miR-142: A Master Regulator in Hematological Malignancies and Therapeutic Opportunities
Abstract
1. Introduction
2. Biogenesis, Regulation, and Functions of miR-142
2.1. Biogenesis of miR-142
2.2. Regulation and Function of miR-142
2.3. Hematopoietic Functions of miR-142
2.3.1. Hematopoiesis
2.3.2. B Cells
2.3.3. T Cells
2.3.4. Natural Killer Cells
2.3.5. Myeloid Cells
2.4. Exosome-Mediated Transport of miR-142
3. The Role of miR-142 in Hematological Malignancies
3.1. Lymphomas
3.1.1. Diffuse Large B-Cell Lymphoma
3.1.2. Follicular Lymphoma
3.1.3. Burkitt Lymphoma
3.1.4. Mucosa-Associated Lymphoid Tissue Lymphoma
3.1.5. Mantle-Cell Lymphoma
3.1.6. Nasal Natural Killer/T-Cell Lymphoma
3.1.7. Cutaneous T-Cell Lymphoma
3.1.8. Adult T-Cell Leukemia/Lymphoma
3.2. Leukemias
3.2.1. Chronic Lymphocytic Leukemia
3.2.2. Chronic Myeloid Leukemia
3.2.3. Acute Lymphocytic Leukemia
3.2.4. Acute Myeloid Leukemia
3.2.5. Acute Promyelocytic Leukemia
4. miR-142 as a Therapeutic Target
4.1. Anti-miRNA Oligonucleotides
4.2. miRNA Sponges
4.3. Circular RNAs
4.4. CRISPR-Cas9 for DNA Knockout and Editing
4.5. Delivery
5. Concluding Remarks and Outlook
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
References
- La Ferlita, A.; Battaglia, R.; Andronico, F.; Caruso, S.; Cianci, A.; Purrello, M.; Di Pietro, C. Non-Coding RNAs in Endometrial Physiopathology. Int. J. Mol. Sci. 2018, 19, 2120. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Makunin, I.V. Non-Coding RNA. Hum. Mol. Genet. 2006, 15, R17–R29. [Google Scholar] [CrossRef] [PubMed]
- Taft, R.J.; Pang, K.C.; Mercer, T.R.; Dinger, M.; Mattick, J.S. Non-Coding RNAs: Regulators of Disease: Non-Coding RNAs: Regulators of Disease. J. Pathol. 2010, 220, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, S.; Babayan, A.; Pantel, K.; Calin, G.A. Clinical Utility of Circulating Non-Coding RNAs—An Update. Nat. Rev. Clin. Oncol. 2018, 15, 541–563. [Google Scholar] [CrossRef]
- Diamantopoulos, M.A.; Tsiakanikas, P.; Scorilas, A. Non-Coding RNAs: The Riddle of the Transcriptome and Their Perspectives in Cancer. Ann. Transl. Med. 2018, 6, 3. [Google Scholar] [CrossRef]
- Novikova, I.V.; Hennelly, S.P.; Sanbonmatsu, K.Y. Sizing up Long Non-Coding RNAs: Do lncRNAs Have Secondary and Tertiary Structure? BioArchitecture 2012, 2, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, R.; Van Roosbroeck, K. miR-155 in Cancer Drug Resistance and as Target for miRNA-Based Therapeutics. Cancer Metastasis Rev. 2018, 37, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wu, H.; Pavlosky, A.; Zou, L.-L.; Deng, X.; Zhang, Z.-X.; Jevnikar, A.M. Regulatory Non-Coding RNA: New Instruments in the Orchestration of Cell Death. Cell Death Dis. 2016, 7, e2333. [Google Scholar] [CrossRef]
- Dragomir, M.P.; Kopetz, S.; Ajani, J.A.; Calin, G.A. Non-Coding RNAs in GI Cancers: From Cancer Hallmarks to Clinical Utility. Gut 2020, 69, 748–763. [Google Scholar] [CrossRef]
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Annese, T.; Tamma, R.; De Giorgis, M.; Ribatti, D. microRNAs Biogenesis, Functions and Role in Tumor Angiogenesis. Front. Oncol. 2020, 10, 581007. [Google Scholar] [CrossRef] [PubMed]
- Nariman-Saleh-Fam, Z.; Saadatian, Z.; Daraei, A.; Mansoori, Y.; Bastami, M.; Tavakkoli-Bazzaz, J. The Intricate Role of miR-155 in Carcinogenesis: Potential Implications for Esophageal Cancer Research. Biomark. Med. 2019, 13, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Lin, C.-C.; Eischen, C.M.; Bandyopadhyay, S.; Zhao, Z. Concordant Dysregulation of miR-5p and miR-3p Arms of the Same Precursor microRNA May Be a Mechanism in Inducing Cell Proliferation and Tumorigenesis: A Lung Cancer Study. RNA 2015, 21, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, R.; Van Roosbroeck, K.; Calin, G.A. Cell-to-cell Communication: microRNAs as Hormones. Mol. Oncol. 2017, 11, 1673–1686. [Google Scholar] [CrossRef]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA Therapeutics—Challenges and Potential Solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The Role of MicroRNAs in Human Cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Iorio, M.V.; Croce, C.M. MicroRNA Dysregulation in Cancer: Diagnostics, Monitoring and Therapeutics. A Comprehensive Review. EMBO Mol. Med. 2012, 4, 143–159. [Google Scholar] [CrossRef]
- Bayraktar, R.; Ivan, C.; Bayraktar, E.; Kanlikilicer, P.; Kabil, N.N.; Kahraman, N.; Mokhlis, H.A.; Karakas, D.; Rodriguez-Aguayo, C.; Arslan, A.; et al. Dual Suppressive Effect of miR-34a on the FOXM1/eEF2-Kinase Axis Regulates Triple-Negative Breast Cancer Growth and Invasion. Clin. Cancer Res. 2018, 24, 4225–4241. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-Coding RNAs in Disease: From Mechanisms to Therapeutics. Nat. Rev. Genet. 2023, 1–22. [Google Scholar] [CrossRef]
- Pahlavan, Y.; Mohammadi Nasr, M.; Dalir Abdolahinia, E.; Pirdel, Z.; Razi Soofiyani, S.; Siahpoush, S.; Nejati, K. Prominent Roles of microRNA-142 in Cancer. Pathol. Res. Pract. 2020, 216, 153220. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zheng, S.; Yu, K.; Yu, Y.; Yu, C.; Shi, W.; Ge, Q.; Ye, Z.; Shao, Y. Prognostic Value of miR-142 in Solid Tumors: A Meta-Analysis. Biosci. Rep. 2021, 41, BSR20204043. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, W.; Pan, G.; Huang, F.; Yang, J.; Zhang, H.; Zhou, R.; Xu, N. miR-142-3p Modulates Cell Invasion and Migration via PKM2-Mediated Aerobic Glycolysis in Colorectal Cancer. Anal. Cell Pathol. 2021, 2021, 9927720. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Duijf, P.H.G.; Mohammadi, A.; Safarzadeh, E.; Ditzel, H.J.; Gjerstorff, M.F.; Cho, W.C.-S.; Baradaran, B. MiR-142-3p Targets HMGA2 and Suppresses Breast Cancer Malignancy. Life Sci. 2021, 276, 119431. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zou, L.; Lu, F.; Ye, L.; Su, B.; Yang, K.; Lin, M.; Fu, J.; Li, Y. miR-142-5p Promotes Renal Cell Tumorigenesis by Targeting TFAP2B. Oncol. Lett. 2020, 20, 324. [Google Scholar] [CrossRef] [PubMed]
- Morin, R.D.; Assouline, S.; Alcaide, M.; Mohajeri, A.; Johnston, R.L.; Chong, L.; Grewal, J.; Yu, S.; Fornika, D.; Bushell, K.; et al. Genetic Landscapes of Relapsed and Refractory Diffuse Large B-Cell Lymphomas. Clin. Cancer Res. 2016, 22, 2290–2300. [Google Scholar] [CrossRef]
- Trissal, M.C.; Wong, T.N.; Yao, J.-C.; Ramaswamy, R.; Kuo, I.; Baty, J.; Sun, Y.; Jih, G.; Parikh, N.; Berrien-Elliott, M.M.; et al. MIR142 Loss-of-Function Mutations Derepress ASH1L to Increase HOXA Gene Expression and Promote Leukemogenesis. Cancer Res. 2018, 78, 3510–3521. [Google Scholar] [CrossRef]
- Sharma, S. Immunomodulation: A Definitive Role of microRNA-142. Dev. Comp. Immunol. 2017, 77, 150–156. [Google Scholar] [CrossRef]
- Merkerova, M.; Belickova, M.; Bruchova, H. Differential Expression of microRNAs in Hematopoietic Cell Lineages. Eur. J. Haematol. 2008, 81, 304–310. [Google Scholar] [CrossRef]
- Haecker, I.; Gay, L.A.; Yang, Y.; Hu, J.; Morse, A.M.; McIntyre, L.M.; Renne, R. Ago HITS-CLIP Expands Understanding of Kaposi’s Sarcoma-Associated Herpesvirus miRNA Function in Primary Effusion Lymphomas. PLoS Pathog. 2012, 8, e1002884. [Google Scholar] [CrossRef]
- Narasimhan, M.; Patel, D.; Vedpathak, D.; Rathinam, M.; Henderson, G.; Mahimainathan, L. Identification of Novel microRNAs in Post-Transcriptional Control of Nrf2 Expression and Redox Homeostasis in Neuronal, SH-SY5Y Cells. PLoS ONE 2012, 7, e51111. [Google Scholar] [CrossRef] [PubMed]
- Skalsky, R.L.; Corcoran, D.L.; Gottwein, E.; Frank, C.L.; Kang, D.; Hafner, M.; Nusbaum, J.D.; Feederle, R.; Delecluse, H.-J.; Luftig, M.A.; et al. The Viral and Cellular MicroRNA Targetome in Lymphoblastoid Cell Lines. PLoS Pathog. 2012, 8, e1002484. [Google Scholar] [CrossRef] [PubMed]
- Gottwein, E.; Corcoran, D.L.; Mukherjee, N.; Skalsky, R.L.; Hafner, M.; Nusbaum, J.D.; Shamulailatpam, P.; Love, C.L.; Dave, S.S.; Tuschl, T.; et al. Viral MicroRNA Targetome of KSHV-Infected Primary Effusion Lymphoma Cell Lines. Cell Host Microbe 2011, 10, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhang, P.; Zhou, L.; Yin, B.; Pan, H.; Peng, X. Clock-Controlled Mir-142-3p Can Target Its Activator, Bmal1. BMC Mol. Biol. 2012, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-W.; Zeng, Z.; Zhu, W.-X.; Fu, G.-H. MiR-142-3p Functions as a Tumor Suppressor by Targeting CD133, ABCG2, and Lgr5 in Colon Cancer Cells. J. Mol. Med. 2013, 91, 989–1000. [Google Scholar] [CrossRef]
- Wang, X.-S.; Gong, J.-N.; Yu, J.; Wang, F.; Zhang, X.-H.; Yin, X.-L.; Tan, Z.-Q.; Luo, Z.-M.; Yang, G.-H.; Shen, C.; et al. MicroRNA-29a and microRNA-142-3p Are Regulators of Myeloid Differentiation and Acute Myeloid Leukemia. Blood 2012, 119, 4992–5004. [Google Scholar] [CrossRef]
- Choi, Y.-C.; Yoon, S.; Jeong, Y.; Yoon, J.; Baek, K. Regulation of Vascular Endothelial Growth Factor Signaling by miR-200b. Mol. Cells 2011, 32, 77–82. [Google Scholar] [CrossRef]
- Grosswendt, S.; Filipchyk, A.; Manzano, M.; Klironomos, F.; Schilling, M.; Herzog, M.; Gottwein, E.; Rajewsky, N. Unambiguous Identification of miRNA:Target Site Interactions by Different Types of Ligation Reactions. Mol. Cell 2014, 54, 1042–1054. [Google Scholar] [CrossRef]
- Manikandan, P.; Vijayakumar, R.; Alshehri, B.; Senthilkumar, S.; Al-Aboody, M.S.; Haribaskar, R.; Veluchamy, A. Long Non-Coding RNAs Act as Novel Therapeutic Targets by Regulating Molecular Networks Associated with Ischemic Stroke. J. King Saud Univ.-Sci. 2022, 34, 102119. [Google Scholar] [CrossRef]
- Shrestha, A.; Mukhametshina, R.T.; Taghizadeh, S.; Vásquez-Pacheco, E.; Cabrera-Fuentes, H.; Rizvanov, A.; Mari, B.; Carraro, G.; Bellusci, S. MicroRNA-142 Is a Multifaceted Regulator in Organogenesis, Homeostasis, and Disease: miR-142 in Organogenesis, Homeostasis, and Disease. Dev. Dyn. 2017, 246, 285–290. [Google Scholar] [CrossRef]
- Bejerano, G.; Pheasant, M.; Makunin, I.; Stephen, S.; Kent, W.J.; Mattick, J.S.; Haussler, D. Ultraconserved Elements in the Human Genome. Science 2004, 304, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Liu, J.; Wei, J.; Yuan, H.; Zhang, T.; Bishopric, N.H. Repression of miR-142 by P300 and MAPK Is Required for Survival Signalling via Gp130 during Adaptive Hypertrophy. EMBO Mol. Med. 2012, 4, 617–632. [Google Scholar] [CrossRef]
- Su, Q.; Liu, Y.; Lv, X.-W.; Ye, Z.-L.; Sun, Y.-H.; Kong, B.-H.; Qin, Z.-B. Inhibition of lncRNA TUG1 Upregulates miR-142-3p to Ameliorate Myocardial Injury during Ischemia and Reperfusion via Targeting HMGB1- and Rac1-Induced Autophagy. J. Mol. Cell Cardiol. 2019, 133, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Tang, J.; He, H.; Cheng, P.; Chen, C. MiR-142-5p Promotes Bone Repair by Maintaining Osteoblast Activity. J. Bone Miner. Metab. 2017, 35, 255–264. [Google Scholar] [CrossRef]
- Song, J.; Kim, Y.-K. Identification of the Role of miR-142-5p in Alzheimer’s Disease by Comparative Bioinformatics and Cellular Analysis. Front. Mol. Neurosci. 2017, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Kétszeri, M.; Kirsch, A.; Frauscher, B.; Moschovaki-Filippidou, F.; Mooslechner, A.A.; Kirsch, A.H.; Schabhuettl, C.; Aringer, I.; Artinger, K.; Pregartner, G.; et al. MicroRNA-142-3p Improves Vascular Relaxation in Uremia. Atherosclerosis 2019, 280, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Li, X. miR-142-5p Regulates the Progression of Diabetic Retinopathy by Targeting IGF1. Int. J. Immunopathol. Pharmacol. 2020, 34, 205873842090904. [Google Scholar] [CrossRef]
- Li, Y.; Gao, M.; Xu, L.-N.; Yin, L.-H.; Qi, Y.; Peng, J.-Y. MicroRNA-142-3p Attenuates Hepatic Ischemia/Reperfusion Injury via Targeting of Myristoylated Alanine-Rich C-Kinase Substrate. Pharmacol. Res. 2020, 156, 104783. [Google Scholar] [CrossRef]
- Carraro, G.; Shrestha, A.; Rostkovius, J.; Contreras, A.; Chao, C.-M.; El Agha, E.; MacKenzie, B.; Dilai, S.; Guidolin, D.; Taketo, M.M.; et al. miR-142-3p Balances Proliferation and Differentiation of Mesenchymal Cells during Lung Development. Development 2014, 141, 1272–1281. [Google Scholar] [CrossRef]
- Zhang, T.; Ji, C.; Shi, R. miR-142-3p Promotes Pancreatic β Cell Survival through Targeting FOXO1 in Gestational Diabetes Mellitus. Int. J. Clin. Exp. Pathol. 2019, 12, 1529–1538. [Google Scholar]
- Sun, Y.; Sun, J.; Tomomi, T.; Nieves, E.; Mathewson, N.; Tamaki, H.; Evers, R.; Reddy, P. PU.1-Dependent Transcriptional Regulation of miR-142 Contributes to Its Hematopoietic Cell–Specific Expression and Modulation of IL-6. J. Immunol. 2013, 190, 4005–4013. [Google Scholar] [CrossRef] [PubMed]
- Poller, W.C.; Nahrendorf, M.; Swirski, F.K. Hematopoiesis and Cardiovascular Disease. Circ. Res. 2020, 126, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shen, W.; Yang, S.; Hu, F.; Li, H.; Zhu, T.-H. miR-223 and miR-142 Attenuate Hematopoietic Cell Proliferation, and miR-223 Positively Regulates miR-142 through LMO2 Isoforms and CEBP-β. Cell Res. 2010, 20, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Wedel, A.; Lömsziegler-Heitbrock, H.W. The C/EBP Family of Transcription Factors. Immunobiology 1995, 193, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Sun, W.; Yang, S.; Du, J.; Zhai, C.-L.; Wang, Z.-Q.; Zhang, J.; Zhu, T.-H. Downregulation of microRNA-142 by Proto-Oncogene LMO2 and Its Co-Factors. Leukemia 2008, 22, 1067–1071. [Google Scholar] [CrossRef][Green Version]
- Ramkissoon, S.H.; Mainwaring, L.A.; Ogasawara, Y.; Keyvanfar, K.; Philip McCoy, J.; Sloand, E.M.; Kajigaya, S.; Young, N.S. Hematopoietic-Specific microRNA Expression in Human Cells. Leuk. Res. 2006, 30, 643–647. [Google Scholar] [CrossRef]
- Xie, H.; Ye, M.; Feng, R.; Graf, T. Stepwise Reprogramming of B Cells into Macrophages. Cell 2004, 117, 663–676. [Google Scholar] [CrossRef]
- Laiosa, C.V.; Stadtfeld, M.; Xie, H.; de Andres-Aguayo, L.; Graf, T. Reprogramming of Committed T Cell Progenitors to Macrophages and Dendritic Cells by C/EBPα and PU.1 Transcription Factors. Immunity 2006, 25, 731–744. [Google Scholar] [CrossRef]
- Lekstrom-Himes, J.; Xanthopoulos, K.G. Biological Role of the CCAAT/Enhancer-Binding Protein Family of Transcription Factors. J. Biol. Chem. 1998, 273, 28545–28548. [Google Scholar] [CrossRef]
- Wiekmeijer, A.-S.; Pike-Overzet, K.; Brugman, M.H.; van Eggermond, M.C.J.A.; Cordes, M.; de Haas, E.F.E.; Li, Y.; Oole, E.; van IJcken, W.F.J.; Egeler, R.M.; et al. Overexpression of LMO2 Causes Aberrant Human T-Cell Development in Vivo by Three Potentially Distinct Cellular Mechanisms. Exp. Hematol. 2016, 44, 838–849.e9. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Neuberg, D.S.; Staunton, J.; Loh, M.L.; Huard, C.; Raimondi, S.C.; Behm, F.G.; Pui, C.-H.; Downing, J.R.; Gilliland, D.G.; et al. Gene Expression Signatures Define Novel Oncogenic Pathways in T Cell Acute Lymphoblastic Leukemia. Cancer Cell 2002, 1, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Hacein-Bey-Abina, S.; Von Kalle, C.; Schmidt, M.; McCormack, M.P.; Wulffraat, N.; Leboulch, P.; Lim, A.; Osborne, C.S.; Pawliuk, R.; Morillon, E.; et al. LMO2 -Associated Clonal T Cell Proliferation in Two Patients after Gene Therapy for SCID-X1. Science 2003, 302, 415–419. [Google Scholar] [CrossRef]
- Kramer, N.J.; Wang, W.-L.; Reyes, E.Y.; Kumar, B.; Chen, C.-C.; Ramakrishna, C.; Cantin, E.M.; Vonderfecht, S.L.; Taganov, K.D.; Chau, N.; et al. Altered Lymphopoiesis and Immunodeficiency in miR-142 Null Mice. Blood 2015, 125, 3720–3730. [Google Scholar] [CrossRef] [PubMed]
- Koralov, S.B.; Muljo, S.A.; Galler, G.R.; Krek, A.; Chakraborty, T.; Kanellopoulou, C.; Jensen, K.; Cobb, B.S.; Merkenschlager, M.; Rajewsky, N.; et al. Dicer Ablation Affects Antibody Diversity and Cell Survival in the B Lymphocyte Lineage. Cell 2008, 132, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Belver, L.; de Yébenes, V.G.; Ramiro, A.R. MicroRNAs Prevent the Generation of Autoreactive Antibodies. Immunity 2010, 33, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Guo, K.; Zeng, Q.; Huo, J.; Lam, K.-P. The RNase III Enzyme Dicer Is Essential for Germinal Center B-Cell Formation. Blood 2012, 119, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Haupt, S.; Prots, I.; Thümmler, K.; Kremmer, E.; Lipsky, P.E.; Schulze-Koops, H.; Skapenko, A. miR-142-3p Is Involved in CD25 + CD4 T Cell Proliferation by Targeting the Expression of Glycoprotein A Repetitions Predominant. J. Immunol. 2013, 190, 6579–6588. [Google Scholar] [CrossRef]
- Sun, Y.; Varambally, S.; Maher, C.A.; Cao, Q.; Chockley, P.; Toubai, T.; Malter, C.; Nieves, E.; Tawara, I.; Wang, Y.; et al. Targeting of microRNA-142-3p in Dendritic Cells Regulates Endotoxin-Induced Mortality. Blood 2011, 117, 6172–6183. [Google Scholar] [CrossRef]
- Khan, W.N. B Cell Receptor and BAFF Receptor Signaling Regulation of B Cell Homeostasis. J. Immunol. 2009, 183, 3561–3567. [Google Scholar] [CrossRef]
- Muljo, S.A.; Ansel, K.M.; Kanellopoulou, C.; Livingston, D.M.; Rao, A.; Rajewsky, K. Aberrant T Cell Differentiation in the Absence of Dicer. J. Exp. Med. 2005, 202, 261–269. [Google Scholar] [CrossRef]
- Sun, Y.; Oravecz-Wilson, K.; Mathewson, N.; Wang, Y.; McEachin, R.; Liu, C.; Toubai, T.; Wu, J.; Rossi, C.; Braun, T.; et al. Mature T Cell Responses Are Controlled by microRNA-142. J. Clin. Investig. 2015, 125, 2825–2840. [Google Scholar] [CrossRef] [PubMed]
- Hatzioannou, A.; Boumpas, A.; Papadopoulou, M.; Papafragkos, I.; Varveri, A.; Alissafi, T.; Verginis, P. Regulatory T Cells in Autoimmunity and Cancer: A Duplicitous Lifestyle. Front. Immunol. 2021, 12, 731947. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of Peripheral CD4+CD25− Naive T Cells to CD4+CD25+ Regulatory T Cells by TGF-β Induction of Transcription Factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-L.; Ouyang, C.; Graham, N.M.; Zhang, Y.; Cassady, K.; Reyes, E.Y.; Xiong, M.; Davis, A.M.; Tang, K.; Zeng, D.; et al. microRNA-142 Guards against Autoimmunity by Controlling Treg Cell Homeostasis and Function. PLOS Biol. 2022, 20, e3001552. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Overacre-Delgoffe, A.E.; Chikina, M.; Dadey, R.E.; Yano, H.; Brunazzi, E.A.; Shayan, G.; Horne, W.; Moskovitz, J.M.; Kolls, J.K.; Sander, C.; et al. Interferon-γ Drives Treg Fragility to Promote Anti-Tumor Immunity. Cell 2017, 169, 1130–1141.e11. [Google Scholar] [CrossRef]
- Stokic-Trtica, V.; Diefenbach, A.; Klose, C.S.N. NK Cell Development in Times of Innate Lymphoid Cell Diversity. Front. Immunol. 2020, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Lodoen, M.B.; Lanier, L.L. Natural Killer Cells as an Initial Defense against Pathogens. Curr. Opin. Immunol. 2006, 18, 391–398. [Google Scholar] [CrossRef]
- Sivori, S.; Pende, D.; Quatrini, L.; Pietra, G.; Della Chiesa, M.; Vacca, P.; Tumino, N.; Moretta, F.; Mingari, M.C.; Locatelli, F.; et al. NK Cells and ILCs in Tumor Immunotherapy. Mol. Asp. Med. 2021, 80, 100870. [Google Scholar] [CrossRef]
- Klose, C.S.N.; Flach, M.; Möhle, L.; Rogell, L.; Hoyler, T.; Ebert, K.; Fabiunke, C.; Pfeifer, D.; Sexl, V.; Fonseca-Pereira, D.; et al. Differentiation of Type 1 ILCs from a Common Progenitor to All Helper-like Innate Lymphoid Cell Lineages. Cell 2014, 157, 340–356. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Sun, Y.; Neal, C.; Ireland, A.; Trissal, M.C.; Sullivan, R.P.; Wagner, J.A.; Leong, J.W.; Wong, P.; Mah-Som, A.Y.; et al. MicroRNA-142 Is Critical for the Homeostasis and Function of Type 1 Innate Lymphoid Cells. Immunity 2019, 51, 479–490.e6. [Google Scholar] [CrossRef] [PubMed]
- Nanbakhsh, A.; Malarkannan, S. The Role of microRNAs in NK Cell Development and Function. Cells 2021, 10, 2020. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Souza-Fonseca-Guimaraes, F.; Bald, T.; Ng, S.S.; Young, A.; Ngiow, S.F.; Rautela, J.; Straube, J.; Waddell, N.; Blake, S.J.; et al. Tumor Immunoevasion by the Conversion of Effector NK Cells into Type 1 Innate Lymphoid Cells. Nat. Immunol. 2017, 18, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Harabuchi, Y.; Takahara, M.; Kishibe, K.; Nagato, T.; Kumai, T. Extranodal Natural Killer/T-Cell Lymphoma, Nasal Type: Basic Science and Clinical Progress. Front. Pediatr. 2019, 7, 141. [Google Scholar] [CrossRef]
- Gao, F.; He, S.; Jin, A. MiRNAs and lncRNAs in NK Cell Biology and NK/T-Cell Lymphoma. Genes Dis. 2021, 8, 590–602. [Google Scholar] [CrossRef]
- De Kleer, I.; Willems, F.; Lambrecht, B.; Goriely, S. Ontogeny of Myeloid Cells. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef]
- Yáñez, A.; Coetzee, S.G.; Olsson, A.; Muench, D.E.; Berman, B.P.; Hazelett, D.J.; Salomonis, N.; Grimes, H.L.; Goodridge, H.S. Granulocyte-Monocyte Progenitors and Monocyte-Dendritic Cell Progenitors Independently Produce Functionally Distinct Monocytes. Immunity 2017, 47, 890–902.e4. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current Knowledge of Their Composition, Biological Functions, and Diagnostic and Therapeutic Potentials. Biochim. Biophys. Acta BBA Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Pan, B.T.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron Microscopic Evidence for Externalization of the Transferrin Receptor in Vesicular Form in Sheep Reticulocytes. J. Cell Biol. 1985, 101, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of Established Murine Tumors Using a Novel Cell-Free Vaccine: Dendritic Cell Derived Exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of mRNAs and microRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, L.; Qu, Y.; Wang, Y.; Lewis, C.J.; Cobb, B.A.; Takatsu, K.; Boom, W.H.; Dubyak, G.R.; Harding, C.V. Mycobacterium Tuberculosis Synergizes with ATP To Induce Release of Microvesicles and Exosomes Containing Major Histocompatibility Complex Class II Molecules Capable of Antigen Presentation. Infect. Immun. 2010, 78, 5116–5125. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, N.; Lankar, D.; Faure, F.; Regnault, A.; Dumont, C.; Raposo, G.; Hivroz, C. TCR Activation of Human T Cells Induces the Production of Exosomes Bearing the TCR/CD3/ζ Complex. J. Immunol. 2002, 168, 3235–3241. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Pan, B.T. The Fate of the Transferrin Receptor during Maturation of Sheep Reticulocytes in Vitro. Can. J. Biochem. Cell Biol. 1984, 62, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Raposo, G. Exosomes—Vesicular Carriers for Intercellular Communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A Compendium of Exosomal Proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-Stranded DNA in Exosomes: A Novel Biomarker in Cancer Detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef]
- Schageman, J.; Zeringer, E.; Li, M.; Barta, T.; Lea, K.; Gu, J.; Magdaleno, S.; Setterquist, R.; Vlassov, A.V. The Complete Exosome Workflow Solution: From Isolation to Characterization of RNA Cargo. BioMed Res. Int. 2013, 2013, 253957. [Google Scholar] [CrossRef] [PubMed]
- Airola, M.V.; Shanbhogue, P.; Shamseddine, A.A.; Guja, K.E.; Senkal, C.E.; Maini, R.; Bartke, N.; Wu, B.X.; Obeid, L.M.; Garcia-Diaz, M.; et al. Structure of Human nSMase2 Reveals an Interdomain Allosteric Activation Mechanism for Ceramide Generation. Proc. Natl. Acad. Sci. USA 2017, 114, E5549–E5558. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Gutiérrez-Vázquez, C.; Sánchez-Cabo, F.; Pérez-Hernández, D.; Vázquez, J.; Martin-Cofreces, N.; Martinez-Herrera, D.J.; Pascual-Montano, A.; Mittelbrunn, M.; Sánchez-Madrid, F. Sumoylated hnRNPA2B1 Controls the Sorting of miRNAs into Exosomes through Binding to Specific Motifs. Nat. Commun. 2013, 4, 2980. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 Complexes Carry a Population of Circulating microRNAs Independent of Vesicles in Human Plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [PubMed]
- Chevillet, J.R.; Kang, Q.; Ruf, I.K.; Briggs, H.A.; Vojtech, L.N.; Hughes, S.M.; Cheng, H.H.; Arroyo, J.D.; Meredith, E.K.; Gallichotte, E.N.; et al. Quantitative and Stoichiometric Analysis of the microRNA Content of Exosomes. Proc. Natl. Acad. Sci. USA 2014, 111, 14888–14893. [Google Scholar] [CrossRef] [PubMed]
- Guay, C.; Kruit, J.K.; Rome, S.; Menoud, V.; Mulder, N.L.; Jurdzinski, A.; Mancarella, F.; Sebastiani, G.; Donda, A.; Gonzalez, B.J.; et al. Lymphocyte-Derived Exosomal MicroRNAs Promote Pancreatic β Cell Death and May Contribute to Type 1 Diabetes Development. Cell Metab. 2019, 29, 348–361.e6. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, P.; Chen, Q.; Sun, Y.; Shi, M.; Mang, G.; Yu, S.; Zheng, Y.; Li, Z.; Sun, M.; et al. Metabolic Reprogramming Orchestrates CD4+ T-Cell Immunological Status and Restores Cardiac Dysfunction in Autoimmune Induced-Dilated Cardiomyopathy Mice. J. Mol. Cell Cardiol. 2019, 135, 134–148. [Google Scholar] [CrossRef]
- Cai, L.; Chao, G.; Li, W.; Zhu, J.; Li, F.; Qi, B.; Wei, Y.; Chen, S.; Zhou, G.; Lu, X.; et al. Activated CD4+ T Cells-Derived Exosomal miR-142-3p Boosts Post-Ischemic Ventricular Remodeling by Activating Myofibroblast. Aging 2020, 12, 7380–7396. [Google Scholar] [CrossRef]
- Cortes-Troncoso, J.; Jang, S.-I.; Perez, P.; Hidalgo, J.; Ikeuchi, T.; Greenwell-Wild, T.; Warner, B.M.; Moutsopoulos, N.M.; Alevizos, I. T Cell Exosome–Derived miR-142-3p Impairs Glandular Cell Function in Sjögren’s Syndrome. JCI Insight 2020, 5, e133497. [Google Scholar] [CrossRef]
- Guiot, J.; Cambier, M.; Boeckx, A.; Henket, M.; Nivelles, O.; Gester, F.; Louis, E.; Malaise, M.; Dequiedt, F.; Louis, R.; et al. Macrophage-Derived Exosomes Attenuate Fibrosis in Airway Epithelial Cells through Delivery of Antifibrotic miR-142-3p. Thorax 2020, 75, 870–881. [Google Scholar] [CrossRef]
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO Classification of Lymphoid Neoplasms and beyond: Evolving Concepts and Practical Applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. In WHO Classification of Tumours, 4th ed.; Organisation Mondiale de la Santé, Centre International de Recherche sur le Cancer, Eds.; International Agency for Research on Cancer: Lyon, France, 2008; ISBN 978-92-832-2431-0. [Google Scholar]
- Ye, X.; Wang, L.; Nie, M.; Wang, Y.; Dong, S.; Ren, W.; Li, G.; Li, Z.-M.; Wu, K.; Pan-Hammarström, Q. A Single-Cell Atlas of Diffuse Large B Cell Lymphoma. Cell Rep. 2022, 39, 110713. [Google Scholar] [CrossRef] [PubMed]
- Urbanek-Trzeciak, M.O.; Galka-Marciniak, P.; Nawrocka, P.M.; Kowal, E.; Szwec, S.; Giefing, M.; Kozlowski, P. Pan-Cancer Analysis of Somatic Mutations in miRNA Genes. EBioMedicine 2020, 61, 103051. [Google Scholar] [CrossRef] [PubMed]
- Hezaveh, K.; Kloetgen, A.; Bernhart, S.H.; Mahapatra, K.D.; Lenze, D.; Richter, J.; Haake, A.; Bergmann, A.K.; Brors, B.; Burkhardt, B.; et al. Alterations of microRNA and microRNA-Regulated Messenger RNA Expression in Germinal Center B-Cell Lymphomas Determined by Integrative Sequencing Analysis. Haematologica 2016, 101, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Kwanhian, W.; Lenze, D.; Alles, J.; Motsch, N.; Barth, S.; Döll, C.; Imig, J.; Hummel, M.; Tinguely, M.; Trivedi, P.; et al. MicroRNA-142 Is Mutated in about 20% of Diffuse Large B-Cell Lymphoma. Cancer Med. 2012, 1, 141–155. [Google Scholar] [CrossRef]
- Kuriyama, K.; Enomoto, Y.; Suzuki, R.; Watanuki, J.; Hosoi, H.; Yamashita, Y.; Murata, S.; Mushino, T.; Tamura, S.; Hanaoka, N.; et al. Enforced Expression of MIR142, a Target of Chromosome Translocation in Human B-Cell Tumors, Results in B-Cell Depletion. Int. J. Hematol. 2018, 107, 345–354. [Google Scholar] [CrossRef]
- Bouska, A.; Zhang, W.; Gong, Q.; Iqbal, J.; Scuto, A.; Vose, J.; Ludvigsen, M.; Fu, K.; Weisenburger, D.D.; Greiner, T.C.; et al. Combined Copy Number and Mutation Analysis Identifies Oncogenic Pathways Associated with Transformation of Follicular Lymphoma. Leukemia 2017, 31, 83–91. [Google Scholar] [CrossRef]
- Puente, X.S.; Beà, S.; Valdés-Mas, R.; Villamor, N.; Gutiérrez-Abril, J.; Martín-Subero, J.I.; Munar, M.; Rubio-Pérez, C.; Jares, P.; Aymerich, M.; et al. Non-Coding Recurrent Mutations in Chronic Lymphocytic Leukaemia. Nature 2015, 526, 519–524. [Google Scholar] [CrossRef]
- PCAWG Drivers and Functional Interpretation Working Group; PCAWG Structural Variation Working Group; PCAWG Consortium; Rheinbay, E.; Nielsen, M.M.; Abascal, F.; Wala, J.A.; Shapira, O.; Tiao, G.; Hornshøj, H.; et al. Analyses of Non-Coding Somatic Drivers in 2,658 Cancer Whole Genomes. Nature 2020, 578, 102–111. [Google Scholar] [CrossRef]
- Galka-Marciniak, P.; Kanduła, Z.; Tire, A.; Wegorek, W.; Gwozdz-Bak, K.; Handschuh, L.; Giefing, M.; Lewandowski, K.; Kozlowski, P. Mutations in the miR-142 Gene Are Not Common in Myeloproliferative Neoplasms. Sci. Rep. 2022, 12, 10924. [Google Scholar] [CrossRef]
- Thol, F.; Scherr, M.; Kirchner, A.; Shahswar, R.; Battmer, K.; Kade, S.; Chaturvedi, A.; Koenecke, C.; Stadler, M.; Platzbecker, U.; et al. Clinical and Functional Implications of microRNA Mutations in a Cohort of 935 Patients with Myelodysplastic Syndromes and Acute Myeloid Leukemia. Haematologica 2015, 100, e122–e124. [Google Scholar] [CrossRef] [PubMed]
- Ley, T.J.; Miller, C.; Ding, L.; Raphael, B.J.; Mungall, A.J.; Robertson, A.; Heath, S.E. The Cancer Genome Atlas Research Network Genomic and Epigenomic Landscapes of Adult De Novo Acute Myeloid Leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Oyang, L.; Rao, S.; Han, Y.; Luo, X.; Yi, P.; Lin, J.; Xia, L.; Hu, J.; Tan, S.; et al. Rac1, A Potential Target for Tumor Therapy. Front. Oncol. 2021, 11, 674426. [Google Scholar] [CrossRef] [PubMed]
- Gulbins, E.; Brenner, B.; Schlottmann, K.; Koppenhoefer, U.; Linderkamp, O.; Coggeshall, K.M.; Lang, F. Activation of the Ras Signaling Pathway by the CD40 Receptor. J. Immunol. Baltim. Md 1950 1996, 157, 2844–2850. [Google Scholar] [CrossRef]
- Bosco, E.E.; Ni, W.; Wang, L.; Guo, F.; Johnson, J.F.; Zheng, Y. Rac1 Targeting Suppresses P53 Deficiency–Mediated Lymphomagenesis. Blood 2010, 115, 3320–3328. [Google Scholar] [CrossRef] [PubMed]
- Falini, B.; Mason, D.Y. Proteins Encoded by Genes Involved in Chromosomal Alterations in Lymphoma and Leukemia: Clinical Value of Their Detection by Immunocytochemistry. Blood 2002, 99, 409–426. [Google Scholar] [CrossRef]
- Velaithan, R.; Kang, J.; Hirpara, J.L.; Loh, T.; Goh, B.C.; Le Bras, M.; Brenner, C.; Clement, M.-V.; Pervaiz, S. The Small GTPase Rac1 Is a Novel Binding Partner of Bcl-2 and Stabilizes Its Antiapoptotic Activity. Blood 2011, 117, 6214–6226. [Google Scholar] [CrossRef]
- Wagner, A.J.; Small, M.B.; Hay, N. Myc-Mediated Apoptosis Is Blocked by Ectopic Expression of Bcl-2. Mol. Cell Biol. 1993, 13, 2432–2440. [Google Scholar] [CrossRef][Green Version]
- Lawrie, C.H.; Chi, J.; Taylor, S.; Tramonti, D.; Ballabio, E.; Palazzo, S.; Saunders, N.J.; Pezzella, F.; Boultwood, J.; Wainscoat, J.S.; et al. Expression of microRNAs in Diffuse Large B Cell Lymphoma Is Associated with Immunophenotype, Survival and Transformation from Follicular Lymphoma. J. Cell Mol. Med. 2009, 13, 1248–1260. [Google Scholar] [CrossRef]
- Freedman, A.; Jacobsen, E. Follicular Lymphoma: 2020 Update on Diagnosis and Management. Am. J. Hematol. 2020, 95, 316–327. [Google Scholar] [CrossRef]
- Zhou, L.; Bu, Y.; Liang, Y.; Zhang, F.; Zhang, H.; Li, S. Epstein-Barr Virus (EBV)-BamHI-A Rightward Transcript (BART)-6 and Cellular MicroRNA-142 Synergistically Compromise Immune Defense of Host Cells in EBV-Positive Burkitt Lymphoma. Med. Sci. Monit. 2016, 22, 4114–4120. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, M.-M. PTEN Tumor Suppressor Network in PI3K-Akt Pathway Control. Genes Cancer 2010, 1, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Solé, C.; Arnaiz, E.; Lawrie, C.H. MicroRNAs as Biomarkers of B-Cell Lymphoma. Biomark. Insights 2018, 13, 117727191880684. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Suzuki, H.; Tsugawa, H.; Imaeda, H.; Matsuzaki, J.; Hirata, K.; Hosoe, N.; Nakamura, M.; Mukai, M.; Saito, H.; et al. Overexpression of miR-142-5p and miR-155 in Gastric Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma Resistant to Helicobacter Pylori Eradication. PLoS ONE 2012, 7, e47396. [Google Scholar] [CrossRef] [PubMed]
- Tomasini, R.; Seux, M.; Nowak, J.; Bontemps, C.; Carrier, A.; Dagorn, J.-C.; Pébusque, M.-J.; Iovanna, J.L.; Dusetti, N.J. TP53INP1 Is a Novel P73 Target Gene That Induces Cell Cycle Arrest and Cell Death by Modulating P73 Transcriptional Activity. Oncogene 2005, 24, 8093–8104. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.-Y.; Chan, L.-H.; Chai, S.; Tong, M.; Guan, X.-Y.; Lee, N.P.; Yuan, Y.; Xie, D.; Lee, T.K.; Dusetti, N.J.; et al. TP53INP1 Downregulation Activates a P73-Dependent DUSP10/ERK Signaling Pathway to Promote Metastasis of Hepatocellular Carcinoma. Cancer Res. 2017, 77, 4602–4612. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Bellosillo, B.; Ferraro, M.; Seoane, A.; Sánchez-González, B.; Pairet, S.; Pons, A.; Barranco, L.; Vela, M.C.; Gimeno, E.; et al. MicroRNAs 142-3p, miR-155 and miR-203 Are Deregulated in Gastric MALT Lymphomas Compared to Chronic Gastritis. Cancer Genom. Proteom. 2017, 14, 75–82. [Google Scholar] [CrossRef][Green Version]
- Gebauer, N.; Kuba, J.; Senft, A.; Schillert, A.; Bernard, V.; Thorns, C. MicroRNA-150 Is up-Regulated in Extranodal Marginal Zone Lymphoma of MALT Type. Cancer Genom. Proteom. 2014, 11, 51–56. [Google Scholar]
- Zhao, J.-J.; Lin, J.; Lwin, T.; Yang, H.; Guo, J.; Kong, W.; Dessureault, S.; Moscinski, L.C.; Rezania, D.; Dalton, W.S.; et al. microRNA Expression Profile and Identification of miR-29 as a Prognostic Marker and Pathogenetic Factor by Targeting CDK6 in Mantle Cell Lymphoma. Blood 2010, 115, 2630–2639. [Google Scholar] [CrossRef]
- Pérez-Galán, P.; Dreyling, M.; Wiestner, A. Mantle Cell Lymphoma: Biology, Pathogenesis, and the Molecular Basis of Treatment in the Genomic Era. Blood 2011, 117, 26–38. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Chen, F.-F.; Wang, Y.-Q.; Yu, W.; Dong, F.-L.; Zhuang, H.-X. MiR-150 Inhibits Proliferation of Mantle-Cell Lymphoma Cells via Regulation of MET. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12063–12072. [Google Scholar] [CrossRef] [PubMed]
- Motsch, N.; Alles, J.; Imig, J.; Zhu, J.; Barth, S.; Reineke, T.; Tinguely, M.; Cogliatti, S.; Dueck, A.; Meister, G.; et al. MicroRNA Profiling of Epstein-Barr Virus-Associated NK/T-Cell Lymphomas by Deep Sequencing. PLoS ONE 2012, 7, e42193. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Zhang, M. Non-Coding RNAs in Natural Killer/T-Cell Lymphoma. Front. Oncol. 2019, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Avilés, A. Nasal NK/T-Cell Lymphoma. A Comparative Analysis of a Mexican Population with the Other Populations of Latin-America. Mediterr. J. Hematol. Infect. Dis. 2015, 7, e2015052. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.W.; Binte Hanafi, Z.; Chew, L.C.Y.; Mei, Y.; Liu, H. IL-1α Processing, Signaling and Its Role in Cancer Progression. Cells 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.N.; Voronov, E. Is Interleukin-1 a Good or Bad ‘Guy’ in Tumor Immunobiology and Immunotherapy? Immunol. Rev. 2008, 222, 222–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ohyashiki, J.H.; Takaku, T.; Shimizu, N.; Ohyashiki, K. Transcriptional Profiling of Epstein–Barr Virus (EBV) Genes and Host Cellular Genes in Nasal NK/T-Cell Lymphoma and Chronic Active EBV Infection. Br. J. Cancer 2006, 94, 599–608. [Google Scholar] [CrossRef][Green Version]
- Sung, S.S.; Walters, J.A. Increased Cyclic AMP Levels Enhance IL-1 Alpha and IL-1 Beta mRNA Expression and Protein Production in Human Myelomonocytic Cell Lines and Monocytes. J. Clin. Investig. 1991, 88, 1915–1923. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, J.; Lei, Z.; Shen, S.; Li, D.; Shen, G.-X.; Zhang, G.-M.; Feng, Z.-H. miR-142-3p Restricts cAMP Production in CD4+CD25− T Cells and CD4+CD25+ TREG Cells by Targeting AC9 mRNA. EMBO Rep. 2009, 10, 180–185. [Google Scholar] [CrossRef]
- Alles, J.; Hasler, D.; Kazmi, S.; Tesson, M.; Hamilton, A.; Schlegel, L.; Marx, S.; Eichner, N.; Reinhardt, R.; Meister, G.; et al. Epstein-Barr Virus EBER Transcripts Affect miRNA-Mediated Regulation of Specific Targets and Are Processed to Small RNA Species. Non-Coding RNA 2015, 1, 170–191. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and Regulation of Akt/PKB by the Rictor-mTOR Complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Huang, W.-T.; Yang, L.-W.; Lin, C.-W. The PTEN-AKT-mTOR/RICTOR Pathway in Nasal Natural Killer Cell Lymphoma Is Activated by miR-494-3p via PTEN But Inhibited by miR-142-3p via RICTOR. Am. J. Pathol. 2015, 185, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Jebali, A.; Dumaz, N. The Role of RICTOR Downstream of Receptor Tyrosine Kinase in Cancers. Mol. Cancer 2018, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.-H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, M.Z.; Emamzadeh, R.; Mozhgani, S.-H. Deciphering microRNA-mRNA Regulatory Network in Adult T-Cell Leukemia/Lymphoma; the Battle between Oncogenes and Anti-Oncogenes. PLoS ONE 2021, 16, e0247713. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, Y.; Liu, D.; Liu, P.; Li, F.; Zhang, Z.; Zhang, M.; Wang, X.; Zhang, Y.; Sun, X.; et al. Downregulation of miR-142a Contributes to the Enhanced Anti-Apoptotic Ability of Murine Chronic Myelogenous Leukemia Cells. Front. Oncol. 2021, 11, 718731. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Ma, L.; Cao, S.; Gao, W.; Xiong, Q.; Wang, K.; Yang, L. CIAPIN1 Targeted NHE1 and ERK1/2 to Suppress NSCLC Cells’ Metastasis and Predicted Good Prognosis in NSCLC Patients Receiving Pulmonectomy. Oxid. Med. Cell Longev. 2019, 2019, 1970818. [Google Scholar] [CrossRef]
- Klümper, T.; Bruckmueller, H.; Diewock, T.; Kaehler, M.; Haenisch, S.; Pott, C.; Bruhn, O.; Cascorbi, I. Expression Differences of miR-142-5p between Treatment-Naïve Chronic Myeloid Leukemia Patients Responding and Non-Responding to Imatinib Therapy Suggest a Link to Oncogenic ABL2, SRI, cKIT and MCL1 Signaling Pathways Critical for Development of Therapy Resistance. Exp. Hematol. Oncol. 2020, 9, 26. [Google Scholar] [CrossRef]
- Wang, J.-M.; Chao, J.-R.; Chen, W.; Kuo, M.-L.; Yen, J.J.-Y.; Yang-Yen, H.-F. The Antiapoptotic Gene Mcl-1 Is Up-Regulated by the Phosphatidylinositol 3-Kinase/Akt Signaling Pathway through a Transcription Factor Complex Containing CREB. Mol. Cell Biol. 1999, 19, 6195–6206. [Google Scholar] [CrossRef]
- Pathania, S.; Pentikäinen, O.T.; Singh, P.K. A Holistic View on C-Kit in Cancer: Structure, Signaling, Pathophysiology and Its Inhibitors. Biochim. Biophys. Acta BBA-Rev. Cancer 2021, 1876, 188631. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, X.; Jia, H.; Li, D.; Zhang, B.; Zhang, H.; Hong, M.; Jiang, T.; Jiang, Q.; Lu, J.; et al. An Oncogenic Role of miR-142-3p in Human T-Cell Acute Lymphoblastic Leukemia (T-ALL) by Targeting Glucocorticoid Receptor-α and cAMP/PKA Pathways. Leukemia 2012, 26, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Li, D.; Shi, Q.; Hou, H.; Sun, N.; Shen, B. Differential microRNA expression in childhood B-cell precursor acute lymphoblastic leukemia. Pediatr. Hematol. Oncol. 2009, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, N.; Komada, Y.; Hanaki, R.; Morimoto, M.; Ito, T.; Nakato, D.; Hirayama, M. Role of microRNAs in Glucocorticoid-resistant B-cell Precursor Acute Lymphoblastic Leukemia. Oncol. Rep. 2019, 42, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Li, J.; Zheng, D.; Li, Y.; Gao, X.; Xu, C.; Gao, L.; Wang, L.; Yu, L. MicroRNA-142-3p Inhibits Cell Proliferation in Human Acute Lymphoblastic Leukemia by Targeting the MLL-AF4 Oncogene. Mol. Biol. Rep. 2013, 40, 6811–6819. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Go, J.H.; Kim, E.K.; Lee, H.; Lee, W.M.; Cho, C.-S.; Han, K. Mutational Analysis of Extranodal NK/T-Cell Lymphoma Using Targeted Sequencing with a Comprehensive Cancer Panel. Genom. Inform. 2016, 14, 78. [Google Scholar] [CrossRef]
- Bagherani, N.; Smoller, B.R. An Overview of Cutaneous T Cell Lymphomas. F1000Research 2016, 5, 1882. [Google Scholar] [CrossRef]
- Shen, X.; Wang, B.; Li, K.; Wang, L.; Zhao, X.; Xue, F.; Shi, R.; Zheng, J. MicroRNA Signatures in Diagnosis and Prognosis of Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2018, 138, 2024–2032. [Google Scholar] [CrossRef]
- Sandoval, J.; Díaz-Lagares, A.; Salgado, R.; Servitje, O.; Climent, F.; Ortiz-Romero, P.L.; Pérez-Ferriols, A.; Garcia-Muret, M.P.; Estrach, T.; Garcia, M.; et al. MicroRNA Expression Profiling and DNA Methylation Signature for Deregulated MicroRNA in Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2015, 135, 1128–1137. [Google Scholar] [CrossRef]
- Manso, R.; Martínez-Magunacelaya, N.; Eraña-Tomás, I.; Monsalvez, V.; Rodríguez-Peralto, J.L.; Ortiz-Romero, P.-L.; Santonja, C.; Cristóbal, I.; Piris, M.A.; Rodríguez-Pinilla, S.M. Mycosis Fungoides Progression Could Be Regulated by microRNAs. PLoS ONE 2018, 13, e0198477. [Google Scholar] [CrossRef]
- Mehta-Shah, N.; Ratner, L.; Horwitz, S.M. Adult T-Cell Leukemia/Lymphoma. J. Oncol. Pract. 2017, 13, 487–492. [Google Scholar] [CrossRef]
- Bellon, M.; Lepelletier, Y.; Hermine, O.; Nicot, C. Deregulation of microRNA Involved in Hematopoiesis and the Immune Response in HTLV-I Adult T-Cell Leukemia. Blood 2009, 113, 4914–4917. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, K.; Guffanti, A.; Corradin, A.; Sharma, V.K.; De Bellis, G.; Corti, G.; Grassi, A.; Zanovello, P.; Bronte, V.; Ciminale, V.; et al. Small Noncoding RNAs in Cells Transformed by Human T-Cell Leukemia Virus Type 1: A Role for a tRNA Fragment as a Primer for Reverse Transcriptase. J. Virol. 2014, 88, 3612–3622. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Zhao, J.; Qin, S.; Wang, R.; Li, Y.; Wang, Q.; Tan, Y.; Jin, H.; Zhu, F.; Ou, Y.; et al. Over-Expression of Thrombospondin 4 Correlates with Loss of miR-142 and Contributes to Migration and Vascular Invasion of Advanced Hepatocellular Carcinoma. Oncotarget 2017, 8, 23277–23288. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, H.; Huo, W. THBS4 Silencing Regulates the Cancer Stem Cell-like Properties in Prostate Cancer via Blocking the PI3K/Akt Pathway. Prostate 2020, 80, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Almasri, M.; Amer, M.; Ghanej, J.; Mahmoud, A.M.; Gaidano, G.; Moia, R. Druggable Molecular Pathways in Chronic Lymphocytic Leukemia. Life 2022, 12, 283. [Google Scholar] [CrossRef]
- Kipps, T.J.; Stevenson, F.K.; Wu, C.J.; Croce, C.M.; Packham, G.; Wierda, W.G.; O’Brien, S.; Gribben, J.; Rai, K. Chronic Lymphocytic Leukaemia. Nat. Rev. Dis. Primer 2017, 3, 16096. [Google Scholar] [CrossRef]
- Zhu, D.-X.; Zhu, W.; Fang, C.; Fan, L.; Zou, Z.-J.; Wang, Y.-H.; Liu, P.; Hong, M.; Miao, K.-R.; Liu, P.; et al. miR-181a/b Significantly Enhances Drug Sensitivity in Chronic Lymphocytic Leukemia Cells via Targeting Multiple Anti-Apoptosis Genes. Carcinogenesis 2012, 33, 1294–1301. [Google Scholar] [CrossRef]
- Zanette, D.L.; Rivadavia, F.; Molfetta, G.A.; Barbuzano, F.G.; Proto-Siqueira, R.; Falcão, R.P.; Zago, M.A.; Silva-Jr, W.A. miRNA Expression Profiles in Chronic Lymphocytic and Acute Lymphocytic Leukemia. Braz. J. Med. Biol. Res. 2007, 40, 1435–1440. [Google Scholar] [CrossRef]
- Apperley, J.F. Chronic Myeloid Leukaemia. Lancet Lond. Engl. 2015, 385, 1447–1459. [Google Scholar] [CrossRef]
- Cong, X.L.; Li, B.; Yang, R.C.; Feng, S.Z.; Chen, S.J.; Han, Z.C. Enhanced Growth Suppression of Philadephia1 Leukemia Cells by Targeting Bcr3/Abl2 and VEGF through Antisense Strategy. Leukemia 2005, 19, 1517–1524. [Google Scholar] [CrossRef][Green Version]
- Greuber, E.K.; Smith-Pearson, P.; Wang, J.; Pendergast, A.M. Role of ABL Family Kinases in Cancer: From Leukaemia to Solid Tumours. Nat. Rev. Cancer 2013, 13, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.H.; Simpson, M.A.; Koleske, A.J.; Boggon, T.J. Structure of the ABL2/ARG Kinase in Complex with Dasatinib. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pendergast, A.M. The Emerging Role of ABL Kinases in Solid Tumors. Trends Cancer 2015, 1, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-W.; Sun, Y.-M. The IL-6/JAK/STAT3 Pathway: Potential Therapeutic Strategies in Treating Colorectal Cancer (Review). Int. J. Oncol. 2014, 44, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Akgul, C. Mcl-1 Is a Potential Therapeutic Target in Multiple Types of Cancer. Cell Mol. Life Sci. CMLS 2009, 66, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.W.; Lam, C.; Edwards, S.W. Mcl-1; the Molecular Regulation of Protein Function. FEBS Lett. 2010, 584, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, E.; Bonadiman, L.; Greco, A.; Albertini, V.; Negri, T.; Gronchi, A.; Bertulli, R.; Colecchia, M.; Casali, P.G.; Pierotti, M.A.; et al. A New Mutation in the KIT ATP Pocket Causes Acquired Resistance to Imatinib in a Gastrointestinal Stromal Tumor Patient. Gastroenterology 2004, 127, 294–299. [Google Scholar] [CrossRef]
- Chen, L.-T.; Chen, C.-T.; Jiaang, W.-T.; Chen, T.-Y.; Butterfield, J.H.; Shih, N.-Y.; Hsu, J.T.-A.; Lin, H.-Y.; Lin, S.-F.; Tsai, H.-J. BPR1J373, an Oral Multiple Tyrosine Kinase Inhibitor, Targets c-KIT for the Treatment of c-KIT–Driven Myeloid Leukemia. Mol. Cancer Ther. 2016, 15, 2323–2333. [Google Scholar] [CrossRef]
- Weiler, S.R.; Mou, S.; DeBerry, C.S.; Keller, J.R.; Ruscetti, F.W.; Ferris, D.K.; Longo, D.L.; Linnekin, D. JAK2 Is Associated with the C-Kit Proto-Oncogene Product and Is Phosphorylated in Response to Stem Cell Factor. Blood 1996, 87, 3688–3693. [Google Scholar] [CrossRef]
- Tuo, H.; Shu, F.; She, S.; Yang, M.; Zou, X.Q.; Huang, J.; Hu, H.D.; Hu, P.; Ren, H.; Peng, S.F.; et al. Sorcin Induces Gastric Cancer Cell Migration and Invasion Contributing to STAT3 Activation. Oncotarget 2017, 8, 104258–104271. [Google Scholar] [CrossRef]
- Nicholson, K.M.; Anderson, N.G. The Protein Kinase B/Akt Signalling Pathway in Human Malignancy. Cell Signal. 2002, 14, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Sirohi, V.K.; Kumari, S.; Shukla, V.; Manohar, M.; Popli, P.; Dwivedi, A. Sorcin Is Involved during Embryo Implantation via Activating VEGF/PI3K/Akt Pathway in Mice. J. Mol. Endocrinol. 2018, 60, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tan, Y.; Yang, C.; Zhao, C.; Zhao, H.; Wang, J.; Xue, Y.; Han, M.; Qian, L.; Zhao, C. Expression and Clinical Implications of the Soluble Drug Resistance-Related Calcium-Binding Protein (Sorcin) Gene in Leukemia Patients. Zhonghua Xue Ye Xue Za Zhi Zhonghua Xueyexue Zazhi 2002, 23, 293–296. [Google Scholar] [PubMed]
- Warfvinge, R.; Geironson, L.; Sommarin, M.N.E.; Lang, S.; Karlsson, C.; Roschupkina, T.; Stenke, L.; Stentoft, J.; Olsson-Strömberg, U.; Hjorth-Hansen, H.; et al. Single-Cell Molecular Analysis Defines Therapy Response and Immunophenotype of Stem Cell Subpopulations in CML. Blood 2017, 129, 2384–2394. [Google Scholar] [CrossRef] [PubMed]
- Flamant, S.; Ritchie, W.; Guilhot, J.; Holst, J.; Bonnet, M.-L.; Chomel, J.-C.; Guilhot, F.; Turhan, A.G.; Rasko, J.E.J. Micro-RNA Response to Imatinib Mesylate in Patients with Chronic Myeloid Leukemia. Haematologica 2010, 95, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.F.; Moura, L.G.; Tojal, I.; Ambrósio, L.; Pinto-Simões, B.; Hamerschlak, N.; Calin, G.A.; Ivan, C.; Covas, D.T.; Kashima, S.; et al. ApoptomiRs Expression Modulated by BCR–ABL Is Linked to CML Progression and Imatinib Resistance. Blood Cells Mol. Dis. 2014, 53, 47–55. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Moles, R.; Nicot, C. Clinical Significance of microRNAs in Chronic and Acute Human Leukemia. Mol. Cancer 2016, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lyu, C.; Wang, F.; Wang, C.; Wu, F.; Li, X.; Gan, S. Identification of Potential Signatures and Their Functions for Acute Lymphoblastic Leukemia: A Study Based on the Cancer Genome Atlas. Front. Genet. 2021, 12, 656042. [Google Scholar] [CrossRef]
- Terwilliger, T.; Abdul-Hay, M. Acute Lymphoblastic Leukemia: A Comprehensive Review and 2017 Update. Blood Cancer J. 2017, 7, e577. [Google Scholar] [CrossRef]
- Dahlhaus, M.; Roolf, C.; Ruck, S.; Lange, S.; Freund, M.; Junghanss, C. Expression and Prognostic Significance of Hsa-miR-142-3p in Acute Leukemias. Neoplasma 2013, 60, 432–438. [Google Scholar] [CrossRef][Green Version]
- Longjohn, M.N.; Squires, W.R.B.; Christian, S.L. Meta-Analysis of microRNA Profiling Data Does Not Reveal a Consensus Signature for B Cell Acute Lymphoblastic Leukemia. Gene 2022, 821, 146211. [Google Scholar] [CrossRef] [PubMed]
- De Kouchkovsky, I.; Abdul-Hay, M. ‘Acute Myeloid Leukemia: A Comprehensive Review and 2016 Update. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Araki, K.; Bai, J.; Furukawa, I.; Tateishi, K.; Yoshinobu, K.; Usuki, S.; Nimmo, R.A.; Kaname, T.; Yoshihara, M.; et al. A Gain-of-function Mutation in microRNA 142 Is Sufficient to Cause the Development of T-cell Leukemia in Mice. Cancer Sci. 2023, 114, 2821–2834. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, U.; Mamo, A.; Kroon, E.; Jerome, L.; Bijl, J.; Lawrence, H.J.; Humphries, K.; Sauvageau, G. Overexpression of the Myeloid Leukemia-Associated Hoxa9 Gene in Bone Marrow Cells Induces Stem Cell Expansion. Blood 2002, 99, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.; Kasturiarachchi, J.; Datta, P.; Guo, Y.; Deltcheva, E.; James, C.; Brown, J.; May, G.; Anandagoda, N.; Jackson, I.; et al. Mir142 Loss Unlocks IDH2R140-Dependent Leukemogenesis through Antagonistic Regulation of HOX Genes. Sci. Rep. 2021, 11, 6974. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, X.-S.; Yang, G.-H.; Zhai, P.-F.; Xiao, Z.; Xia, L.-Y.; Chen, L.-R.; Wang, Y.; Wang, X.-Z.; Bi, L.-X.; et al. miR-29a and miR-142-3p Downregulation and Diagnostic Implication in Human Acute Myeloid Leukemia. Mol. Biol. Rep. 2012, 39, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, P.P.; Qui, Y.H.; Coombes, K.R.; Zhang, N.; Ruvolo, V.R.; Borthakur, G.; Konopleva, M.; Andreeff, M.; Kornblau, S.M. Low Expression of PP2A Regulatory Subunit B55α Is Associated with T308 Phosphorylation of AKT and Shorter Complete Remission Duration in Acute Myeloid Leukemia Patients. Leukemia 2011, 25, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, P.P.; Ruvolo, V.R.; Jacamo, R.; Burks, J.K.; Zeng, Z.; Duvvuri, S.R.; Zhou, L.; Qiu, Y.; Coombes, K.R.; Zhang, N.; et al. The Protein Phosphatase 2A Regulatory Subunit B55α Is a Modulator of Signaling and microRNA Expression in Acute Myeloid Leukemia Cells. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2014, 1843, 1969–1977. [Google Scholar] [CrossRef]
- Chen, F.-F.; Lou, Y.-J.; Chen, J.; Jin, J.; Zhou, J.-N.; Yin, X.-F.; Zhu, Z.-J.; Hu, C.; Yu, M.-X.; Wang, H.-P.; et al. Absence of miR-142 Mutation in Chinese Patients with Acute Myeloid Leukemia. Leuk. Lymphoma 2014, 55, 2961–2962. [Google Scholar] [CrossRef]
- Yuan, D.-M.; Ma, J.; Fang, W.-B. Identification of Non-Coding RNA Regulatory Networks in Pediatric Acute Myeloid Leukemia Reveals Circ-0004136 Could Promote Cell Proliferation by Sponging miR-142. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9251–9258. [Google Scholar] [CrossRef]
- Grassilli, S.; Nika, E.; Lambertini, E.; Brugnoli, F.; Piva, R.; Capitani, S.; Bertagnolo, V. A Network Including PU.1, Vav1 and miR-142-3p Sustains ATRA-Induced Differentiation of Acute Promyelocytic Leukemia Cells—A Short Report. Cell Oncol. 2016, 39, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liao, Y.; Zhang, C.; Liu, J.; Wang, W.; Huang, J.; Du, Q.; Liu, T.; Zou, Q.; Huang, H.; et al. TAB2 Promotes the Stemness and Biological Functions of Cervical Squamous Cell Carcinoma Cells. Stem Cells Int. 2021, 2021, 6550388. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Therapeutic Advances of miRNAs: A Preclinical and Clinical Update. J. Adv. Res. 2021, 28, 127–138. [Google Scholar] [CrossRef]
- Prinz, F.; Jonas, K.; Balihodzic, A.; Klec, C.; Reicher, A.; Barth, D.A.; Riedl, J.; Gerger, A.; Kiesslich, T.; Mayr, C.; et al. MicroRNA Mimics Can Distort Physiological microRNA Effects on Immune Checkpoints by Triggering an Antiviral Interferon Response. RNA Biol. 2022, 19, 1305–1315. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.-K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.-L.; Kim, T.-Y.; et al. Phase 1 Study of MRX34, a Liposomal miR-34a Mimic, in Patients with Advanced Solid Tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and Activity of microRNA-Loaded Minicells in Patients with Recurrent Malignant Pleural Mesothelioma: A First-in-Man, Phase 1, Open-Label, Dose-Escalation Study. Lancet Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Pel, M.E.; Kirschner, M.B.; Cheng, Y.Y.; Mugridge, N.; Weiss, J.; Williams, M.; Wright, C.; Edelman, J.J.B.; Vallely, M.P.; et al. Restoring Expression of miR-16: A Novel Approach to Therapy for Malignant Pleural Mesothelioma. Ann. Oncol. 2013, 24, 3128–3135. [Google Scholar] [CrossRef]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in Vivo with ‘Antagomirs’. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef]
- Weiler, J.; Hunziker, J.; Hall, J. Anti-miRNA Oligonucleotides (AMOs): Ammunition to Target miRNAs Implicated in Human Disease? Gene Ther. 2006, 13, 496–502. [Google Scholar] [CrossRef]
- Yu, B.; Zhao, X.; Lee, L.J.; Lee, R.J. Targeted Delivery Systems for Oligonucleotide Therapeutics. AAPS J. 2009, 11, 195–203. [Google Scholar] [CrossRef]
- Eckstein, F. Phosphorothioates, Essential Components of Therapeutic Oligonucleotides. Nucleic Acid Ther. 2014, 24, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, F. Phosphorothioate Oligodeoxynucleotides: What Is Their Origin and What Is Unique About Them? Antisense Nucleic Acid Drug Dev. 2000, 10, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Watts, J.K. Oligonucleotide Therapeutics: Chemistry, Delivery and Clinical Progress. Future Med. Chem. 2015, 7, 2221–2242. [Google Scholar] [CrossRef] [PubMed]
- Geary, R.S. Antisense Oligonucleotide Pharmacokinetics and Metabolism. Expert Opin. Drug Metab. Toxicol. 2009, 5, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.Z.; Grundy, J.S.; Geary, R.S. Clinical Pharmacokinetics of Second Generation Antisense Oligonucleotides. Expert Opin. Drug Metab. Toxicol. 2013, 9, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Bramsen, J.B.; Kjems, J. Chemical Modification of Small Interfering RNA. In Antiviral RNAi; van Rij, R.P., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 721, pp. 77–103. ISBN 978-1-61779-036-2. [Google Scholar]
- Whitehead, K.A.; Dahlman, J.E.; Langer, R.S.; Anderson, D.G. Silencing or Stimulation? siRNA Delivery and the Immune System. Ann. Rev. Chem. Biomol. Eng. 2011, 2, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, M.; Feng, X.; Zhu, E.; Wang, B. miR-142a-5p Promoted Osteoblast Differentiation via Targeting Nuclear Factor IA. J. Cell Physiol. 2021, 236, 1810–1821. [Google Scholar] [CrossRef]
- Wahlestedt, C.; Salmi, P.; Good, L.; Kela, J.; Johnsson, T.; Hökfelt, T.; Broberger, C.; Porreca, F.; Lai, J.; Ren, K.; et al. Potent and Nontoxic Antisense Oligonucleotides Containing Locked Nucleic Acids. Proc. Natl. Acad. Sci. USA 2000, 97, 5633–5638. [Google Scholar] [CrossRef]
- Obad, S.; dos Santos, C.O.; Petri, A.; Heidenblad, M.; Broom, O.; Ruse, C.; Fu, C.; Lindow, M.; Stenvang, J.; Straarup, E.M.; et al. Silencing of microRNA Families by Seed-Targeting Tiny LNAs. Nat. Genet. 2011, 43, 371–378. [Google Scholar] [CrossRef]
- Lindow, M.; Kauppinen, S. Discovering the First microRNA-Targeted Drug. J. Cell Biol. 2012, 199, 407–412. [Google Scholar] [CrossRef]
- Janssen, H.L.A.; Reesink, H.W.; Lawitz, E.J.; Zeuzem, S.; Rodriguez-Torres, M.; Patel, K.; van der Meer, A.J.; Patick, A.K.; Chen, A.; Zhou, Y.; et al. Treatment of HCV Infection by Targeting MicroRNA. N. Engl. J. Med. 2013, 368, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-K.; Bär, C.; Thum, T. miR-21, Mediator, and Potential Therapeutic Target in the Cardiorenal Syndrome. Front. Pharmacol. 2020, 11, 726. [Google Scholar] [CrossRef] [PubMed]
- Roblain, Q.; Louis, T.; Yip, C.; Baudin, L.; Struman, I.; Caolo, V.; Lambert, V.; Lecomte, J.; Noël, A.; Heymans, S. Intravitreal Injection of Anti-miRs against miR-142-3p Reduces Angiogenesis and Microglia Activation in a Mouse Model of Laser-Induced Choroidal Neovascularization. Aging 2021, 13, 12359–12377. [Google Scholar] [CrossRef] [PubMed]
- Naseri, Z.; Kazemi Oskuee, R.; Jaafari, M.R.; Forouzandeh, M. Exosome-Mediated Delivery of Functionally Active miRNA-142-3p Inhibitor Reduces Tumorigenicity of Breast Cancer in Vitro and in Vivo. Int. J. Nanomed. 2018, 13, 7727–7747. [Google Scholar] [CrossRef] [PubMed]
- Duijvis, N.W.; Moerland, P.D.; Kunne, C.; Slaman, M.M.W.; van Dooren, F.H.; Vogels, E.W.; de Jonge, W.J.; Meijer, S.L.; Fluiter, K.; te Velde, A.A. Inhibition of miR-142-5P Ameliorates Disease in Mouse Models of Experimental Colitis. PLoS ONE 2017, 12, e0185097. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Zhao, Q.; He, C.; Huang, D.; Liu, J.; Chen, F.; Chen, J.; Liao, J.-Y.; Cui, X.; Zeng, Y.; et al. miR-142-5p and miR-130a-3p Are Regulated by IL-4 and IL-13 and Control Profibrogenic Macrophage Program. Nat. Commun. 2015, 6, 8523. [Google Scholar] [CrossRef] [PubMed]
- Iversen, P. Phosphorodiamidate Morpholino Oligomers. In Antisense Drug Technology; Crooke, S., Ed.; CRC Press: Boca Raton, FL, USA, 2001; ISBN 978-0-8247-0566-4. [Google Scholar]
- Lalwani, M.K.; Sharma, M.; Singh, A.R.; Chauhan, R.K.; Patowary, A.; Singh, N.; Scaria, V.; Sivasubbu, S. Reverse Genetics Screen in Zebrafish Identifies a Role of miR-142a-3p in Vascular Development and Integrity. PLoS ONE 2012, 7, e52588. [Google Scholar] [CrossRef]
- Nimmo, R.; Ciau-Uitz, A.; Ruiz-Herguido, C.; Soneji, S.; Bigas, A.; Patient, R.; Enver, T. miR-142-3p Controls the Specification of Definitive Hemangioblasts during Ontogeny. Dev. Cell 2013, 26, 237–249. [Google Scholar] [CrossRef][Green Version]
- Nielsen, P.E.; Egholm, M.; Buchardt, O. Peptide Nucleic Acid (PNA). A DNA Mimic with a Peptide Backbone. Bioconjug. Chem. 1994, 5, 3–7. [Google Scholar] [CrossRef]
- Englund, E.A.; Appella, D.H. γ-Substituted Peptide Nucleic Acids Constructed fromL-Lysine Are a Versatile Scaffold for Multifunctional Display. Angew. Chem. Int. Ed. 2007, 46, 1414–1418. [Google Scholar] [CrossRef]
- Kim, D.; Song, J.; Kim, S.; Kang, S.-S.; Jin, E.-J. MicroRNA-142-3p Regulates TGF-Β3-Mediated Region-Dependent Chondrogenesis by Regulating ADAM9. Biochem. Biophys. Res. Commun. 2011, 414, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Dzierlega, K.; Yokota, T. Optimization of Antisense-Mediated Exon Skipping for Duchenne Muscular Dystrophy. Gene Ther. 2020, 27, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, H.-Y.; Hao, N.-B.; Tang, B.; Guo, H.; Yong, X.; Dong, H.; Yang, S.-M. microRNA Inhibitors: Natural and Artificial Sequestration of microRNA. Cancer Lett. 2017, 407, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.W.; Bracken, C.P.; Szubert, J.M.; Goodall, G.J. On Measuring miRNAs after Transient Transfection of Mimics or Antisense Inhibitors. PLoS ONE 2013, 8, e55214. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen Recognition by the Innate Immune System. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Dalpke, A.H.; Helm, M. RNA Mediated Toll-like Receptor Stimulation in Health and Disease. RNA Biol. 2012, 9, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Sioud, M. Single-Stranded Small Interfering RNA Are More Immunostimulatory than Their Double-Stranded Counterparts: A Central Role for 2′-Hydroxyl Uridines in Immune Responses. Eur. J. Immunol. 2006, 36, 1222–1230. [Google Scholar] [CrossRef]
- Tay, F.C.; Lim, J.K.; Zhu, H.; Hin, L.C.; Wang, S. Using Artificial microRNA Sponges to Achieve microRNA Loss-of-Function in Cancer Cells. Adv. Drug Deliv. Rev. 2015, 81, 117–127. [Google Scholar] [CrossRef]
- Das, S.; Kohr, M.; Dunkerly-Eyring, B.; Lee, D.I.; Bedja, D.; Kent, O.A.; Leung, A.K.L.; Henao-Mejia, J.; Flavell, R.A.; Steenbergen, C. Divergent Effects of miR-181 Family Members on Myocardial Function Through Protective Cytosolic and Detrimental Mitochondrial microRNA Targets. J. Am. Heart Assoc. 2017, 6, e004694. [Google Scholar] [CrossRef]
- Bak, R.O.; Mikkelsen, J.G. miRNA Sponges: Soaking up miRNAs for Regulation of Gene Expression: MicroRNA Sponges. Wiley Interdiscip. Rev. RNA 2014, 5, 317–333. [Google Scholar] [CrossRef]
- Jung, J.; Yeom, C.; Choi, Y.-S.; Kim, S.; Lee, E.; Park, M.J.; Kang, S.W.; Kim, S.B.; Chang, S. Simultaneous Inhibition of Multiple Oncogenic miRNAs by a Multi-Potent microRNA Sponge. Oncotarget 2015, 6, 20370–20387. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Sun, W.; Okano, K.; Chen, Y.; Zhang, N.; Maeda, T.; Palczewski, K. Sponge Transgenic Mouse Model Reveals Important Roles for the MicroRNA-183 (miR-183)/96/182 Cluster in Postmitotic Photoreceptors of the Retina. J. Biol. Chem. 2011, 286, 31749–31760. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.-H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA Genes Are Transcribed by RNA Polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.J.; Coller, H.A. The Code within the Code: microRNAs Target Coding Regions. Cell Cycle 2010, 9, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Ebert, M.S.; Sharp, P.A. MicroRNA Sponges: Progress and Possibilities. RNA 2010, 16, 2043–2050. [Google Scholar] [CrossRef]
- Bak, R.O.; Hollensen, A.K.; Primo, M.N.; Sørensen, C.D.; Mikkelsen, J.G. Potent microRNA Suppression by RNA Pol II-Transcribed ‘Tough Decoy’ Inhibitors. RNA 2013, 19, 280–293. [Google Scholar] [CrossRef]
- Kluiver, J.; Slezak-Prochazka, I.; Smigielska-Czepiel, K.; Halsema, N.; Kroesen, B.-J.; van den Berg, A. Generation of miRNA Sponge Constructs. Methods 2012, 58, 113–117. [Google Scholar] [CrossRef]
- Yekta, S.; Shih, I.; Bartel, D.P. MicroRNA-Directed Cleavage of HOXB8 mRNA. Science 2004, 304, 594–596. [Google Scholar] [CrossRef]
- Kawamata, T.; Seitz, H.; Tomari, Y. Structural Determinants of miRNAs for RISC Loading and Slicer-Independent Unwinding. Nat. Struct. Mol. Biol. 2009, 16, 953–960. [Google Scholar] [CrossRef]
- Mcintyre, G.J.; Yu, Y.H.; Tran, A.; Jaramillo, A.B.; Arndt, A.J.; Millington, M.L.; Boyd, M.P.; Elliott, F.A.; Shen, S.W.; Murray, J.M.; et al. Cassette Deletion in Multiple shRNA Lentiviral Vectors for HIV-1 and Its Impact on Treatment Success. Virol. J. 2009, 6, 184. [Google Scholar] [CrossRef][Green Version]
- Bennett, D.; Sakurai, F.; Shimizu, K.; Matsui, H.; Tomita, K.; Suzuki, T.; Katayama, K.; Kawabata, K.; Mizuguchi, H. Further Reduction in Adenovirus Vector-Mediated Liver Transduction without Largely Affecting Transgene Expression in Target Organ by Exploiting MicroRNA-Mediated Regulation and the Cre-loxP Recombination System. Mol. Pharm. 2012, 9, 3452–3463. [Google Scholar] [CrossRef] [PubMed]
- Sonda, N.; Simonato, F.; Peranzoni, E.; Calì, B.; Bortoluzzi, S.; Bisognin, A.; Wang, E.; Marincola, F.M.; Naldini, L.; Gentner, B.; et al. miR-142-3p Prevents Macrophage Differentiation during Cancer-Induced Myelopoiesis. Immunity 2013, 38, 1236–1249. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, T.; Ozaki, Y.; Iba, H. Vectors Expressing Efficient RNA Decoys Achieve the Long-Term Suppression of Specific microRNA Activity in Mammalian Cells. Nucleic Acids Res. 2009, 37, e43. [Google Scholar] [CrossRef] [PubMed]
- Mullokandov, G.; Baccarini, A.; Ruzo, A.; Jayaprakash, A.D.; Tung, N.; Israelow, B.; Evans, M.J.; Sachidanandam, R.; Brown, B.D. High-Throughput Assessment of microRNA Activity and Function Using microRNA Sensor and Decoy Libraries. Nat. Methods 2012, 9, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-T.; Coca-Prados, M. Electron Microscopic Evidence for the Circular Form of RNA in the Cytoplasm of Eukaryotic Cells. Nature 1979, 280, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs Are a Large Class of Animal RNAs with Regulatory Potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA Circles Function as Efficient microRNA Sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Patop, I.L.; Wüst, S.; Kadener, S. Past, Present, and Future of Circ RNA s. EMBO J. 2019, 38, e100836. [Google Scholar] [CrossRef]
- Rybak-Wolf, A.; Stottmeister, C.; Glažar, P.; Jens, M.; Pino, N.; Giusti, S.; Hanan, M.; Behm, M.; Bartok, O.; Ashwal-Fluss, R.; et al. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell 2015, 58, 870–885. [Google Scholar] [CrossRef]
- Liang, D.; Wilusz, J.E. Short Intronic Repeat Sequences Facilitate Circular RNA Production. Genes Dev. 2014, 28, 2233–2247. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs Are Abundant, Conserved, and Associated with ALU Repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Bachmayr-Heyda, A.; Reiner, A.T.; Auer, K.; Sukhbaatar, N.; Aust, S.; Bachleitner-Hofmann, T.; Mesteri, I.; Grunt, T.W.; Zeillinger, R.; Pils, D. Correlation of Circular RNA Abundance with Proliferation—Exemplified with Colorectal and Ovarian Cancer, Idiopathic Lung Fibrosis, and Normal Human Tissues. Sci. Rep. 2015, 5, 8057. [Google Scholar] [CrossRef]
- Peng, L.; Sang, H.; Wei, S.; Li, Y.; Jin, D.; Zhu, X.; Li, X.; Dang, Y.; Zhang, G. circCUL2 Regulates Gastric Cancer Malignant Transformation and Cisplatin Resistance by Modulating Autophagy Activation via miR-142-3p/ROCK2. Mol. Cancer 2020, 19, 156. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, N.; Jiang, X.; Wang, J.; Dong, J.; Gao, Y. FUS-induced Circular RNA ZNF609 Promotes Tumorigenesis and Progression via Sponging miR-142-3p in Lung Cancer. J. Cell Physiol. 2021, 236, 79–92. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, X.; Zou, A.; Mai, Z.; Huang, Z.; Sun, L.; Zhao, J. circIGHG-Induced Epithelial-to-Mesenchymal Transition Promotes Oral Squamous Cell Carcinoma Progression via miR-142-5p/IGF2BP3 Signaling. Cancer Res. 2021, 81, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Wang, H.; Sun, G.; Xu, P.; Lv, J.; Zhang, X.; Zhang, L.; Wang, S.; Cao, J.; Xia, Y.; Xuan, Z.; et al. Circular RNA TMEM87A Promotes Cell Proliferation and Metastasis of Gastric Cancer by Elevating ULK1 via Sponging miR-142-5p. J. Gastroenterol. 2021, 56, 125–138. [Google Scholar] [CrossRef]
- Katheder, N.S.; Khezri, R.; O’Farrell, F.; Schultz, S.W.; Jain, A.; Rahman, M.M.; Schink, K.O.; Theodossiou, T.A.; Johansen, T.; Juhász, G.; et al. Microenvironmental Autophagy Promotes Tumour Growth. Nature 2017, 541, 417–420. [Google Scholar] [CrossRef]
- Ma, X.-L.; Zhan, T.-C.; Hu, J.-P.; Zhang, C.-L.; Zhu, K.-P. Doxorubicin-Induced Novel circRNA_0004674 Facilitates Osteosarcoma Progression and Chemoresistance by Upregulating MCL1 through miR-142-5p. Cell Death Discov. 2021, 7, 309. [Google Scholar] [CrossRef]
- Chen, C.; Sarnow, P. Internal Ribosome Entry Sites Tests with Circular mRNAs. In Protein Synthesis; Humana Press: New Jersey, NJ, USA, 1998; Volume 77, pp. 355–364. ISBN 978-0-89603-397-9. [Google Scholar]
- Ochi, A.; Umekage, S.; Kikuchi, Y. Non-Enzymatic in Vitro Production of Circular Hammerhead Ribozyme Targeting the Template Region of Human Telomerase RNA. Nucleic Acids Symp. Ser. 2009, 53, 275–276. [Google Scholar] [CrossRef][Green Version]
- Umekage, S.; Kikuchi, Y. In Vitro and in Vivo Production and Purification of Circular RNA Aptamer. J. Biotechnol. 2009, 139, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Wesselhoeft, R.A.; Kowalski, P.S.; Anderson, D.G. Engineering Circular RNA for Potent and Stable Translation in Eukaryotic Cells. Nat. Commun. 2018, 9, 2629. [Google Scholar] [CrossRef] [PubMed]
- Lavenniah, A.; Luu, T.D.A.; Li, Y.P.; Lim, T.B.; Jiang, J.; Ackers-Johnson, M.; Foo, R.S.-Y. Engineered Circular RNA Sponges Act as miRNA Inhibitors to Attenuate Pressure Overload-Induced Cardiac Hypertrophy. Mol. Ther. 2020, 28, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Bak, R.O.; Hollensen, A.K.; Mikkelsen, J.G. Managing MicroRNAs with Vector-Encoded Decoy-Type Inhibitors. Mol. Ther. 2013, 21, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, K.; Shi, Z.; Zhang, A.; Jia, Z.; Wang, G.; Pu, P.; Kang, C.; Han, L. A Lentivirus-Mediated miR-23b Sponge Diminishes the Malignant Phenotype of Glioma Cells in Vitro and in Vivo. Oncol. Rep. 2014, 31, 1573–1580. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Chen, L.-L. The Biogenesis, Functions, and Challenges of Circular RNAs. Mol. Cell 2018, 71, 428–442. [Google Scholar] [CrossRef]
- Breuer, J.; Rossbach, O. Production and Purification of Artificial Circular RNA Sponges for Application in Molecular Biology and Medicine. Methods Protoc. 2020, 3, 42. [Google Scholar] [CrossRef]
- Obi, P.; Chen, Y.G. The Design and Synthesis of Circular RNAs. Methods 2021, 196, 85–103. [Google Scholar] [CrossRef]
- Chen, Y.G.; Kim, M.V.; Chen, X.; Batista, P.J.; Aoyama, S.; Wilusz, J.E.; Iwasaki, A.; Chang, H.Y. Sensing Self and Foreign Circular RNAs by Intron Identity. Mol. Cell 2017, 67, 228–238.e5. [Google Scholar] [CrossRef]
- Chen, Y.G.; Chen, R.; Ahmad, S.; Verma, R.; Kasturi, S.P.; Amaya, L.; Broughton, J.P.; Kim, J.; Cadena, C.; Pulendran, B.; et al. N6-Methyladenosine Modification Controls Circular RNA Immunity. Mol. Cell 2019, 76, 96–109.e9. [Google Scholar] [CrossRef]
- Wesselhoeft, R.A.; Kowalski, P.S.; Parker-Hale, F.C.; Huang, Y.; Bisaria, N.; Anderson, D.G. RNA Circularization Diminishes Immunogenicity and Can Extend Translation Duration In Vivo. Mol. Cell 2019, 74, 508–520.e4. [Google Scholar] [CrossRef] [PubMed]
- Basavappa, M.G.; Cherry, S. Going in Circles: The Black Box of Circular RNA Immunogenicity. Mol. Cell 2019, 76, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Tao, H.; Zhu, H.; Pan, Y.; Li, P.; Liang, H.; Zhang, B.; Song, J. Circular RNA circUBE2J2 Acts as the Sponge of microRNA-370-5P to Suppress Hepatocellular Carcinoma Progression. Cell Death Dis. 2021, 12, 985. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Xu, C.; Liu, Y.; Hu, Y.; Wu, H. Circular RNA Hsa_circ_0001649 Inhibits Hepatocellular Carcinoma Progression via Multiple miRNAs Sponge. Aging 2019, 11, 3362–3375. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome Engineering Using the CRISPR-Cas9 System. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Mildner, A.; Chapnik, E.; Varol, D.; Aychek, T.; Lampl, N.; Rivkin, N.; Bringmann, A.; Paul, F.; Boura-Halfon, S.; Hayoun, Y.S.; et al. MicroRNA-142 Controls Thymocyte Proliferation. Eur. J. Immunol. 2017, 47, 1142–1152. [Google Scholar] [CrossRef]
- Roberts, L.B.; Jowett, G.M.; Read, E.; Zabinski, T.; Berkachy, R.; Selkirk, M.E.; Jackson, I.; Niazi, U.; Anandagoda, N.; Araki, M.; et al. MicroRNA-142 Critically Regulates Group 2 Innate Lymphoid Cell Homeostasis and Function. J. Immunol. 2021, 206, 2725–2739. [Google Scholar] [CrossRef]
- Anderson, K.R.; Haeussler, M.; Watanabe, C.; Janakiraman, V.; Lund, J.; Modrusan, Z.; Stinson, J.; Bei, Q.; Buechler, A.; Yu, C.; et al. CRISPR Off-Target Analysis in Genetically Engineered Rats and Mice. Nat. Methods 2018, 15, 512–514. [Google Scholar] [CrossRef]
- Weisheit, I.; Kroeger, J.A.; Malik, R.; Klimmt, J.; Crusius, D.; Dannert, A.; Dichgans, M.; Paquet, D. Detection of Deleterious On-Target Effects after HDR-Mediated CRISPR Editing. Cell Rep. 2020, 31, 107689. [Google Scholar] [CrossRef]
- Gough, V.; Gersbach, C.A. Immunity to Cas9 as an Obstacle to Persistent Genome Editing. Mol. Ther. 2020, 28, 1389–1391. [Google Scholar] [CrossRef]
- Burmistrz, M.; Krakowski, K.; Krawczyk-Balska, A. RNA-Targeting CRISPR–Cas Systems and Their Applications. Int. J. Mol. Sci. 2020, 21, 1122. [Google Scholar] [CrossRef] [PubMed]
- Bouard, D.; Alazard-Dany, D.; Cosset, F.-L. Viral Vectors: From Virology to Transgene Expression. Br. J. Pharmacol. 2009, 157, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Zacchigna, S.; Zentilin, L.; Giacca, M. Adeno-Associated Virus Vectors as Therapeutic and Investigational Tools in the Cardiovascular System. Circ. Res. 2014, 114, 1827–1846. [Google Scholar] [CrossRef] [PubMed]
- McClements, M.E.; MacLaren, R.E. Adeno-Associated Virus (AAV) Dual Vector Strategies for Gene Therapy Encoding Large Transgenes. Yale J. Biol. Med. 2017, 90, 611–623. [Google Scholar] [PubMed]
- Selot, R.; Hareendran, S.; Jayandharan, G. Developing Immunologically Inert Adeno-Associated Virus (AAV) Vectors for Gene Therapy: Possibilities and Limitations. Curr. Pharm. Biotechnol. 2014, 14, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Mingozzi, F.; High, K.A. Immune Responses to AAV Vectors: Overcoming Barriers to Successful Gene Therapy. Blood 2013, 122, 23–36. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, X.; Wright, J.F.; High, K.A.; Couto, L.; Qu, G. PEG-Modulated Column Chromatography for Purification of Recombinant Adeno-Associated Virus Serotype 9. J. Virol. Methods 2011, 173, 99–107. [Google Scholar] [CrossRef]
- Keeler, A.M.; Flotte, T.R. Recombinant Adeno-Associated Virus Gene Therapy in Light of Luxturna (and Zolgensma and Glybera): Where Are We, and How Did We Get Here? Annu. Rev. Virol. 2019, 6, 601–621. [Google Scholar] [CrossRef]
- Gándara, C.; Affleck, V.; Stoll, E.A. Manufacture of Third-Generation Lentivirus for Preclinical Use, with Process Development Considerations for Translation to Good Manufacturing Practice. Hum. Gene Ther. Methods 2018, 29, 1–15. [Google Scholar] [CrossRef]
- Labbé, R.P.; Vessillier, S.; Rafiq, Q.A. Lentiviral Vectors for T Cell Engineering: Clinical Applications, Bioprocessing and Future Perspectives. Viruses 2021, 13, 1528. [Google Scholar] [CrossRef] [PubMed]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Ban, E.; Kwon, T.-H.; Kim, A. Delivery of Therapeutic miRNA Using Polymer-Based Formulation. Drug Deliv. Transl. Res. 2019, 9, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Höbel, S.; Aigner, A. Polyethylenimines for siRNA and miRNA Delivery in Vivo. WIREs Nanomed. Nanobiotechnology 2013, 5, 484–501. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Tian, H.; Guo, Y.; Li, Y.; Guo, Z.; Zhu, X.; Chen, X. miRNA Oligonucleotide and Sponge for miRNA-21 Inhibition Mediated by PEI-PLL in Breast Cancer Therapy. Acta Biomater. 2015, 25, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Biray Avcı, Ç.; Özcan, İ.; Balcı, T.; Özer, Ö.; Gündüz, C. Design of Polyethylene Glycol-Polyethylenimine Nanocomplexes as Non-Viral Carriers: Mir-150 Delivery to Chronic Myeloid Leukemia Cells: PEG-PEI Nanocomplexes for miRNA Delivery. Cell Biol. Int. 2013, 37, 1205–1214. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-Based Nanoparticles: An Overview of Biomedical Applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Kapadia, C.H.; Luo, B.; Dang, M.N.; Irvin-Choy, N.; Valcourt, D.M.; Day, E.S. Polymer Nanocarriers for MicroRNA Delivery. J. Appl. Polym. Sci. 2020, 137, 48651. [Google Scholar] [CrossRef]
- Cai, C.; Xie, Y.; Wu, L.; Chen, X.; Liu, H.; Zhou, Y.; Zou, H.; Liu, D.; Zhao, Y.; Kong, X.; et al. PLGA-Based Dual Targeted Nanoparticles Enhance miRNA Transfection Efficiency in Hepatic Carcinoma. Sci. Rep. 2017, 7, 46250. [Google Scholar] [CrossRef]
- Moraes, F.C.; Pichon, C.; Letourneur, D.; Chaubet, F. miRNA Delivery by Nanosystems: State of the Art and Perspectives. Pharmaceutics 2021, 13, 1901. [Google Scholar] [CrossRef]
- Golan, T.; Khvalevsky, E.Z.; Hubert, A.; Gabai, R.M.; Hen, N.; Segal, A.; Domb, A.; Harari, G.; David, E.B.; Raskin, S.; et al. RNAi Therapy Targeting KRAS in Combination with Chemotherapy for Locally Advanced Pancreatic Cancer Patients. Oncotarget 2015, 6, 24560–24570. [Google Scholar] [CrossRef] [PubMed]
- Sarett, S.M.; Nelson, C.E.; Duvall, C.L. Technologies for Controlled, Local Delivery of siRNA. J. Control. Release Off. J. Control. Release Soc. 2015, 218, 94–113. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.L.; Paoletti, C.; Campisi, M.; Osaki, T.; Adriani, G.; Kamm, R.D.; Mattu, C.; Chiono, V. MicroRNA Delivery through Nanoparticles. J. Control. Release 2019, 313, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Scheideler, M.; Vidakovic, I.; Prassl, R. Lipid Nanocarriers for microRNA Delivery. Chem. Phys. Lipids 2020, 226, 104837. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA Vaccine Delivery Using Lipid Nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro Story and the Clinical Translation of Nanomedicines Containing Nucleic Acid-Based Drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ishihara, H. Difference in the Lipid Nanoparticle Technology Employed in Three Approved siRNA (Patisiran) and mRNA (COVID-19 Vaccine) Drugs. Drug Metab. Pharmacokinet. 2021, 41, 100424. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the Cold Reality of mRNA Vaccine Stability. J. Pharm. Sci. 2021, 110, 997–1001. [Google Scholar] [CrossRef]
- Debacker, A.J.; Voutila, J.; Catley, M.; Blakey, D.; Habib, N. Delivery of Oligonucleotides to the Liver with GalNAc: From Research to Registered Therapeutic Drug. Mol. Ther. 2020, 28, 1759–1771. [Google Scholar] [CrossRef]
- Schachner-Nedherer, A.-L.; Werzer, O.; Zimmer, A. A Protocol To Characterize Peptide-Based Drug Delivery Systems for miRNAs. ACS Omega 2019, 4, 7014–7022. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs Are Transported in Plasma and Delivered to Recipient Cells by High-Density Lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, X.; Zhang, X.; Liu, B.; Huang, L. Nanoparticles Modified with Tumor-Targeting scFv Deliver siRNA and miRNA for Cancer Therapy. Mol. Ther. 2010, 18, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Baek, S.C.; Lee, Y.-Y.; Bastiaanssen, C.; Kim, J.; Kim, H.; Kim, V.N. A Quantitative Map of Human Primary microRNA Processing Sites. Mol. Cell 2021, 81, 3422–3439.e11. [Google Scholar] [CrossRef] [PubMed]
- Arif, K.M.T.; Elliott, E.K.; Haupt, L.M.; Griffiths, L.R. Regulatory Mechanisms of Epigenetic miRNA Relationships in Human Cancer and Potential as Therapeutic Targets. Cancers 2020, 12, 2922. [Google Scholar] [CrossRef]
- Chiou, G.-Y.; Chien, C.-S.; Wang, M.-L.; Chen, M.-T.; Yang, Y.-P.; Yu, Y.-L.; Chien, Y.; Chang, Y.-C.; Shen, C.-C.; Chio, C.-C.; et al. Epigenetic Regulation of the miR142-3p/Interleukin-6 Circuit in Glioblastoma. Mol. Cell 2013, 52, 693–706. [Google Scholar] [CrossRef]
- Andreopoulos, B.; Anastassiou, D. Integrated Analysis Reveals Hsa-miR-142 as a Representative of a Lymphocyte-Specific Gene Expression and Methylation Signature. Cancer Inform. 2012, 11, CIN.S9037. [Google Scholar] [CrossRef]
- Li, Y.; He, Q.; Wen, X.; Hong, X.; Yang, X.; Tang, X.; Zhang, P.; Lei, Y.; Sun, Y.; Zhang, J.; et al. EZH2-DNMT1-Mediated Epigenetic Silencing of miR-142-3p Promotes Metastasis through Targeting ZEB2 in Nasopharyngeal Carcinoma. Cell Death Differ. 2019, 26, 1089–1106. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Chen, Y.-B.; Yue, H.-R.; Zhou, X.-J.; Ma, H.-Y.; Wang, X.; Cao, X.-C.; Yu, Y. PAX5-miR-142 Feedback Loop Promotes Breast Cancer Proliferation by Regulating DNMT1 and ZEB1. Mol. Med. 2023, 29, 89. [Google Scholar] [CrossRef]
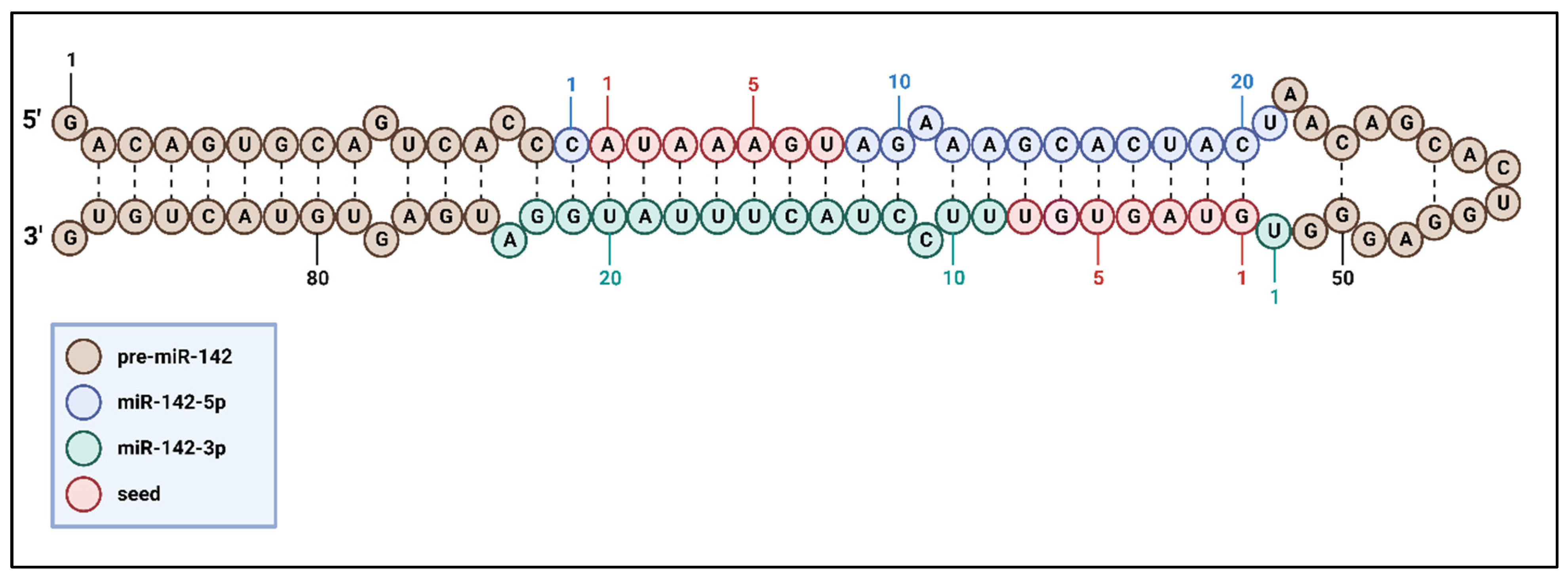
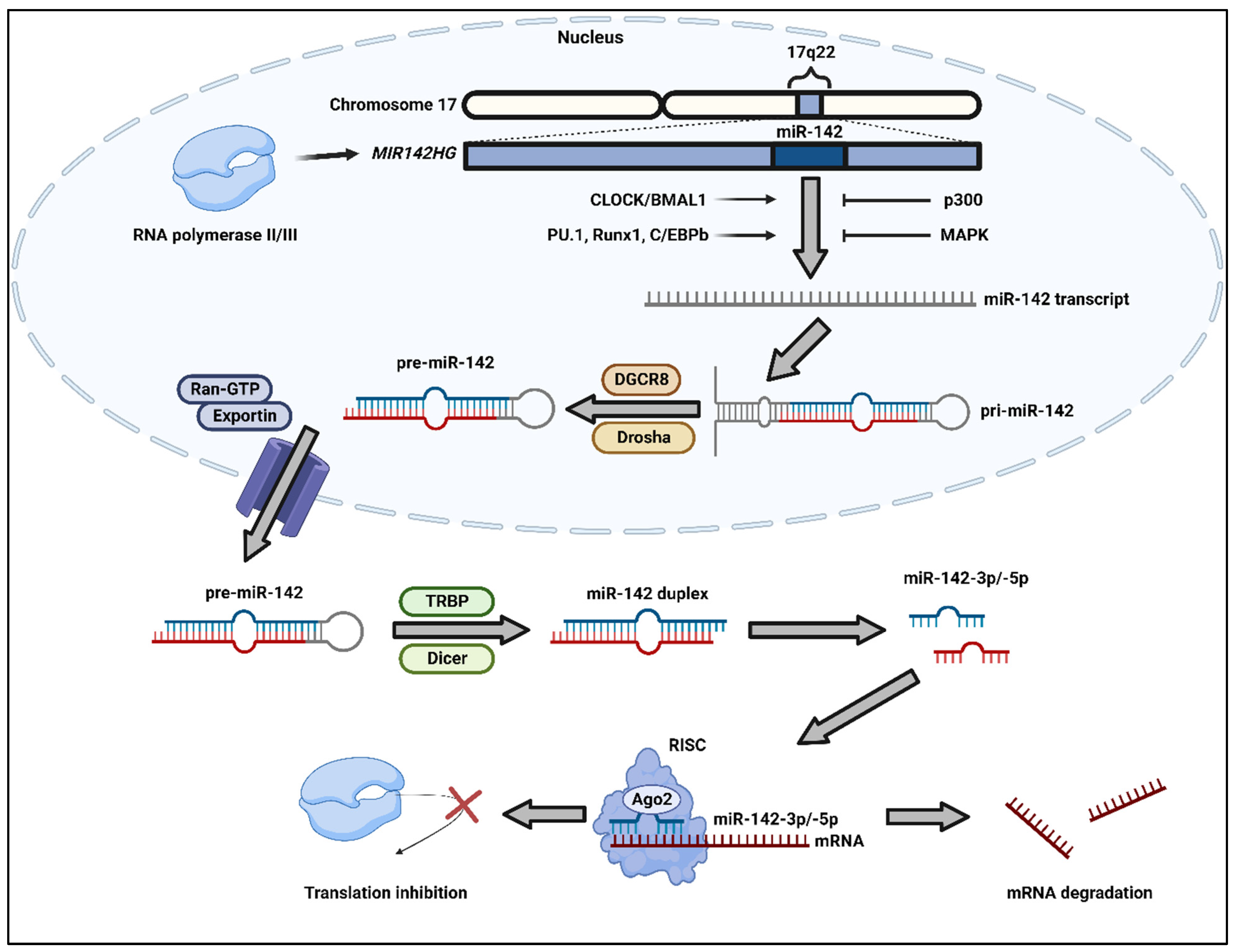
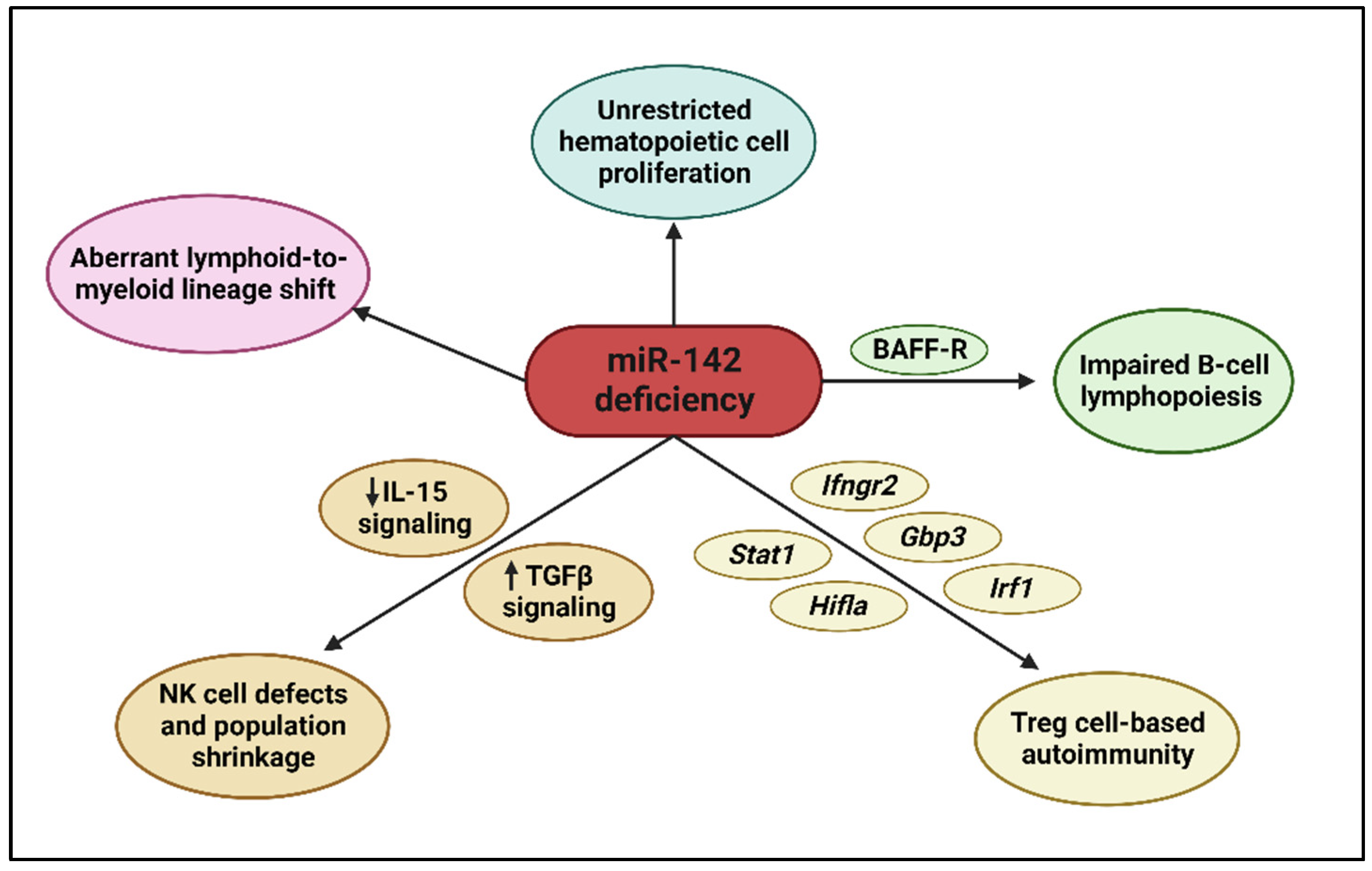
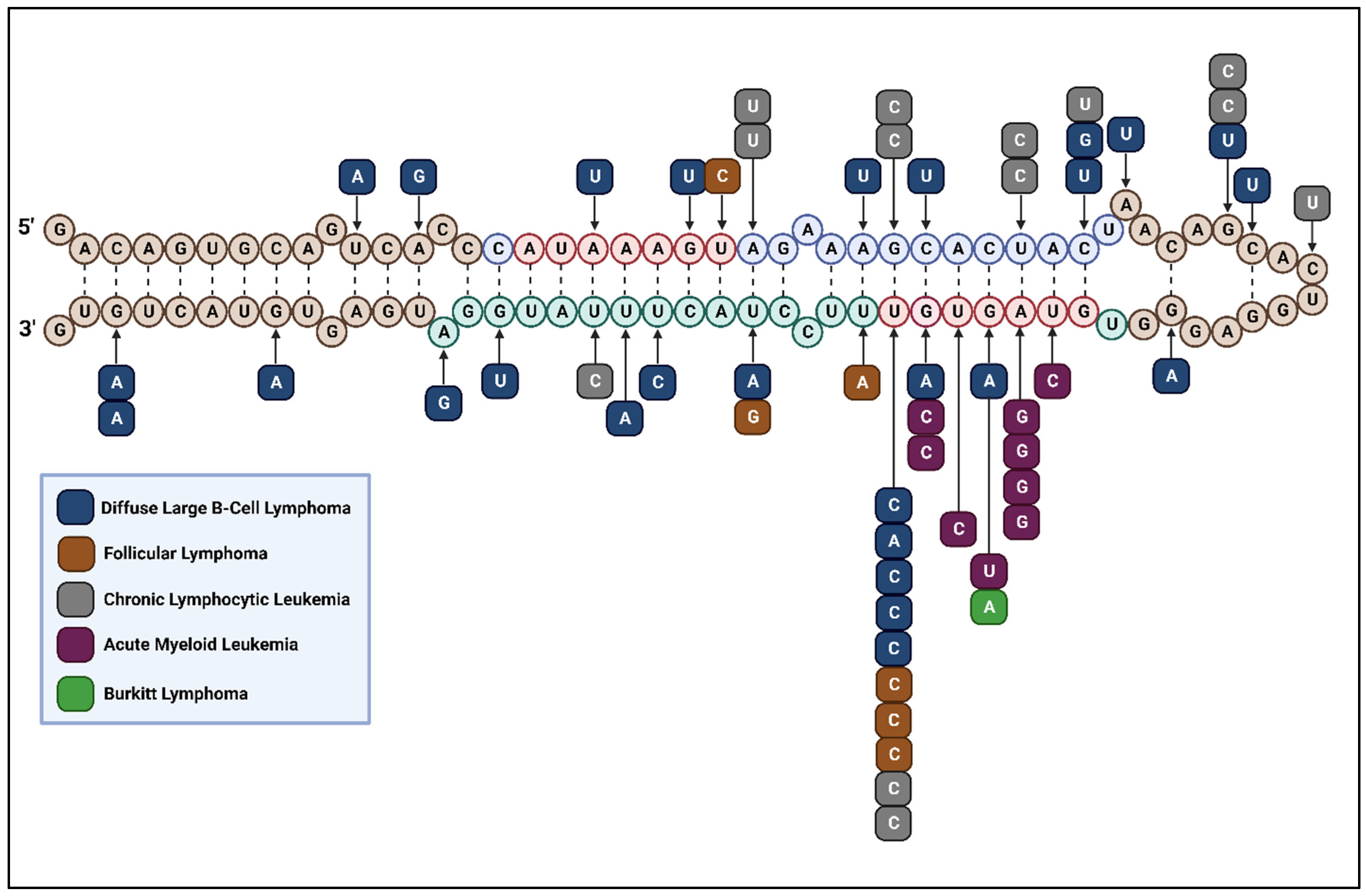
| Cancer Type | Cancer Subtype | Mutation Position | Position Reference | Occurrence of Mutation in Each Position | Reference |
|---|---|---|---|---|---|
| Lymphoma | DLBCL | Positions 13, 21 | miR-142-3p | 1/37 cases | [114] |
| Position 6 | miR-142-3p seed | ||||
| Positions 80, 85 | pre-miR-142 | ||||
| Position 20 | miR-142-5p | 2/37 cases | |||
| Position 7 | miR-142-3p seed | 3/37 cases | |||
| Position 4 | miR-142-3p seed | 1/19 cases | [115] | ||
| Position 17 | miR-142-3p | ||||
| Position 50 | pre-miR-142 | ||||
| Positions 3, 6 | miR-142-5p seed | 1/56 cases | [116] | ||
| Positions 13, 15 | miR-142-5p | ||||
| Positions 16, 23 | miR-142-3p | ||||
| Positions 11, 13, 37, 41, 42, 85 | pre-miR-142 | ||||
| Position 7 | miR-142-3p seed | 2/56 cases | |||
| t(8;17)(q24;q22) | pre-miR-142 | 1/1 case | [117] | ||
| FL | Position 7 | miR-142-5p seed | 1/12 aggressive cases | [118] | |
| Position 7 | miR-142-3p seed | 2/12 aggressive cases | |||
| Position 7 | miR-142-3p seed | 1/21 cases | [115] | ||
| Positions 9, 13 | miR-142-3p | ||||
| BL | Position 4 | miR-142-3p seed | 1/5 cell lines | [114] | |
| Leukemia | CLL | Positions 9, 14, 18, 20 | miR-142-5p | 1/452 cases | [119] |
| Position 18 | miR-142-3p | ||||
| Position 7 | miR-142-3p seed | ||||
| Positions 41, 44 | pre-miR-142 | ||||
| Positions 9, 14 | miR-142-5p | 1/90 cases | [120] | ||
| Position 41 | pre-miR-142 | ||||
| Position 14 | miR-142-5p | 1/210 cases | [121] | ||
| Position 7 | miR-142-3p seed | ||||
| AML | Positions 2, 3, 4 | miR-142-3p seed | 1/416 cases | [122] | |
| Position 5 | miR-142-3p seed | 1/200 adult cases | [123] | ||
| Positions 3, 6 | miR-142-3p seed | 2/200 adult cases | |||
| Position 3 | miR-142-3p seed | 1/149 cases | [114] |
| Cancer Type | Cancer Subtype | Mir-142 Strand | Mutation/Altered Expression in miR-142 | Validated Direct Cancer Targets/Relevant Pathway | Identified Affected Function | Reference |
|---|---|---|---|---|---|---|
| Non-Hodgkin lymphoma | DLBCL | -3p | Loss-of-function mutation | RAC1/RAC1 pathway | Increased cell proliferation; decreased cell apoptosis | [116,124] |
| FL | -3p | Loss-of-function mutation | RAC1/RAC1 pathway | Increased cell proliferation; decreased cell apoptosis | [116,118,124] | |
| BL | -5p | Upregulated expression in EBV+ | PTEN/PI3K-AKT pathway | Decreased cell apoptosis | [132,133] | |
| MALT Lymphoma | -5p | Upregulated expression in H. pylori eradication resistant | TP53INP1 | Decreased cell apoptosis | [135] | |
| NKTCL | -3p | Downregulated expression in EBV+ | IL1A, AC9; RICTOR/IL-1; PI3K-AKT pathway | Increased cell growth, mobility, and proliferation; decreased cell apoptosis | [143,146,150,153,154] | |
| ATLL | -3p | Downregulated expression | THBS4 | Increased tumor angiogenesis, cell migration, and vascular invasion | [156] | |
| Leukemia | CML | -3p; -5p | Downregulated expression | CIAPIN1/tyrosine kinase-Ras pathway | Decreased cell apoptosis | [157,158] |
| -5p | Downregulated in imatinib nonresponsive patients | ABL2, MCL1, cKIT, SRI/GM-CSF, IL-3, PI3K-AKT, JAK-STAT, Src family kinase, MAPK pathways | Increased cell invasion, metastasis, proliferation, cytoskeleton rearrangement, and imatinib and multi-drug resistance; decreased cell apoptosis | [159,160,161] | ||
| ALL | -3p | Upregulated expression in T-ALL | AC9, GRα/cAMP-PKA pathway | Increased cell proliferation; GC resistance | [150,162] | |
| -3p | Upregulated expression in pre-B-ALL | GRα | Increased GC resistance | [162,163,164] | ||
| -3p | Downregulated expression in t(4;11)(q21;q23) B-ALL | MLL-AF4 | Increased cell proliferation; decreased cell apoptosis | [165] | ||
| AML | -3p | Loss-of-function mutation | ASHL1 | Loss of lymphoid potential, maintenance of myeloid potential, expansion of myeloblasts and immature hematopoietic cells (in IDH2R140Q/IDH2R172K+) | [27] | |
| APL | -3p | Downregulated expression | CCNT2, TAB2 | Decreased monocytic and granulocytic differentiation | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Paul, D.; Calin, G.A.; Bayraktar, R. miR-142: A Master Regulator in Hematological Malignancies and Therapeutic Opportunities. Cells 2024, 13, 84. https://doi.org/10.3390/cells13010084
Huang W, Paul D, Calin GA, Bayraktar R. miR-142: A Master Regulator in Hematological Malignancies and Therapeutic Opportunities. Cells. 2024; 13(1):84. https://doi.org/10.3390/cells13010084
Chicago/Turabian StyleHuang, Wilson, Doru Paul, George A. Calin, and Recep Bayraktar. 2024. "miR-142: A Master Regulator in Hematological Malignancies and Therapeutic Opportunities" Cells 13, no. 1: 84. https://doi.org/10.3390/cells13010084
APA StyleHuang, W., Paul, D., Calin, G. A., & Bayraktar, R. (2024). miR-142: A Master Regulator in Hematological Malignancies and Therapeutic Opportunities. Cells, 13(1), 84. https://doi.org/10.3390/cells13010084









