.C.; Liu, L.; Kang, X.; Shen, X.Q.; Yang, Y.F.; et al. Bi-allelic BRWD1 variants cause male infertility with asthenoteratozoospermia and likely primary ciliary dyskinesia. Hum. Genet. 2021, 140, 761–773. [Google Scholar] [CrossRef]
- Liu, C.; Tu, C.; Wang, L.; Wu, H.; Houston, B.J.; Mastrorosa, F.K.; Zhang, W.; Shen, Y.; Wang, J.; Tian, S.; et al. Deleterious variants in X-linked CFAP47 induce asthenoteratozoospermia and primary male infertility. Am. J. Hum. Genet. 2021, 108, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tian, S.; Nie, H.; Tu, C.; Liu, C.; Li, Y.; Li, D.; Yang, X.; Meng, L.; Hu, T.; et al. CFAP65 is required in the acrosome biogenesis and mitochondrial sheath assembly during spermiogenesis. Hum. Mol. Genet. 2021, 30, 2240–2254. [Google Scholar] [CrossRef]
- Ma, H.; Li, T.; Xie, X.; Jiang, L.; Ye, J.; Gong, C.; Jiang, H.; Fan, S.; Zhang, H.; Shi, B.; et al. RAD51AP2 is required for efficient meiotic recombination between X and Y chromosomes. Sci. Adv. 2022, 8, eabk1789. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, G.; Zhao, Y.; Gao, X.M.; Wang, J. Regulation of the Development and Function of B Cells by ZBTB Transcription Factors. Front. Immunol. 2018, 9, 580. [Google Scholar] [CrossRef]
- Maeda, T. Regulation of hematopoietic development by ZBTB transcription factors. Int. J. Hematol. 2016, 104, 310–323. [Google Scholar] [CrossRef]
- Siggs, O.M.; Beutler, B. The BTB-ZF transcription factors. Cell Cycle 2012, 11, 3358–3369. [Google Scholar] [CrossRef] [PubMed]
- Song, H.W.; Wilkinson, M.F. Transcriptional control of spermatogonial maintenance and differentiation. Semin. Cell Dev. Biol. 2014, 30, 14–26. [Google Scholar] [CrossRef]
- Doolittle, M.L.; Calabrese, G.M.; Mesner, L.D.; Godfrey, D.A.; Maynard, R.D.; Ackert-Bicknell, C.L.; Farber, C.R. Genetic analysis of osteoblast activity identifies Zbtb40 as a regulator of osteoblast activity and bone mass. PLoS Genet. 2020, 16, e1008805. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, Y.; Zhong, C.; Guo, Y.; Li, S.; Lin, H.; Liu, X. Transcriptome profiling of laser-captured germ cells and functional characterization of zbtb40 during 17alpha-methyltestosterone-induced spermatogenesis in orange-spotted grouper (Epinephelus coioides). BMC Genom. 2020, 21, 73. [Google Scholar] [CrossRef] [PubMed]
- Rappold, G.A. The pseudoautosomal regions of the human sex chromosomes. Hum. Genet. 1993, 92, 315–324. [Google Scholar] [CrossRef]
- O’Flynn O’Brien, K.L.; Varghese, A.C.; Agarwal, A. The genetic causes of male factor infertility: A review. Fertil. Steril. 2010, 93, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Casamonti, E. Spermatogenic failure and the Y chromosome. Hum. Genet. 2017, 136, 637–655. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, J.M.; Lapin, C.D.; Jarvi, K.A. Genetics of CFTR and male infertility. Transl. Androl. Urol. 2021, 10, 1391–1400. [Google Scholar] [CrossRef]
- Tuttelmann, F.; Laan, M.; Grigorova, M.; Punab, M.; Sober, S.; Gromoll, J. Combined effects of the variants FSHB -211G>T and FSHR 2039A>G on male reproductive parameters. J. Clin. Endocrinol. Metab. 2012, 97, 3639–3647. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; He, T.T.; Gao, X.M.; Zhao, Y.; Wang, J. ZBTB Transcription Factors: Key Regulators of the Development, Differentiation and Effector Function of T Cells. Front. Immunol. 2021, 12, 713294. [Google Scholar] [CrossRef]
- Chen, W.; Iida, S.; Louie, D.C.; Dalla-Favera, R.; Chaganti, R.S. Heterologous promoters fused to BCL6 by chromosomal translocations affecting band 3q27 cause its deregulated expression during B-cell differentiation. Blood 1998, 91, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Costoya, J.A.; Hobbs, R.M.; Barna, M.; Cattoretti, G.; Manova, K.; Sukhwani, M.; Orwig, K.E.; Wolgemuth, D.J.; Pandolfi, P.P. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 2004, 36, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Furu, K.; Klungland, A. Tzfp represses the androgen receptor in mouse testis. PLoS ONE 2013, 8, e62314. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, H.; Yin, S.; Zhang, Y.; Yang, W.; Zheng, W.; Wang, L.; Wang, Z.; Bukhari, I.; Cooke, H.J.; et al. Specific deficiency of Plzf paralog, Zbtb20, in Sertoli cells does not affect spermatogenesis and fertility in mice. Sci. Rep. 2014, 4, 7062. [Google Scholar] [CrossRef]
- Mei, B.; Wang, Y.; Ye, W.; Huang, H.; Zhou, Q.; Chen, Y.; Niu, Y.; Zhang, M.; Huang, Q. LncRNA ZBTB40-IT1 modulated by osteoporosis GWAS risk SNPs suppresses osteogenesis. Hum. Genet. 2019, 138, 151–166. [Google Scholar] [CrossRef]
- Chen, H.; He, C.; Wang, C.; Wang, X.; Ruan, F.; Yan, J.; Yin, P.; Wang, Y.; Yan, S. RAD51 supports DMC1 by inhibiting the SMC5/6 complex during meiosis. Plant Cell 2021, 33, 2869–2882. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Gao, Q.; Zheng, W.; Yin, S.; Wang, L.; Zhong, L.; Ali, A.; Khan, T.; Hao, Q.; Fang, H.; et al. MOF influences meiotic expansion of H2AX phosphorylation and spermatogenesis in mice. PLoS Genet. 2018, 14, e1007300. [Google Scholar] [CrossRef]
- Liu, L.; Franco, S.; Spyropoulos, B.; Moens, P.B.; Blasco, M.A.; Keefe, D.L. Irregular telomeres impair meiotic synapsis and recombination in mice. Proc. Natl. Acad. Sci. USA 2004, 101, 6496–6501. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Tuttelmann, F.; Wyrwoll, M.J.; Kliesch, S.; Lopes, A.M.; Goncalves, J.; Boyden, S.E.; Woste, M.; Hotaling, J.M.; Consortium, G.; et al. Disruption of human meiotic telomere complex genes TERB1, TERB2 and MAJIN in men with non-obstructive azoospermia. Hum. Genet. 2021, 140, 217–227. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Chen, J.; Wang, L.; Nie, L.; Long, J.; Chang, H.; Wu, J.; Huang, C.; Lei, M. The meiotic TERB1-TERB2-MAJIN complex tethers telomeres to the nuclear envelope. Nat. Commun. 2019, 10, 564. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Zhou, M.; He, Q.; He, Z. Zbtb40 Deficiency Leads to Morphological and Phenotypic Abnormalities of Spermatocytes and Spermatozoa and Causes Male Infertility. Cells 2023, 12, 1264. https://doi.org/10.3390/cells12091264
Cui Y, Zhou M, He Q, He Z. Zbtb40 Deficiency Leads to Morphological and Phenotypic Abnormalities of Spermatocytes and Spermatozoa and Causes Male Infertility. Cells. 2023; 12(9):1264. https://doi.org/10.3390/cells12091264
Chicago/Turabian StyleCui, Yinghong, Mingqing Zhou, Quanyuan He, and Zuping He. 2023. "Zbtb40 Deficiency Leads to Morphological and Phenotypic Abnormalities of Spermatocytes and Spermatozoa and Causes Male Infertility" Cells 12, no. 9: 1264. https://doi.org/10.3390/cells12091264
APA StyleCui, Y., Zhou, M., He, Q., & He, Z. (2023). Zbtb40 Deficiency Leads to Morphological and Phenotypic Abnormalities of Spermatocytes and Spermatozoa and Causes Male Infertility. Cells, 12(9), 1264. https://doi.org/10.3390/cells12091264




