Cognitive Impairment Is Associated with AMPAR Glutamatergic Dysfunction in a Mouse Model of Neuronal Methionine Synthase Deficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Collection
2.2. RNA Analysis and Quantitative RT-PCR Analysis
2.3. Protein Extraction and Wes Protein Analyses
2.4. Behavioral Tests
2.5. LC-MS/MS Analyses
2.6. Tissue Measurments and Immunohistochemistry
2.7. Protein Interactions
2.8. Statistical Analysis
3. Results
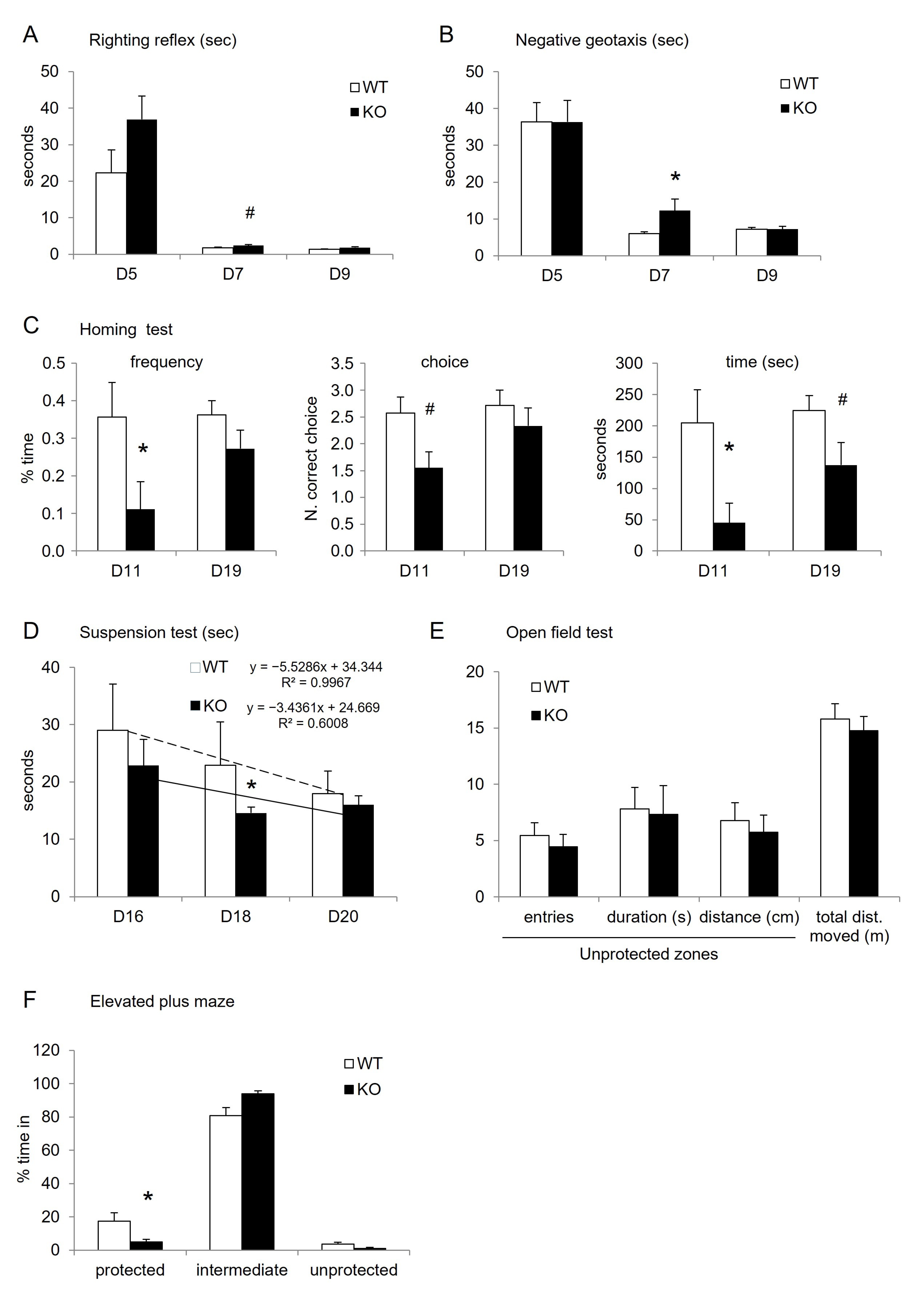
3.1. Physiological Characterization of the Mouse Model
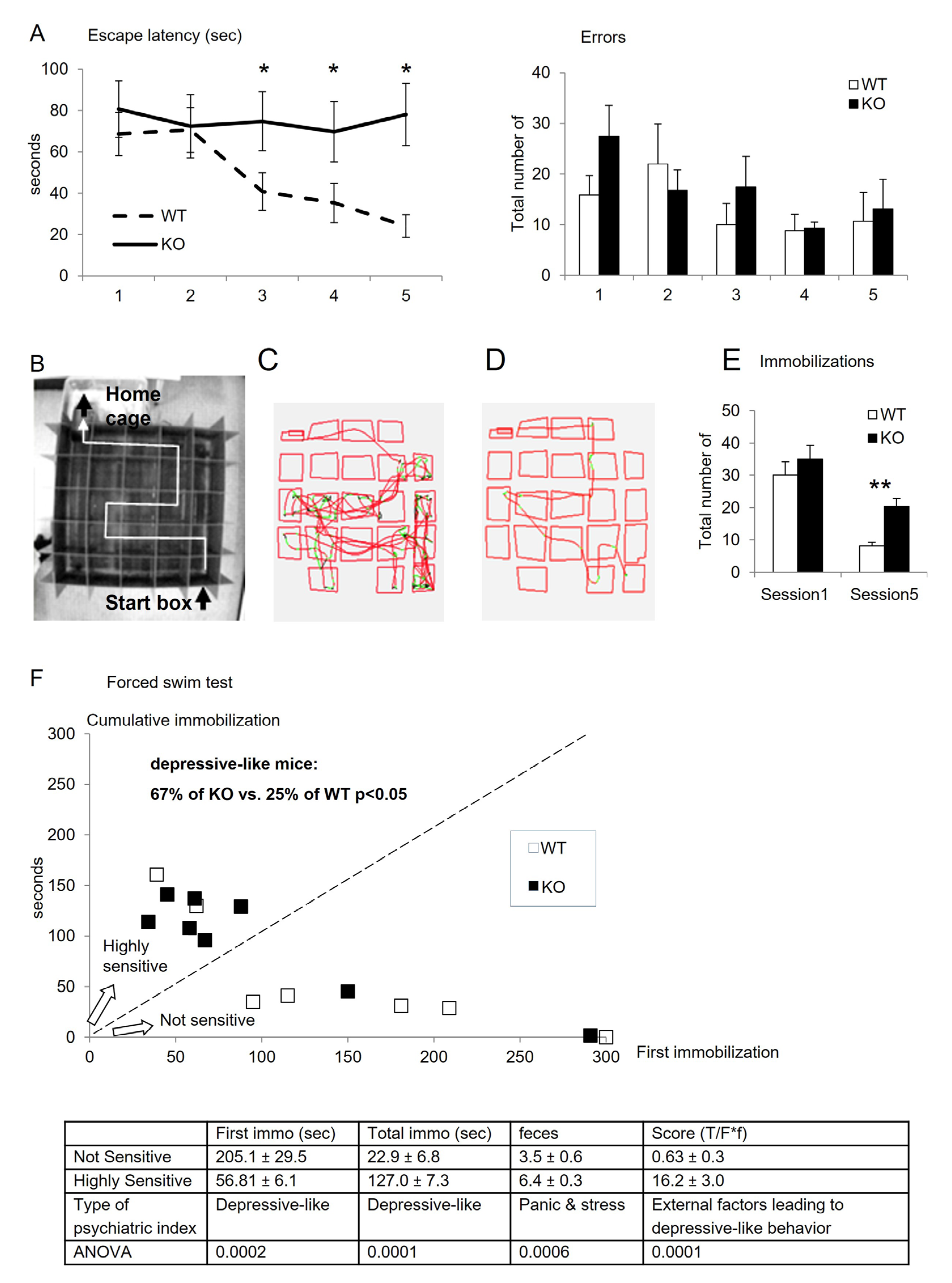
3.2. Behavioral Characterization
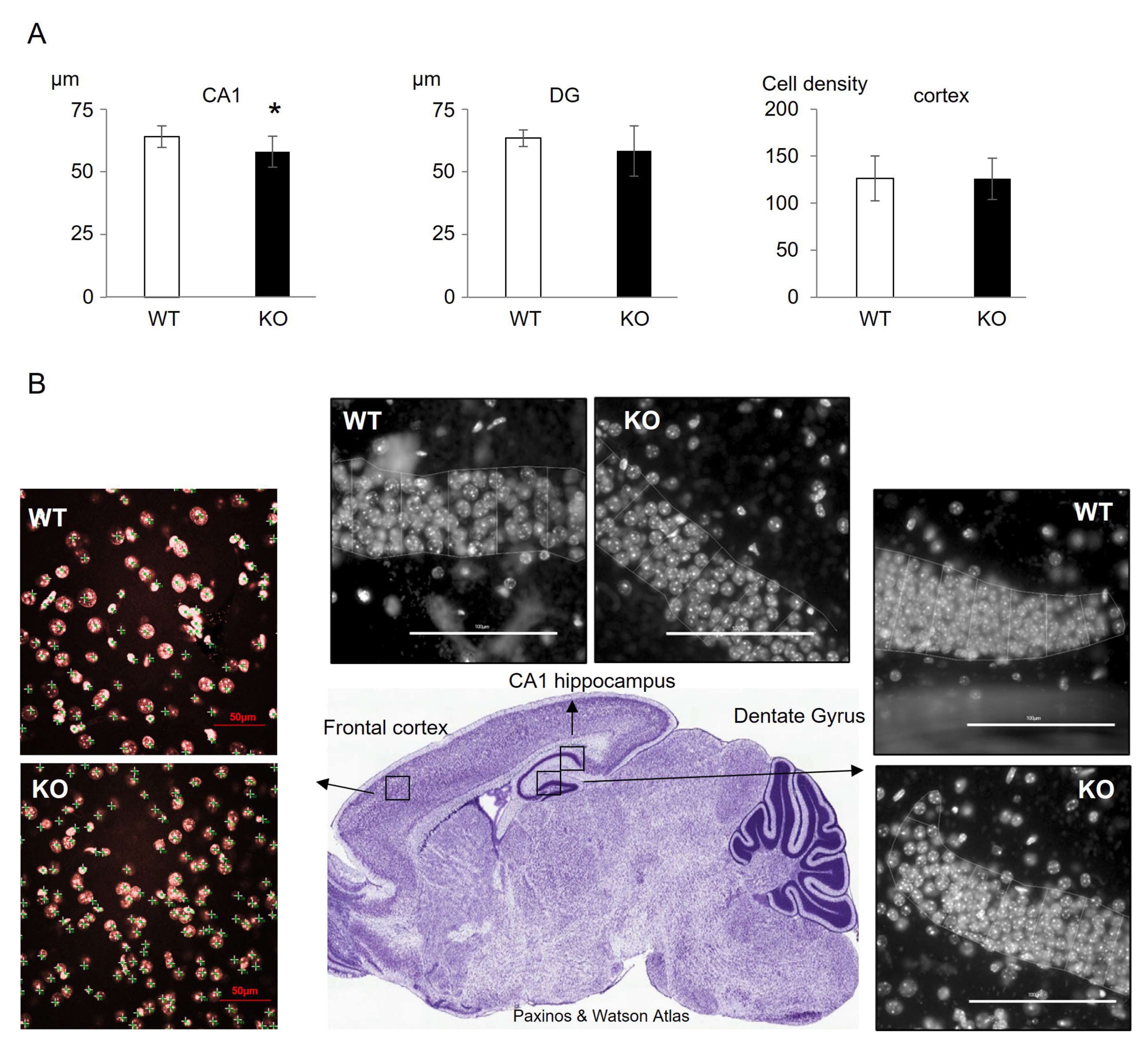
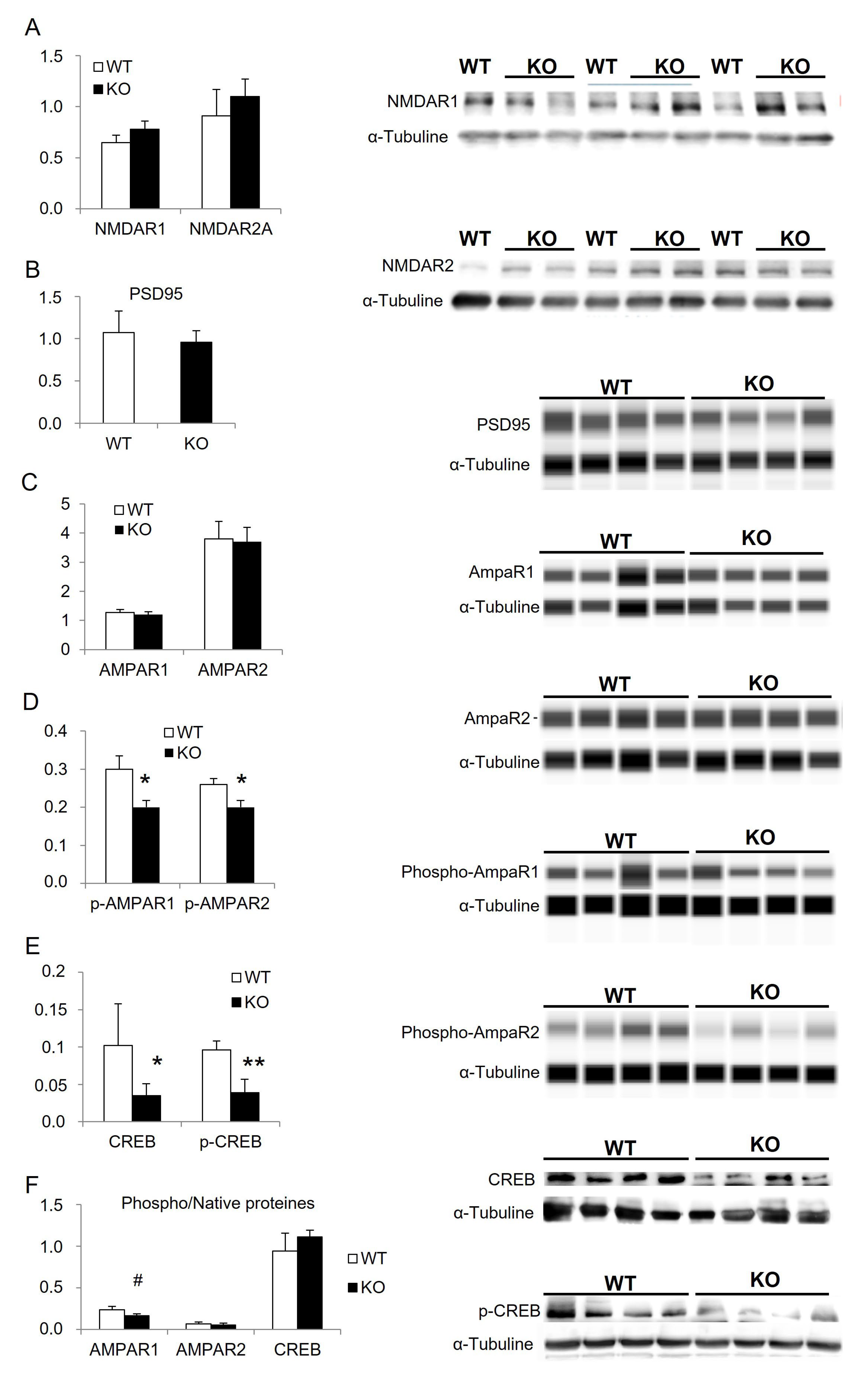
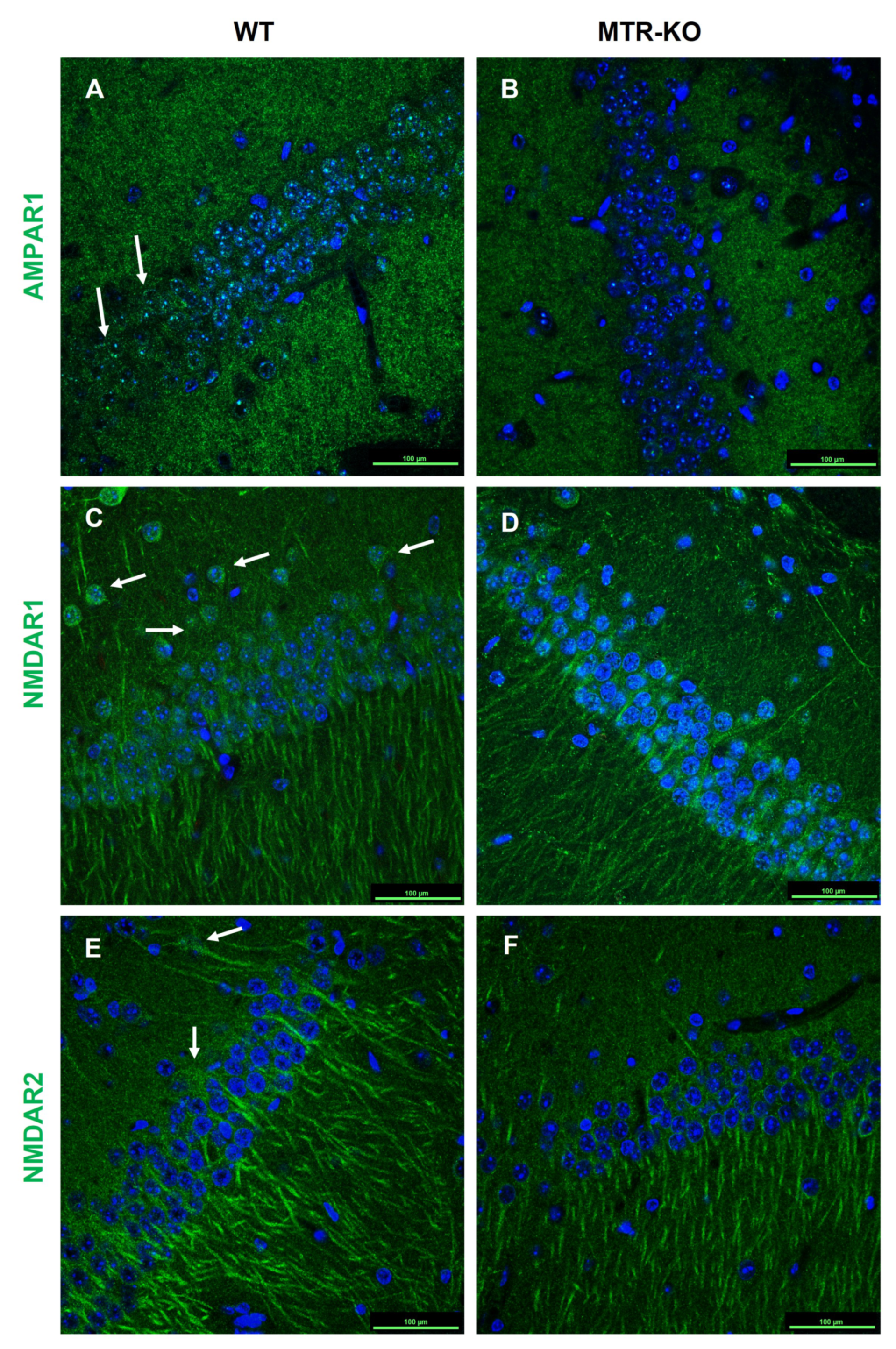
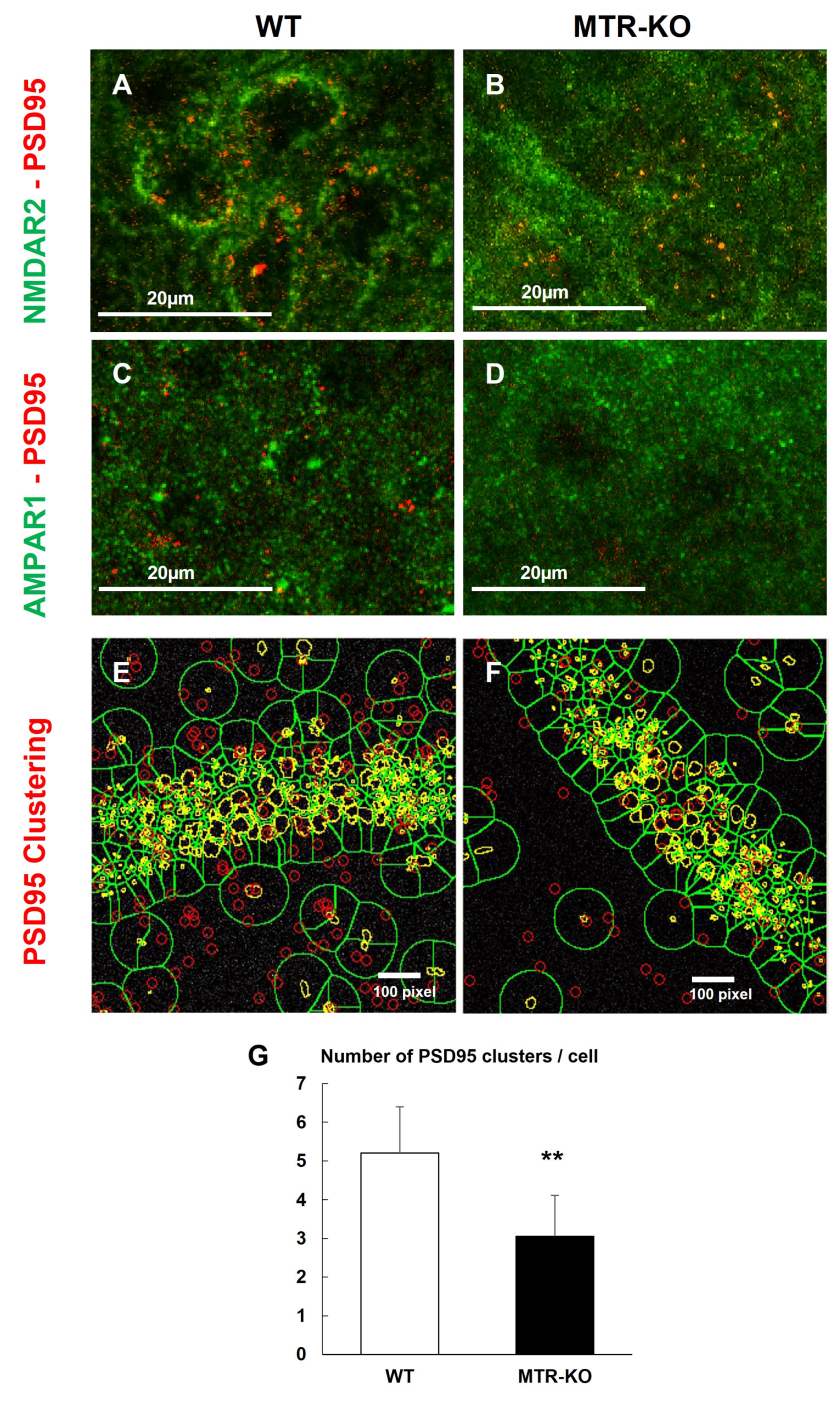
3.3. Status of Cognitive Brain Sub-Regions
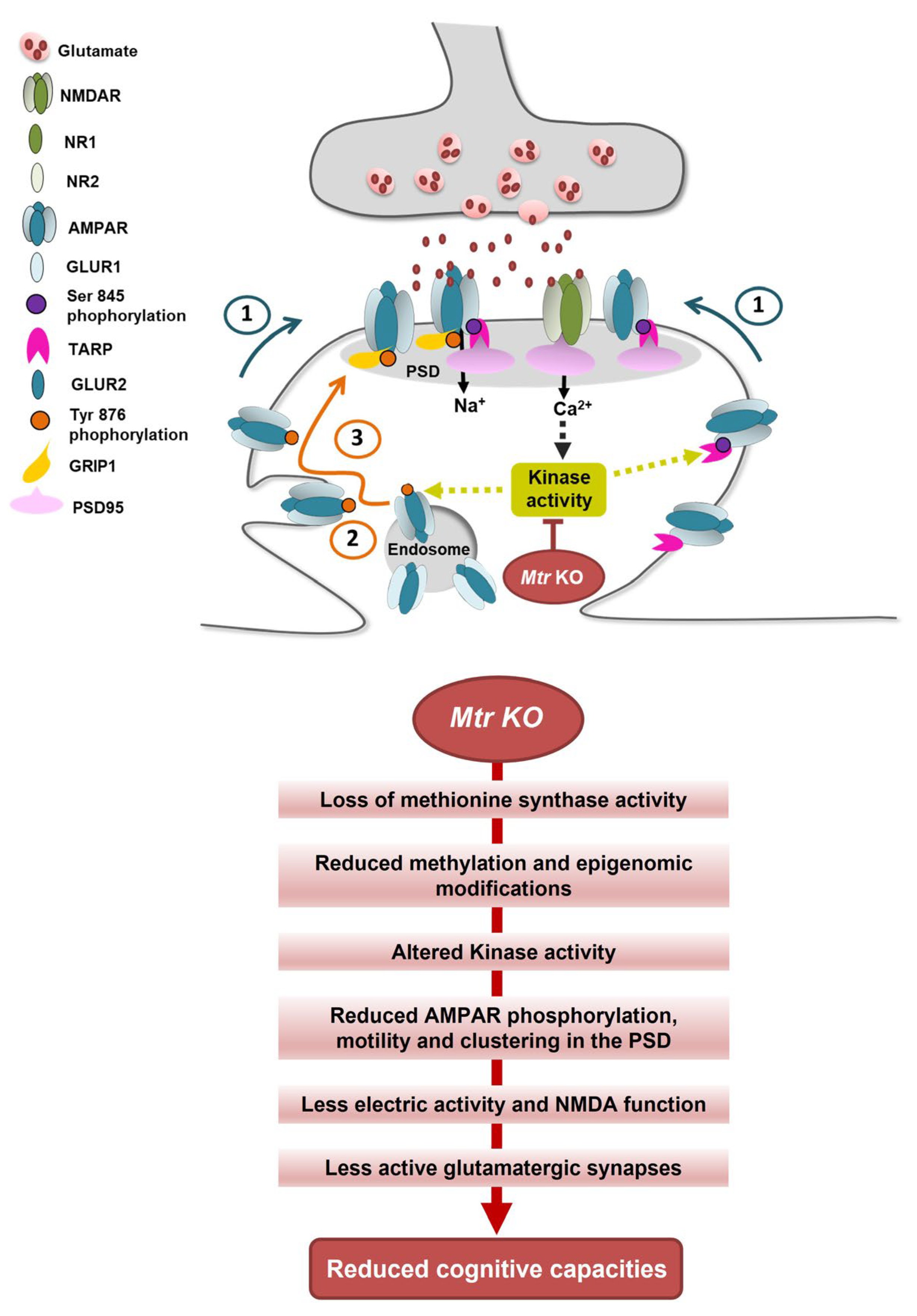
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Pei, L.; Li, R.; Zhang, W.; Yang, H.; Li, Y.; Guo, Y.; Tan, P.; Han, J.J.; Zheng, X.; et al. Spina bifida in fetus is associated with an altered pattern of DNA methylation in placenta. J. Hum. Genet. 2015, 60, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Podobinska, M.; Szablowska-Gadomska, I.; Augustyniak, J.; Sandvig, I.; Sandvig, A.; Buzanska, L. Epigenetic Modulation of Stem Cells in Neurodevelopment: The Role of Methylation and Acetylation. Front. Cell Neurosci. 2017, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Fofou-Caillierez, M.B.; Guéant-Rodriguez, R.M.; Alberto, J.M.; Chéry, C.; Josse, T.; Gérard, P.; Forges, T.; Foliguet, B.; Feillet, F.; Guéant, J.L. Vitamin B-12 and liver activity and expression of methionine synthase are decreased in fetuses with neural tube defects. Am. J. Clin. Nutr. 2019, 109, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Pourié, G.; Guéant, J.L.; Quadros, E.V. Behavioral profile of vitamin B12 deficiency: A reflection of impaired brain development, neuronal stress and altered neuroplasticity. Vitam. Horm. 2022, 119, 377–404. [Google Scholar] [PubMed]
- Mitchell, L.E.; Adzick, N.S.; Melchionne, J.; Pasquariello, P.S.; Sutton, L.N.; Whitehead, A.S. Spina bifida. Lancet 2004, 364, 1885–1895. [Google Scholar] [CrossRef]
- McGarel, C.; Pentieva, K.; Strain, J.J.; McNulty, H. Emerging roles for folate and related B-vitamins in brain health across the lifecycle. Proc. Nutr. Soc. 2015, 74, 46–55. [Google Scholar] [CrossRef]
- Battaglia-Hsu, S.F.; Ghemrawi, R.; Coelho, D.; Dreumont, N.; Mosca, P.; Hergalant, S.; Gauchotte, G.; Sequeira, J.M.; Ndiongue, M.; Houlgatte, R.; et al. Inherited disorders of cobalamin metabolism disrupt nucleocytoplasmic transport of mRNA through impaired methylation/phosphorylation of ELAVL1/HuR. Nucleic Acids Res. 2018, 46, 7844–7857. [Google Scholar] [CrossRef]
- Kong, Q.; Yu, M.; Zhang, M.; Wei, C.; Gu, H.; Yu, S.; Sun, W.; Li, N.; Zhou, Y. Conditional Dnmt3b deletion in hippocampal dCA1 impairs recognition memory. Mol. Brain 2020, 13, 1–4. [Google Scholar] [CrossRef]
- Guéant, J.L.; Guéant-Rodriguez, R.M.; Kosgei, V.J.; Coelho, D. Causes and consequences of impaired methionine synthase activity in acquired and inherited disorders of vitamin B12 metabolism. Crit. Rev. Biochem. Mol. Biol. 2022, 57, 133–155. [Google Scholar] [CrossRef]
- Kerek, R.; Geoffroy, A.; Bison, A.; Martin, N.; Akchiche, N.; Pourié, G.; Helle, D.; Guéant, J.L.; Bossenmeyer-Pourié, C.; Daval, J.L. Early methyl donor deficiency may induce persistent brain defects by reducing Stat3 signaling targeted by miR-124. Cell Death Dis. 2013, 4, e755. [Google Scholar] [CrossRef]
- Greene, N.D.; Copp, A.J. Neural tube defects. Annu. Rev. Neurosci. 2014, 37, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Puckett, R.E.; Lubin, F.D. Epigenetic mechanisms in experience-driven memory formation and behavior. Epigenomics 2011, 3, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, A.; Oussalah, A.; Lamireau, N.; Théron, M.; Julien, M.; Mergnac, J.P.; Augay, B.; Deniaud, P.; Alix, T.; Frayssinoux, M.; et al. Clinical, phenotypic and genetic landscape of case reports with genetically proven inherited disorders of vitamin B12 metabolism: A meta-analysis. Cell Rep. Med. 2022, 3, 100670. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.; Pentieva, K.; Hoey, L.; Ward, M. Homocysteine, B-vitamins and CVD. Proc. Nutr. Soc. 2008, 67, 232–237. [Google Scholar] [CrossRef] [PubMed]
- McCleery, J.; Abraham, R.P.; Denton, D.A.; Rutjes, A.W.; Chong, L.Y.; Al-Assaf, A.S.; Griffith, D.J.; Rafeeq, S.; Yaman, H.; Malik, M.A.; et al. Vitamin and mineral supplementation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2018, 11, CD011905. [Google Scholar] [CrossRef] [PubMed]
- Markun, S.; Gravestock, I.; Jäger, L.; Rosemann, T.; Pichierri, G.; Burgstaller, J.M. Effects of Vitamin B12 Supplementation on Cognitive Function, Depressive Symptoms, and Fatigue: A Systematic Review, Meta-Analysis, and Meta-Regression. Nutrients 2021, 13, 923. [Google Scholar] [CrossRef]
- McEwen, B.S. Redefining neuroendocrinology: Epigenetics of brain-body communication over the life course. Front. Neuroendocr. 2018, 49, 8–30. [Google Scholar] [CrossRef]
- McEwen, B.S.; Bulloch, K. Epigenetic impact of the social and physical environment on brain and body. Metabolism 2019, 100, 153941. [Google Scholar] [CrossRef]
- Sloan, J.L.; Carrillo, N.; Adams, D.; Venditti, C.P. Disorders of Intracellular Cobalamin Metabolism. In Gene Reviews; Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2008. [Google Scholar]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- Blaise, S.A.; Nédélec, E.; Schroeder, H.; Alberto, J.M.; Bossenmeyer-Pourié, C.; Guéant, J.L.; Daval, J.L. Gestational vitamin B deficiency leads to homocysteine-associated brain apoptosis and alters neurobehavioral development in rats. Am. J. Pathol. 2007, 170, 667–679. [Google Scholar] [CrossRef]
- Huemer, M.; Diodato, D.; Martinelli, D.; Olivieri, G.; Blom, H.; Gleich, F.; Kölker, S.; Kožich, V.; Morris, A.A.; Seifert, B.; et al. Phenotype, treatment practice and outcome in the cobalamin-dependent remethylation disorders and MTHFR deficiency: Data from the E-HOD registry. J. Inherit. Metab. Dis. 2019, 42, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Scholl-Bürgi, S.; Hadaya, K.; Kern, I.; Beer, R.; Seppi, K.; Fowler, B.; Baumgartner, M.R.; Karall, D. Three new cases of late-onset cblC defect and review of the literature illustrating when to consider inborn errors of metabolism beyond infancy. Orphanet J. Rare Dis. 2014, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Baydas, G.; Koz, S.T.; Tuzcu, M.; Nedzvetsky, V.S.; Etem, E. Effects of maternal hyperhomocysteinemia induced by high methionine diet on the learning and memory performance in offspring. Int. J. Dev. Neurosci. 2007, 25, 133–139. [Google Scholar] [CrossRef]
- Agrawal, A.; Ilango, K.; Singh, P.K.; Karmakar, D.; Singh, G.P.; Kumari, R.; Dubey, G.P. Age dependent levels of plasma homocysteine and cognitive performance. Behav. Brain. Res. 2015, 283, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, A.; Saber-Cherif, L.; Pourié, G.; Helle, D.; Umoret, R.; Guéant, J.L.; Bossenmeyer-Pourié, C.; Daval, J.L. Developmental Impairments in a Rat Model of Methyl Donor Deficiency: Effects of a Late Maternal Supplementation with Folic Acid. Int. J. Mol. Sci. 2019, 20, 973. [Google Scholar] [CrossRef]
- Román, G.C.; Mancera-Páez, O.; Bernal, C. Epigenetic Factors in Late-Onset Alzheimer’s Disease: MTHFR and CTH Gene Polymorphisms, Metabolic Transsulfuration and Methylation Pathways, and B Vitamins. Int. J. Mol. Sci. 2019, 20, 319. [Google Scholar] [CrossRef] [PubMed]
- Maurice, N.; Olry, J.C.; Cariou, R.; Dervilly-Pinel, G.; Le Bizec, B.; Travel, A.; Jondreville, C.; Schroeder, H. Short-term effects of a perinatal exposure to the HBCDD α-isomer in rats: Assessment of early motor and sensory development, spontaneous locomotor activity and anxiety in pups. Neurotoxicol. Teratol. 2015, 52, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Pourié, G.; Martin, N.; Bossenmeyer-Pourié, C.; Akchiche, N.; Guéant-Rodriguez, R.M.; Geoffroy, A.; Jeannesson, E.; El Hajj Chehadeh, S.; Mimoun, K.; Brachet, P.; et al. Folate- and vitamin B12-deficient diet during gestation and lactation alters cerebellar synapsin expression via impaired influence of estrogen nuclear receptor α. FASEB J. 2015, 29, 3713–3725. [Google Scholar] [CrossRef]
- Hassan, Z.; Coelho, D.; Kokten, T.; Alberto, J.M.; Umoret, R.; Daval, J.L.; Guéant, J.L.; Bossenmeyer-Pourié, C.; Pourié, G. Brain Susceptibility to Methyl Donor Deficiency: From Fetal Programming to Aging Outcome in Rats. Int. J. Mol. Sci. 2019, 20, 5692. [Google Scholar] [CrossRef]
- Kalyan, G.B.; Mittal, M.; Jain, R. Compromised Vitamin B12 Status of Indian Infants and Toddlers. Food Nutr. Bull. 2020, 41, 430–437. [Google Scholar] [CrossRef]
- Rubini, E.; Baijens, I.M.M.; Horánszky, A.; Schoenmakers, S.; Sinclair, K.D.; Zana, M.; Dinnyés, A.; Steegers-Theunissen, R.P.M.; Rousian, M. Maternal One-Carbon Metabolism during the Periconceptional Period and Human Foetal Brain Growth: A Systematic Review. Genes 2021, 12, 1634. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006, 5, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Watkins, D.; Rosenblatt, D.S. Vitamin B(12) and birth defects. Mol. Genet. Metab. 2009, 98, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, M.R. Vitamin-responsive disorders: Cobalamin, folate, biotin, vitamins B1 and E. Handb. Clin. Neurol. 2013, 113, 1799–1810. [Google Scholar]
- Bobrowski-Khoury, N.; Ramaekers, V.T.; Sequeira, J.M.; Quadros, E.V. Folate Receptor Alpha Autoantibodies in Autism Spectrum Disorders: Diagnosis, Treatment and Prevention. J. Pers. Med. 2021, 11, 710. [Google Scholar] [CrossRef]
- Guéant, J.L.; Caillerez-Fofou, M.; Battaglia-Hsu, S.; Alberto, J.M.; Freund, J.N.; Dulluc, I.; Adjalla, C.; Maury, F.; Merle, C.; Nicolas, J.P.; et al. Molecular and cellular effects of vitamin B12 in brain, myocardium and liver through its role as co-factor of methionine synthase. Biochimie 2013, 95, 1033–1040. [Google Scholar] [CrossRef]
- Hannibal, L.; Blom, H.J. Homocysteine and disease: Causal associations or epiphenomenons? Mol. Aspects. Med. 2017, 53, 36–42. [Google Scholar] [CrossRef]
- Abu Ahmad, N.; Raizman, M.; Weizmann, N.; Wasek, B.; Arning, E.; Bottiglieri, T.; Tirosh, O.; Troen, A.M. Betaine attenuates pathology by stimulating lipid oxidation in liver and regulating phospholipid metabolism in brain of methionine-choline-deficient rats. FASEB J. 2019, 33, 9334–9349. [Google Scholar] [CrossRef]
- Muratore, C.R.; Hodgson, N.W.; Trivedi, M.S.; Abdolmaleky, H.M.; Persico, A.M.; Lintas, C.; De la Monte, S.; Deth, R.C. Age-dependent decrease and alternative splicing of methionine synthase mRNA in human cerebral cortex and an accelerated decrease in autism. PLoS ONE 2013, 8, e56927. [Google Scholar] [CrossRef]
- Black, M.M. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr. Bull. 2008, 29, S126–S131. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Arnold, C.; Battaglia-Hsu, S.F.; Pourié, G.; Trinh, I.; Bassila, C.; Rashka, C.; Wiedemann, A.; Flayac, J.; Robert, A.; et al. SIRT1 activation rescues the mislocalization of RNA-binding proteins and cognitive defects induced by inherited cobalamin disorders. Metabolism 2019, 101, 153992. [Google Scholar] [CrossRef] [PubMed]
- Esnafoglu, E.; Ozturan, D.D. The relationship of severity of depression with homocysteine, folate, vitamin B12, and vitamin D levels in children and adolescents. Child Adolesc. Ment. Health 2020, 25, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, O.V.; Kanekar, S.; D’Anci, K.E.; Renshaw, P.F. Factors influencing behavior in the forced swim test. Physiol. Behav. 2013, 118, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, H.; Matsuzawa, D.; Ishii, D.; Matsuda, S.; Kawai, K.; Mashimo, Y.; Sutoh, C.; Shimizu, E. Methyl-donor deficiency in adolescence affects memory and epigenetic status in the mouse hippocampus. Genes Brain Behav. 2015, 14, 301–309. [Google Scholar] [CrossRef]
- Lee, H.J.; Zheng, J.J. PDZ domains and their binding partners: Structure, specificity, and modification. Cell Commun. Signal 2010, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Sweatt, J.D. Dynamic DNA methylation controls glutamate receptor trafficking and synaptic scaling. J. Neurochem. 2016, 137, 312–330. [Google Scholar] [CrossRef]
- Diering, G.H.; Huganir, R.L. The AMPA Receptor Code of Synaptic Plasticity. Neuron 2018, 100, 314–329. [Google Scholar] [CrossRef]
- Coley, A.A.; Gao, W.J. PSD-95 deficiency disrupts PFC-associated function and behavior during neurodevelopment. Sci. Rep. 2019, 9, 9486. [Google Scholar] [CrossRef]
- Hayashi, T.; Huganir, R.L. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J. Neurosci. 2004, 24, 6152–6160. [Google Scholar] [CrossRef]
- Matsuda, S.; Kakegawa, W.; Budisantoso, T.; Nomura, T.; Kohda, K.; Yuzaki, M. Stargazin regulates AMPA receptor trafficking through adaptor protein complexes during long-term depression. Nat. Commun. 2013, 4, 2759. [Google Scholar] [CrossRef]
- Choquet, D. Linking Nanoscale Dynamics of AMPA Receptor Organization to Plasticity of Excitatory Synapses and Learning. J. Neurosci. 2018, 38, 9318–9329. [Google Scholar] [CrossRef] [PubMed]
- Buonarati, O.R.; Hammes, E.A.; Watson, J.F.; Greger, I.H.; Hell, J.W. Mechanisms of postsynaptic localization of AMPA-type glutamate receptors and their regulation during long-term potentiation. Sci. Signal 2019, 12, eaar6889. [Google Scholar] [CrossRef] [PubMed]
- Akchiche, N.; Bossenmeyer-Pourié, C.; Pourié, G.; Koziel, V.; Nédélec, E.; Guéant, J.L.; Daval, J.L. Differentiation and neural integration of hippocampal neuronal progenitors: Signaling pathways sequentially involved. Hippocampus 2010, 20, 949–961. [Google Scholar] [CrossRef]
- Geoffroy, A.; Kerek, R.; Pourié, G.; Helle, D.; Guéant, J.L.; Daval, J.L.; Bossenmeyer-Pourié, C. Late Maternal Folate Supplementation Rescues from Methyl Donor Deficiency-Associated Brain Defects by Restoring Let-7 and miR-34 Pathways. Mol. Neurobiol. 2017, 54, 5017–5033. [Google Scholar] [CrossRef]
- Jadavji, N.M.; Deng, L.; Malysheva, O.; Caudill, M.A.; Rozen, R. MTHFR deficiency or reduced intake of folate or choline in pregnant mice results in impaired short-term memory and increased apoptosis in the hippocampus of wild-type offspring. Neuroscience 2015, 300, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; Sharma, V.; Kumar, V.; Nag, T.C.; Wadhwa, S. Activity-dependent synaptic plasticity modulates the critical phase of brain development. Brain Dev. 2016, 38, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Dreumont, N.; Mimoun, K.; Pourié, C.; Quadros, E.V.; Alberto, J.M.; Umoret, R.; Helle, D.; Robert, A.; Daval, J.L.; Guéant, J.L.; et al. Glucocorticoid Receptor Activation Restores Learning Memory by Modulating Hippocampal Plasticity in a Mouse Model of Brain Vitamin B12 Deficiency. Mol. Neurobiol. 2021, 58, 1024–1035. [Google Scholar] [CrossRef]

| Brain | Cortex | Hippocampus | ||||
|---|---|---|---|---|---|---|
| ANOVA qPCR WT vs. KO | F(1,6) = 58.7; p = 0.0003 | F(1,6) = 28.9; p = 0.0017 | F(1,6) = 59.2; p = 0.0003 | |||
| ANOVA MS protein | ||||||
| WT vs. KO | F(1,6) = 47.6; p = 0.0005 | F(1,6) = 54.7; p = 0.0003 | F(1,6) = 44.2; p = 0.0006 | |||
| ANOVA metabolites | Hcy | Meth | SAM | SAH | ||
| WT vs. KO | F(1,6) = 7.0; p = 0.0384 | F(1,6) = 8.7; p = 0.0254 | F(1,6) = 10.3; p = 0.0184 | F(1,6) = 16.0; p = 0.0071 | ||
| D5 | D7 | D9 | |
|---|---|---|---|
| ANOVA RR test WT vs. KO | F(1,14) = 1.3; p = 0.2725 | F(1,14) = 9.2; p = 0.0090 | F(1,14) = 2.8; p = 0.1140 |
| ANOVA NG test | |||
| WT vs. KO | F(1,6) = 0.0; p = 0.9980 | F(1,14) = 4.0; p = 0.0645 | F(1,6) = 0.06; p = 0.8076 |
| ANOVA Homing | D11 | D19 | |
| WT vs. KO frequency | F(1,14) = 5.7; p = 0.0313 | F(1,6) = 0.7; p = 0.4168 | |
| WT vs. KO choice | F(1,14) = 4.5; p = 0.0529 | F(1,6) = 1.9; p = 0.1864 | |
| WT vs. KO time | F(1,14) = 7.4; p = 0.0165 | F(1,6) = 3.6; p = 0.0787 | |
| Suspension test two-way ANOVA | ddl | F value | p value |
| genotype | 1 | 4.368 | 0.0429 |
| days | 2 | 1.716 | 0.1924 |
| genotype × days | 2 | 1.243 | 0.2991 |
| Suspension test one-way ANOVA | D16 | D18 | D20 |
| F(1,14 ddl) | F(1,13 ddl) | F(1,14 ddl) | |
| WT vs. KO | F = 0.487, p = 0.4969 | F = 5.389, p = 0.037 | F = 0.255, p = 0.6215 |
| Water Maze Test | ||||||||
| Two-Way ANOVA | ddl | F Value | p Value | |||||
| genotype | 1 | 40.887 | <0.0001 | |||||
| days | 4 | 1.925 | 0.1151 | |||||
| genotype × days | 4 | 1.669 | 0.1661 | |||||
| ANOVA WT sessions | ||||||||
| F(4,40) = 7.436 | S2 | S3 | S4 | S5 | ||||
| S1 | ns | 0.0037 | 0.0009 | 0.0001 | ||||
| S2 | 0.0126 | 0.0034 | 0.0005 | |||||
| S3 | ns | ns | ||||||
| S4 | ns | |||||||
| ANOVA F(1,15 ddl) | S1 | S2 | S3 | S4 | S5 | |||
| WT vs. KO | F = 1.978, p = 0.180 | F = 1.9, p = 0.1789 | F = 10.8, p = 0.0050 | F = 13.4, p = 0.0023 | F = 34.4, p < 0.0001 | |||
| ANOVA Proteins | p-AMPAR1 | p-AMPAR2 | CREB | p-CREB |
|---|---|---|---|---|
| WT vs. KO | F(1,6) = 26.0, p = 0.0022 | F(1,6) = 9.6, p = 0.0213 | F(1,6) = 28.9, p = 0.0017 | F(1,6) = 94.7, p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, Z.; Coelho, D.; Bossenmeyer-Pourié, C.; Matmat, K.; Arnold, C.; Savladori, A.; Alberto, J.-M.; Umoret, R.; Guéant, J.-L.; Pourié, G. Cognitive Impairment Is Associated with AMPAR Glutamatergic Dysfunction in a Mouse Model of Neuronal Methionine Synthase Deficiency. Cells 2023, 12, 1267. https://doi.org/10.3390/cells12091267
Hassan Z, Coelho D, Bossenmeyer-Pourié C, Matmat K, Arnold C, Savladori A, Alberto J-M, Umoret R, Guéant J-L, Pourié G. Cognitive Impairment Is Associated with AMPAR Glutamatergic Dysfunction in a Mouse Model of Neuronal Methionine Synthase Deficiency. Cells. 2023; 12(9):1267. https://doi.org/10.3390/cells12091267
Chicago/Turabian StyleHassan, Ziad, David Coelho, Carine Bossenmeyer-Pourié, Karim Matmat, Carole Arnold, Aurélie Savladori, Jean-Marc Alberto, Rémy Umoret, Jean-Louis Guéant, and Grégory Pourié. 2023. "Cognitive Impairment Is Associated with AMPAR Glutamatergic Dysfunction in a Mouse Model of Neuronal Methionine Synthase Deficiency" Cells 12, no. 9: 1267. https://doi.org/10.3390/cells12091267
APA StyleHassan, Z., Coelho, D., Bossenmeyer-Pourié, C., Matmat, K., Arnold, C., Savladori, A., Alberto, J.-M., Umoret, R., Guéant, J.-L., & Pourié, G. (2023). Cognitive Impairment Is Associated with AMPAR Glutamatergic Dysfunction in a Mouse Model of Neuronal Methionine Synthase Deficiency. Cells, 12(9), 1267. https://doi.org/10.3390/cells12091267







