Preconditioning of MSCs for Acute Neurological Conditions: From Cellular to Functional Impact—A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Results and Discussion
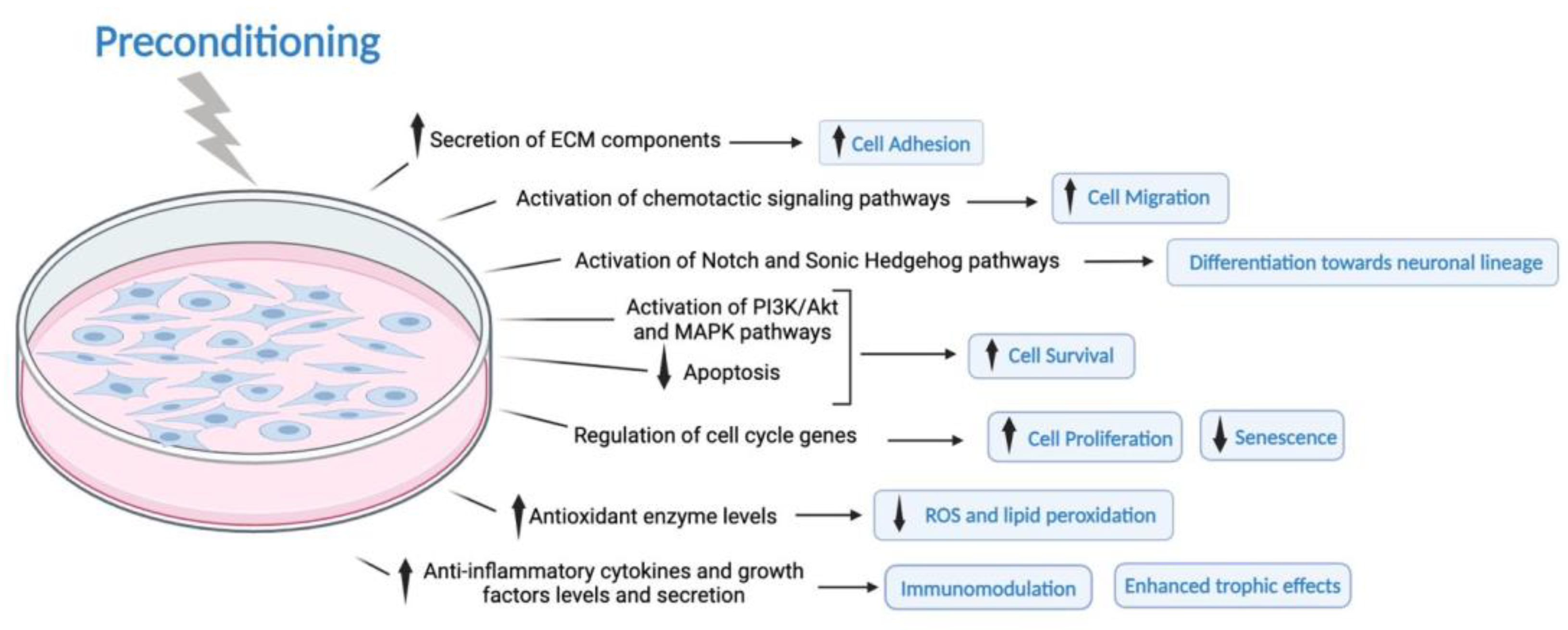
3.1. Effect of the Preconditioning Method on the MSCs Phenotype and Characteristics
| Ref. | Type of Preconditioning | Preconditioning | Pathology | Type of Study | Source | Administration Time (dpi) | Duration (h) | Dose Quantity Intensity |
|---|---|---|---|---|---|---|---|---|
| [10] | Biomechanical Forces | Microfluidic devices | Traumatic Brain Injury | in vitro and in vivo | Human BM | 1 or 3 | 3, 6 or 8 | 15 dyne/cm2 |
| [11] | Chemical substances | Curcumin | Ischemia and reperfusion | in vitro | Rat BM | - | 2 | 1, 5, 10 or 20 μM |
| [12] | Chemical substances | Astaxanthin | Ischemic stroke | in vitro | Human AD | - | ? | 2–128 μM |
| [13] | Chemical substances | Calpain inhibitor (MDL28170) and hypoxia cm or tunicamycin | Spinal Cord Injury | in vitro and in vivo | Rat BM | 7 | 1 MDL28170 and 24 hypoxia cm or tunicamycin | 1–10 µM MDL28170 and hypoxia cm (0%) or 0, 1, 3, 10 mg/mL tunicamycin |
| [14] | Chemical substances | Roxadustat | Ischemic stroke | in vitro and in vivo | Rat BM | 1 | 24 | 10 μmol/L |
| [15] | Chemical substances | Fasudil | Spinal Cord Injury | in vitro and in vivo | Rat BM | 7 | 12, 24, 36, 48, and 72 | 3, 10, 30, and 100 μmol/L |
| [16] | Chemical substances | Isoflurane | Ischemic stroke | in vitro and in vivo | Rat BM | - | 2, 4, 6, 12, and 24 | 1–10% |
| [17] | Chemical substances | Artemisinin | Ischemic stroke | in vitro | Rat BM | - | 24 | 0.1–100 μM |
| [18] | Chemical substances | Sevoflurane | Ischemia and reperfusion | in vitro | Rat BM | - | 2 | 2% |
| [19] | Chemical substances | Hydrogen sulfide | Ischemic stroke | in vitro and in vivo | Rat BM | 1 | 5, 15, 30, 60, 120, and 240 min | 0.1, 0.5, 1, 5, 10 and 50 μM in vitro and 1 μM in vivo |
| [20] | Chemical substances | Modulation of autophagy with rapamycin and 3-MA | N/A | in vitro | Human AD | - | 1, 4, 12, 24, and 48 | Rapa 500 nM or 3-MA 5 mM |
| [21] | Chemical substances | Rosmarinic acid | Ischemic stroke | in vitro | Rat AD | - | 4 or 24 | 0.2–6 μM |
| [22] | Chemical substances | Lycopene and hypoxia | Ischemic stroke | in vitro | Mouse BM | - | 1 with lycopene and 6 of hypoxia with lycopene | 1 h with lycopene (0, 1, 2, 5, 10, 20, 50 µM) and 6 h of lycopene with/without 20 µM LY294002 (PI3K/Akt inhibitors) |
| [23] | Culture scaffolds/3D culture | Graphene Oxide-Substrate | Peripheral nerve injury | in vitro | Human AD | - | 72 | - |
| [24] | Culture scaffolds/3D culture | Encapsulated in 3D hydrogels derived from human fibrin or platelet lysate | N/A | in vitro | Human Wharton’s Jelly | - | Duration of the culture | - |
| [25] | Different culture supplementation | Platelet lysate and G-CSF | Ischemic stroke | in vitro and in vivo | Human BM | 7 | ? | 5% HPL + 0.1 μM G-CSF |
| [26] | Different culture supplementation | Growth medium with neuregulin1-beta1, bFGF, PDGF-AA and forskolin | Peripheral nerve injury | in vitro and in vivo | Human AD | 0 | 2 weeks | 200 ng/mL neuregulin1-beta1, 10 ng/mL bFGF, 5 ng/mL PDGF-AA, and 14 mM forskolin |
| [27] | Different culture supplementation | bFGF, B27 and kanamycin | N/A | in vitro | Human AD and UC | - | 7 days | 0.1 to mg/mL |
| [9] | Different oxygen pressure | Hypoxia | Ischemic stroke | in vitro and in vivo | Rat BM | 1 | 0, 4, 8, 12, and 24 | 1% O2 |
| [28] | Different oxygen pressure | Hypoxia | Ischemia and reperfusion | in vitro | Rat BM | - | 24 | 1% O2 |
| [29] | Different oxygen pressure | Hypoxia | Ischemic stroke | in vitro | Human BM | 0 | 24 | 1% O2 |
| [30] | Different oxygen pressure | Hypoxia | Ischemic stroke | in vitro | Mouse BM | - | 24 | 0.5% O2 |
| [31] | Different oxygen pressure | Hypoxia | Ischemic stroke | in vitro and in vivo | Rat BM | 1 | 24, 48, 72 | 0.5% O2 |
| [32] | Different oxygen pressure | Hypoxia | Spinal Cord Injury | in vitro and in vivo | Rat BM | 0 | 48 or 72 | 1% O2 |
| [24] | Different oxygen pressure | Physioxia | N/A | in vitro | Human Wharton’s Jelly | - | Duration of the culture | 5% O2 |
| [33] | Different oxygen pressure | Hypoxia | N/A | in vitro | Human UC | - | 24 | 1% O2 |
| [34] | Different oxygen pressure | Hypoxia | N/A | in vitro | Canine BM | - | 6, 12, and 24 | 1% O2 |
| [35] | Exposure to lesion mediators | Cerebral tissue extracts from TBI rats | Traumatic Brain Injury | in vitro | Human BM | - | - | 20% TBI tissue extract supernatant |
| [36] | Exposure to lesion mediators | Stroke patient serum | Ischemic stroke | in vitro and in vivo | Human BM | 1 | - | 10% |
| [37] | Exposure to lesion mediators | SCI patient plasma | Spinal Cord Injury | in vitro and in vivo | Human BM | 0 and once a week for 8 weeks | Duration of the culture | 10% |
| [38] | Exposure to lesion mediators | Activated microglia | Ischemic stroke | in vitro | Rat BM | - | 24 | ? |
| [39] | Inflammatory factors | IL-1α, IL-1β, TNF-α or IFN-γ | N/A | in vitro | Human BM | - | 24 | 1, 10, 50 or 100 ng/mL |
| [40] | Inflammatory factors | Recombinant human IFN-γ | Periventricular leukomalacia | in vitro and in vivo | Human UC | 0 | 24 | 10 ng/mL |
| [41] | Ultrasound and magnetic fields | Electromagnetic field | N/A | in vitro | Human BM | - | 24, 72, 120 | 60 Hz |
| [42] | Ultrasound and magnetic fields | Low intensity pulsed ultrasound | Spinal Cord Injury | in vitro and in vivo | Rat BM | 7 | 72 | 10, 30, 50, 70 mW/cm2, 3 min/d |
| [43] | Ultrasound and magnetic fields | Low frequency pulsed electromagnetic field | Crush-injured nerve | in vitro and in vivo | Rat BM | 0 | 1 | 50 Hz, 1 mT |
3.1.1. Extrinsic Factors
Chemical Substances
Inflammatory Factors
Ultrasounds and Electromagnetic Fields
Manipulation of Cell Culture Supplementation
Exposure to Lesion Mediators
3.1.2. Low Oxygen Pressure
3.1.3. Culture Scaffolds/3D Culture/Biomechanical Forces
3.2. Effect of the Preconditioning Method on the Therapeutic Potential of MSCs to Treat Ischemic Brain Conditions
| Ref. | Type of Preconditioning | Preconditioning | Pathology | Type of Study | Species | Source | Administration Time (dpi) | MSC/Secretome Dose | Duration (h) | Dose Quantity Intensity | ↓ Lesion Extension (vs. Naive) | Functional Improv. (vs. Naive) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [14] | Chemical substances | Roxadustat | Ischemic stroke | in vitro and in vivo | Adult male Sprague–Dawley rats | Rat BM | 1 | 5 × 105 cells | 24 | 10 μmol/L |  | n.e. |
| [16] | Chemical substances | Isoflurane | Ischemic stroke | in vitro and in vivo | Rats | Rat BM | - | 2 × 106 cells | 2, 4, 6, 12 and 24 | 1–10% | n.e. |  |
| [19] | Chemical substances | Hydrogen sulfide donor | Ischemic stroke | in vitro and in vivo | Adult male Wistar rats | Rat BM | 1 | 2 × 106 cells | 5, 15, 30, 60, 120 and 240 min | 0.1, 0.5, 1, 5, 10 and 50 μM in vitro and 1 μM in vivo |  |  |
| [46] | Chemical substances and growth factors | Deferoxamine | Perinatal Asphyxia | in vivo | Adult female Wistar rats | Human AD | 2 h after birth and P7 | 16 µL of secretome (containing 6 µg of protein from 2 × 105 MSCs) | 48 | 400 µM | n.e. | n.e. |
| [25] | Different culture supplementation | HPL and G-CSF | Ischemic stroke | in vitro and in vivo | Adult male Sprague–Dawley rats | Human BM | 7 | 5 × 105 cells | ? | 5% HPL + 0.1 μM G-CSF |  | n.e. |
| [47] | Different oxygen pressure | Hypoxia | Ischemic stroke | in vivo | Adult male Sprague–Dawley rats | Rat BM | 0.5 and every 2 days for 28 days | CM from MSC cultured in 80% confluence | 12 | 3% O2 |  |  |
| [9] | Different oxygen pressure | Hypoxia | Ischemic stroke | in vitro and in vivo | Adult male Sprague–Dawley rats | Rat BM | 1 | 2 × 106 cells | 0, 4, 8, 12, and 24 | 1% O2 |  |  |
| [48] | Different oxygen pressure | Hypoxia | Hemorrhagic stroke | in vivo | Adult male C57BL/6 mice | Rat BM | 3 and 7 | 106 cells | 24 | 0.1–0.3% | n.e. | n.e. |
| [31] | Different oxygen pressure | Hypoxia | Ischemic stroke | in vitro and in vivo | Adult male Wistar rats | Rat BM | 1 | 106 cells | 24, 48, 72 | 0.5% O2 | n.e. |  |
| [49] | Different oxygen pressure | Hypoxia | Ischemic stroke | in vivo | Adult male C57BL/6 mice | Rat BM | 3, once a day for 3 days | 106 cells | 24 | 0.1–0.3% | n.e. | n.e. |
| [50] | Different oxygen pressure | Hypoxia | Ischemic stroke | in vivo | Adult male C57BL/6 mice | Rat BM | 1 | 106 cells | 24 | 0.1–0.3% |  | n.e. |
| [51] | Different oxygen pressure | Hypoxia | Neonatal stroke | in vivo | P7 male Wistar rats | Rat BM | 6 h | 106 cells | 24 | 0.1–0.3% | n.e. | n.e. |
| [36] | Exposure to lesion mediators | Stroke patient serum | Ischemic stroke | in vitro and in vivo | Adult male Sprague–Dawley rats | Human BM | 1 | 2 × 106 cells | - | 10% |  |  |
| [40] | Inflammatory factors | Recombinant human IFN-γ | Periventricular leukomalacia | in vitro and in vivo | P4 Sprague Dawley rats | Human UC | 0 | 106 cells | 24 | 10 ng/mL |  | n.e. |
| [46] | Inflammatory factors | TNF-α+IFN-γ | Perinatal Asphyxia | in vivo | Adult female Wistar rats | Human AD | 2 h after birth and P7 | 16 µL of secretome (containing 6 µg of protein from 2 × 105 MSCs) | 48 | 10 ng/mL TNF-α and 15 ng/mL IFN-γ | n.e. | n.e. |
 —yes;
—yes;  —no; ↓—decrease; ?—not reported.
—no; ↓—decrease; ?—not reported.3.2.1. Manipulation of Cell Culture Supplementation
3.2.2. Extrinsic Factors
3.2.3. Different Oxygen Pressure
3.3. Effect of the Preconditioning Method on the Therapeutic Potential of MSCs for SCI and Other Traumatic Injuries Affecting the Nervous System
| Ref. | Type of Preconditioning | Preconditioning | Pathology | Type of Study | Species | Source | Administration Time (dpi) | MSC/Secretome Dose | Duration (h) | Dose Quantity Intensity | ↓ Lesion Extension (vs. Naive) | Functional Improv. (vs. Naive) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [10] | Biomecha-nical forces | Microfluidic devices | Traumatic Brain Injury | in vitro and in vivo | Adult male Sprague–Dawley rats | Human BM | 1 or 3 | 107 cells/kg | 3, 6 or 8 | 15 dyne/cm2 |  | n.e. |
| [55] | Chemical substances | All-Trans Retinoic Acid | Spinal Cord Injury | in vivo | Adult male Wistar rats | Mouse BM | 1 | 3 × 105 cells | 24 | 1 µM ATRA |  |  |
| [13] | Chemical substances | Calpain inhibitor (MDL28170) and hypoxia cm or tunicamycin | Spinal Cord Injury | in vitro and in vivo | Adult male Sprague–Dawley rats | Rat BM | 7 | 106 cells | 1 MDL28170 and 24 hypoxia- CM or tunicamycin | 1–10 µM MDL28170 and hypoxia cm (0%) or 0, 1, 3, 10 mg/mL tunicamycin | n.e. |  |
| [15] | Chemical substances | Fasudil | Spinal Cord Injury | in vitro and in vivo | Adult female Sprague Dawley rats | Rat BM | 7 | - | 12, 24, 36, 48, and 72 | 3, 10, 30, and 100 μmol/L |  | n.e. |
| [56] | Chemical substances | Melatonin | Spinal Cord Injury | in vivo | Adult male Sprague–Dawley rats | Mouse AD | 7 | Not specified | 24 | 5 μM |  |  |
| [57] | Chemical substances | Calpain inhibitor | Traumatic Brain Injury | in vivo | Adult male Sprague–Dawley rats | Rat BM | 1 | 105 cells | - | 1.0 μL of 50 mM |  |  |
| [58] | Chemical substances | Valproic acid and AMD3100 | Spinal Cord Injury | in vivo | Adult male Sprague–Dawley rats | Human BM | 7 | 106 cells | 3 for valproic acid and 6 for AMD3100 | 2.5 mmol/L of valproic acid and 20 umol/L of AMD3100 |  |  |
| [59] | Culture scaffolds/3D culture | 3D-printed collagen/silk fibroin/secretome derived from bFGF-pretreated MSCs | Traumatic Brain Injury | in vivo | Dogs | Human UC | 0 | Not specified | bFGF 24 | N/A |  |  |
| [60] | Culture scaffolds/3D culture | Collagen scaffold | Spinal Cord Injury | in vivo and clinical trial | Adult female Sprague–Dawley rats, female beagle canines aged 1 year old, and forty patients | Human UC | 0 | 106 cells in rats, 107 cells in beagles, and 4 × 107 cells in humans | 7 days | - | n.e. | n.e. |
| [26] | Different culture supplementation | Growth medium with neuregulin1-beta1, bFGF, PDGF-AA, and forskolin | Peripheral nerve injury | in vitro and in vivo | Adult female Sprague Dawley rats | Human AD | 0 | 2 × 106 cells | 2 weeks | 200 ng/mL neuregulin1-beta1, 10 ng/mL bFGF, 5 ng/mL PDGF-AA, and 14 mM forskolin | no | n.e. |
| [61] | Different oxygen pressure | Hypoxia | Traumatic Brain Injury | in vivo | Adult male C57BL/6 mice | Mouse BM | 1 | 2 × 106 cells | 8 | 1% O2 |  |  |
| [32] | Different oxygen pressure | Hypoxia | Spinal Cord Injury | in vitro and in vivo | Adult male Sprague–Dawley rats | Rat BM | 0 | 2 × 106 cells | 48 or 72 | 1% O2 | n.e. | n.e. |
| [62] | Different oxygen pressure | Hypoxia | Traumatic Brain Injury | in vivo | Adult male C57BL/6 mice | Mouse BM | 0.5 for 3 days | CM from 2 × 106 cells | 24 | 0.5% O2 |  |  |
| [63] | Different oxygen pressure | Hypoxia | Traumatic Brain Injury | in vivo | Adult male Sprague–Dawley rats | Human AD | 7 | Secretome (Not specified) | 24 | 5% O2 | n.e. | n.e. |
| [64] | Different oxygen pressure | Hypoxia | Spinal Cord Injury | in vivo | Adult male Sprague–Dawley rats | Rat BM | 2 prior to ischemia/reperfusion | 5 × 105 cells | 24 | 3% O2 |  |  |
| [65] | Exposure to lesion mediators | TBI tissue extracts | Traumatic Brain Injury | in vivo | Adult male Sprague–Dawley rats | Human UC | 0 | CM from 106 cells | 24 | ? |  |  |
| [37] | Exposure to lesion mediators | SCI patient plasma | Spinal Cord Injury | in vitro and in vivo | Adult female Sprague Dawley rats | Human BM | 0 and once a week for 8 weeks | CM from 106 cells | Duration of the culture | 10% | n.e. |  |
| [42] | Ultrasound and magnetic fields | Low-intensity pulsed ultrasound | Spinal Cord Injury | in vitro and in vivo | Adult female Wistar rats | Rat BM | 7 | 5 × 105 cells | 72 | 10, 30, 50, 70 mW/cm2, 3 min/d |  |  |
| [43] | Ultrasound and magnetic fields | Low-frequency pulsed electromagnetic field | Crush-injured nerve | in vitro and in vivo | Adult male Sprague–Dawley rats | Rat BM | 0 | 106 cells | 1 | 50 Hz, 1 mT |  | n.e. |
 —yes;
—yes;  —no; ↓—decrease; ?—not reported.
—no; ↓—decrease; ?—not reported.3.3.1. Extrinsic Factors
3.3.2. Differential Oxygen Pressure
3.3.3. Culture Scaffolds and Biomechanical Forces
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3-MA | 3-Methyladenine |
| AD | adipose tissue |
| ATRA | all trans retinoic acid |
| BDNF | brain-derived neurotrophic factor |
| bFGF | basic fibroblast growth factor |
| BM | bone marrow |
| CM | conditioned medium |
| COX2 | cyclooxygenase-2 |
| DFX | deferoxamine |
| DFX-MSCs | MSCs incubated with DFX |
| EGF | endothelial growth factor |
| EPO | erythropoietin |
| G-CSF | granulocyte-colony stimulating factor |
| GDNF | glial cell-derived neurotrophic factor |
| GFAP | Glial Fibrillary Acidic Protein |
| HIF | hypoxia-inducible factor |
| HP | hypoxia preconditioning |
| HP-MSCs | MSCs subjected to HP |
| Iba1 | ionized calcium binding adaptor molecule 1 |
| IFN | interferon |
| IFN-γ-UC-MSCs | UC-MSCs stimulated with IFN-γ |
| IL | interleukin |
| LDH | lactate dehydrogenase |
| LPS | liposaccharide |
| MAP-2 | microtubule-associated protein 2 |
| MCAO | middle cerebral artery occlusion |
| mNSS | modified neurological severity score |
| MSCs | mesenchymal stem cells |
| NGF | neural growth factor |
| NRF2 | Nuclear Erythroid 2-Related Factor 2 |
| NRLM-MSCs | MSCs incubated with bFGF, EGF, plasma from SCI patients |
| OGD | oxygen and glucose deprivation |
| PD | placenta-derived |
| PI3K | phosphoinositide-3 kinase |
| PL | platelet lysate |
| ROS | reactive oxygen species |
| SCI | spinal cord injury |
| TBI | traumatic brain injury |
| TGF | transforming growth factor |
| TNF | tumor necrosis factor |
| UC | umbilical cord |
| UC-MSCs-CM | CM from naive UC-MSCs |
| VEGF | vascular endothelial growth factor |
References
- Alessandrini, M.; Preynat-Seauve, O.; De Bruin, K.; Pepper, M.S. Stem cell therapy for neurological disorders. S. Afr. Med. J. 2019, 109, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef] [PubMed]
- Skok, M. Mesenchymal stem cells as a potential therapeutic tool to cure cognitive impairment caused by neuroinflammation. World J. Stem Cells 2021, 13, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Chang, Y.H.; Shyu, W.C.; Lin, S.Z. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplant. 2015, 24, 339–347. [Google Scholar] [CrossRef]
- Li, Y.; Hu, G.; Cheng, Q. Implantation of human umbilical cord mesenchymal stem cells for ischemic stroke: Perspectives and challenges. Front. Med. 2015, 9, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Domingues, C.; Geraldo, A.M.; Anjo, S.I.; Matos, A.; Almeida, C.; Caramelo, I.; Lopes-da-Silva, J.A.; Paiva, A.; Carvalho, J.; Pires das Neves, R.; et al. Cofilin-1 Is a Mechanosensitive Regulator of Transcription. Front. Cell Dev. Biol. 2020, 8, 678. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.G.; Panchalingam, K.M.; Anjo, S.I.; Manadas, B.; Pereira, R.; Sousa, N.; Salgado, A.J.; Behie, L.A. Do hypoxia/normoxia culturing conditions change the neuroregulatory profile of Wharton Jelly mesenchymal stem cell secretome? Stem Cell Res. Ther. 2015, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, Y.; Shen, L.; Ding, W.; Chen, X.; Wu, E.; Cai, K.; Wang, G. Hypoxic Preconditioning Augments the Therapeutic Efficacy of Bone Marrow Stromal Cells in a Rat Ischemic Stroke Model. Cell. Mol. Neurobiol. 2017, 37, 1115–1129. [Google Scholar] [CrossRef]
- Diaz, M.F.; Vaidya, A.B.; Evans, S.M.; Lee, H.J.; Aertker, B.M.; Alexander, A.J.; Price, K.M.; Ozuna, J.A.; Liao, G.P.; Aroom, K.R.; et al. Biomechanical Forces Promote Immune Regulatory Function of Bone Marrow Mesenchymal Stromal Cells. Stem Cells 2017, 35, 1259–1272. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Yang, Y.; Zhang, W.; Luo, L.; Han, F.; Guan, H.; Tao, K.; Hu, D. Curcumin pretreatment protects against hypoxia/reoxgenation injury via improvement of mitochondrial function, destabilization of HIF-1α and activation of Epac1-Akt pathway in rat bone marrow mesenchymal stem cells. Biomed. Pharmacother. 2019, 109, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Barzegari, A.; Dehnad, A.; Barar, J.; Omidi, Y. Astaxanthin protects mesenchymal stem cells from oxidative stress by direct scavenging of free radicals and modulation of cell signaling. Chem. Biol. Interact. 2021, 333, 109324. [Google Scholar] [CrossRef]
- Wang, C.; Shi, D.; Song, X.; Chen, Y.; Wang, L.; Zhang, X. Calpain inhibitor attenuates ER stress-induced apoptosis in injured spinal cord after bone mesenchymal stem cells transplantation. Neurochem. Int. 2016, 97, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, X.; Yao, C.; Bingwa, L.A.; Wang, H.; Lin, Z.; Jin, K.; Zhuge, Q.; Yang, S. Transplantation of Roxadustat-preconditioned bone marrow stromal cells improves neurological function recovery through enhancing grafted cell survival in ischemic stroke rats. CNS Neurosci. Ther. 2022, 28, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; He, J.; Chen, M.; Luo, D.; Lin, D. Fasudil Promotes BMSC Migration via Activating the MAPK Signaling Pathway and Application in a Model of Spinal Cord Injury. Stem Cells Int. 2018, 2018, 9793845. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Q.F.; Yan, J.; Hu, R.; Jiang, H. Isoflurane Preconditioning Promotes the Survival and Migration of Bone Marrow Stromal Cells. Cell. Physiol. Biochem. 2015, 36, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhao, X.; Li, S.; Xing, X.; Wang, H.; Lazarovici, P.; Zheng, W. Protective mechanism of artemisinin on rat bone marrow-derived mesenchymal stem cells against apoptosis induced by hydrogen peroxide via activation of c-Raf-Erk1/2-p90(rsk)-CREB pathway. Stem Cell Res. Ther. 2019, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Fang, B.; Zhao, X.; Zhang, G.; Ma, H. Preconditioning of mesenchymal stem cells by sevoflurane to improve their therapeutic potential. PLoS ONE 2014, 9, e90667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, S.; Li, T.; Yuan, L.; Liu, H.; Wang, X.; Wang, F.; Wang, S.; Hao, A.; Liu, D.; et al. Preconditioning of bone marrow mesenchymal stem cells with hydrogen sulfide improves their therapeutic potential. Oncotarget 2016, 7, 58089–58104. [Google Scholar] [CrossRef]
- Wise, R.M.; Al-Ghadban, S.; Harrison, M.A.A.; Sullivan, B.N.; Monaco, E.R.; Aleman, S.J.; Donato, U.M.; Bunnell, B.A. Short-Term Autophagy Preconditioning Upregulates the Expression of COX2 and PGE2 and Alters the Immune Phenotype of Human Adipose-Derived Stem Cells In Vitro. Cells 2022, 11, 1376. [Google Scholar] [CrossRef]
- Ghorbani, A.; Sadeghnia, H.R.; Afshari, A.R.; Hosseini, A. Rosmarinic Acid Protects Adipose Tissue-Derived Mesenchymal Stem Cells in Nutrient-Deficient Conditions. Prev. Nutr. Food Sci. 2019, 24, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xue, F.; Xu, S.Z.; Wang, X.W.; Tong, X.; Lin, X.J. Lycopene protects bone marrow mesenchymal stem cells against ischemia-induced apoptosis in vitro. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1625–1631. [Google Scholar] [PubMed]
- Llewellyn, S.H.; Faroni, A.; Iliut, M.; Bartlam, C.; Vijayaraghavan, A.; Reid, A.J. Graphene Oxide Substrate Promotes Neurotrophic Factor Secretion and Survival of Human Schwann-Like Adipose Mesenchymal Stromal Cells. Adv. Biol. 2021, 5, e2000271. [Google Scholar] [CrossRef] [PubMed]
- Lech, W.; Sarnowska, A.; Kuczynska, Z.; Dabrowski, F.; Figiel-Dabrowska, A.; Domanska-Janik, K.; Buzanska, L.; Zychowicz, M. Biomimetic microenvironmental preconditioning enhance neuroprotective properties of human mesenchymal stem cells derived from Wharton’s Jelly (WJ-MSCs). Sci. Rep. 2020, 10, 16946. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Saito, H.; Ito, M.; Shichinohe, H.; Houkin, K.; Kuroda, S. Platelet lysate and granulocyte-colony stimulating factor serve safe and accelerated expansion of human bone marrow stromal cells for stroke therapy. Transl. Stroke Res. 2014, 5, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Kingham, P.J.; Kolar, M.K.; Novikova, L.N.; Novikov, L.N.; Wiberg, M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 2014, 23, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.A.; Fraga, J.S.; Grãos, M.; Neves, N.M.; Reis, R.L.; Gimble, J.M.; Sousa, N.; Salgado, A.J. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res. Ther. 2012, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xu, Z.; Qu, G.; Wang, H.; Lin, L.; Li, X.; Xie, X.; Lei, Y.; He, X.; Chen, Y.; et al. Hypoxic Preconditioning Enhances the Efficacy of Mesenchymal Stem Cells-Derived Conditioned Medium in Switching Microglia toward Anti-inflammatory Polarization in Ischemia/Reperfusion. Cell. Mol. Neurobiol. 2021, 41, 505–524. [Google Scholar] [CrossRef]
- Kim, Y.S.; Noh, M.Y.; Cho, K.A.; Kim, H.; Kwon, M.S.; Kim, K.S.; Kim, J.; Koh, S.H.; Kim, S.H. Hypoxia/Reoxygenation-Preconditioned Human Bone Marrow-Derived Mesenchymal Stromal Cells Rescue Ischemic Rat Cortical Neurons by Enhancing Trophic Factor Release. Mol. Neurobiol. 2015, 52, 792–803. [Google Scholar] [CrossRef]
- Theus, M.H.; Wei, L.; Cui, L.; Francis, K.; Hu, X.; Keogh, C.; Yu, S.P. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp. Neurol. 2008, 210, 656–670. [Google Scholar] [CrossRef]
- Wei, L.; Fraser, J.L.; Lu, Z.Y.; Hu, X.; Yu, S.P. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol. Dis. 2012, 46, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wu, F.; Xue, E.; Huang, L.; Yan, P.; Pan, X.; Zhou, Y. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival by inducing HIF-1α in injured neuronal cells derived exosomes culture system. Cell Death Dis. 2019, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.M.; Klose, K.; Bieback, K.; Korinth, D.; Schneider, M.; Seifert, M.; Choi, Y.H.; Kurtz, A.; Falk, V.; Stamm, C. Hypoxic Preconditioning Increases Survival and Pro-Angiogenic Capacity of Human Cord Blood Mesenchymal Stromal Cells In Vitro. PLoS ONE 2015, 10, e0138477. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, X.L.; Cheng, Q.G.; Lu, S.S.; Xu, X.Q.; Zu, Q.Q.; Liu, S. G-CSF and hypoxic conditioning improve the proliferation, neural differentiation and migration of canine bone marrow mesenchymal stem cells. Exp. Ther. Med. 2016, 12, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Katakowski, M.; Li, Y.; Lu, D.; Wang, L.; Zhang, L.; Chen, J.; Xu, Y.; Gautam, S.; Mahmood, A.; et al. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: Growth factor production. J. Neurosci. Res. 2002, 69, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Moon, G.J.; Cho, Y.H.; Kim, D.H.; Sung, J.H.; Son, J.P.; Kim, S.; Cha, J.M.; Bang, O.Y. Serum-mediated Activation of Bone Marrow-derived Mesenchymal Stem Cells in Ischemic Stroke Patients: A Novel Preconditioning Method. Cell Transplant. 2018, 27, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Tsai, M.J.; Hsieh, N.; Lo, M.J.; Lee, M.J.; Cheng, H.; Huang, W.C. The superiority of conditioned medium derived from rapidly expanded mesenchymal stem cells for neural repair. Stem Cell Res. Ther. 2019, 10, 390. [Google Scholar] [CrossRef]
- Lv, B.; Li, F.; Fang, J.; Xu, L.; Sun, C.; Han, J.; Hua, T.; Zhang, Z.; Feng, Z.; Wang, Q.; et al. Activated Microglia Induce Bone Marrow Mesenchymal Stem Cells to Produce Glial Cell-Derived Neurotrophic Factor and Protect Neurons Against Oxygen-Glucose Deprivation Injury. Front. Cell. Neurosci. 2016, 10, 283. [Google Scholar] [CrossRef]
- Redondo-Castro, E.; Cunningham, C.; Miller, J.; Martuscelli, L.; Aoulad-Ali, S.; Rothwell, N.J.; Kielty, C.M.; Allan, S.M.; Pinteaux, E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res. Ther. 2017, 8, 79. [Google Scholar] [CrossRef]
- Morioka, C.; Komaki, M.; Taki, A.; Honda, I.; Yokoyama, N.; Iwasaki, K.; Iseki, S.; Morio, T.; Morita, I. Neuroprotective effects of human umbilical cord-derived mesenchymal stem cells on periventricular leukomalacia-like brain injury in neonatal rats. Inflamm. Regen. 2017, 37, 1. [Google Scholar] [CrossRef]
- Urnukhsaikhan, E.; Cho, H.; Mishig-Ochir, T.; Seo, Y.K.; Park, J.K. Pulsed electromagnetic fields promote survival and neuronal differentiation of human BM-MSCs. Life Sci. 2016, 151, 130–138. [Google Scholar] [CrossRef]
- Ning, G.Z.; Song, W.Y.; Xu, H.; Zhu, R.S.; Wu, Q.L.; Wu, Y.; Zhu, S.B.; Li, J.Q.; Wang, M.; Qu, Z.G.; et al. Bone marrow mesenchymal stem cells stimulated with low-intensity pulsed ultrasound: Better choice of transplantation treatment for spinal cord injury: Treatment for SCI by LIPUS-BMSCs transplantation. CNS Neurosci. Ther. 2019, 25, 496–508. [Google Scholar] [CrossRef]
- Seo, N.; Lee, S.H.; Ju, K.W.; Woo, J.; Kim, B.; Kim, S.; Jahng, J.W.; Lee, J.H. Low-frequency pulsed electromagnetic field pretreated bone marrow-derived mesenchymal stem cells promote the regeneration of crush-injured rat mental nerve. Neural Regen. Res. 2018, 13, 145–153. [Google Scholar] [CrossRef]
- Ding, Q.; Wang, Q.; Deng, J.; Gu, Q.; Hu, S.; Li, Y.; Su, B.; Zeng, Y.; Xiong, L. Sevoflurane preconditioning induces rapid ischemic tolerance against spinal cord ischemia/reperfusion through activation of extracellular signal-regulated kinase in rabbits. Anesth. Analg. 2009, 109, 1263–1272. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, C.S.; Fu, J. Forcing stem cells to behave: A biophysical perspective of the cellular microenvironment. Annu. Rev. Biophys. 2012, 41, 519–542. [Google Scholar] [CrossRef]
- Farfán, N.; Carril, J.; Redel, M.; Zamorano, M.; Araya, M.; Monzón, E.; Alvarado, R.; Contreras, N.; Tapia-Bustos, A.; Quintanilla, M.E.; et al. Intranasal Administration of Mesenchymal Stem Cell Secretome Reduces Hippocampal Oxidative Stress, Neuroinflammation and Cell Death, Improving the Behavioral Outcome Following Perinatal Asphyxia. Int. J. Mol. Sci. 2020, 21, 7800. [Google Scholar] [CrossRef]
- Jiang, R.H.; Wu, C.J.; Xu, X.Q.; Lu, S.S.; Zu, Q.Q.; Zhao, L.B.; Wang, J.; Liu, S.; Shi, H.B. Hypoxic conditioned medium derived from bone marrow mesenchymal stromal cells protects against ischemic stroke in rats. J. Cell. Physiol. 2019, 234, 1354–1368. [Google Scholar] [CrossRef]
- Sun, J.; Wei, Z.Z.; Gu, X.; Zhang, J.Y.; Zhang, Y.; Li, J.; Wei, L. Intranasal delivery of hypoxia-preconditioned bone marrow-derived mesenchymal stem cells enhanced regenerative effects after intracerebral hemorrhagic stroke in mice. Exp. Neurol. 2015, 272, 78–87. [Google Scholar] [CrossRef]
- Chau, M.J.; Deveau, T.C.; Gu, X.; Kim, Y.S.; Xu, Y.; Yu, S.P.; Wei, L. Delayed and repeated intranasal delivery of bone marrow stromal cells increases regeneration and functional recovery after ischemic stroke in mice. BMC Neurosci. 2018, 19, 20. [Google Scholar] [CrossRef]
- Wei, N.; Yu, S.P.; Gu, X.; Taylor, T.M.; Song, D.; Liu, X.F.; Wei, L. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant. 2013, 22, 977–991. [Google Scholar] [CrossRef]
- Wei, Z.Z.; Gu, X.; Ferdinand, A.; Lee, J.H.; Ji, X.; Ji, X.M.; Yu, S.P.; Wei, L. Intranasal delivery of bone marrow mesenchymal stem cells improved neurovascular regeneration and rescued neuropsychiatric deficits after neonatal stroke in rats. Cell Transplant. 2015, 24, 391–402. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Kimura, H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem. Int. 2013, 63, 492–497. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Z.; Zhan, J.; Zhang, Q.; Wang, J.; Zhang, Q.; Xian, X.; Luan, Q.; Hao, A. Hydrogen sulfide promotes proliferation and neuronal differentiation of neural stem cells and protects hypoxia-induced decrease in hippocampal neurogenesis. Pharmacol. Biochem. Behav. 2014, 116, 55–63. [Google Scholar] [CrossRef]
- Gholaminejhad, M.; Jameie, S.B.; Abdi, M.; Abolhassani, F.; Mohammed, I.; Hassanzadeh, G. All-Trans Retinoic Acid-Preconditioned Mesenchymal Stem Cells Improve Motor Function and Alleviate Tissue Damage After Spinal Cord Injury by Inhibition of HMGB1/NF-κB/NLRP3 Pathway Through Autophagy Activation. J. Mol. Neurosci. 2022, 72, 947–962. [Google Scholar] [CrossRef]
- Naeimi, A.; Zaminy, A.; Amini, N.; Balabandi, R.; Golipoor, Z. Effects of melatonin-pretreated adipose-derived mesenchymal stem cells (MSC) in an animal model of spinal cord injury. BMC Neurosci. 2022, 23, 65. [Google Scholar] [CrossRef]
- Hu, J.; Chen, L.; Huang, X.; Wu, K.; Ding, S.; Wang, W.; Wang, B.; Smith, C.; Ren, C.; Ni, H.; et al. Calpain inhibitor MDL28170 improves the transplantation-mediated therapeutic effect of bone marrow-derived mesenchymal stem cells following traumatic brain injury. Stem Cell Res. Ther. 2019, 10, 96. [Google Scholar] [CrossRef]
- Chen, L.; Cui, X.; Wu, Z.; Jia, L.; Yu, Y.; Zhou, Q.; Hu, X.; Xu, W.; Luo, D.; Liu, J.; et al. Transplantation of bone marrow mesenchymal stem cells pretreated with valproic acid in rats with an acute spinal cord injury. Biosci. Trends 2014, 8, 111–119. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Wei, P.; Hao, L.; Zhong, L.; Zhong, K.; Liu, C.; Liu, P.; Feng, Q.; Wang, S.; et al. 3D-printed collagen/silk fibroin/secretome derived from bFGF-pretreated HUCMSCs scaffolds enhanced therapeutic ability in canines traumatic brain injury model. Front. Bioeng. Biotechnol. 2022, 10, 995099. [Google Scholar] [CrossRef]
- Deng, W.S.; Ma, K.; Liang, B.; Liu, X.Y.; Xu, H.Y.; Zhang, J.; Shi, H.Y.; Sun, H.T.; Chen, X.Y.; Zhang, S. Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regen. Res. 2020, 15, 1686–1700. [Google Scholar] [CrossRef]
- Yuan, X.; Luo, Q.; Shen, L.; Chen, J.; Gan, D.; Sun, Y.; Ding, L.; Wang, G. Hypoxic preconditioning enhances the differentiation of bone marrow stromal cells into mature oligodendrocytes via the mTOR/HIF-1α/VEGF pathway in traumatic brain injury. Brain Behav. 2020, 10, e01675. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Chio, C.C.; Cheong, C.U.; Chao, C.M.; Cheng, B.C.; Lin, M.T. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin. Sci. 2013, 124, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Diao, Y.F.; Wang, J.; Liang, J.; Xu, H.H.; Zhao, M.L.; Zheng, B.; Luan, Z.; Wang, J.J.; Yang, X.P.; et al. Intravenously Infusing the Secretome of Adipose-Derived Mesenchymal Stem Cells Ameliorates Neuroinflammation and Neurological Functioning After Traumatic Brain Injury. Stem Cells Dev. 2020, 29, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fang, B.; Tan, Z.; Zhang, D.; Ma, H. Hypoxic preconditioning increases the protective effect of bone marrow mesenchymal stem cells on spinal cord ischemia/reperfusion injury. Mol. Med. Rep. 2016, 13, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wei, M.G.; Liang, J.; Xu, H.H.; Wang, J.J.; Wang, J.; Yang, X.P.; Lv, F.F.; Wang, K.Q.; Duan, J.H.; et al. Injury-preconditioning secretome of umbilical cord mesenchymal stem cells amplified the neurogenesis and cognitive recovery after severe traumatic brain injury in rats. J. Neurochem. 2020, 153, 230–251. [Google Scholar] [CrossRef] [PubMed]
- Gottlicher, M.; Minucci, S.; Zhu, P.; Kramer, O.H.; Schimpf, A.; Giavara, S.; Sleeman, J.P.; Lo Coco, F.; Nervi, C.; Pelicci, P.G.; et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001, 20, 6969–6978. [Google Scholar] [CrossRef]
- Tsai, L.K.; Leng, Y.; Wang, Z.; Leeds, P.; Chuang, D.M. The mood stabilizers valproic acid and lithium enhance mesenchymal stem cell migration via distinct mechanisms. Neuropsychopharmacology 2010, 35, 2225–2237. [Google Scholar] [CrossRef] [PubMed]
- Pourjafar, M.; Saidijam, M.; Mansouri, K.; Ghasemibasir, H.; Karimi Dermani, F.; Najafi, R. All-trans retinoic acid preconditioning enhances proliferation, angiogenesis and migration of mesenchymal stem cell in vitro and enhances wound repair in vivo. Cell Prolif. 2017, 50, e12315. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrenho, I.; Ferreira, S.A.; Baltazar, G. Preconditioning of MSCs for Acute Neurological Conditions: From Cellular to Functional Impact—A Systematic Review. Cells 2024, 13, 845. https://doi.org/10.3390/cells13100845
Serrenho I, Ferreira SA, Baltazar G. Preconditioning of MSCs for Acute Neurological Conditions: From Cellular to Functional Impact—A Systematic Review. Cells. 2024; 13(10):845. https://doi.org/10.3390/cells13100845
Chicago/Turabian StyleSerrenho, Inês, Susana Alves Ferreira, and Graça Baltazar. 2024. "Preconditioning of MSCs for Acute Neurological Conditions: From Cellular to Functional Impact—A Systematic Review" Cells 13, no. 10: 845. https://doi.org/10.3390/cells13100845
APA StyleSerrenho, I., Ferreira, S. A., & Baltazar, G. (2024). Preconditioning of MSCs for Acute Neurological Conditions: From Cellular to Functional Impact—A Systematic Review. Cells, 13(10), 845. https://doi.org/10.3390/cells13100845







