Therapeutic Effects of Combined Treatment with the AEA Hydrolysis Inhibitor PF04457845 and the Substrate Selective COX-2 Inhibitor LM4131 in the Mouse Model of Neuropathic Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Chronic Constriction Injury Surgery
2.4. Assessment of Nociceptive Behavior
2.4.1. Hot Plate Test
2.4.2. Von Frey Test
2.5. Drug Treatment
2.6. QRT-PCR
2.7. Immunostaining
2.8. ELISA Assay
2.9. Statistical Analysis
3. Results
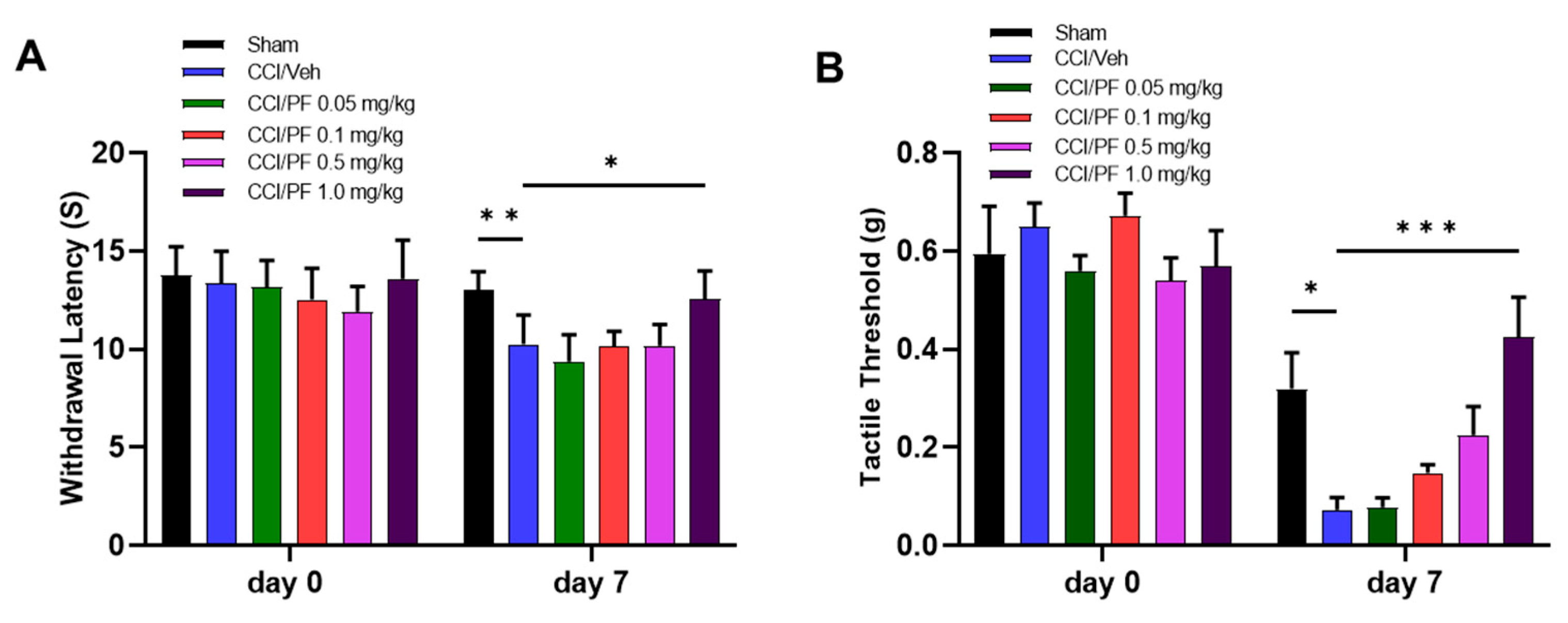
3.1. PF04457845 Dose-Dependently Attenuated CCI-Induced Thermal Hyperalgesia and Mechanical Allodynia
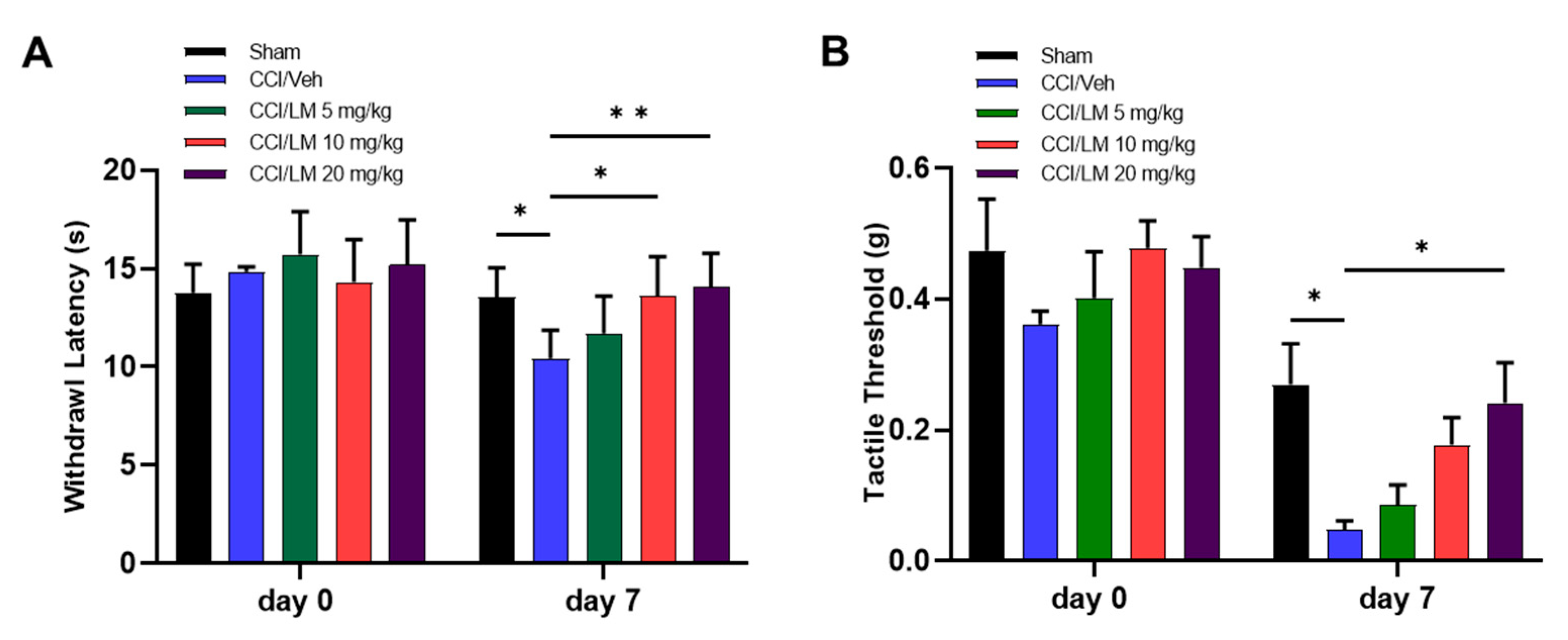
3.2. Concentration Dependent Effect of LM4131 in the CCI Induced Mechanical Allodynia and Thermal Hyperalgesia
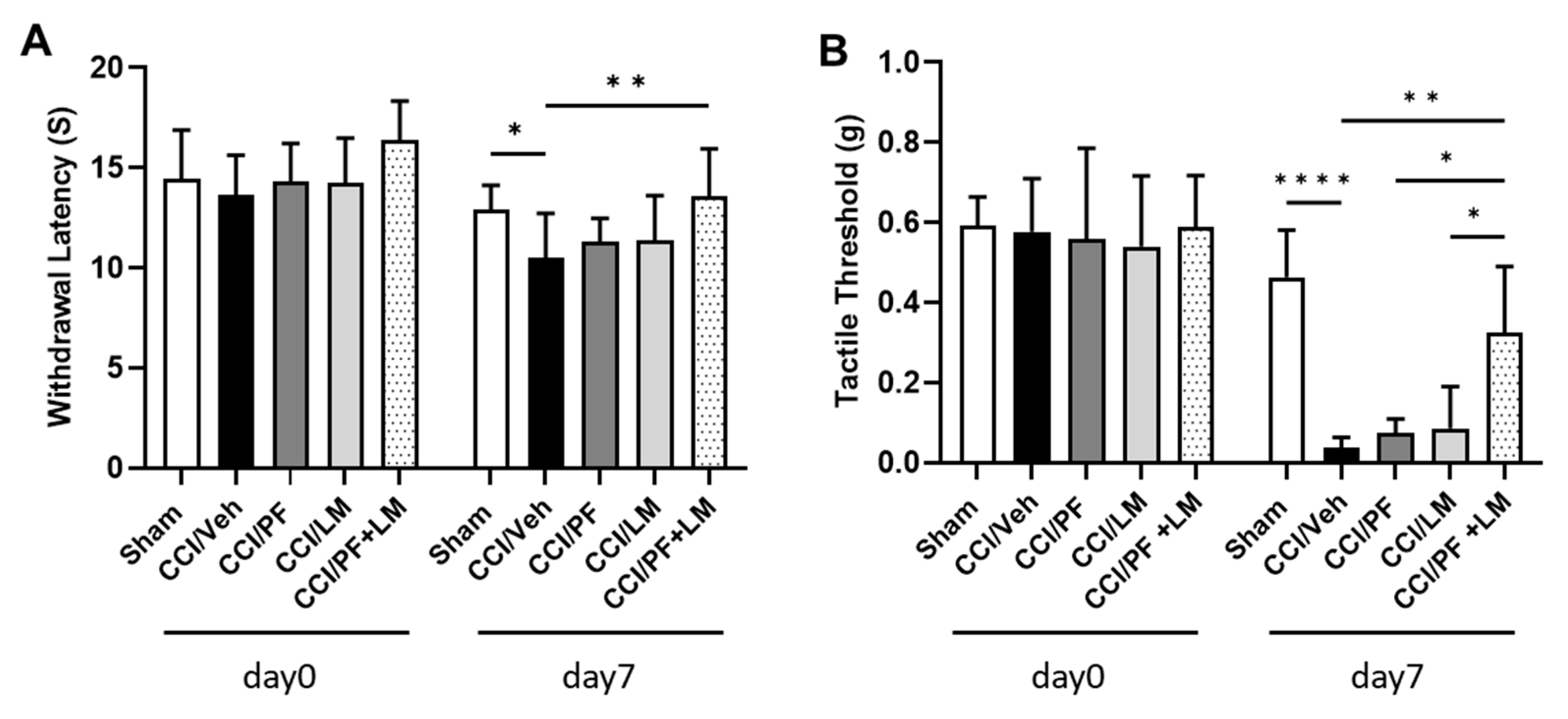
3.3. Co-Treatment with Subeffective Doses of LM4131 and PF04457845 Suppressed Neuropathic Pain
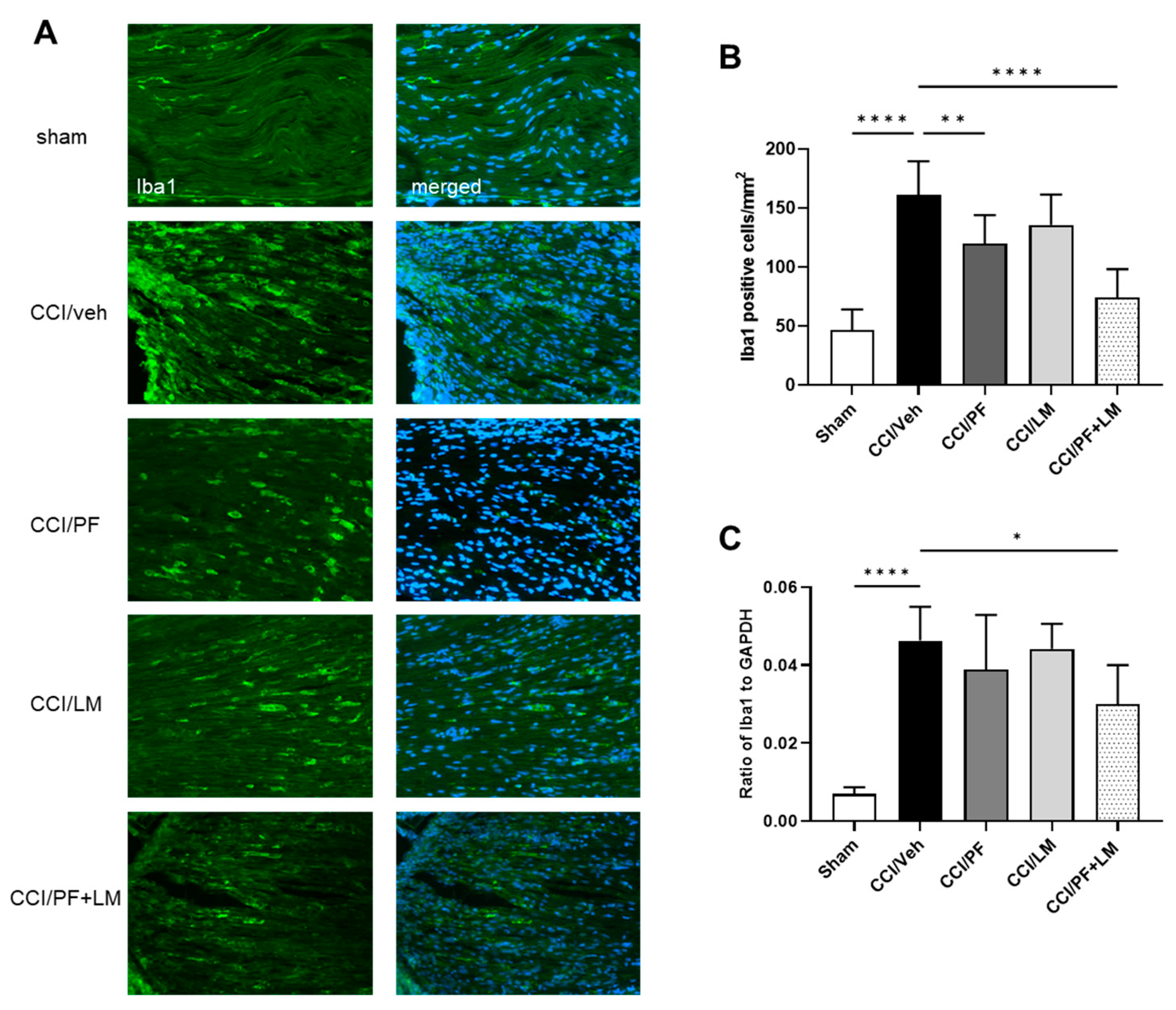
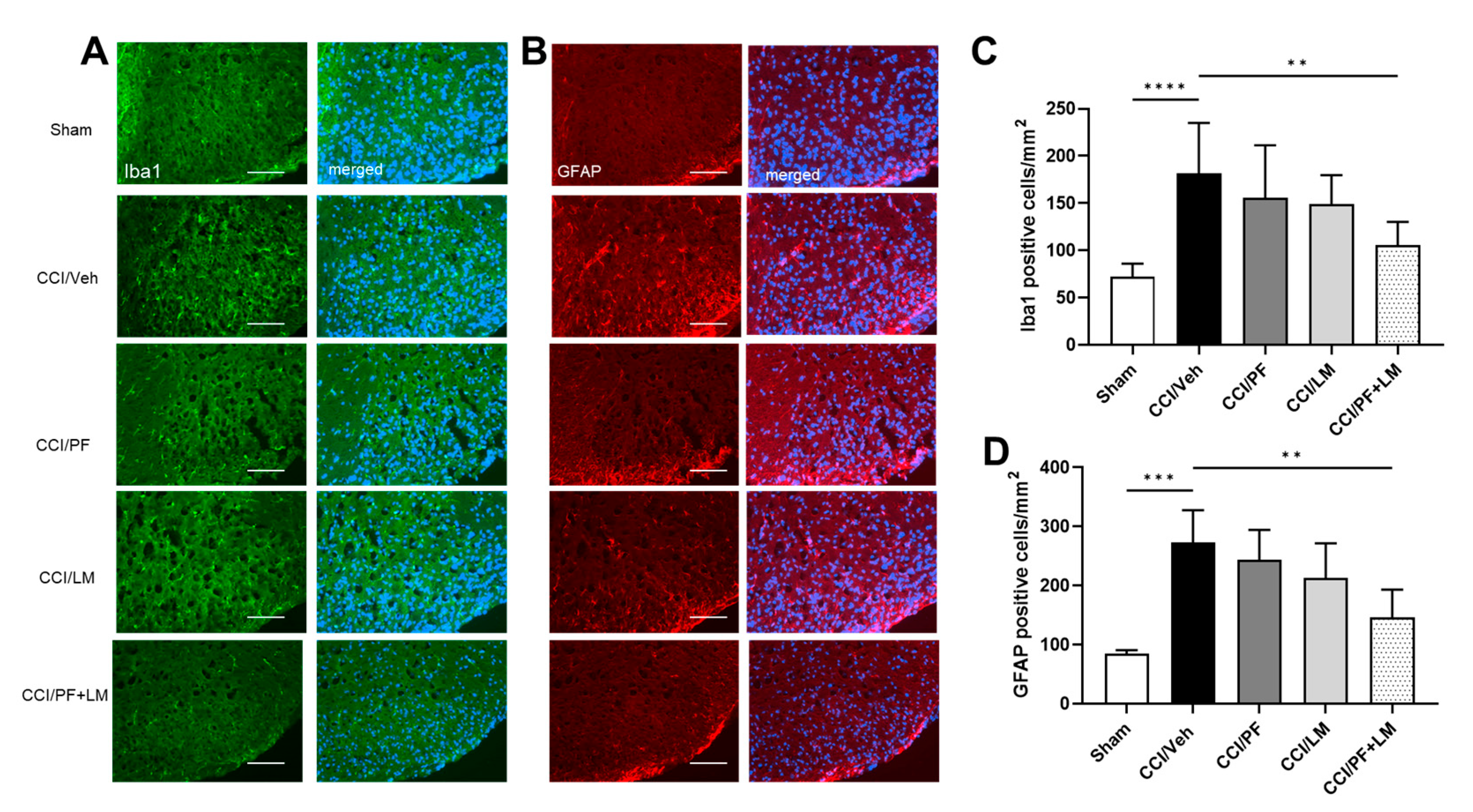
3.4. Combination of PF04457845 and LM4131 Suppressed Inflammatory Response in Sciatic Nerve and Spinal Cord
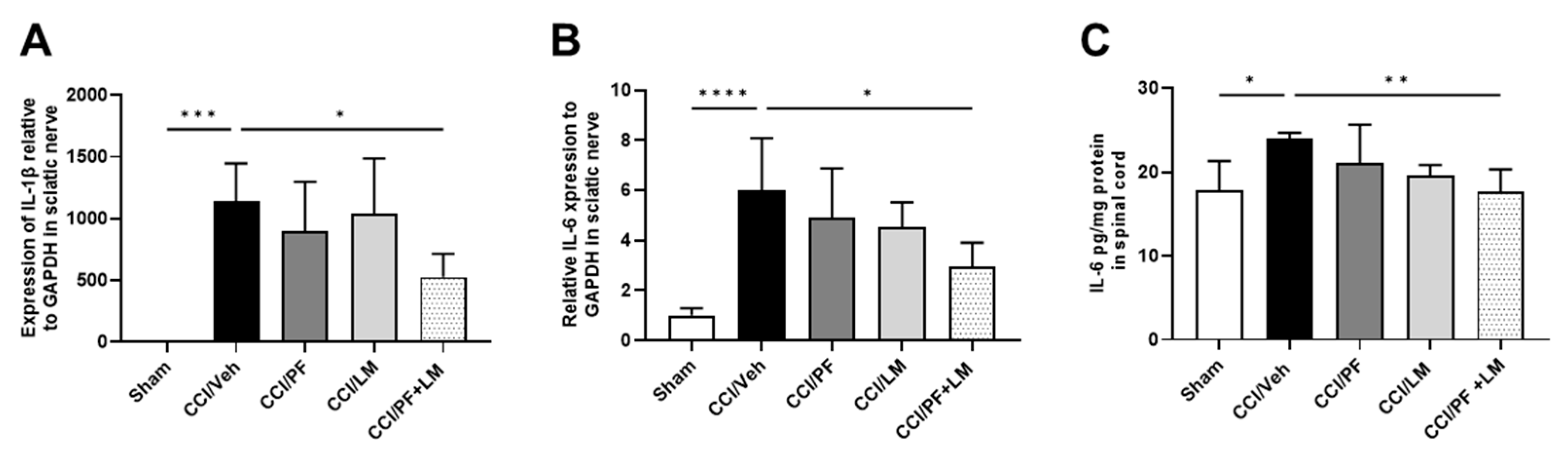
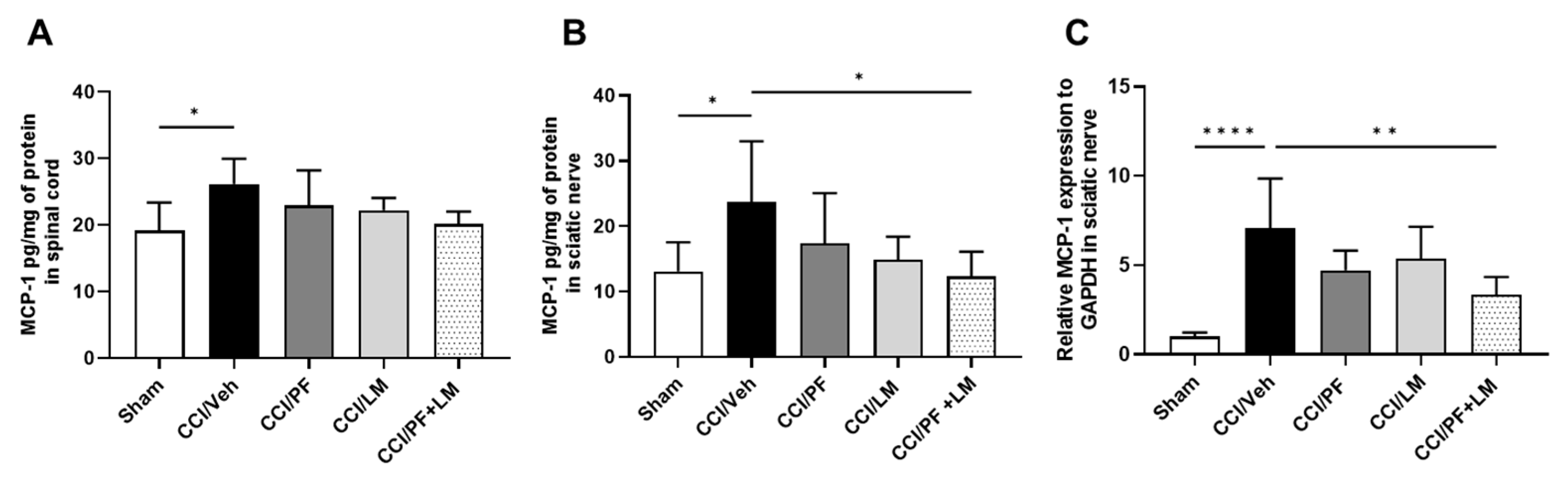
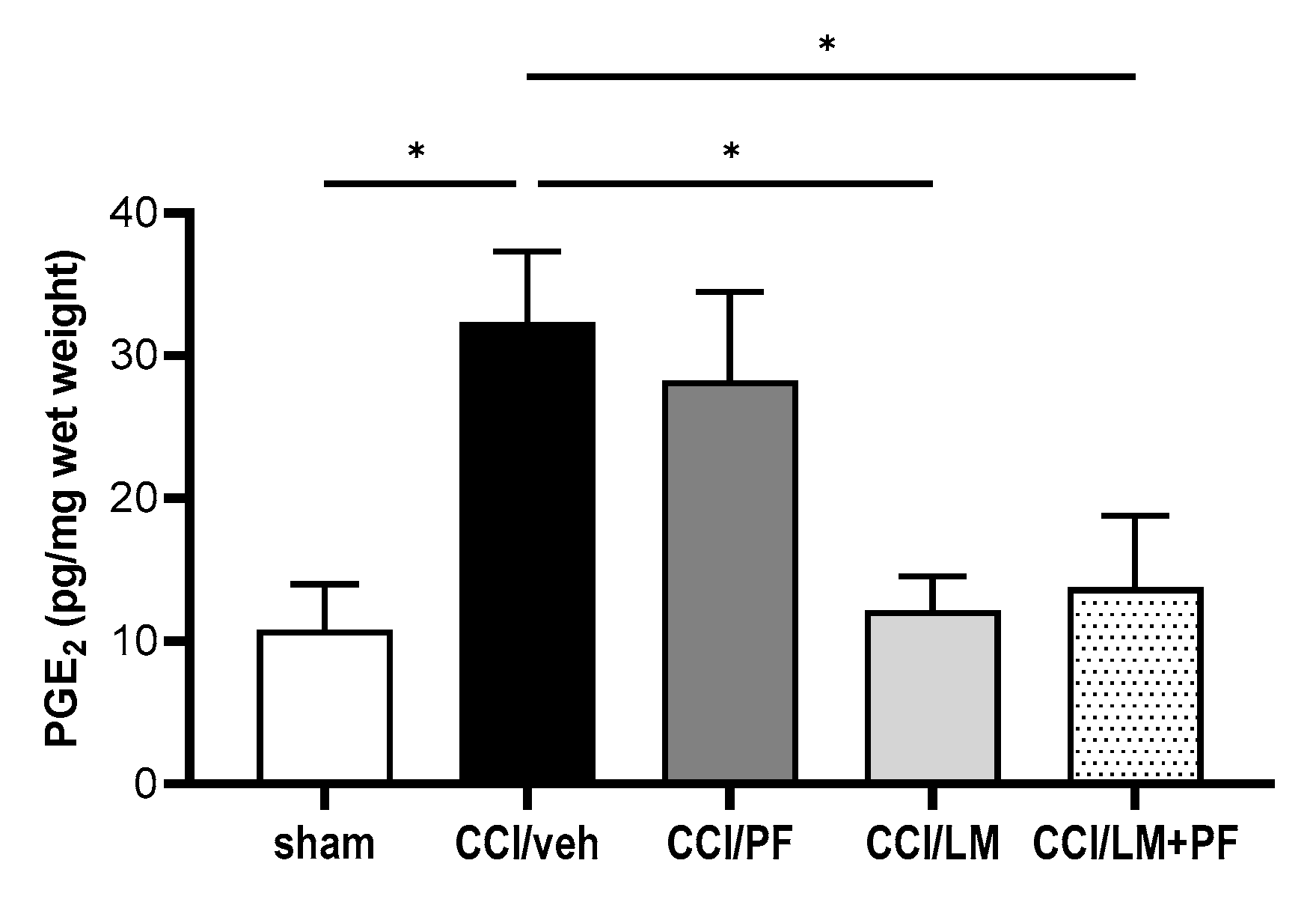
3.5. PF04457845 and LM4131 Co-Treatment Attenuated the Inflammatory Cytokines/Chemokines Levels in the Sciatic Nerve and Spinal Cord
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donvito, G.; Nass, S.; Wilkerson, J.; Curry, Z.; Schurman, L.; Kinsey, S.G.; Lichtman, A.H. The Endogenous Cannabinoid System: A Budding Source of Targets for Treating Inflammatory and Neuropathic Pain. Neuropsychopharmacology 2018, 43, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Rahn, E.J.; Hohmann, A.G. Cannabinoids as pharmacotherapies for neuropathic pain: From the bench to the bedside. Neurother. J. Am. Soc. Exp. NeuroTher. 2009, 6, 713–737. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol. 2009, 156, 397–411. [Google Scholar] [CrossRef]
- Manzanares, J.; Julian, M.; Carrascosa, A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr. Neuropharmacol. 2006, 4, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, S.G.; Chapman, V.; Finn, D.P.; Hohmann, A.G.; Neugebauer, V. The cannabinoid system and pain. Neuropharmacology 2017, 124, 105–120. [Google Scholar] [CrossRef]
- Hwang, J.; Adamson, C.; Butler, D.; Janero, D.R.; Makriyannis, A.; Bahr, B.A. Enhancement of endocannabinoid signaling by fatty acid amide hydrolase inhibition: A neuroprotective therapeutic modality. Life Sci. 2010, 86, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Savinainen, J.R.; Saario, S.M.; Laitinen, J.T. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol. 2012, 204, 267–276. [Google Scholar] [CrossRef]
- Ueda, N.; Tsuboi, K.; Uyama, T.; Ohnishi, T. Biosynthesis and degradation of the endocannabinoid 2-arachidonoylglycerol. Biofactors 2011, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Toczek, M.; Malinowska, B. Enhanced endocannabinoid tone as a potential target of pharmacotherapy. Life Sci. 2018, 204, 20–45. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. Inhibitors of endocannabinoid breakdown for pain: Not so FA(AH)cile, after all. Pain 2012, 153, 1785–1786. [Google Scholar] [CrossRef]
- Huggins, J.P.; Smart, T.S.; Langman, S.; Taylor, L.; Young, T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to in-duce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain 2012, 153, 1837–1846. [Google Scholar]
- Scarpelli, R.; Sasso, O.; Piomelli, D.A. Double Whammy: Targeting Both Fatty Acid Amide Hydrolase (FAAH) and Cy-clooxygenase (COX) To Treat Pain and Inflammation. ChemMedChem 2016, 11, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Rouzer, C.A.; Marnett, L.J. Endocannabinoid Oxygenation by Cyclooxygenases, Lipoxygenases, and Cytochromes P450: Cross-Talk between the Eicosanoid and Endocannabinoid Signaling Pathways. Chem. Rev. 2011, 111, 5899–5921. [Google Scholar] [CrossRef]
- Gatta, L.; Piscitelli, F.; Giordano, C.; Boccella, S.; Lichtman, A.H.; Maione, S.; Di Marzo, V. Discovery of prostamide F2alpha and its role in inflam-matory pain and dorsal horn nociceptive neuron hyperexcitability. PLoS ONE 2012, 7, e31111. [Google Scholar] [CrossRef] [PubMed]
- Sang, N.; Zhang, J.; Chen, C. COX-2 oxidative metabolite of endocannabinoid 2-AG enhances excitatory glutamatergic synaptic transmission and induces neurotoxicity. J. Neurochem. 2007, 102, 1966–1977. [Google Scholar] [CrossRef]
- Duggan, K.C.; Hermanson, D.J.; Musee, J.; Prusakiewicz, J.J.; Scheib, J.L.; Carter, B.D.; Banerjee, S.; Oates, J.A.; Marnett, L.J. (R)-Profens are substrate-selective inhibitors of endocannabinoid oxygenation by COX-2. Nat. Chem. Biol. 2011, 7, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Bishay, P.; Schmidt, H.; Marian, C.; Häussler, A.; Wijnvoord, N.; Ziebell, S.; Metzner, J.; Koch, M.; Myrczek, T.; Bechmann, I.; et al. R-Flurbiprofen Reduces Neuropathic Pain in Rodents by Restoring Endogenous Cannabinoids. PLoS ONE 2010, 5, e10628. [Google Scholar] [CrossRef]
- Schmitz, K.; de Bruin, N.; Bishay, P.; Männich, J.; Häussler, A.; Altmann, C.; Ferreirós, N.; Lotsch, J.; Ultsch, A.; Parnham, M.J.; et al. R-flurbiprofen attenuates experimental autoimmune encephalomyelitis in mice. EMBO Mol. Med. 2014, 6, 1398–1422. [Google Scholar] [CrossRef]
- Hermanson, D.J.; Hartley, N.D.; Gamble-George, J.; Brown, N.; Shonesy, B.C.; Kingsley, P.J.; Colbran, R.J.; Reese, J.; Marnett, L.J.; Patel, S. Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation. Nat. Neurosci. 2013, 16, 1291–1298. [Google Scholar] [CrossRef]
- Jones, M.; Wen, J.; Selvaraj, P.; Tanaka, M.; Moran, S.; Zhang, Y. Therapeutic Effect of the Substrate-Selective COX-2 Inhibitor IMMA in the Animal Model of Chronic Constriction Injury. Front. Pharmacol. 2018, 9, 1481. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, D.J.; Gamble-George, J.C.; Marnett, L.J.; Patel, S. Substrate-selective COX-2 inhibition as a novel strategy for therapeutic endocannabinoid augmentation. Trends Pharmacol. Sci. 2014, 35, 358–367. [Google Scholar] [CrossRef]
- Grim, T.W.; Ghosh, S.; Hsu, K.L.; Cravatt, B.F.; Kinsey, S.G.; Lichtman, A.H. Combined inhibition of FAAH and COX produces en-hanced anti-allodynic effects in mouse neuropathic and inflammatory pain models. Pharmacol. Biochem. Behav. 2014, 124, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Beaulieu, P. Antihyperalgesic effects of local injections of anandamide, ibuprofen, rofecoxib and their com-binations in a model of neuropathic pain. Neuropharmacology 2006, 50, 814–823. [Google Scholar] [CrossRef]
- Malek, N.; Starowicz, K. Dual-Acting Compounds Targeting Endocannabinoid and Endovanilloid Systems—A Novel Treatment Option for Chronic Pain Management. Front. Pharmacol. 2016, 7, 257. [Google Scholar] [CrossRef]
- Wen, J.; Jones, M.; Tanaka, M.; Selvaraj, P.; Symes, A.J.; Cox, B.; Zhang, Y. WWL70 protects against chronic constriction injury-induced neuropathic pain in mice by cannabinoid receptor-independent mechanisms. J. Neuroinflammation 2018, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Smits, H.; Ultenius, C.; Deumens, R.; Koopmans, G.C.; Honig, W.; van Kleef, M.; Linderoth, B.; Joosten, E. Effect of spinal cord stimulation in an animal model of neuropathic pain relates to degree of tactile “allodynia”. Neuroscience 2006, 143, 541–546. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Ahn, K.; Smith, S.E.; Liimatta, M.B.; Beidler, D.; Sadagopan, N.; Dudley, D.T.; Young, T.; Wren, P.; Zhang, Y.; Swaney, S.; et al. Mechanistic and pharmacological characterization of PF-04457845: A highly potent and selective fatty acid amide hydrolase inhibitor that reduces inflammatory and nonin-flammatory pain. J. Pharmacol. Exp. Ther. 2011, 338, 114–124. [Google Scholar] [CrossRef]
- Ji, R.-R.; Chamessian, A.; Zhang, Y.-Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Lichtman, A.H. The endogenous cannabinoid system and its role in nociceptive behavior. J. Neurobiol. 2004, 61, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Price, D.D.; Lu, J.; Keniston, L.; Mayer, D.J. Two distinctive antinociceptive systems in rats with pathological pain. Neurosci. Lett. 2000, 280, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Sagar, D.R.; Gaw, A.G.; Okine, B.N.; Woodhams, S.G.; Wong, A.; A Kendall, D.; Chapman, V. Dynamic Regulation of the Endocannabinoid System: Implications for Analgesia. Mol. Pain 2009, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.; Haroutounian, S.; Hohmann, A.G.; Krane, E.; Liao, J.; Macleod, M.; Segelcke, D.; Sena, C.; Thomas, J.; Vollert, J.; et al. Systematic review and meta-analysis of cannabinoids, cannabis-based medicines, and endocannabinoid system modulators tested for antinociceptive effects in animal models of injury-related or pathological persistent pain. Pain 2021, 162, S26–S44. [Google Scholar] [CrossRef]
- Morgan, A.J.; Kingsley, P.J.; Mitchener, M.M.; Altemus, M.; Patrick, T.A.; Gaulden, A.D.; Marnett, L.J.; Patel, S. Detection of Cyclooxygenase-2-Derived Oxygenation Products of the Endogenous Cannabinoid 2-Arachidonoylglycerol in Mouse Brain. ACS Chem. Neurosci. 2018, 9, 1552–1559. [Google Scholar] [CrossRef]
- Carullo, G.; Galligano, F.; Aiello, F. Structure-activity relationships for the synthesis of selective cyclooxygenase 2 inhibi-tors: An overview (2009–2016). Medchemcomm 2016, 8, 492–500. [Google Scholar] [CrossRef]
- Starowicz, K.; Finn, D.P. Cannabinoids and Pain: Sites and Mechanisms of Action. Adv. Pharmacol. 2017, 80, 437–475. [Google Scholar]
- Drew, G.M.; Lau, B.K.; Vaughan, C.W. Substance P drives endocannabinoid-mediated disinhibition in a midbrain de-scending analgesic pathway. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 7220–7229. [Google Scholar] [CrossRef]
- Mitrirattanakul, S.; Ramakul, N.; Guerrero, A.V.; Matsuka, Y.; Ono, T.; Iwase, H.; Mackie, K.; Faull, K.F.; Spigelman, I. Site-specific increases in peripheral cannabinoid receptors and their endogenous ligands in a model of neuropathic pain. Pain 2006, 126, 102–114. [Google Scholar] [CrossRef]
- Caprioli, A.; Coccurello, R.; Rapino, C.; Di Serio, S.; Di Tommaso, M.; Vertechy, M.; Vacca, V.; Battista, N.; Pavone, F.; Maccarrone, M.; et al. The Novel Reversible Fatty Acid Amide Hydrolase Inhibitor ST4070 Increases Endocannabinoid Brain Levels and Counteracts Neuropathic Pain in Different Animal Models. J. Pharmacol. Exp. Therapeutics 2012, 342, 188–195. [Google Scholar] [CrossRef]
- Russo, R.; Loverme, J.; La Rana, G.; Compton, T.R.; Parrott, J.; Duranti, A.; Tontini, A.; Mor, M.; Tarzia, G.; Calignano, A.; et al. The fatty acid amide hydrolase inhibitor URB597 (cyclo-hexylcarbamic acid 3’-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J. Pharmacol. Exp. Ther. 2007, 322, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Schlosburg, J.E.; Carlson, B.L.A.; Ramesh, D.; Abdullah, R.A.; Long, J.Z.; Cravatt, B.F.; Lichtman, A.H. Inhibitors of Endocannabinoid-Metabolizing Enzymes Reduce Precipitated Withdrawal Responses in THC-Dependent Mice. AAPS J. 2009, 11, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Mika, J.; Zychowska, M.; Popiolek-Barczyk, K.; Rojewska, E.; Przewlocka, B. Importance of glial activation in neuropathic pain. Eur. J. Pharmacol. 2013, 716, 106–119. [Google Scholar] [CrossRef]
- Oprée, A.; Kress, M. Involvement of the Proinflammatory Cytokines Tumor Necrosis Factor-α, IL-1β, and IL-6 But Not IL-8 in the Development of Heat Hyperalgesia: Effects on Heat-Evoked Calcitonin Gene-Related Peptide Release from Rat Skin. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 6289–6293. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, R.; Tilley, D.M.; Vogel, L.; Benyamin, R. The Role of Glia and the Immune System in the Development and Maintenance of Neuropathic Pain. Pain Pract. 2010, 10, 167–184. [Google Scholar] [CrossRef]
- Honore, P.; Wade, C.L.; Zhong, C.; Harris, R.R.; Wu, C.; Ghayur, T.; Iwakura, Y.; Decker, M.W.; Faltynek, C.; Sullivan, J.; et al. Interleukin-1alphabeta gene-deficient mice show reduced nociceptive sensitivity in models of inflammatory and neuropathic pain but not post-operative pain. Behav. Brain Res. 2006, 167, 355–364. [Google Scholar] [CrossRef]
- Johnston, I.N.; Milligan, E.D.; Wieseler-Frank, J.; Frank, M.G.; Zapata, V.; Campisi, J.; Langer, S.; Martin, D.; Green, P.; Fleshner, M.; et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J. Neurosci. Off. J. of Soc. Neurosci. 2004, 24, 7353–7365. [Google Scholar] [CrossRef]
- Wolf, G.; Gabay, E.; Tal, M.; Yirmiya, R.; Shavit, Y. Genetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in mice. Pain 2006, 120, 315–324. [Google Scholar] [CrossRef]
- Zhou, Y.-Q.; Liu, Z.; Liu, Z.-H.; Chen, S.-P.; Li, M.; Shahveranov, A.; Ye, D.-W.; Tian, Y.-K. Interleukin-6: An emerging regulator of pathological pain. J. Neuroinflammation 2016, 13, 1–9. [Google Scholar] [CrossRef]
- DeLeo, J.A.; Colburn, R.W.; Nichols, M.; Malhotra, A. Interleukin-6-Mediated Hyperalgesia/Allodynia and Increased Spinal IL-6 Expression in a Rat Mononeuropathy Model. J. Interf. Cytokine Res. 1996, 16, 695–700. [Google Scholar] [CrossRef]
- Murphy, P.G.; Ramer, M.S.; Borthwick, L.; Gauldie, J.; Richardson, P.M.; Bisby, M.A. Endogenous interleukin-6 contributes to hy-persensitivity to cutaneous stimuli and changes in neuropeptides associated with chronic nerve constriction in mice. Eur. J. Neurosci. 1999, 11, 2243–2253. [Google Scholar] [CrossRef]
- Arruda, J.L.; Colburn, R.W.; Rickman, A.J.; Rutkowski, M.D.; A DeLeo, J. Increase of interleukin-6 mRNA in the spinal cord following peripheral nerve injury in the rat: Potential role of IL-6 in neuropathic pain. Mol. Brain Res. 1998, 62, 228–235. [Google Scholar] [CrossRef]
- Ramer, M.S.; Murphy, P.G.; Richardson, P.M.; Bisby, M.A. Spinal nerve lesion-induced mechanoallodynia and adren-ergic sprouting in sensory ganglia are attenuated in interleukin-6 knockout mice. Pain 1998, 78, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, X.Q.; Echeverry, S.; Mogil, J.S.; De Koninck, Y.; Rivest, S. Expression of CCR2 in both resident and bone mar-row-derived microglia plays a critical role in neuropathic pain. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 12396–12406. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-J.; Ji, R.-R. Chemokines, neuronal–glial interactions, and central processing of neuropathic pain. Pharmacol. Ther. 2010, 126, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Thacker, M.A.; Clark, A.K.; Marchand, F.; McMahon, S.B. Pathophysiology of Peripheral Neuropathic Pain: Immune Cells and Molecules. Anesthesia Analg. 2007, 105, 838–847. [Google Scholar] [CrossRef]
- Echeverry, S.; Shi, X.Q.; Rivest, S.; Zhang, J. Peripheral nerve injury alters blood-spinal cord barrier functional and mo-lecular integrity through a selective inflammatory pathway. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 10819–10828. [Google Scholar] [CrossRef]
- Buisseret, B.; Alhouayek, M.; Guillemot-Legris, O.; Muccioli, G.G. Endocannabinoid and Prostanoid Crosstalk in Pain. Trends Mol. Med. 2019, 25, 882–896. [Google Scholar] [CrossRef]
- Gamble-George, J.C.; Baldi, R.; Halladay, L.; Kocharian, A.; Hartley, N.; Silva, C.G.; Roberts, H.; Haymer, A.; Marnett, L.J.; Holmes, A.; et al. Cyclooxygenase-2 inhibition reduces stress-induced affective pathology. eLife 2016, 5, e14137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, J.; Sackett, S.; Tanaka, M.; Zhang, Y. Therapeutic Effects of Combined Treatment with the AEA Hydrolysis Inhibitor PF04457845 and the Substrate Selective COX-2 Inhibitor LM4131 in the Mouse Model of Neuropathic Pain. Cells 2023, 12, 1275. https://doi.org/10.3390/cells12091275
Wen J, Sackett S, Tanaka M, Zhang Y. Therapeutic Effects of Combined Treatment with the AEA Hydrolysis Inhibitor PF04457845 and the Substrate Selective COX-2 Inhibitor LM4131 in the Mouse Model of Neuropathic Pain. Cells. 2023; 12(9):1275. https://doi.org/10.3390/cells12091275
Chicago/Turabian StyleWen, Jie, Scott Sackett, Mikiei Tanaka, and Yumin Zhang. 2023. "Therapeutic Effects of Combined Treatment with the AEA Hydrolysis Inhibitor PF04457845 and the Substrate Selective COX-2 Inhibitor LM4131 in the Mouse Model of Neuropathic Pain" Cells 12, no. 9: 1275. https://doi.org/10.3390/cells12091275
APA StyleWen, J., Sackett, S., Tanaka, M., & Zhang, Y. (2023). Therapeutic Effects of Combined Treatment with the AEA Hydrolysis Inhibitor PF04457845 and the Substrate Selective COX-2 Inhibitor LM4131 in the Mouse Model of Neuropathic Pain. Cells, 12(9), 1275. https://doi.org/10.3390/cells12091275





