One Stage Masquelets Technique: Evaluation of Different Forms of Membrane Filling with and without Bone Marrow Mononuclear Cells (BMC) in Large Femoral Bone Defects in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animal Care and Group Setup
2.3. BMC Isolation and Scaffold Seeding
2.4. Surgical Procedure
2.5. µCT (Microcomputertomographie)
2.6. Mechanical Testing
2.7. Histology
2.8. SEM
2.9. Statistics
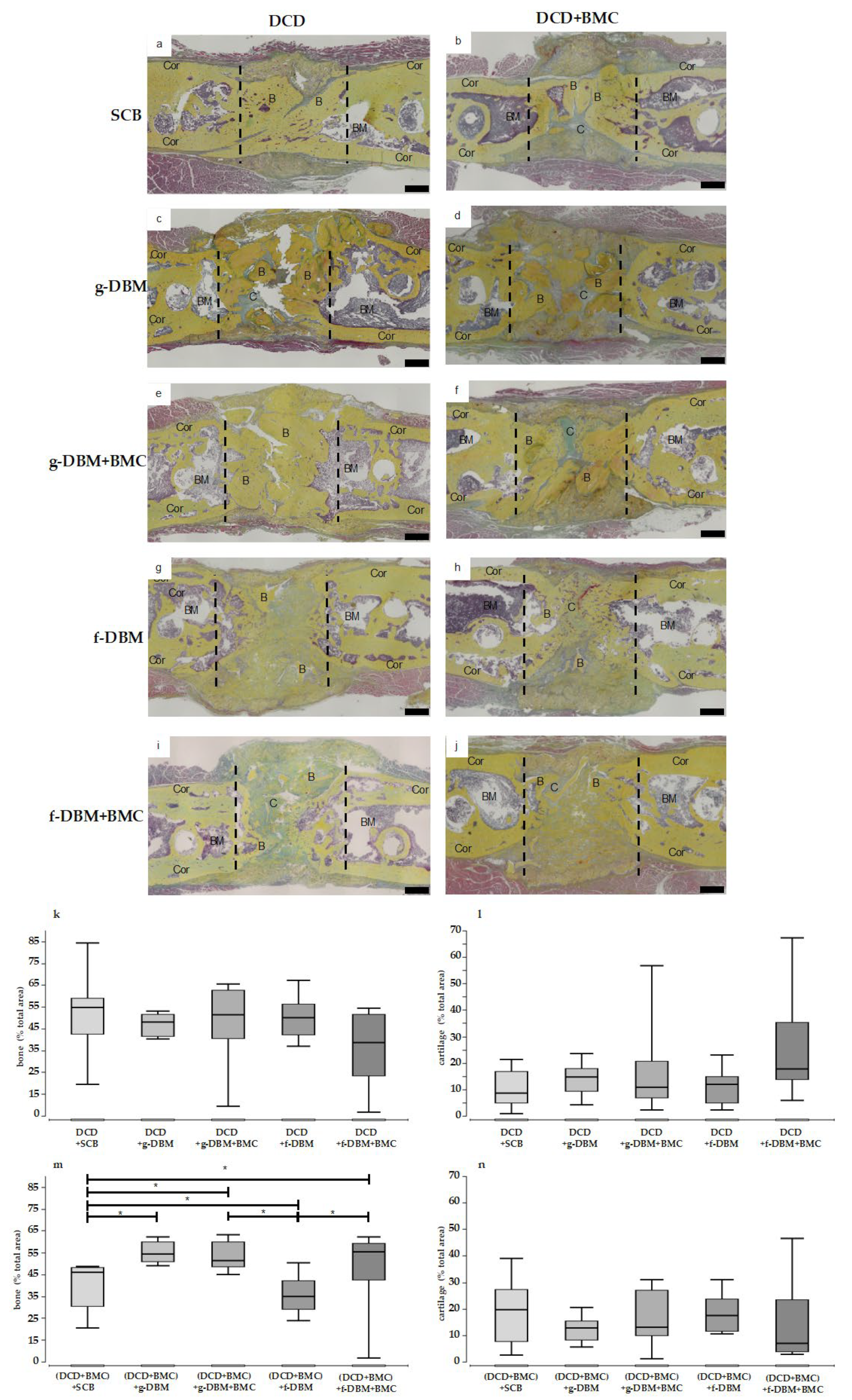
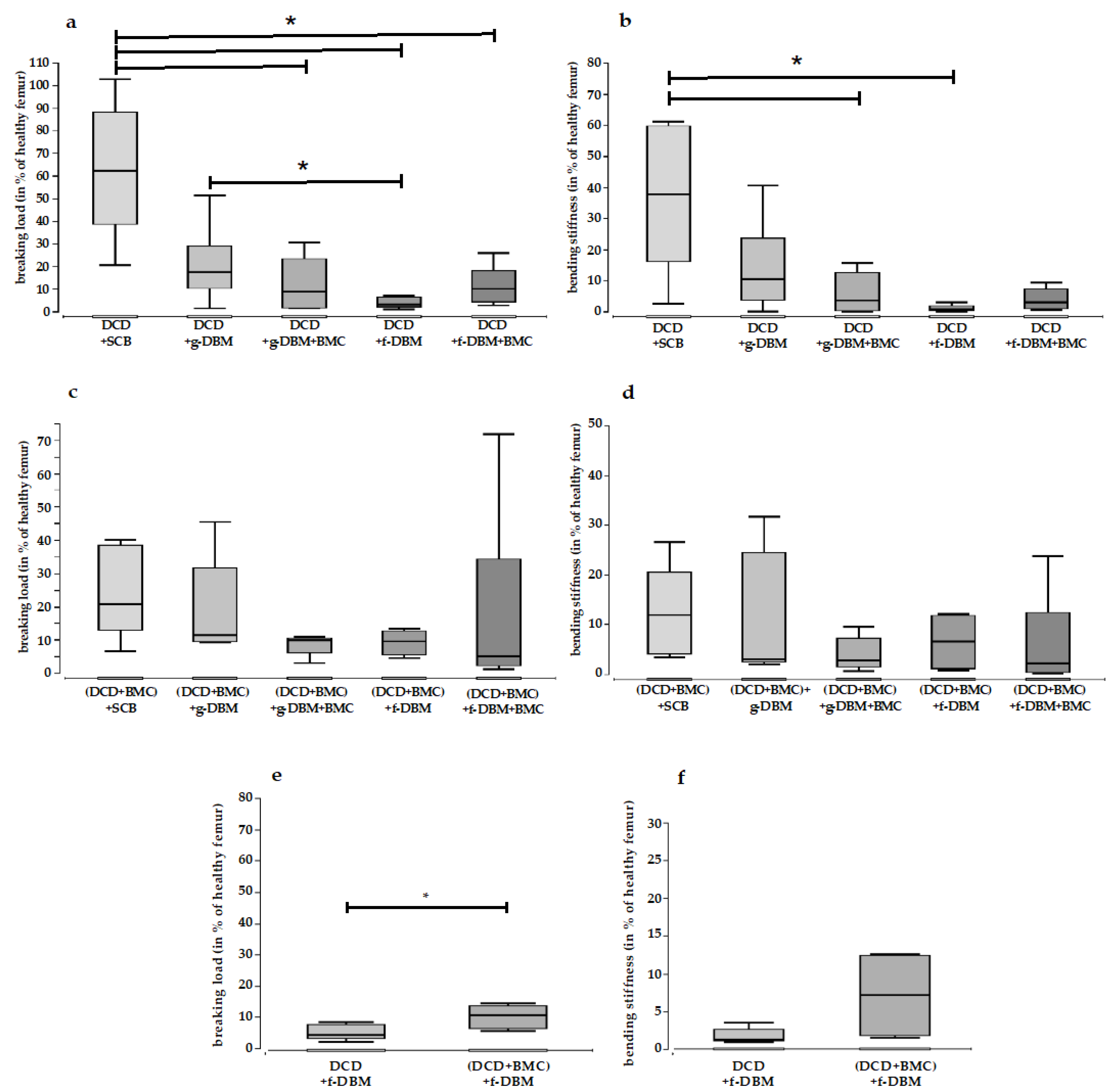
3. Results
3.1. Animal Care and Complications
3.2. Cell Seeding and Seeding Efficacy
3.3. Bone Formation—Bone Score and Bone Mineral Density
3.4. Mechanical Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pelissier, P.; Bollecker, V.; Martin, D.; Baudet, J. Foot reconstruction with the bi-Masquelet procedure. Ann. Chir. Plast. Esthet. 2002, 47, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Masquelet, A.C. Muscle reconstruction in reconstructive surgery: Soft tissue repair and long bone reconstruction. Langenbeck’s Arch. Surg. 2003, 388, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Paradowski, P.T.; Sadzikowski, K.; Majewski, P.; Szczepaniec, M. Multi-stage treatment for malunion and avascular necrosis of the femoral head following reverse oblique pertrochanteric fracture: A case report and literature review. Trauma Case Rep. 2022, 41, 100684. [Google Scholar] [CrossRef] [PubMed]
- Chloros, G.D.; Kanakaris, N.K.; Harwood, P.J.; Giannoudis, P.V. Induced membrane technique for acute bone loss and nonunion management of the tibia. OTA Int. Open Access J. Orthop. Trauma 2022, 5, e170. [Google Scholar] [CrossRef]
- Biau, D.J.; Pannier, S.; Masquelet, A.C.; Glorion, C. Case report: Reconstruction of a 16-cm diaphyseal defect after Ewing’s resection in a child. Clin. Orthop. Relat. Res. 2009, 467, 572–577. [Google Scholar] [CrossRef]
- Janko, M.; Sahm, J.; Schaible, A.; Brune, J.C.; Bellen, M.; Schröder, K.; Seebach, C.; Marzi, I.; Henrich, D.; Schroder, K.; et al. Comparison of three different types of scaffolds preseeded with human bone marrow mononuclear cells on the bone healing in a femoral critical size defect model of the athymic rat. J. Tissue Eng. Regen. Med. 2017, 12, 653–666. [Google Scholar] [CrossRef]
- Wildemann, B.; Kadow-Romacker, A.; Haas, N.P.; Schmidmaier, G. Quantification of various growth factors in different demineralized bone matrix preparations. J. Biomed. Mater. Res. Part A 2007, 81A, 437–442. [Google Scholar] [CrossRef]
- Hammoudeh, J.A.; Fahradyan, A.; Gould, D.J.; Liang, F.; Imahiyerobo, T.; Urbinelli, L.; Nguyen, J.A.T.; Magee, W.; Yen, S.; Urata, M.M. A comparative analysis of recombinant human bone morphogenetic protein-2 with a demineralized bone matrix versus iliac crest bone graft for secondary alveolar bone grafts in patients with cleft lip and palate: Review of 501 cases. Plast. Reconstr. Surg. 2017, 140, 318e–325e. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Orth, C.; Willershausen, I.; Thimm, B.W.; Hoffmann, C.; Rasic, A.; Sader, R.A.; Unger, R.E.; Peters, F.; et al. Influence of β-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater. 2010, 6, 4476–4487. [Google Scholar] [CrossRef]
- Jensen, S.S.; Aaboe, M.; Janner, S.F.M.; Saulacic, N.; Bornstein, M.M.; Bosshardt, D.D.; Buser, D. Influence of particle size of deproteinized bovine bone mineral on new bone formation and implant stability after simultaneous sinus floor elevation: A histomorphometric study in minipigs. Clin. Implant. Dent. Relat. Res. 2015, 17, 274–285. [Google Scholar] [CrossRef]
- Sun, J.S.; Tsuang, Y.H.; Liao, C.J.; Liu, H.C.; Hang, Y.S.; Lin, F.H. The effect of sintered β-dicalcium pyrophosphate particle size on newborn wistar rat osteoblasts. Artif. Organs 1999, 23, 331–338. [Google Scholar] [CrossRef]
- Leiblein, M.; Winkenbach, A.; Koch, E.; Schaible, A.; Büchner, H.; Marzi, I.; Henrich, D.; Nau, C. Impact of scaffold granule size use in Masquelet technique on periosteal reaction: A study in rat femur critical size bone defect model. Eur. J. Trauma Emerg. Surg. 2020, 48, 679–687. [Google Scholar] [CrossRef]
- Leiblein, M.; Koch, E.; Winkenbach, A.; Schaible, A.; Nau, C.; Büchner, H.; Schröder, K.; Marzi, I.; Henrich, D. Size matters: Effect of granule size of the bone graft substitute (Herafill®) on bone healing using Masquelet’s induced membrane in a critical size defect model in the rat’s femur. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1469–1482. [Google Scholar] [CrossRef]
- Seebach, C.; Schultheiss, J.; Wilhelm, K.; Frank, J.; Henrich, D. Comparison of six bone-graft substitutes regarding to cell seeding efficiency, metabolism and growth behaviour of human mesenchymal stem cells (MSC) in vitro. Injury 2010, 41, 731–738. [Google Scholar] [CrossRef]
- Schultheiss, J.; Seebach, C.; Henrich, D.; Wilhelm, K.; Barker, J.H.; Frank, J. Mesenchymal stem cell (MSC) and endothelial progenitor cell (EPC) growth and adhesion in six different bone graft substitutes. Eur. J. Trauma Emerg. Surg. 2011, 37, 635–644. [Google Scholar] [CrossRef]
- Henrich, D.; Verboket, R.R.; Schaible, A.; Kontradowitz, K.; Oppermann, E.; Brune, J.C.; Nau, C.; Meier, S.; Bonig, H.; Marzi, I.; et al. Characterization of bone marrow mononuclear cells on biomaterials for bone tissue engineering in vitro. Biomed. Res. Int. 2015, 2015, 762407. [Google Scholar]
- Seebach, C.; Henrich, D.; Schaible, A.; Relja, B.; Jugold, M.; Bönig, H.; Marzi, I. Cell-Based Therapy by Implanted Human Bone Marrow-Derived Mononuclear Cells Improved Bone Healing of Large Bone Defects in Rats. Tissue Eng. Part A 2015, 21, 1565–1578. [Google Scholar] [CrossRef]
- Verboket, R.; Leiblein, M.; Seebach, C.; Nau, C.; Janko, M.; Bellen, M.; Bonig, H.; Henrich, D.; Marzi, I.; Bönig, H.; et al. Autologous cell-based therapy for treatment of large bone defects: From bench to bedside. Eur. J. Trauma Emerg. Surg. 2018, 44, 649–665. [Google Scholar] [CrossRef]
- Verboket, R.D.; Söhling, N.; Heilani, M.; Fremdling, C.; Schaible, A.; Schröder, K.; Brune, J.C.; Marzi, I.; Henrich, D. The Induced Membrane Technique—The Filling Matters: Evaluation of Different Forms of Membrane Filling with and without Bone Marrow Mononuclear Cells (BMC) in Large Femoral Bone Defects in Rats. Biomedicines 2022, 10, 642. [Google Scholar] [CrossRef]
- Nau, C.; If, T.D.; Seebach, C.; Trumm, A.; Schaible, A.; Kontradowitz, K.; Meier, S.; Buechner, H.; Marzi, I.; Henrich, D. Alteration of Masquelet’s induced membrane characteristics by different kinds of antibiotic enriched bone cement in a critical size defect model in the rat’s femur. Injury 2016, 47, 325–334. [Google Scholar] [CrossRef]
- Gindraux, F.; Loisel, F.; Bourgeois, M.; Oudina, K.; Melin, M.; de Billy, B.; Sergent, P.; Leclerc, G.; Petite, H.; Auber, F.; et al. Induced membrane maintains its osteogenic properties even when the second stage of Masquelet’s technique is performed later. Eur. J. Trauma Emerg. Surg. 2020, 46, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Gessmann, J.; Rosteius, T.; Baecker, H.; Sivalingam, K.; Peter, E.; Schildhauer, T.A.; Köller, M. Is the bioactivity of induced membranes time dependent? Eur. J. Trauma Emerg. Surg. 2021, 48, 3051–3061. [Google Scholar] [CrossRef] [PubMed]
- Owston, H.E.; Moisley, K.M.; Tronci, G.; Russell, S.J.; Giannoudis, P.V.; Jones, E. Induced periosteum-mimicking membrane with cell barrier and multipotential stromal cell (MSC) homing functionalities. Int. J. Mol. Sci. 2020, 21, 5233. [Google Scholar] [CrossRef] [PubMed]
- Verboket, R.D.; Leiblein, M.; Janko, M.; Schaible, A.; Brune, J.C.; Schröder, K.; Heilani, M.; Fremdling, C.; Busche, Y.; Irrle, T.; et al. From two stages to one: Acceleration of the induced membrane (Masquelet) technique using human acellular dermis for the treatment of non-infectious large bone defects. Eur. J. Trauma Emerg. Surg. 2020, 46, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Pruss, A.; Göbel, U.B.; Pauli, G.; Kao, M.; Seibold, M.; Mönig, H.J.; Hansen, A.; von Versen, R. Peracetic acid-ethanol treatment of allogeneic avital bone tissue transplants--a reliable sterilization method. Ann. Transplant. 2003, 8, 34–42. [Google Scholar]
- Henrich, D.; Seebach, C.; Verboket, R.; Schaible, A.; Marzi, I.; Bonig, H. The osteo-inductive activity of bone-marrow-derived mononuclear cells resides within the CD14+ population and is independent of the CD34+ population. Eur. Cell. Mater. 2018, 35, 165–177. [Google Scholar] [CrossRef]
- Verboket, R.D.; Irrle, T.; Busche, Y.; Schaible, A.; Schröder, K.; Brune, J.C.; Marzi, I.; Nau, C.; Henrich, D. Fibrous demineralized bone matrix (Dbm) improves bone marrow mononuclear cell (bmc)-supported bone healing in large femoral bone defects in rats. Cells 2021, 10, 1249. [Google Scholar] [CrossRef]
- Keller, T.S.; Mao, Z.; Spengler, D.M. Young’s modulus, bending strength, and tissue physical properties of human compact bone. J. Orthop. Res. 1990, 8, 592–603. [Google Scholar] [CrossRef]
- Garvey, W.; Fathi, A.; Bigelow, F.; Carpenter, B.; Jimenez, C. Improved movat pentachrome stain. Biotech. Histochem. 1986, 61, 60–62. [Google Scholar] [CrossRef]
- Han, Z.; Bhavsar, M.; Leppik, L.; Oliveira, K.M.C.; Barker, J.H. Histological Scoring Method to Assess Bone Healing in Critical Size Bone Defect Models. Tissue Eng. Part C Methods 2018, 24, 272–279. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Y.; Sun, Y.; Qi, X.; Tian, L.; Zhao, Y.; Xu, Y.; Liu, X. Masquelet technique combined with artificial dermis for the treatment of bone and soft tissue defects in rabbits. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2019, 33, 578–585. [Google Scholar]
- Hisatome, T.; Yasunaga, Y.; Yanada, S.; Tabata, Y.; Ikada, Y.; Ochi, M. Neovascularization and bone regeneration by implantation of autologous bone marrow mononuclear cells. Biomaterials 2005, 26, 4550–4556. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, Y.; Zhang, C. The effect of bone marrow mononuclear cells on vascularization and bone regeneration in steroid-induced osteonecrosis of the femoral head. Jt. Bone Spine 2009, 76, 685–690. [Google Scholar] [CrossRef]
- Eldesoqi, K.; Henrich, D.; El-Kady, A.M.; Arbid, M.S.; Abd El-Hady, B.M.; Marzi, I.; Seebach, C. Safety evaluation of a bioglass-polylactic acid composite scaffold seeded with progenitor cells in a rat skull critical-size bone defect. PLoS ONE 2014, 9, e87642. [Google Scholar] [CrossRef]
- Nau, C.; Simon, S.; Schaible, A.; Seebach, C.; Schröder, K.; Marzi, I.; Henrich, D. Influence of the induced membrane filled with syngeneic bone and regenerative cells on bone healing in a critical size defect model of the rat’s femur. Injury 2018, 49, 1721–1731. [Google Scholar] [CrossRef]
- Ogle, M.E.; Segar, C.E.; Sridhar, S.; Botchwey, E.A. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp. Biol. Med. 2016, 241, 1084–1097. [Google Scholar] [CrossRef]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of Monocytes, Macrophages, and Dendritic Cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Kähling, C.; Wilhelm, K.; Tami, A.E.; Alini, M.; Marzi, I. Endothelial Progenitor Cells and Mesenchymal Stem Cells Seeded onto β-TCP Granules Enhance Early Vascularization and Bone Healing in a Critical-Sized Bone Defect in Rats. Tissue Eng. Part A 2010, 16, 1961–1970. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; Van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef]
- Hoff, P.; Gaber, T.; Strehl, C.; Jakstadt, M.; Hoff, H.; Schmidt-Bleek, K.; Lang, A.; Röhner, E.; Huscher, D.; Matziolis, G.; et al. A pronounced inflammatory activity characterizes the early fracture healing phase in immunologically restricted patients. Int. J. Mol. Sci. 2017, 18, 583. [Google Scholar] [CrossRef]
- Schlundt, C.; Reinke, S.; Geissler, S.; Bucher, C.H.; Giannini, C.; Märdian, S.; Dahne, M.; Kleber, C.; Samans, B.; Baron, U.; et al. Individual effector/regulator T cell ratios impact bone regeneration. Front. Immunol. 2019, 10, 1954. [Google Scholar] [CrossRef] [PubMed]
- Söhling, N.; Leiblein, M.; Schaible, A.; Janko, M.; Schwäble, J.; Seidl, C.; Brune, J.; Nau, C.; Marzi, I.; Henrich, D.; et al. First Human Leucocyte Antigen (HLA) Response and Safety Evaluation of Fibrous Demineralized Bone Matrix in a Critical Size Femoral Defect Model of the Sprague-Dawley Rat. Materials 2020, 13, 3120. [Google Scholar] [CrossRef] [PubMed]
- Söhling, N.; Ondreka, M.; Kontradowitz, K.; Reichel, T.; Marzi, I.; Henrich, D. Early immune response in foreign body reaction is im-plant/material specific. Materials 2022, 15, 2195. [Google Scholar] [CrossRef] [PubMed]
- Gruskin, E.; Doll, B.A.; Futrell, F.W.; Schmitz, J.P.; Hollinger, J.O. Demineralized bone matrix in bone repair: History and use. Adv. Drug Deliv. Rev. 2012, 64, 1063–1077. [Google Scholar] [CrossRef]
- Zhang, M.; Powers, R.M.; Wolfinbarger, L. Effect(s) of the Demineralization Process on the Osteoinductivity of Demineralized Bone Matrix. J. Periodontol. 1997, 68, 1085–1092. [Google Scholar] [CrossRef]
- Katz, J.M.; Nataraj, C.; Jaw, R.; Deigl, E.; Bursac, P. Demineralized bone matrix as an osteoinductive biomaterial and in vitro predictors of its biological potential. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 127–134. [Google Scholar] [CrossRef]
- Kular, J.; Tickner, J.; Chim, S.M.; Xu, J. An overview of the regulation of bone remodelling at the cellular level. Clin. Biochem. 2012, 45, 863–873. [Google Scholar] [CrossRef]
- Tachi, K.; Takami, M.; Sato, H.; Mochizuki, A.; Zhao, B.; Miyamoto, Y.; Tsukasaki, H.; Inoue, T.; Shintani, S.; Koike, T.; et al. Enhancement of bone morphogenetic protein-2-induced ectopic bone formation by transforming growth factor-β1. Tissue Eng. Part A 2011, 17, 597–606. [Google Scholar] [CrossRef]
- Khan, S.N.; Cammisa, F.P.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef]
- Urist, M.R.; Dawson, E. Intertransverse process fusion with the aid of chemosterilized autolyzed antigen-extracted allogeneic (AAA) bone. Clin. Orthop. Relat. Res. 1981, 154, 97–113. [Google Scholar] [CrossRef]
- Ghimire, S.; Miramini, S.; Edwards, G.; Rotne, R.; Xu, J.; Ebeling, P.; Zhang, L. The investigation of bone fracture healing under intramembranous and endochondral ossification. Bone Rep. 2021, 14, 100740. [Google Scholar] [CrossRef]
- Weber, F.E. Reconsidering Osteoconduction in the Era of Additive Manufacturing. Tissue Eng. Part B Rev. 2019, 25, 375–386. [Google Scholar] [CrossRef]
- Schouten, C.C.; Hartman, E.H.M.; Spauwen, P.H.M.; Jansen, J.A. DBM induced ectopic bone formation in the rat: The importance of surface area. J. Mater. Sci. Mater. Med. 2005, 16, 149–152. [Google Scholar] [CrossRef]
- Sanaei, R.; Abu, J.; Nazari, M.; Zuki, M.A.B.; Allaudin, Z.N. Evaluation of Osteogenic Potentials of Avian Demineralized Bone Matrix in the Healing of Osseous Defects in Pigeons. Vet. Surg. 2015, 44, 603–612. [Google Scholar] [CrossRef]
- Söhling, N.; Neijhoft, J.; Nienhaus, V.; Acker, V.; Harbig, J.; Menz, F.; Ochs, J.; Verboket, R.D.; Ritz, U.; Blaeser, A.; et al. 3D-Printing of Hierarchically Designed and Osteoconductive Bone Tissue Engineering Scaffolds. Materials 2020, 13, 1836. [Google Scholar] [CrossRef]
- Söhling, N.; Al Zoghool, S.; Schätzlein, E.; Neijhoft, J.; Oliveira, K.M.C.; Leppik, L.; Ritz, U.; Dörsam, E.; Frank, J.; Marzi, I.; et al. In vitro Evaluation of a 20% Bioglass-Containing 3D printable PLA Composite for Bone Tissue Engineering. Int. J. Bioprinting 2022, 8, 65–81. [Google Scholar] [CrossRef]
- Viateau, V.; Bensidhoum, M.; Guillemin, G.; Petite, H.; Hannouche, D.; Anagnostou, F.; Pélissier, P. Use of the Induced Membrane Technique for Bone Tissue Engineering Purposes: Animal Studies. Orthop. Clin. N. Am. 2010, 41, 49–56. [Google Scholar] [CrossRef]
- Tolstunov, L.; Hamrick, J.F.E.; Broumand, V.; Shilo, D.; Rachmiel, A. Bone Augmentation Techniques for Horizontal and Vertical Alveolar Ridge Deficiency in Oral Implantology. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 163–191. [Google Scholar] [CrossRef]




| Material | Decellularized Dermis (DCD) | Decellularized Dermis + Bone Mononuclear Cells (DCD + BMC) |
|---|---|---|
| Syngenic cancellous Bone (SCB) | n = 10 (group 1) | n = 10 (group 2) |
| DBM granules (GDBM) | n = 10 (group 3) | n = 10 (group 4) |
| DBM granules + BMC (GDBM +BMC) | n = 10 (group 5) | n = 10 (group 6) |
| fibrous demineralized bone matrix (f-DBM) | n = 10 (group 7) | n = 10 (group 8) |
| fibrous demineralized bonematrix + BMC (f-DBM + BMC) | n = 10 (group 9) | n = 10 (group 10) |
| sum | 50 | 50 |
| Material | DCD | DCD + BMC |
|---|---|---|
| SCB | 0.74 (±4.2%) | 0.76 (±4.8%) |
| g-DBM | 0.64 (±4.1%)) | 0.58 (±3.4%)) |
| g-DBM + BMC | 0.70 (±4.8%) | 0.67 (±5.1%) |
| f-DBM | 0.67 (±4.1%) | 0.59 (±5.6%) |
| f-DBM + BMC | 0.58 (±3.4%) | 0.51 (±5.8%) |
| Material | DCD | DCD + BMC |
|---|---|---|
| Syngeneic cancellous Bone (SCB) | 22 | 21 |
| DBM granules (GDBM) | 19 | 18 |
| DBM granules + BMC (GDBM +BMC) | 18 | 18 |
| fibrous demineralized bone matrix (f-DBM) | 21 | 20 |
| Breaking Load % (Median) | Bending Stiffness % (Median) | |
|---|---|---|
| DCD + SCB | 62.1 | 27.8 |
| DCD + g-DBM | 17.4 | 10.5 |
| DCD + g-DBM | 8.5 | 3.4 |
| DCD + (g-DBM + BMC) | 3.1 | 0.6 |
| DCD + (f-DBM + BMC) | 10.0 | 2.7 |
| (DCD + BMC) + SCB | 20.8 | 11.8 |
| (DCD + BMC) + g-DBM | 11.2 | 3.0 |
| (DCD + BMC) + (g-DBM + BMC) | 9.8 | 2.7 |
| (DCD + BMC) + f-DBM | 9.6 | 6.5 |
| (DCD + BMC) + (f-DBM + BMC) | 5.1 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Söhling, N.; Heilani, M.; Fremdling, C.; Schaible, A.; Schröder, K.; Brune, J.C.; Eras, V.; Nau, C.; Marzi, I.; Henrich, D.; et al. One Stage Masquelets Technique: Evaluation of Different Forms of Membrane Filling with and without Bone Marrow Mononuclear Cells (BMC) in Large Femoral Bone Defects in Rats. Cells 2023, 12, 1289. https://doi.org/10.3390/cells12091289
Söhling N, Heilani M, Fremdling C, Schaible A, Schröder K, Brune JC, Eras V, Nau C, Marzi I, Henrich D, et al. One Stage Masquelets Technique: Evaluation of Different Forms of Membrane Filling with and without Bone Marrow Mononuclear Cells (BMC) in Large Femoral Bone Defects in Rats. Cells. 2023; 12(9):1289. https://doi.org/10.3390/cells12091289
Chicago/Turabian StyleSöhling, Nicolas, Myriam Heilani, Charlotte Fremdling, Alexander Schaible, Katrin Schröder, Jan C. Brune, Volker Eras, Christoph Nau, Ingo Marzi, Dirk Henrich, and et al. 2023. "One Stage Masquelets Technique: Evaluation of Different Forms of Membrane Filling with and without Bone Marrow Mononuclear Cells (BMC) in Large Femoral Bone Defects in Rats" Cells 12, no. 9: 1289. https://doi.org/10.3390/cells12091289
APA StyleSöhling, N., Heilani, M., Fremdling, C., Schaible, A., Schröder, K., Brune, J. C., Eras, V., Nau, C., Marzi, I., Henrich, D., & Verboket, R. D. (2023). One Stage Masquelets Technique: Evaluation of Different Forms of Membrane Filling with and without Bone Marrow Mononuclear Cells (BMC) in Large Femoral Bone Defects in Rats. Cells, 12(9), 1289. https://doi.org/10.3390/cells12091289








