Establishment and Characterization of Cell Lines from Canine Metastatic Osteosarcoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origins of Tumor and Normal Cells and Cell Culture
2.2. Preparation and Maintenance of Cell Culture
2.3. Immunohistochemistry Staining of OSA Cells
2.4. OSA Cells and Osteoblasts Doubling Time
2.5. Migration Assay
2.6. Gene Expression Profiling of BR, BZ and LK Cells
2.7. Gene Expression and Pathway Analysis Using the ROSALIND® Platform
2.8. Validation of NanoString Analysis
2.9. Western Blot Analysis
3. Results
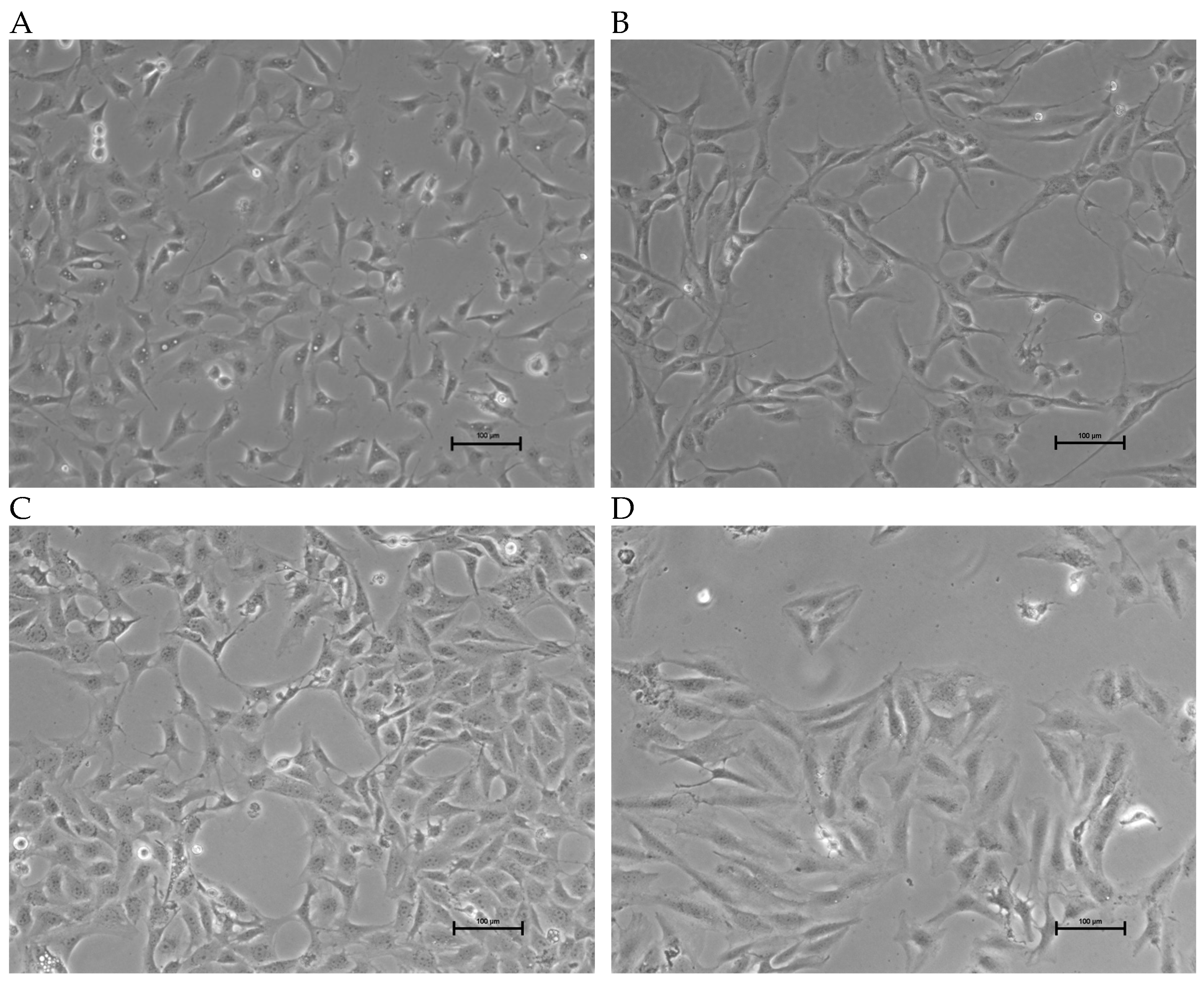
3.1. Characteristics of Established OSA Cell Lines
3.2. Normal Canine Osteoblast Cell Strains
3.3. NanoString Gene Expression and Pathway Analysis
3.4. Epithelial Mesenchymal Transition (EMT) Pathway
3.5. Cell Cycle
3.6. Cytokines
3.7. Immune Checkpoints and Targetable Targets on OSA Cells
3.8. PTEN and p16INK4A
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Withrow, S.J.; MacEwen, E.G. (Eds.) Small Animal Clinical Oncology, 3rd ed.; W. B. Saunders: Philadelphia, PA, USA, 2001. [Google Scholar]
- Rowell, J.L.; McCarthy, D.O.; Alvarez, C.E. Dog models of naturally occurring cancer. Trends Mol. Med. 2011, 17, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Anfinsen, K.P.; Grotmol, T.; Bruland, O.S.; Jonasdottir, T.J. Breed-specific incidence rates of canine primary bone tumors—A population based survey of dogs in Norway. Can. J. Vet. Res. 2011, 75, 209–215. [Google Scholar] [PubMed]
- Yang, Y.T.; Yuzbasiyan-Gurkan, V. Sorafenib and Doxorubicin Show Synergistic Effects in Human and Canine Osteosarcoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 9345. [Google Scholar] [CrossRef] [PubMed]
- Laschi, M.; Bernardini, G.; Geminiani, M.; Ghezzi, L.; Amato, L.; Braconi, D.; Millucci, L.; Frediani, B.; Spreafico, A.; Franchi, A.; et al. Establishment of Four New Human Primary Cell Cultures from Chemo-Naive Italian Osteosarcoma Patients. J. Cell. Physiol. 2015, 230, 2718–2727. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Mannheimer, J.D.; Tawa, G.; Gerhold, D.; Braisted, J.; Sayers, C.M.; McEachron, T.A.; Meltzer, P.; Mazcko, C.; Beck, J.A.; LeBlanc, A.K. Transcriptional profiling of canine osteosarcoma identifies prognostic gene expression signatures with translational value for humans. Commun. Biol. 2023, 6, 856. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, A.; Yamamoto, K.; Lan, N.T.; Uchida, K.; Yamaguchi, R.; Hayashi, T.; Tateyama, S. Establishment and characterization of a cell line, MCO-Y4, derived from canine mammary gland osteosarcoma. J. Vet. Med. Sci. 2006, 68, 1047–1053. [Google Scholar] [CrossRef]
- Meyer, F.R.L.; Walter, I. Establishment and Characterization of New Canine and Feline Osteosarcoma Primary Cell Lines. Vet. Sci. 2016, 3, 9. [Google Scholar] [CrossRef]
- Barroga, E.F.; Kadosawa, T.; Okumura, M.; Fujinaga, T. Establishment and characterization of the growth and pulmonary metastasis of a highly lung metastasizing cell line from canine osteosarcoma in nude mice. J. Vet. Med. Sci. 1999, 61, 361–367. [Google Scholar] [CrossRef]
- Hong, S.H.; Kadosawa, T.; Mochizuki, M.; Matsunaga, S.; Nishimura, R.; Sasaki, N. Establishment and Characterization of Two Cell Lines Derived from Canine Spontaneous Osteosarcoma. J. Vet. Med. Sci. 1998, 60, 757–760. [Google Scholar] [CrossRef]
- Gillette, J.M.; Gibbs, C.P.; Nielsen-Preiss, S.M. Establishment and characterization of OS 99-1, a cell line derived from a highly aggressive primary human osteosarcoma. Vitr. Cell. Dev. Biol. Anim. 2008, 44, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Souza, C.; Oliveira, I.D.; de Oliveira, R.; de Seixas Alves, M.T.; Petrilli, A.S.; Toledo, S.R. Establishment and cytogenetic characterization of a cell line from a pulmonary metastasis of osteosarcoma. Cytotechnology 2013, 65, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Blattmann, C.; Thiemann, M.; Stenzinger, A.; Roth, E.K.; Dittmar, A.; Witt, H.; Lehner, B.; Renker, E.; Jugold, M.; Eichwald, V.; et al. Establishment of a patient-derived orthotopic osteosarcoma mouse model. J. Transl. Med. 2015, 13, 136. [Google Scholar] [CrossRef]
- Pereira, B.P.; Zhou, Y.; Gupta, A.; Leong, D.T.; Aung, K.Z.; Ling, L.; Pho, R.W.; Galindo, M.; Salto-Tellez, M.; Stein, G.S.; et al. Runx2, p53, and pRB status as diagnostic parameters for deregulation of osteoblast growth and differentiation in a new pre-chemotherapeutic osteosarcoma cell line (OS1). J. Cell. Physiol. 2009, 221, 778–788. [Google Scholar] [CrossRef]
- Neupane, M.; Chang, C.C.; Kiupel, M.; Yuzbasiyan-Gurkan, V. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng. Part A 2008, 14, 1007–1015. [Google Scholar] [CrossRef]
- Abarrategi, A.; Tornin, J.; Martinez-Cruzado, L.; Hamilton, A.; Martinez-Campos, E.; Rodrigo, J.P.; Gonzalez, M.V.; Baldini, N.; Garcia-Castro, J.; Rodriguez, R. Osteosarcoma: Cells-of-Origin, Cancer Stem Cells, and Targeted Therapies. Stem Cells Int. 2016, 2016, 3631764. [Google Scholar] [CrossRef]
- SketchAndCalc. Available online: https://www.sketchandcalc.com (accessed on 25 May 2023).
- Magee, K.; Marsh, I.R.; Turek, M.M.; Grudzinski, J.; Aluicio-Sarduy, E.; Engle, J.W.; Kurzman, I.D.; Zuleger, C.L.; Oseid, E.A.; Jaskowiak, C.; et al. Safety and feasibility of an in situ vaccination and immunomodulatory targeted radionuclide combination immuno-radiotherapy approach in a comparative (companion dog) setting. PLoS ONE 2021, 16, e0255798. [Google Scholar] [CrossRef]
- NanoString nCounter® Canine IO Panel. Available online: https://nanostring.com/products/ncounter-assays-panels/oncology/canine-io/ (accessed on 9 July 2023).
- Available online: https://www.rosalind.bio (accessed on 11 May 2023).
- Danaher, P.; Warren, S.; Dennis, L.; D’Amico, L.; White, A.; Disis, M.L.; Geller, M.A.; Odunsi, K.; Beechem, J.; Fling, S.P. Gene expression markers of Tumor Infiltrating Leukocytes. J. Immunother. Cancer 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Christian, H. Cran-Package fpc. Available online: https://cran.r-project.org/web/packages/fpc/index.html (accessed on 5 April 2023).
- Alexa, A.; Rahnenführer, J. topGO: Enrichment Analysis for Gene Ontology, R package version 1.38.1.; Bioconductor: Saarbrücken, Germany, 2019.
- Mitchell, A.L.; Attwood, T.K.; Babbitt, P.C.; Blum, M.; Bork, P.; Bridge, A.; Brown, S.D.; Chang, H.Y.; El-Gebali, S.; Fraser, M.I.; et al. InterPro in 2019: Improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 2019, 47, D351–D360. [Google Scholar] [CrossRef]
- Geer, L.Y.; Marchler-Bauer, A.; Geer, R.C.; Han, L.; He, J.; He, S.; Liu, C.; Shi, W.; Bryant, S.H. The NCBI BioSystems database. Nucleic Acids Res. 2010, 38, D492–D496. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdottir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef] [PubMed]
- Slenter, D.N.; Kutmon, M.; Hanspers, K.; Riutta, A.; Windsor, J.; Nunes, N.; Melius, J.; Cirillo, E.; Coort, S.L.; Digles, D.; et al. WikiPathways: A multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res. 2018, 46, D661–D667. [Google Scholar] [CrossRef] [PubMed]
- Malkov, V.A.; Serikawa, K.A.; Balantac, N.; Watters, J.; Geiss, G.; Mashadi-Hossein, A.; Fare, T. Multiplexed measurements of gene signatures in different analytes using the Nanostring nCounter Assay System. BMC Res. Notes 2009, 2, 80. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Flinn, I.; Taylor, M.H.; Sikic, B.I.; Brody, J.; Nemunaitis, J.; Feldman, A.; Hawthorne, T.R.; Rawls, T.; Keler, T.; et al. Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, for hematologic malignancies. Blood Adv. 2020, 4, 1917–1926. [Google Scholar] [CrossRef]
- Tang, X.Y.; Sun, Y.; Zhang, A.; Hu, G.L.; Cao, W.; Wang, D.H.; Zhang, B.; Chen, H. Third-generation CD28/4-1BB chimeric antigen receptor T cells for chemotherapy relapsed or refractory acute lymphoblastic leukaemia: A non-randomised, open-label phase I trial protocol. BMJ Open 2016, 6, e013904. [Google Scholar] [CrossRef]
- Tan, T.J.; Ang, W.X.G.; Wang, W.W.; Chong, H.S.; Tan, S.H.; Cheong, R.; Chia, J.W.; Syn, N.L.; Shuen, W.H.; Ba, R.; et al. A phase I study of an adenoviral vector delivering a MUC1/CD40-ligand fusion protein in patients with advanced adenocarcinoma. Nat. Commun. 2022, 13, 6453. [Google Scholar] [CrossRef]
- Samant, M.; Ziemniak, J.; Paolini, J.F. First-in-Human Phase 1 Randomized Trial with the Anti-CD40 Monoclonal Antibody KPL-404: Safety, Tolerability, Receptor Occupancy, and Suppression of T-Cell-Dependent Antibody Response. J. Pharmacol. Exp. Ther. 2023, 387, 306–314. [Google Scholar] [CrossRef]
- Judge, S.J.; Yanagisawa, M.; Sturgill, I.R.; Bateni, S.B.; Gingrich, A.A.; Foltz, J.A.; Lee, D.A.; Modiano, J.F.; Monjazeb, A.M.; Culp, W.T.N.; et al. Blood and tissue biomarker analysis in dogs with osteosarcoma treated with palliative radiation and intra-tumoral autologous natural killer cell transfer. PLoS ONE 2020, 15, e0224775. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.H.; He, A.R.; Doi, T.; Levy, R.; Bhatia, S.; Pishvaian, M.J.; Cesari, R.; Chen, Y.; Davis, C.B.; Huang, B.; et al. Phase I Study of Single-Agent Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Patients with Advanced Cancer. Clin. Cancer Res. 2018, 24, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.; Haynes, C.; Marable, J.; Pundkar, C.; Nance, R.L.; Bedi, D.; Agarwal, P.; Suryawanshi, A.S.; Mishra, A.; Smith, B.F.; et al. Development of OX40 agonists for canine cancer immunotherapy. iScience 2022, 25, 105158. [Google Scholar] [CrossRef] [PubMed]
- Ammons, D.; Hopkins, L.; Cronise, K.; Kurihara, J.; Regan, D.; Dow, S. Single-cell RNA sequencing reveals the cellular and molecular heterogeneity of treatment-naive primary osteosarcoma in dogs. Res. Sq. 2023. preprint. [Google Scholar] [CrossRef]
- Patel, M.R.; Naing, A.; III, H.A.B.; Lin, C.-C.; Curigliano, G.; Thistlethwaite, F.; Minchom, A.R.; Ascierto, P.A.; Braud, F.G.D.; Cecchini, M.; et al. A phase 1/2 open-label study of KY1044, an anti-ICOS antibody with dual mechanism of action, as single agent and in combination with atezolizumab, in adult patients with advanced malignancies. J. Clin. Oncol. 2021, 39, 2624. [Google Scholar] [CrossRef]
- Lim, E.A.; Bendell, J.C.; Falchook, G.S.; Bauer, T.M.; Drake, C.G.; Choe, J.H.; George, D.J.; Karlix, J.L.; Ulahannan, S.; Sachsenmeier, K.F.; et al. Phase Ia/b, Open-Label, Multicenter Study of AZD4635 (an Adenosine A2A Receptor Antagonist) as Monotherapy or Combined with Durvalumab, in Patients with Solid Tumors. Clin. Cancer Res. 2022, 28, 4871–4884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Black, R.G.; Kohli, K.; Hayes, B.J.; Miller, C.; Koehne, A.; Schroeder, B.A.; Abrams, K.; Schulte, B.C.; Alexiev, B.A.; et al. B7-H3 Specific CAR T Cells for the Naturally Occurring, Spontaneous Canine Sarcoma Model. Mol. Cancer Ther. 2022, 21, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Schilder, R.J.; Powderly, J.D.; Park, H.; Bilen, M.A.; McKean, M.; May, R.; Feng, H.; Yao, S.; Keegan, P.; Naing, A. Phase Ia dose-escalation study of the anti-BTLA antibody icatolimab as a monotherapy in patients with advanced solid tumor. J. Clin. Oncol. 2022, 40, 2643. [Google Scholar] [CrossRef]
- Mason, N.J.; Chester, N.; Xiong, A.; Rotolo, A.; Wu, Y.; Yoshimoto, S.; Glassman, P.; Gulendran, G.; Siegel, D.L. Development of a fully canine anti-canine CTLA4 monoclonal antibody for comparative translational research in dogs with spontaneous tumors. MAbs 2021, 13, 2004638. [Google Scholar] [CrossRef]
- Ikeda, N.; Kato, D.; Tsuboi, M.; Yoshitake, R.; Eto, S.; Yoshimoto, S.; Shinada, M.; Kamoto, S.; Hashimoto, Y.; Takahashi, Y.; et al. Detection of indoleamine 2,3-dioxygenase 1-expressing cells in canine normal and tumor tissues. J. Vet. Med. Sci. 2021, 83, 1885–1890. [Google Scholar] [CrossRef]
- Peng, X.; Zhao, Z.; Liu, L.; Bai, L.; Tong, R.; Yang, H.; Zhong, L. Targeting Indoleamine Dioxygenase and Tryptophan Dioxygenase in Cancer Immunotherapy: Clinical Progress and Challenges. Drug Des. Devel. Ther. 2022, 16, 2639–2657. [Google Scholar] [CrossRef]
- Ligon, J.A.; Choi, W.; Cojocaru, G.; Fu, W.; Hsiue, E.H.; Oke, T.F.; Siegel, N.; Fong, M.H.; Ladle, B.; Pratilas, C.A.; et al. Pathways of immune exclusion in metastatic osteosarcoma are associated with inferior patient outcomes. J. Immunother. Cancer 2021, 9, e001772. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, Q.; Zhang, C.; She, J.; Cao, S.; Cao, M.; Zhang, N.; Adiila, A.V.; Zhong, J.; Yao, C.; et al. Identification and Validation of CYBB, CD86, and C3AR1 as the Key Genes Related to Macrophage Infiltration of Gastric Cancer. Front. Mol. Biosci. 2021, 8, 756085. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Moreno, V.; Morgensztern, D.; Curigliano, G.; Rutkowski, P.; Trigo, J.M.; Calvo, A.; Kowalski, D.; Cortinovis, D.; Plummer, R.; et al. First-in-human, open-label, phase 1/2 study of the monoclonal antibody programmed cell death protein-1 (PD-1) inhibitor cetrelimab (JNJ-63723283) in patients with advanced cancers. Cancer Chemother. Pharmacol. 2022, 89, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, L.; Wu, C.; Huang, L.; Ruan, Y.; Xue, W. Tumor-derived Exosomes Induced M2 Macrophage Polarization and Promoted the Metastasis of Osteosarcoma Cells Through Tim-3. Arch. Med. Res. 2021, 52, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Mo, S.; Sun, Z.; Lu, Z.; Yu, S.; Chen, J.; Xiang, Y. Analysis of the immune checkpoint V-domain Ig-containing suppressor of T-cell activation (VISTA) in endometrial cancer. Mod. Pathol. 2022, 35, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Matsuyama, A.; Mutsaers, A.J.; Woods, J.P. Retrospective evaluation of toceranib (Palladia) treatment for canine metastatic appendicular osteosarcoma. Can. Vet. J. 2017, 58, 1059–1064. [Google Scholar]
- Sayles, L.C.; Breese, M.R.; Koehne, A.L.; Leung, S.G.; Lee, A.G.; Liu, H.Y.; Spillinger, A.; Shah, A.T.; Tanasa, B.; Straessler, K.; et al. Genome-Informed Targeted Therapy for Osteosarcoma. Cancer Discov. 2019, 9, 46–63. [Google Scholar] [CrossRef]
- Musser, M.L.; Berger, E.P.; Tripp, C.D.; Clifford, C.A.; Bergman, P.J.; Johannes, C.M. Safety evaluation of the canine osteosarcoma vaccine, live Listeria vector. Vet. Comp. Oncol. 2021, 19, 92–98. [Google Scholar] [CrossRef]
- Ebb, D.; Meyers, P.; Grier, H.; Bernstein, M.; Gorlick, R.; Lipshultz, S.E.; Krailo, M.; Devidas, M.; Barkauskas, D.A.; Siegal, G.P.; et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: A report from the children’s oncology group. J. Clin. Oncol. 2012, 30, 2545–2551. [Google Scholar] [CrossRef]
- Doyle, H.A.; Gee, R.J.; Masters, T.D.; Gee, C.R.; Booth, C.J.; Peterson-Roth, E.; Koski, R.A.; Helfand, S.C.; Price, L.; Bascombe, D.; et al. Vaccine-induced ErbB (EGFR/HER2)-specific immunity in spontaneous canine cancer. Transl. Oncol. 2021, 14, 101205. [Google Scholar] [CrossRef]
- Igase, M.; Nemoto, Y.; Itamoto, K.; Tani, K.; Nakaichi, M.; Sakurai, M.; Sakai, Y.; Noguchi, S.; Kato, M.; Tsukui, T.; et al. A pilot clinical study of the therapeutic antibody against canine PD-1 for advanced spontaneous cancers in dogs. Sci. Rep. 2020, 10, 18311. [Google Scholar] [CrossRef] [PubMed]
- Boye, K.; Longhi, A.; Guren, T.; Lorenz, S.; Naess, S.; Pierini, M.; Taksdal, I.; Lobmaier, I.; Cesari, M.; Paioli, A.; et al. Pembrolizumab in advanced osteosarcoma: Results of a single-arm, open-label, phase 2 trial. Cancer Immunol. Immunother. 2021, 70, 2617–2624. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.C.; Cam, H.; Phelps, D.A.; Saraf, A.J.; Bid, H.K.; Cam, M.; London, C.A.; Winget, S.A.; Arnold, M.A.; Brandolini, L.; et al. IL-6 and CXCL8 mediate osteosarcoma-lung interactions critical to metastasis. JCI Insight 2018, 3, e99791. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.M.; Barger, A.M.; Fredrickson, R.L.; Fitzsimmons, D.; Garrett, L.D. Investigating CXCR4 expression in canine appendicular osteosarcoma. J. Vet. Intern. Med. 2008, 22, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Byrum, M.L.; Pondenis, H.C.; Fredrickson, R.L.; Wycislo, K.L.; Fan, T.M. Downregulation of CXCR4 Expression and Functionality After Zoledronate Exposure in Canine Osteosarcoma. J. Vet. Intern. Med. 2016, 30, 1187–1196. [Google Scholar] [CrossRef]
- Gardner, H.L.; Sivaprakasam, K.; Briones, N.; Zismann, V.; Perdigones, N.; Drenner, K.; Facista, S.; Richholt, R.; Liang, W.; Aldrich, J.; et al. Canine osteosarcoma genome sequencing identifies recurrent mutations in DMD and the histone methyltransferase gene SETD2. Commun. Biol. 2019, 2, 266. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, X.; Tang, J.; Hu, F.; Li, D.; Song, H.; Chen, J.; Guo, J.; Li, R.; Lin, Y.; et al. ISG15 is a potential therapeutic target for osteosarcoma: A comprehensive analysis based on bioinformatics and in vitro experiments. Am. J. Transl. Res. 2023, 15, 817–833. [Google Scholar]
- Yang, Y.; Zhang, Y.; Qu, X.; Xia, J.; Li, D.; Li, X.; Wang, Y.; He, Z.; Li, S.; Zhou, Y.; et al. Identification of differentially expressed genes in the development of osteosarcoma using RNA-seq. Oncotarget 2016, 7, 87194–87205. [Google Scholar] [CrossRef]
- Aljohani, A.I.; Joseph, C.; Kurozumi, S.; Mohammed, O.J.; Miligy, I.M.; Green, A.R.; Rakha, E.A. Myxovirus resistance 1 (MX1) is an independent predictor of poor outcome in invasive breast cancer. Breast Cancer Res. Treat. 2020, 181, 541–551. [Google Scholar] [CrossRef]
- Gong, W.; Donnelly, C.R.; Heath, B.R.; Bellile, E.; Donnelly, L.A.; Taner, H.F.; Broses, L.; Brenner, J.C.; Chinn, S.B.; Ji, R.R.; et al. Cancer-specific type-I interferon receptor signaling promotes cancer stemness and effector CD8+ T-cell exhaustion. Oncoimmunology 2021, 10, 1997385. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, C.; Gao, L.; Wang, Y.; Gao, X.; Tang, L.; Zhang, K.; Li, Z.; Han, J.; Xiao, J. NPNT promotes early-stage bone metastases in breast cancer by regulation of the osteogenic niche. J. Bone Oncol. 2018, 13, 91–96. [Google Scholar] [CrossRef]
- Steigedal, T.S.; Toraskar, J.; Redvers, R.P.; Valla, M.; Magnussen, S.N.; Bofin, A.M.; Opdahl, S.; Lundgren, S.; Eckhardt, B.L.; Lamar, J.M.; et al. Nephronectin is Correlated with Poor Prognosis in Breast Cancer and Promotes Metastasis via its Integrin-Binding Motifs. Neoplasia 2018, 20, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Kahai, S.; Lee, S.C.; Seth, A.; Yang, B.B. Nephronectin promotes osteoblast differentiation via the epidermal growth factor-like repeats. FEBS Lett. 2010, 584, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Righi, A.; Gambarotti, M.; Sbaraglia, M.; Sisto, A.; Ferrari, S.; Dei Tos, A.P.; Picci, P. p16 expression as a prognostic and predictive marker in high-grade localized osteosarcoma of the extremities: An analysis of 357 cases. Hum. Pathol. 2016, 58, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, C.; Guo, Z.; Fu, Y.; Yu, X.; Liu, B.; Zhou, H.; Wang, J.; Li, W.; Pang, Q. P16 protein expression as a useful predictive biomarker for neoadjuvant chemotherapy response in patients with high-grade osteosarcoma: A systematic meta-analysis under guideline of PRISMA. Medicine 2017, 96, e6714. [Google Scholar] [CrossRef]
- Murphy, B.G.; Mok, M.Y.; York, D.; Rebhun, R.; Woolard, K.D.; Hillman, C.; Dickinson, P.; Skorupski, K. Evaluation of P16 expression in canine appendicular osteosarcoma. BMC Vet. Res. 2017, 13, 189. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.S.; Jaworski, L.; Kisseberth, W.C. Immunohistochemical detection of p53, PTEN, Rb, and p16 in canine osteosarcoma using tissue microarray. J. Vet. Diagn. Investig. 2018, 30, 504–509. [Google Scholar] [CrossRef]
- Karlsson, E.K.; Sigurdsson, S.; Ivansson, E.; Thomas, R.; Elvers, I.; Wright, J.; Howald, C.; Tonomura, N.; Perloski, M.; Swofford, R.; et al. Genome-wide analyses implicate 33 loci in heritable dog osteosarcoma, including regulatory variants near CDKN2A/B. Genome Biol. 2013, 14, R132. [Google Scholar] [CrossRef]
- Letko, A.; Minor, K.M.; Norton, E.M.; Marinescu, V.D.; Drogemuller, M.; Ivansson, E.; Megquier, K.; Noh, H.J.; Starkey, M.; Friedenberg, S.G.; et al. Genome-Wide Analyses for Osteosarcoma in Leonberger Dogs Reveal the CDKN2A/B Gene Locus as a Major Risk Locus. Genes 2021, 12, 1964. [Google Scholar] [CrossRef]
- Xi, Y.; Chen, Y. PTEN Plays Dual Roles As a Tumor Suppressor in Osteosarcoma Cells. J. Cell. Biochem. 2017, 118, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.A.; Forest, T.; Smith, C. Tumor suppressor PTEN is mutated in canine osteosarcoma cell lines and tumors. Vet. Pathol. 2002, 39, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Angstadt, A.Y.; Motsinger-Reif, A.; Thomas, R.; Kisseberth, W.C.; Guillermo Couto, C.; Duval, D.L.; Nielsen, D.M.; Modiano, J.F.; Breen, M. Characterization of canine osteosarcoma by array comparative genomic hybridization and RT-qPCR: Signatures of genomic imbalance in canine osteosarcoma parallel the human counterpart. Genes Chromosomes Cancer 2011, 50, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Su, X.; Xia, Q.; Wang, J.; Kan, S. Expression of NF-kappaB and PTEN in osteosarcoma and its clinical significance. Oncol. Lett. 2017, 14, 6744–6748. [Google Scholar] [CrossRef] [PubMed]
- Loftus, J.P.; Cavatorta, D.; Bushey, J.J.; Levine, C.B.; Sevier, C.S.; Wakshlag, J.J. The 5-lipoxygenase inhibitor tepoxalin induces oxidative damage and altered PTEN status prior to apoptosis in canine osteosarcoma cell lines. Vet. Comp. Oncol. 2016, 14, e17–e30. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.J.; Wu, N.; Liu, Y.; Shu, K.J.; Zou, X.; Zhang, R.X.; Pi, C.J.; He, B.C.; Ke, Z.Y.; Chen, L.; et al. Evodiamine inhibits the proliferation of human osteosarcoma cells by blocking PI3K/Akt signaling. Oncol. Rep. 2015, 34, 1388–1396. [Google Scholar] [CrossRef]
- Sui, W.; Zhang, Y.; Wang, Z.; Wang, Z.; Jia, Q.; Wu, L.; Zhang, W. Antitumor effect of a selective COX-2 inhibitor, celecoxib, may be attributed to angiogenesis inhibition through modulating the PTEN/PI3K/Akt/HIF-1 pathway in an H(2)(2) murine hepatocarcinoma model. Oncol. Rep. 2014, 31, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Cable, M.G.; Randall, R.L. Characterizing Osteosarcoma Through PTEN and PI3K: What p53 and Rb1 Can’t Tell Us. In Osteosarcoma-Biology, Behavior and Mechanisms; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef]
- Mason, N.J.; Gnanandarajah, J.S.; Engiles, J.B.; Gray, F.; Laughlin, D.; Gaurnier-Hausser, A.; Wallecha, A.; Huebner, M.; Paterson, Y. Immunotherapy with a HER2-Targeting Listeria Induces HER2-Specific Immunity and Demonstrates Potential Therapeutic Effects in a Phase I Trial in Canine Osteosarcoma. Clin. Cancer Res. 2016, 22, 4380–4390. [Google Scholar] [CrossRef]
- Riggs, J.L.; McAllister, R.M.; Lennette, E.H. Immunofluorescent studies of RD-114 virus replication in cell culture. J. Gen. Virol. 1974, 25, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, M.; Lechowski, R.; Zabielska, K. What do we know about canine osteosarcoma treatment? Review. Vet. Res. Commun. 2015, 39, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Kito, F.; Oyama, R.; Sakumoto, M.; Takahashi, M.; Shiozawa, K.; Qiao, Z.; Sakamoto, H.; Hirose, T.; Setsu, N.; Yoshida, A.; et al. Establishment and characterization of novel patient-derived osteosarcoma xenograft and cell line. Vitr. Cell. Dev. Biol. Anim. 2018, 54, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Fenger, J.M.; Roberts, R.D.; Iwenofu, O.H.; Bear, M.D.; Zhang, X.; Couto, J.I.; Modiano, J.F.; Kisseberth, W.C.; London, C.A. MiR-9 is overexpressed in spontaneous canine osteosarcoma and promotes a metastatic phenotype including invasion and migration in osteoblasts and osteosarcoma cell lines. BMC Cancer 2016, 16, 784. [Google Scholar] [CrossRef]





| Cell Line | Sex | Age | Breed | Location | Note |
|---|---|---|---|---|---|
| BR | N/A | N/A | Mongrel | Lung metastasis | N/A |
| BZ | Male | 8 years | German shepherd | Zygomatic arch | Underwent two cycles of carboplatin |
| LK | Male | 3 years | Doberman pinscher | Lung metastasis | Euthanized 4 months after diagnosis with OSA. This patient underwent two cycles of carboplatin, one cycle of cyclophosphamide and two cycles of doxorubicin. |
| Cell Line | Doubling Time (Hours) |
|---|---|
| BR | 14.8 |
| BZ | 20.1 |
| LK | 16.4 |
| Name | Log2 Fold Change | p-Adj | BR | BZ | LK |
|---|---|---|---|---|---|
| ISG15 | 9.1 | 3.00 × 10−8 | 3.3 | 10.6 | 4.6 |
| MX1 | 7.7 | 2.87 × 10−8 | 3.1 | 9.2 | 3.4 |
| CXCL8 | 7.2 | 2.54 × 10−9 | 7.2 | 7.6 | 6.6 |
| IL6 | 6.6 | 1.11 × 10−6 | 7.6 | 4.1 | 2.5 |
| CXCL10 | 6.3 | 0.000259 | 5.1 | 8.2 | 6.8 |
| NPNT | 6.1 | 5.31 × 10−8 | 5.9 | 5.1 | 6.9 |
| LOC102154078 | 6 | 4.70 × 10−6 | 7.4 | 4.2 | 3.1 |
| ISG20 | 5.9 | 5.48 × 10−7 | 4.9 | 6.4 | 4.8 |
| CXCR4 | 5.4 | 1.22 × 10−5 | 6.5 | 4.6 | 3 |
| ARG2 | 5 | 5.62 × 10−12 | 4.5 | 4.7 | 5.6 |
| TNFSF18 | 4.7 | 3.22 × 10−5 | 5 | 5.3 | 2.5 |
| CYFIP2 | 4.6 | 4.48 × 10−10 | 4.2 | 5.1 | 4.2 |
| THBS1 | −3.8 | 2.48 × 10−6 | −2.8 | −5.6 | −4.3 |
| ICAM2 | −3.9 | 0.000172 | −3.6 | −4.6 | 2.2 |
| CCL23 | −3.9 | 0.04431 | −2.9 | −4.4 | −4.4 |
| ADAMTS2 | −3.9 | 5.48 × 10−7 | −3 | −5.5 | −4.1 |
| PTEN | −4.1 | 0.026195 | −0.2 | −2.4 | −9.8 |
| CDKN2B | −4.2 | 0.000564 | −6 | −4.3 | −6 |
| APOE | −4.5 | 0.000116 | −2.7 | −5.7 | −4.7 |
| LCN2 | −4.7 | 3.28 × 10−6 | −4.8 | −6 | −3.9 |
| PDPN | −4.9 | 3.10 × 10−5 | −7.6 | −3.6 | −8.1 |
| IL1R2 | −5.1 | 1.05 × 10−5 | −4.7 | −5.2 | −5.8 |
| CFD | −5.7 | 3.71 × 10−5 | −5.2 | −6 | −6 |
| CDKN2A | −5.7 | 0.000478 | −6.5 | −3.8 | −7 |
| Name | Alias | OSA avg | BR | BZ | LK | Current Clinical Trial/Reference in Literature |
|---|---|---|---|---|---|---|
| CD27 | - | 1.7 | 2 | 1.7 | 1.3 | Ansell [33] |
| CD28 | - | 0.8 | 1 | 1.2 | 0.2 | Tang [34] |
| CD40LG | CD154|TNFSF5 | 0.9 | 1.1 | 1.6 | 0.1 | Tan [35] |
| CD40 | TNFRSF5 | 5.3 | 5.3 | 5.5 | 5 | Samant [36] |
| IL2RB | CD122 | 1.7 | 1.8 | 2.2 | 1.3 | Judge [37] |
| TNFRSF9 | CD137, 4-1BB | 1.3 | 1.2 | 2.1 | 0.7 | Segal [38] |
| TNFRSF4 | OX40 | 3.1 | 3.1 | 3.1 | 3.1 | Ruiz [39] |
| TNFRSF18 | GITR | 3.1 | 3.1 | 2.9 | 3.4 | Ammons [40] |
| ICOS | - | 0.7 | 1.2 | 1.3 | −0.3 | Patel [41] |
| ADORA2A | A2AR | 7.3 | 6.6 | 8.6 | 6.8 | Lim [42] |
| CD276 | B7-H3 | 6.8 | 7.4 | 5.8 | 7 | Zhang [43] |
| BTLA | - | 1.6 | 1.8 | 1.6 | 1.5 | Schilder AACR meeting [44] |
| CTLA4 | CD152 | 3 | 3.2 | 3 | 2.6 | Mason [45] |
| IDO1 | INDO | 4.5 | 2.3 | 8.3 | 2.9 | Ikeda [46] |
| IDO2 | INDOL1 | 2.3 | 2.4 | 2.4 | 2.2 | Peng [47] |
| LAG3 | - | 3.1 | 3.4 | 2.9 | 3 | Ligon [48] |
| CYBB | NOX2 | 2 | 2 | 2.3 | 1.6 | Chen [49] |
| CD274 | PD-L1 | 3.6 | 4.1 | 3.6 | 3 | Felip E [50] |
| HAVCR2 | TIM3 | 3.3 | 3.1 | 3.3 | 3.5 | Cheng [51] |
| VSIR | VISTA | 5.5 | 5.8 | 5.2 | 5.5 | Zong [52] |
| KIT | c-KIT | 3.1 | 4.2 | 2.9 | 2.3 | Kim [53] |
| VEGFA | VEGF | 9 | 9.7 | 7.6 | 9.6 | Sayles [54] |
| ERBB2 | HER-2|c-erbB-2 | 6.7 | 7.1 | 6.6 | 6.6 | Musser [55] Ebb [56] |
| EGFR | - | 7.2 | 7.7 | 7.6 | 6.4 | Doyle [57] |
| PDCD1 | PD1 | 2.8 | 2.5 | 3.5 | 2.4 | Igase [58] Boye [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.-T.; Engleberg, A.I.; Yuzbasiyan-Gurkan, V. Establishment and Characterization of Cell Lines from Canine Metastatic Osteosarcoma. Cells 2024, 13, 25. https://doi.org/10.3390/cells13010025
Yang Y-T, Engleberg AI, Yuzbasiyan-Gurkan V. Establishment and Characterization of Cell Lines from Canine Metastatic Osteosarcoma. Cells. 2024; 13(1):25. https://doi.org/10.3390/cells13010025
Chicago/Turabian StyleYang, Ya-Ting, Alexander I. Engleberg, and Vilma Yuzbasiyan-Gurkan. 2024. "Establishment and Characterization of Cell Lines from Canine Metastatic Osteosarcoma" Cells 13, no. 1: 25. https://doi.org/10.3390/cells13010025
APA StyleYang, Y.-T., Engleberg, A. I., & Yuzbasiyan-Gurkan, V. (2024). Establishment and Characterization of Cell Lines from Canine Metastatic Osteosarcoma. Cells, 13(1), 25. https://doi.org/10.3390/cells13010025







