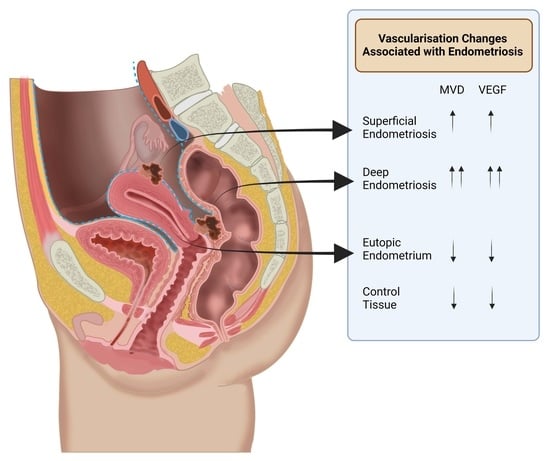
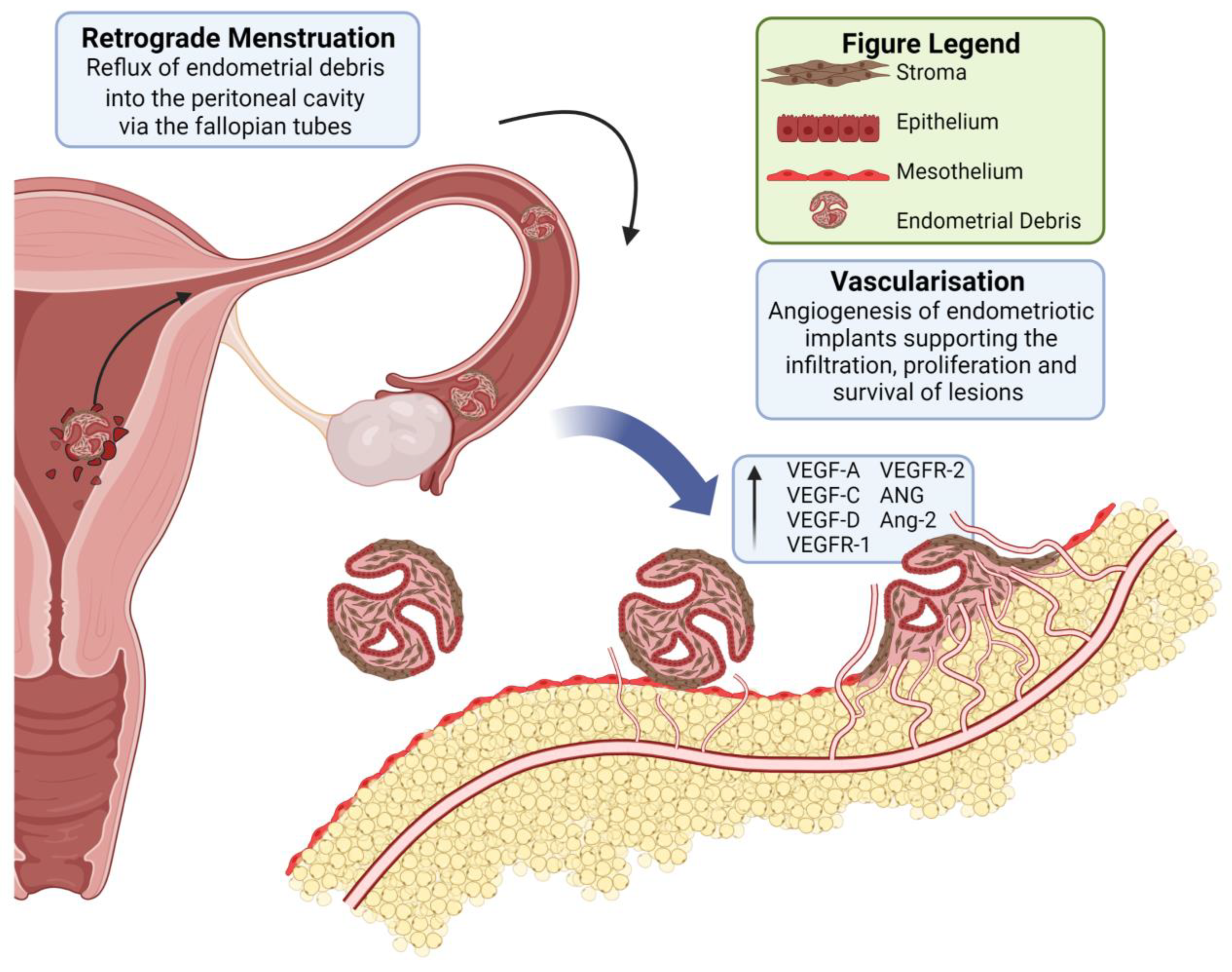
Vascularisation in Deep Endometriosis: A Systematic Review with Narrative Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic Search
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction and Synthesis
2.5. Bias Analysis
3. Results
3.1. Microvessel Density
3.2. Vascular Endothelial Growth Factor (VEGF) and Angiogenesis-Related Signalling Molecules
3.3. HIF-1A Expression
4. Discussion
4.1. Microvessel Density
4.2. VEGF
4.3. HIF-1A
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, S.; Bergqvist, A.; Chapron, C.; D’Hooghe, T.; Dunselman, G.; Greb, R.; Hummelshoj, L.; Prentice, A.; Saridogan, E. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. 2005, 20, 2698–2704. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Endometriosis: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng73 (accessed on 2 March 2022).
- Laganà, A.S.; Vitale, S.G.; Salmeri, F.M.; Triolo, O.; Ban Frangež, H.; Vrtačnik-Bokal, E.; Stojanovska, L.; Apostolopoulos, V.; Granese, R.; Sofo, V. Unus pro omnibus, omnes pro uno: A novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med. Hypotheses 2017, 103, 10–20. [Google Scholar] [CrossRef]
- Sourial, S.; Tempest, N.; Hapangama, D.K. Theories on the pathogenesis of endometriosis. Int. J. Reprod. Med. 2014, 2014, 179515. [Google Scholar] [CrossRef]
- Sampson, J.A. Heterotopic or misplaced endometrial tissue. Am. J. Obstet. Gynecol. 1925, 10, 649–664. [Google Scholar] [CrossRef]
- Sampson, J.A. Perforating hemorrhagic (chocolate) cysts of the ovary: Their importance and especially their relation to pelvic adenomas of endometrial type (adenomyoma of the uterus, rectovaginal septum, sigmoid, etc.). Arch. Surg. 1921, 3, 245–323. [Google Scholar] [CrossRef]
- Sasson, I.E.; Taylor, H.S. Stem Cells and the Pathogenesis of Endometriosis. Ann. N. Y. Acad. Sci. 2008, 1127, 106–115. [Google Scholar] [CrossRef]
- Hill, C.J.; Fakhreldin, M.; Maclean, A.; Dobson, L.; Nancarrow, L.; Bradfield, A.; Choi, F.; Daley, D.; Tempest, N.; Hapangama, D.K. Endometriosis and the fallopian tubes: Theories of origin and clinical implications. J. Clin. Med. 2020, 9, 1905. [Google Scholar] [CrossRef]
- Halme, J.; Hammond, M.G.; Hulka, J.F.; Raj, S.G.; Talbert, L.M. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 1984, 64, 151–154. [Google Scholar]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef]
- Warzecha, D.; Szymusik, I.; Wielgos, M.; Pietrzak, B. The Impact of Endometriosis on the Quality of Life and the Incidence of Depression-A Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 3641. [Google Scholar] [CrossRef] [PubMed]
- Sperschneider, M.L.; Hengartner, M.P.; Kohl-Schwartz, A.; Geraedts, K.; Rauchfuss, M.; Woelfler, M.M.; Haeberlin, F.; von Orelli, S.; Eberhard, M.; Maurer, F.; et al. Does endometriosis affect professional life? A matched case-control study in Switzerland, Germany and Austria. BMJ Open 2019, 9, e019570. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Martin, D. Treatment of deeply infiltrating endometriosis. Curr. Opin. Obs. Gynecol. 1994, 6, 231–241. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Meuleman, C.; Demeyere, S.; Lesaffre, E.; Cornillie, F.J. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil. Steril. 1991, 55, 759–765. [Google Scholar] [CrossRef]
- Fauconnier, A.; Chapron, C.; Dubuisson, J.B.; Vieira, M.; Dousset, B.; Bréart, G. Relation between pain symptoms and the anatomic location of deep infiltrating endometriosis. Fertil. Steril. 2002, 78, 719–726. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis. In Biology of Endothelial Cells. Developments in Cardiovascular Medicine; Springer: Boston, MA, USA, 1984; pp. 412–428. [Google Scholar]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Asahara, T.; Kawamoto, A. Endothelial progenitor cells for postnatal vasculogenesis. Am. J. Physiol.-Cell Physiol. 2004, 287, C572–C579. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Vollmar, B.; Menger, M.D. Inosculation: Connecting the life-sustaining pipelines. Tissue Eng. Part B Rev. 2009, 15, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Demir, R.; Yaba, A.; Huppertz, B. Vasculogenesis and angiogenesis in the endometrium during menstrual cycle and implantation. Acta Histochem. 2010, 112, 203–214. [Google Scholar] [CrossRef]
- Healy, D.; Rogers, P.; Hii, L.; Wingfield, M. Angiongenesis: A new theory for endometriosis. Hum. Reprod. Update 1998, 4, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.L.L.; Reis, F.M.; Taylor, R.N. Angiogenesis and endometriosis. Obs. Gynecol. Int. 2013, 2013, 859619. [Google Scholar] [CrossRef]
- Nagy, J.A.; Dvorak, A.M.; Dvorak, H.F. VEGF-A and the induction of pathological angiogenesis. Annu. Rev. Pathol. Mech. Dis. 2007, 2, 251–275. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr. Drug Targets 2010, 11, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, G.; Cohen, T.; Gengrinovitch, S.; Poltorak, Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999, 13, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. VEGF-A: A critical regulator of blood vessel growth. Eur. Cytokine Netw. 2009, 20, 158–163. [Google Scholar] [CrossRef]
- Lohela, M.; Bry, M.; Tammela, T.; Alitalo, K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 2009, 21, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Blancher, C.; Harris, A.L. The molecular basis of the hypoxia response pathway: Tumour hypoxia as a therapy target. Cancer Metastasis Rev. 1998, 17, 187–194. [Google Scholar] [CrossRef]
- Serrano-Oviedo, L.; Giménez-Bachs, J.M.; Nam-Cha, S.Y.; Cimas, F.J.; García-Cano, J.; Sánchez-Prieto, R.; Salinas-Sánchez, A.S. Implication of VHL, ERK5, and HIF-1alpha in clear cell renal cell carcinoma: Molecular basis. Urol. Oncol. 2017, 35, 114.e15–114.e22. [Google Scholar] [CrossRef]
- Zhang, C.C.; Sadek, H.A. Hypoxia and Metabolic Properties of Hematopoietic Stem Cells. Antioxid. Redox Signal. 2013, 20, 1891–1901. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Basic mechanisms of vascularization in endometriosis and their clinical implications. Hum. Reprod. Update 2018, 24, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLOS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Jackson, J.L.; Kuriyama, A.; Anton, A.; Choi, A.; Fournier, J.-P.; Geier, A.-K.; Jacquerioz, F.; Kogan, D.; Scholcoff, C.; Sun, R. The accuracy of Google Translate for abstracting data from non–English-language trials for systematic reviews. Ann. Intern. Med. 2019, 171, 677–679. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, j.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 2 March 2023).
- Vinci, G.; Arkwright, S.; Audebourg, A.; Radenen, B.; Chapron, C.; Borghese, B.; Dousset, B.; Mehats, C.; Vaiman, D.; Vacher-Lavenu, M.C.; et al. Correlation Between the Clinical Parameters and Tissue Phenotype in Patients Affected by Deep-Infiltrating Endometriosis. Reprod. Sci. 2016, 23, 1258–1268. [Google Scholar] [CrossRef]
- Stratopoulou, C.A.; Camboni, A.; Donnez, J.; Dolmans, M.-M. Identifying Common Pathogenic Features in Deep Endometriotic Nodules and Uterine Adenomyosis. J. Clin. Med. 2021, 10, 4585. [Google Scholar] [CrossRef]
- Signorile, P.G.; Campioni, M.; Vincenzi, B.; D’Avino, A.; Baldi, A. Rectovaginal septum endometriosis: An immunohistochemical analysis of 62 cases. In Vivo 2009, 23, 459–464. [Google Scholar] [PubMed]
- Robin, B.; Planeix, F.; Sastre-Garau, X.; Pichon, C.; Olesen, T.K.; Gogusev, J.; Ghinea, N. Follicle-Stimulating Hormone Receptor Expression in Endometriotic Lesions and the Associated Vasculature: An Immunohistochemical Study. Reprod. Sci. 2016, 23, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, D.; Mastronardi, M.; Mabrouk, M.; Cafagna, G.; Salucci, P.; Arena, A.; Iodice, R.; Borghese, G.; Casadio, P.; Del Forno, S.; et al. Rectosigmoid Endometriosis Vascular Patterns at Intraoperative Indocyanine Green Angiography and their Correlation with Clinicopathological Data. Surg. Innov. 2020, 27, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.E.; Abrao, M.S.; Berardo, P.T.; Takiya, C.M.; Nasciutti, L.E. Vascular density and distribution of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) are significantly higher in patients with deeply infiltrating endometriosis affecting the rectum. Fertil. Steril. 2008, 90, 148–155. [Google Scholar] [CrossRef]
- Kim, H.O.; Yang, K.M.; Kang, I.S.; Koong, M.K.; Kim, H.S.; Zhang, X.; Kim, I. Expression of CD44s, vascular endothelial growth factor, matrix metalloproteinase-2 and Ki-67 in peritoneal, rectovaginal and ovarian endometriosis. J. Reprod. Med. 2007, 52, 207–213. [Google Scholar]
- Yerlikaya, G.; Balendran, S.; Pröstling, K.; Reischer, T.; Birner, P.; Wenzl, R.; Kuessel, L.; Streubel, B.; Husslein, H. Comprehensive study of angiogenic factors in women with endometriosis compared to women without endometriosis. Eur. J. Obs. Gynecol. Reprod. Biol. 2016, 204, 88–98. [Google Scholar] [CrossRef]
- Jondet, M.; Vacher-Lavenu, M.C.; Chapron, C. Image analysis measurements of the microvascularisation in endometrium, superficial and deep endometriotic tissues. Angiogenesis 2006, 9, 177–182. [Google Scholar] [CrossRef]
- Filippi, I.; Carrarelli, P.; Luisi, S.; Batteux, F.; Chapron, C.; Naldini, A.; Petraglia, F. Different Expression of Hypoxic and Angiogenic Factors in Human Endometriotic Lesions. Reprod. Sci. 2016, 23, 492–497. [Google Scholar] [CrossRef]
- Van Langendonckt, A.; Punyadeera, C.; Kamps, R.; Dunselman, G.; Klein-Hitpass, L.; Schurgers, L.J.; Squifflet, J.; Donnez, J.; Groothuis, P. Identification of novel antigens in blood vessels in rectovaginal endometriosis. Mol. Hum. Reprod. 2007, 13, 875–886. [Google Scholar] [CrossRef]
- Ramón, L.A.; Braza-Boïls, A.; Gilabert-Estellés, J.; Gilabert, J.; España, F.; Chirivella, M.; Estellés, A. microRNAs expression in endometriosis and their relation to angiogenic factors. Hum. Reprod. 2011, 26, 1082–1090. [Google Scholar] [CrossRef]
- Perricos, A.; Wenzl, R.; Husslein, H.; Eiwegger, T.; Gstoettner, M.; Weinhaeusel, A.; Beikircher, G.; Kuessel, L. Does the Use of the “Proseek R Multiplex Oncology I Panel” on Peritoneal Fluid Allow a Better Insight in the Pathophysiology of Endometriosis, and in Particular Deep-Infiltrating Endometriosis? J. Clin. Med. 2020, 9, 2009. [Google Scholar] [CrossRef]
- Bourlev, V.; Iljasova, N.; Adamyan, L.; Larsson, A.; Olovsson, M. Signs of reduced angiogenic activity after surgical removal of deeply infiltrating endometriosis. Fertil. Steril. 2010, 94, 52–57. [Google Scholar] [CrossRef]
- Keichel, S.; Barcena de Arellano, M.L.; Reichelt, U.; Riedlinger, W.F.; Schneider, A.; Köhler, C.; Mechsner, S. Lymphangiogenesis in deep infiltrating endometriosis. Hum. Reprod. 2011, 26, 2713–2720. [Google Scholar] [CrossRef]
- Mabrouk, M.; Arena, A.; Moro, E.; Raimondo, D.; Seracchioli, R. Deep Infiltrating Endometriosis and Spontaneous Hemoperitoneum: A Life-Threatening Situation Treated by Laparoscopy. J. Minim. Invasive Gynecol. 2020, 27, 579. [Google Scholar] [CrossRef]
- Machado, D.E.; Berardo, P.T.; Palmero, C.Y.; Nasciutti, L.E. Higher expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) and metalloproteinase-9 (MMP-9) in a rat model of peritoneal endometriosis is similar to cancer diseases. J. Exp. Clin. Cancer Res. CR 2010, 29, 4. [Google Scholar] [CrossRef] [PubMed]
- Ramon, L.; Gilabert-Estelles, J.; Castello, R.; Gilabert, J.; Espana, F.; Romeu, A.; Chirivella, M.; Aznar, J.; Estellés, A. mRNA analysis of several components of the plasminogen activator and matrix metalloproteinase systems in endometriosis using a real-time quantitative RT–PCR assay. Hum. Reprod. 2005, 20, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Eyster, K.M.; Boles, A.L.; Brannian, J.D.; Hansen, K.A. DNA microarray analysis of gene expression markers of endometriosis. Fertil. Steril. 2002, 77, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Maclean, A.; Kamal, A.; Adishesh, M.; Alnafakh, R.; Tempest, N.; Hapangama, D.K. Human uterine biopsy: Research value and common pitfalls. Int. J. Reprod. Med. 2020, 2020, 9275360. [Google Scholar] [CrossRef] [PubMed]
- Hapangama, D.; Kamal, A.; Bulmer, J. Estrogen receptor β: The guardian of the endometrium. Hum. Reprod. Update 2015, 21, 174–193. [Google Scholar] [CrossRef]
- Kamal, A.; Tempest, N.; Parkes, C.; Alnafakh, R.; Makrydima, S.; Adishesh, M.; Hapangama, D.K. Hormones and endometrial carcinogenesis. Horm. Mol. Biol. Clin. Investig. 2016, 25, 129–148. [Google Scholar] [CrossRef]
- Maclean, A.; Adishesh, M.; Button, L.; Richards, L.; Alnafakh, R.; Newton, E.; Drury, J.; Hapangama, D. The effect of pre-analytical variables on downstream application and data analysis of human endometrial biopsies. Hum. Reprod. Open 2022, 2022, hoac026. [Google Scholar] [CrossRef]
- Tempest, N.; Jansen, M.; Baker, A.M.; Hill, C.J.; Hale, M.; Magee, D.; Treanor, D.; Wright, N.A.; Hapangama, D.K. Histological 3D reconstruction and in vivo lineage tracing of the human endometrium. J. Pathol. 2020, 251, 440–451. [Google Scholar] [CrossRef]
- Tempest, N.; Hill, C.J.; Maclean, A.; Marston, K.; Powell, S.G.; Al-Lamee, H.; Hapangama, D.K. Novel microarchitecture of human endometrial glands: Implications in endometrial regeneration and pathologies. Hum. Reprod. Update 2022, 28, 153–171. [Google Scholar] [CrossRef]
- Fassbender, A.; Rahmioglu, N.; Vitonis, A.F.; Viganò, P.; Giudice, L.C.; D’Hooghe, T.M.; Hummelshoj, L.; Adamson, G.D.; Becker, C.M.; Missmer, S.A.; et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: IV. Tissue collection, processing, and storage in endometriosis research. Fertil. Steril. 2014, 102, 1244–1253. [Google Scholar] [CrossRef]
- Becker, C.M.; Laufer, M.R.; Stratton, P.; Hummelshoj, L.; Missmer, S.A.; Zondervan, K.T.; Adamson, G.D. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: I. Surgical phenotype data collection in endometriosis research. Fertil. Steril. 2014, 102, 1213–1222. [Google Scholar] [CrossRef]
- Hlatky, L.; Hahnfeldt, P.; Folkman, J. Clinical application of antiangiogenic therapy: Microvessel density, what it does and doesn’t tell us. J. Natl. Cancer Inst. 2002, 94, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Xiao, J.; Chen, Q. Solving the Puzzle: What Is the Role of Progestogens in Neovascularization? Biomolecules 2021, 11, 1686. [Google Scholar] [CrossRef] [PubMed]
- Gezer, A.; Oral, E. Progestin Therapy in Endometriosis. Women’s Health 2015, 11, 643–652. [Google Scholar] [CrossRef]
- Nisolle, M.; Casanas-Roux, F.; Anaf, V.; Mine, J.M.; Donnez, J. Morphometric Study of the Stromal Vascularization in Peritoneal Endometriosis. Fertil. Steril. 1993, 59, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Canis, M.; Pouly, J.-L.; Darcha, C. Soft matrices inhibit cell proliferation and inactivate the fibrotic phenotype of deep endometriotic stromal cells in vitro. Hum. Reprod. 2016, 31, 541–553. [Google Scholar] [CrossRef]
- Zlatska, A.V.; Rodnichenko, A.E.; Gubar, O.S.; Zubov, D.O.; Novikova, S.N.; Vasyliev, R.G. Endometrial stromal cells: Isolation, expansion, morphological and functional properties. Exp. Oncol. 2017, 39, 197–202. [Google Scholar] [CrossRef]
- Zhu, H.; Hou, C.C.; Luo, L.F.; Hu, Y.J.; Yang, W.X. Endometrial stromal cells and decidualized stromal cells: Origins, transformation and functions. Gene 2014, 551, 1–14. [Google Scholar] [CrossRef]
- Skobe, M.; Hawighorst, T.; Jackson, D.G.; Prevo, R.; Janes, L.; Velasco, P.; Riccardi, L.; Alitalo, K.; Claffey, K.; Detmar, M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 2001, 7, 192–198. [Google Scholar] [CrossRef]
- George, M.L.; Tutton, M.G.; Janssen, F.; Arnaout, A.; Abulafi, A.M.; Eccles, S.A.; Swift, R.I. Vegf-a, vegf-c, and vegf-d in colorectal cancer progression. Neoplasia 2001, 3, 420–427. [Google Scholar] [CrossRef]
- Su, J.-L.; Yen, C.; Chen, P.; Chuang, S.; Hong, C.; Kuo, I.; Chen, H.; Hung, M.-C.; Kuo, M. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br. J. Cancer 2007, 96, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yasuoka, H.; Tsujimoto, M.; Imabun, S.; Nakahara, M.; Nakao, K.; Nakamura, M.; Mori, I.; Kakudo, K. Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Breast Cancer Res. Treat. 2005, 91, 125–132. [Google Scholar] [CrossRef]
- Wu, M.H.; Chen, K.F.; Lin, S.C.; Lgu, C.W.; Tsai, S.J. Aberrant expression of leptin in human endometriotic stromal cells is induced by elevated levels of hypoxia inducible factor-1alpha. Am. J. Pathol. 2007, 170, 590–598. [Google Scholar] [CrossRef]
- Wu, M.-H.; Hsiao, K.-Y.; Tsai, S.-J. Hypoxia: The force of endometriosis. J. Obstet. Gynaecol. Res. 2019, 45, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef]
- Martínez-Aguilar, R.; Kershaw, L.E.; Reavey, J.J.; Critchley, H.O.; Maybin, J.A. Hypoxia and reproductive health: The presence and role of hypoxia in the endometrium. Reproduction 2021, 161, F1–F17. [Google Scholar] [CrossRef] [PubMed]
- Goteri, G.; Lucarini, G.; Zizzi, A.; Rubini, C.; Di Primio, R.; Tranquilli, A.L.; Ciavattini, A. Proangiogenetic molecules, hypoxia-inducible factor-1alpha and nitric oxide synthase isoforms in ovarian endometriotic cysts. Virchows Arch. 2010, 456, 703–710. [Google Scholar] [CrossRef]
- Becker, C.M.; Rohwer, N.; Funakoshi, T.; Cramer, T.; Bernhardt, W.; Birsner, A.; Folkman, J.; D’Amato, R.J. 2-methoxyestradiol inhibits hypoxia-inducible factor-1{alpha} and suppresses growth of lesions in a mouse model of endometriosis. Am. J. Pathol. 2008, 172, 534–544. [Google Scholar] [CrossRef] [PubMed]

| Author (Year) | Focus of Study | Experimental Technique | Number of Patients | Hormonal Treatment | Phase of Menstrual Cycle | Relevant Findings of Study | |||
|---|---|---|---|---|---|---|---|---|---|
| Superficial Endometriosis | DE | Control (Description of Control Cohort) | Total | ||||||
| Robin 2016 [41] | MVD | Immunohistochemistry FSHR marker | 8 | 186 | 17 (healthy uterine tissue obtained from patients undergoing benign gynaecological procedures. Histological examination confirmed the absence of endometrial pathology) | 211 | Not Stated | Matched proliferative and secretory phases. Statistical analysis of samples was undertaken according to menstrual phase | Increased FSHR-positive MVD in DE and superficial endometriosis lesions compared to control samples. Similar MVD in DE and superficial endometriosis * |
| Stratopoulou 2021 [39] | MVD VEGF | Immunohistochemistry VEGF, CD31 and αSMA staining of microvessels | 0 | 13 | 14 (healthy uterine tissue obtained from patients undergoing benign gynaecological procedures. Histological examination confirmed the absence of endometrial pathology) | 41 | None had hormone therapy for at least three months prior to surgery | Samples were collected throughout proliferative, secretory and menstrual phases in an unmatched manner. No statistical analysis of samples from different menstrual phases was undertaken | Increased CD31-positive MVD in DE compared to control tissue. No difference in VEGF staining intensity between DE, superficial endometriosis and control tissue * |
| Machado 2008 [43] | MVD VEGF | Immunohistochemistry assessment of blood vessels using VEGF, vWF and Flk-1 | 10 | 20 | 20 (healthy uterine tissue obtained from patients undergoing benign gynaecological procedures. Histological examination confirmed the absence of endometrial pathology) 12 (normal tissues of ovary (n = 4), bladder (n = 4) and rectum (n = 4) were obtained from these organs beside the endometriotic lesions) | 62 | None of the patients received hormonal treatment for at least three months before the study | Matched proliferative and secretory phases. Statistical analysis of samples was undertaken according to menstrual phase. 51 | Increased wVF-positive MVD in DE compared to control endometrium (in both proliferative and secretory phases) and control rectal tissue |
| Keichel 2011 [52] | VEGF | Immunohistochemistry VEGF-C & VEGF-D | 0 | 38 | 13 (tumour-free marginal border of the resection part of patients with colon cancer) 10 (unaffected vagina taken during hysterectomy due to benign diseases) | 61 | Patients receiving and not receiving hormonal contraceptives were recruited for this study | Samples were collected throughout proliferative, secretory and menstrual phases in an unmatched manner. No statistical analysis of samples from different menstrual phases was undertaken | Increased staining of VEGF-C and VEGF-D and lymph vessel density in DE |
| Signorile 2009 [40] | MVD | Immunohistochemistry CD34, PR, ER markers | 6 | 56 | 0 | 62 | Not Stated | Not Stated | Intense CD34 staining associated with DE lesions compared to superficial endometriosis. ER and PR expression in DE lesions correlated to the degree of vascularisation |
| Jondet 2006 [46] | MVD | Immunohistochemistry assessment of blood vessels using CD31 | 32 | 34 | 0 | 66 | Study included both progestin- and non-progestin-treated women | Not Stated | Increased CD31 staining associated with DE lesions compared to superficial endometriosis Progestin therapy significantly reduces MVD in both eutopic endometrium and endometriotic lesions |
| Vinci 2016 [38] | MVD | Immunohistochemistry CD31 staining of vessels within endometriotic “hot spots” | 0 | 113 | 0 | 113 | All were treated with GnRH agonists at least six months before surgery | Not Stated | CD31 staining was found to be “intense” in 42% of the DE tissue samples, “medium” in 32% and “weak” in 26% of DE “hot spots.” |
| Raimondo 2020 [42] | MVD | Intraoperative Indocyanine green angiography assessment of endometriotic lesions. Immunohistochemistry assessment of endometriotic biopsies using CD31 | 0 | 30 | 0 | 30 | All women assumed progestin therapy in the three months before surgery | Not Stated | IGA assessment identified 60% of the DE lesions were found to be hypovascular, while the remaining 40% were deemed to be hypervascular Using CD31 staining, hypovascular lesions were demonstrated to have a significantly lower MVD than hypervascular lesions |
| Kim 2007 [44] | VEGF | Immunohistochemistry of stroma using CD44, VEGF and Ki-67 | 51 | 11 | 0 | 62 | None of the patients received hormonal treatment for at least six months before the study | Not Stated | No difference in VEGF staining between stages I/II and III/IV of endometriosis lesions * |
| Author (Year) | Focus of Study | Experimental Technique | Number of Patients | Hormonal Treatment | Phase of Menstrual Cycle | Relevant Findings of Study | |||
|---|---|---|---|---|---|---|---|---|---|
| Superficial Endometriosis | DE | Control (Description of Control Cohort) | Total | ||||||
| Van Langendonckt 2007 [48] | MVD | Laser Capture Microdissection and qPCR | 0 | 28 | 20 (eutopic endometrium obtained from recruited patients with DE. No histological evidence of endometrial pathology was identified in the control tissue as assessed by a pathologist) | 28 | Patients were not receiving hormonal treatment at the time of tissue collection | Samples were collected throughout proliferative, secretory and menstrual phases in an unmatched manner. No statistical analysis of samples from different menstrual phases was undertaken | Significantly raised expression of MGP in DE microvessels compared with vessels collected from control tissues |
| Perricos 2020 [50] | VEGF | Untargeted analysis of 92 cancer-related proteins (including VEGF-D) using the Proseek Multiplex Oncology I Cancer Panel | 34 | 19 | 31 (Peritoneal fluid obtained from patients undergoing benign gynaecological procedures without macroscopic evidence of endometriosis at the time of surgery) | 84 | Not Stated | Samples were collected throughout proliferative and secretory phases in an unmatched manner. No statistical analysis of samples from different menstrual phases was undertaken | Increased VEGF-D in PF of DE patients compared to superficial endometriosis |
| Bourlev 2010 [51] | VEGF | Peritoneal and serum fluid analysis of VEGF-A, VEGFR-1 and Ang-2 using ELISA | 0 | 32 | 21 (serum and peritoneal fluid collected from healthy patients undergoing laparoscopic sterilisation) | 53 | None of the patients received hormonal treatment for at least three months before the study | Samples were collected throughout proliferative, secretory and menstrual phases in an unmatched manner. No statistical analysis of samples from different menstrual phases was undertaken | Increased concentration of VEGF-A in serum and peritoneal fluid of patients with DE compared to controls. Surgery to remove DE lesions significantly lowered serum VEGF-A |
| Ramón 2011 2011 [49] | VEGF | VEGF-A mRNA expression was assessed using qPCR. VEGF-A protein levels were quantified by ELISA | 45 | 13 | 38 (healthy uterine tissue obtained from patients undergoing benign gynaecological procedures. Histological examination confirmed the absence of endometrial pathology) | 96 | None of the patients received hormonal treatment for at least three months before the study | Samples were collected throughout proliferative and secretory phases. Statistical analysis of samples was undertaken according to menstrual phase | Increased levels of VEGF-A of DE lesions compared with control tissue * |
| Filippi 2016 [47] | VEGF HIF-1A | VEGF-A and HIF-1A expression was assessed using qPCR | 16 | 11 | 15 (healthy uterine tissue obtained from patients undergoing hysteroscopy) | 42 | None of the patients received hormonal treatment for at least three months before the study | Proliferative samples only | No significant difference in the VEGF-A mRNA levels in DE lesions compared to control endometrium * |
| Yerlikaya 2016 [45] | VEGF HIF-1A | VEGFA, VEGFR2, HIF-1A and ANG mRNA expression was analysed using qPCR | 23 | 38 | 53 (healthy uterine tissue obtained from patients undergoing benign gynaecological procedures. Histological examination confirmed the absence of endometrial pathology) | 114 | Not Stated | Samples were collected throughout proliferative and secretory phases. Statistical analysis of samples was undertaken according to menstrual phase | Increased VEGF-A mRNA expression levels in DE versus control endometrium and eutopic endometrium * |
| Paper Reference | Adequate Case Definition? | Representativeness of the Cases? | Selection of Controls? | Definition of Controls? | Comparability of Cases and Controls on the Basis of the Design or Analysis? | Ascertainment of Exposure? | Same Method of Ascertainment for Cases and Controls? | Non-Response Rate? | Selection | Comparability | Exposure | Total Score | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vinci 2016 [38] | * | 0 | * | * | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 | Poor |
| Van Langendonckt 2007 [48] | * | * | * | * | * | * | * | * | 4 | 1 | 3 | 8 | Good |
| Raimondo 2020 [42] | * | * | * | 0 | * | * | * | * | 3 | 1 | 3 | 7 | Good |
| Signorile 2009 [40] | * | * | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | Poor |
| Robin 2016 [41] | * | * | * | * | * | 0 | * | 3 | 1 | 2 | 6 | Good | |
| Jondet 2006 [46] | * | 0 | * | * | 0 | * | * | * | 3 | 0 | 3 | 6 | Poor |
| Stramiddleoulou 2021 [39] | * | * | * | * | * | * | * | * | 4 | 1 | 3 | 8 | Good |
| Machado 2008 [43] | * | 0 | * | * | * | * | * | * | 3 | 1 | 3 | 7 | Good |
| Keichel 2011 [52] | * | * | * | * | ** | * | * | * | 4 | 2 | 3 | 9 | Good |
| Ramón 2011 [49] | * | * | * | * | * | * | 0 | * | 4 | 1 | 2 | 7 | Good |
| Perricos 2020 [50] | * | * | * | * | * | * | 0 | * | 4 | 1 | 2 | 7 | Good |
| Bourlev 2010 [51] | * | 0 | * | * | ** | * | * | * | 3 | 2 | 3 | 8 | Good |
| Filippi 2016 [47] | * | * | * | * | * | * | * | * | 4 | 1 | 3 | 8 | Good |
| Yerlikaya 2016 [45] | * | 0 | * | 0 | ** | * | * | * | 2 | 3 | 3 | 7 | Good |
| Kim 2007 [44] | * | 0 | * | 0 | * | * | * | * | 2 | 1 | 3 | 6 | Fair |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powell, S.G.; Sharma, P.; Masterson, S.; Wyatt, J.; Arshad, I.; Ahmed, S.; Lash, G.; Cross, M.; Hapangama, D.K. Vascularisation in Deep Endometriosis: A Systematic Review with Narrative Outcomes. Cells 2023, 12, 1318. https://doi.org/10.3390/cells12091318
Powell SG, Sharma P, Masterson S, Wyatt J, Arshad I, Ahmed S, Lash G, Cross M, Hapangama DK. Vascularisation in Deep Endometriosis: A Systematic Review with Narrative Outcomes. Cells. 2023; 12(9):1318. https://doi.org/10.3390/cells12091318
Chicago/Turabian StylePowell, Simon G., Priyanka Sharma, Samuel Masterson, James Wyatt, Ilyas Arshad, Shakil Ahmed, Gendie Lash, Michael Cross, and Dharani K. Hapangama. 2023. "Vascularisation in Deep Endometriosis: A Systematic Review with Narrative Outcomes" Cells 12, no. 9: 1318. https://doi.org/10.3390/cells12091318
APA StylePowell, S. G., Sharma, P., Masterson, S., Wyatt, J., Arshad, I., Ahmed, S., Lash, G., Cross, M., & Hapangama, D. K. (2023). Vascularisation in Deep Endometriosis: A Systematic Review with Narrative Outcomes. Cells, 12(9), 1318. https://doi.org/10.3390/cells12091318