hPSC-Derived Astrocytes at the Forefront of Translational Applications in Neurological Disorders
Abstract
1. Introduction
2. In Vivo Development and Heterogeneity of Astrocytes
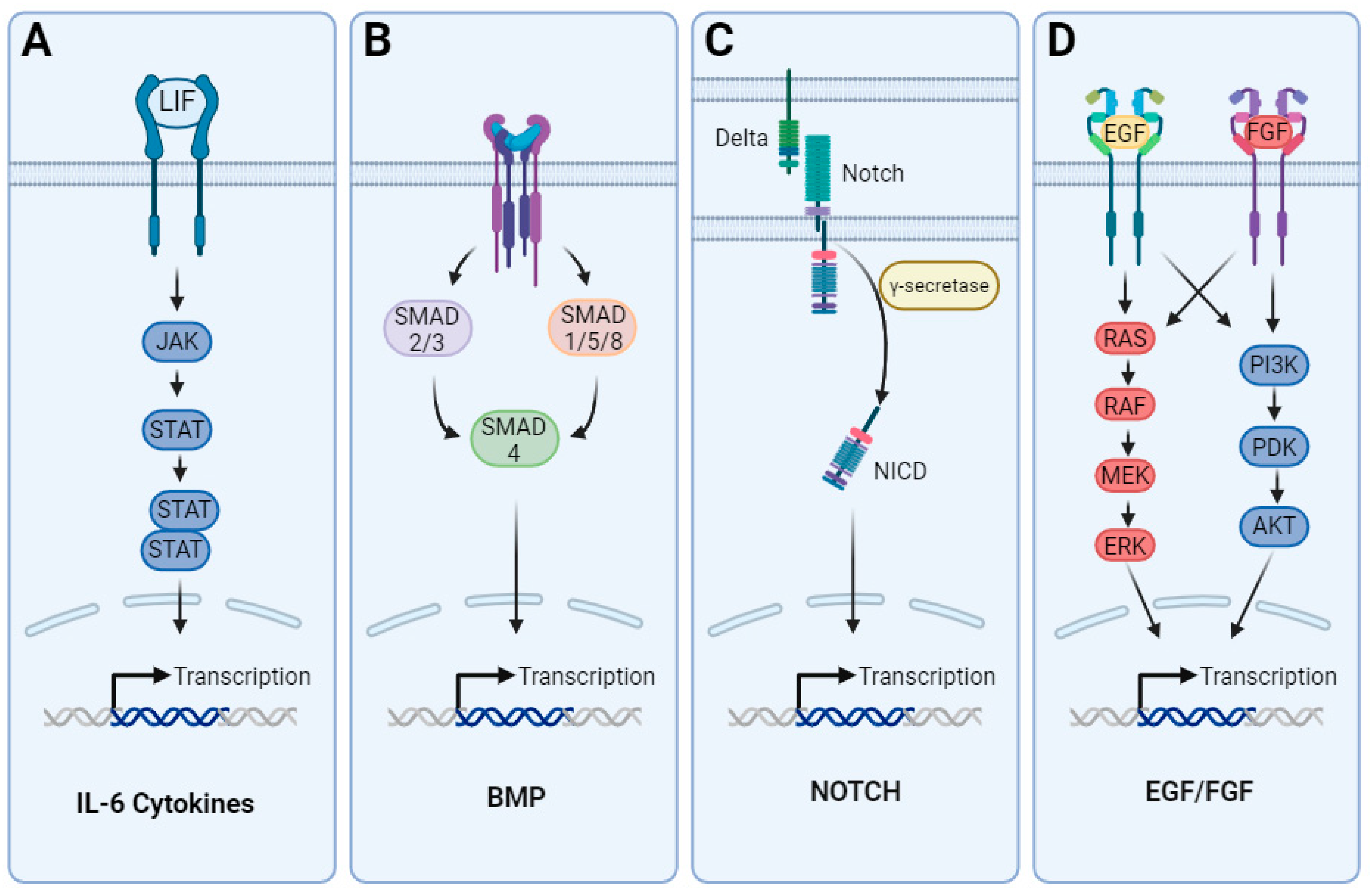
3. Differentiation of Astrocytes from hPSCs
4. hPSC-Derived Astrocytes for Modeling Rare and Common Neurological Disorders
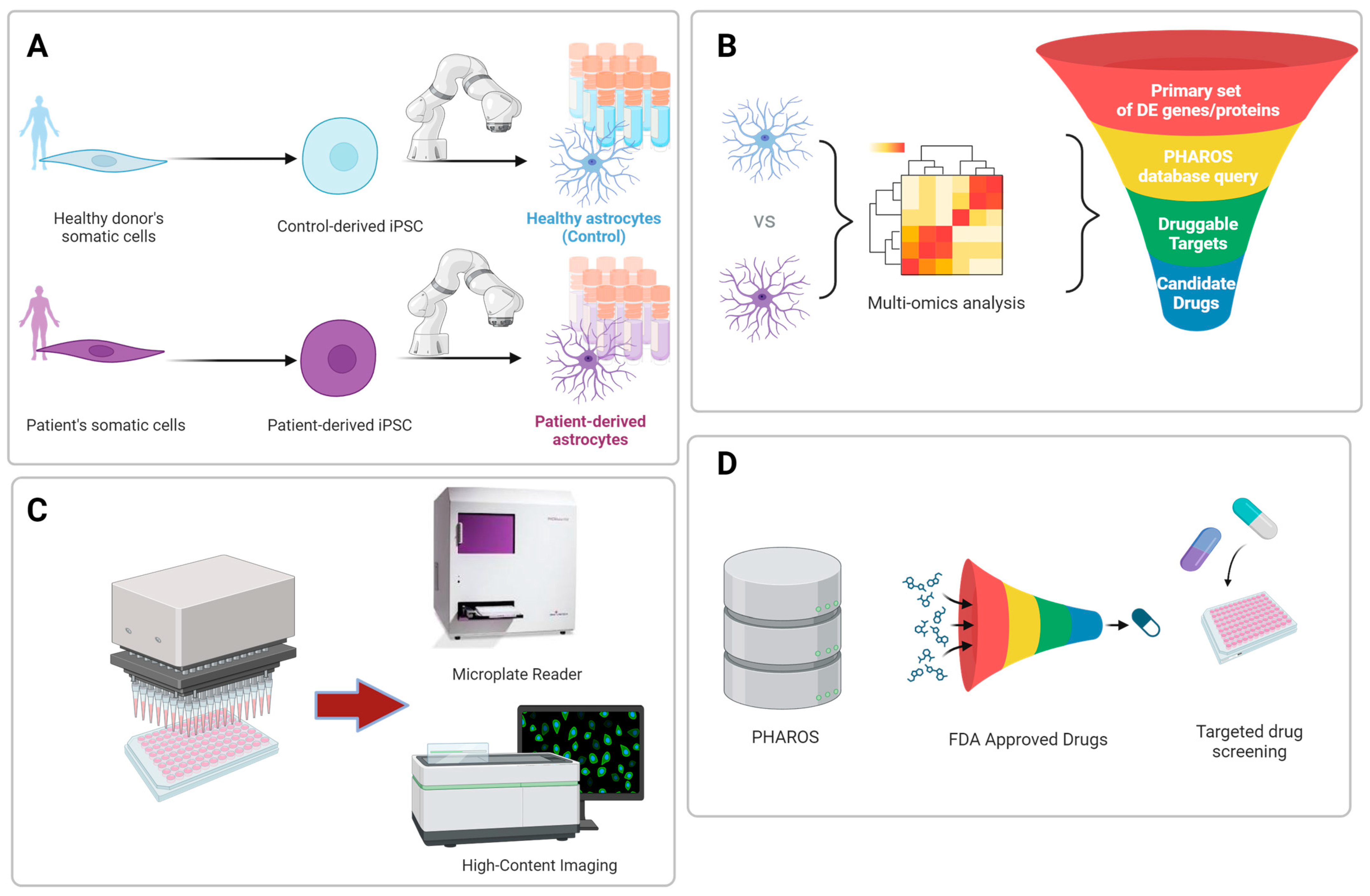
5. Advancements in Biomanufacturing of Human Astrocytes for Disease Modeling and Drug Screening
6. High-Throughput Cell-Based Functional Astrocyte Assays
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| ALDH1L1 | Cytosolic 10-formyltetrahydrofolate dehydrogenase |
| ALS | Amyotrophic Lateral Sclerosis |
| AQP-4 | Aquaporin 4 |
| AQP-4 | Aquaporin-4 |
| APOE4 | Apolipoprotein E4 |
| ASD | Autism Spectrum Disorder |
| ATP | Adenosine triphosphate |
| BBB | Blood–Brain Barrier |
| cAMP | Cyclic adenosine monophosphate |
| Cas 9 | CRISPR-associated protein 9 |
| CD44 | Cluster of differentiation 44 |
| CNTF | Ciliary Neurotrophic Factor |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| CT-1 | Cardiotrophin-1 |
| E | Day of embryonic development of the mouse embryo |
| EGF | Epidermal Growth Factor |
| FABP7 | Fatty acid binding protein 7/brain lipid binding protein |
| FDA | United States Food and Drug Administration |
| FGF-(2) | Fibroblast Growth Factor (2) |
| FBS | Fetal Bovine Serum |
| GFAP | Glial Fibrillary Acidic Protein |
| GLT-1 | Glutamate transporter 1 |
| gp130 | Glycoprotein Gp 130 |
| IL-1alpha | Interleukin–1 alpha |
| IL-6 | Interleukin-6 |
| iPSC | Induced pluripotent stem cell |
| JAK | Janus kinases |
| LIF | Leukemia inhibitory factor |
| LIFR-β | Leukemia inhibitory factor receptor beta |
| LRRK2 | Leucine rich repeat kinase 2 |
| MS | Multiple Sclerosis |
| NFIA | Nuclear Factor I-a |
| NFIB | Nuclear Factor I-b |
| NG2 | Neural/glial antigen 2 |
| NPC | Neural progenitor cell |
| NCATS | National Center for Advancing Translational Sciences |
| OPC | Oligodendrocyte Precursor Cell |
| PD | Parkinson’s Disease |
| PSEN1/2 | Presenilin-1/2 |
| RGC | Radial glia cell |
| SOX9 | SRY-Box Transcription Factor 9 |
| S100B | S100 calcium-binding protein B |
| SMAD | Small mothers against decapentaplegic intracellular signaling proteins |
| STAT | Signal Transducer and Activator of Transcription |
| TDP-43 | Transactive response DNA binding protein of 43 kDa |
| TNF | Tumor Necrosis Factor |
References
- Chaboub, L.S.; Deneen, B. Developmental origins of astrocyte heterogeneity: The final frontier of CNS development. Dev. Neurosci. 2012, 34, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marin, V.; Garcia-Lopez, P.; Freire, M. Cajal’s contributions to glia research. Trends Neurosci. 2007, 30, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Sloan, S.A.; Barres, B.A. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr. Opin. Neurobiol. 2014, 27, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Stogsdill, J.A.; Ramirez, J.; Liu, D.; Kim, Y.H.; Baldwin, K.T.; Enustun, E.; Ejikeme, T.; Ji, R.R.; Eroglu, C. Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 2017, 551, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, M.; Wang, F.; Windrem, M.; Wang, S.; Shanz, S.; Xu, Q.; Oberheim, N.A.; Bekar, L.; Betstadt, S.; et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 2013, 12, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Wheeler, M.A.; Quintana, F.J. Function and therapeutic value of astrocytes in neurological diseases. Nat. Rev. Drug Discov. 2022, 21, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Banker, G.A. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science 1980, 209, 809–810. [Google Scholar] [CrossRef] [PubMed]
- Belanger, M.; Allaman, I.; Magistretti, P.J. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef]
- Foo, L.C.; Allen, N.J.; Bushong, E.A.; Ventura, P.B.; Chung, W.S.; Zhou, L.; Cahoy, J.D.; Daneman, R.; Zong, H.; Ellisman, M.H.; et al. Development of a method for the purification and culture of rodent astrocytes. Neuron 2011, 71, 799–811. [Google Scholar] [CrossRef]
- Ridet, J.L.; Malhotra, S.K.; Privat, A.; Gage, F.H. Reactive astrocytes: Cellular and molecular cues to biological function. Trends Neurosci. 1997, 20, 570–577. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.G.; Nahmou, M.; Toth, A.B.; Heo, L.; Tanasa, B.; Dalal, R.; Yan, W.; Nallagatla, P.; Xia, X.; Hay, S.; et al. A molecular switch for neuroprotective astrocyte reactivity. Nature 2024, 626, 574–582. [Google Scholar] [CrossRef]
- Guttenplan, K.A.; Weigel, M.K.; Prakash, P.; Wijewardhane, P.R.; Hasel, P.; Rufen-Blanchette, U.; Munch, A.E.; Blum, J.A.; Fine, J.; Neal, M.C.; et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature 2021, 599, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhauser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Al-Dalahmah, O.; Sosunov, A.A.; Shaik, A.; Ofori, K.; Liu, Y.; Vonsattel, J.P.; Adorjan, I.; Menon, V.; Goldman, J.E. Single-nucleus RNA-seq identifies Huntington disease astrocyte states. Acta Neuropathol. Commun. 2020, 8, 19. [Google Scholar] [CrossRef]
- Grubman, A.; Chew, G.; Ouyang, J.F.; Sun, G.; Choo, X.Y.; McLean, C.; Simmons, R.K.; Buckberry, S.; Vargas-Landin, D.B.; Poppe, D.; et al. A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 2019, 22, 2087–2097. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, I.D.; Lim, J.; Moon, J.S. Pathological phenotypes of astrocytes in Alzheimer’s disease. Exp. Mol. Med. 2024, 56, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Ahlsen, G.; Rosengren, L.; Belfrage, M.; Palm, A.; Haglid, K.; Hamberger, A.; Gillberg, C. Glial fibrillary acidic protein in the cerebrospinal fluid of children with autism and other neuropsychiatric disorders. Biol. Psychiatry 1993, 33, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Johnson, A.B.; Boespflug-Tanguy, O.; Rodriguez, D.; Goldman, J.E.; Messing, A. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat. Genet. 2001, 27, 117–120. [Google Scholar] [CrossRef]
- Habib, N.; McCabe, C.; Medina, S.; Varshavsky, M.; Kitsberg, D.; Dvir-Szternfeld, R.; Green, G.; Dionne, D.; Nguyen, L.; Marshall, J.L.; et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 2020, 23, 701–706. [Google Scholar] [CrossRef]
- Frost, G.R.; Li, Y.M. The role of astrocytes in amyloid production and Alzheimer’s disease. Open Biol. 2017, 7, 170228. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.J.; Yeh, C.Y.; Terzieva, S.; Olabarria, M.; Kulijewicz-Nawrot, M.; Verkhratsky, A. Complex and region-specific changes in astroglial markers in the aging brain. Neurobiol. Aging 2014, 35, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Gonzalez, P.; Mato, S.; Chara, J.C.; Verkhratsky, A.; Matute, C.; Cavaliere, F. Astrocytic atrophy as a pathological feature of Parkinson’s disease with LRRK2 mutation. NPJ Park. Dis. 2021, 7, 31. [Google Scholar] [CrossRef]
- Levine, J.B.; Kong, J.; Nadler, M.; Xu, Z. Astrocytes interact intimately with degenerating motor neurons in mouse amyotrophic lateral sclerosis (ALS). Glia 1999, 28, 215–224. [Google Scholar] [CrossRef]
- Itoh, N.; Itoh, Y.; Tassoni, A.; Ren, E.; Kaito, M.; Ohno, A.; Ao, Y.; Farkhondeh, V.; Johnsonbaugh, H.; Burda, J.; et al. Cell-specific and region-specific transcriptomics in the multiple sclerosis model: Focus on astrocytes. Proc. Natl. Acad. Sci. USA 2018, 115, E302–E309. [Google Scholar] [CrossRef]
- Black, J.A.; Newcombe, J.; Waxman, S.G. Astrocytes within multiple sclerosis lesions upregulate sodium channel Nav1.5. Brain 2010, 133, 835–846. [Google Scholar] [CrossRef]
- Diaz-Castro, B.; Gangwani, M.R.; Yu, X.; Coppola, G.; Khakh, B.S. Astrocyte molecular signatures in Huntington’s disease. Sci. Transl. Med. 2019, 11, eaaw8546. [Google Scholar] [CrossRef]
- Toker, L.; Mancarci, B.O.; Tripathy, S.; Pavlidis, P. Transcriptomic Evidence for Alterations in Astrocytes and Parvalbumin Interneurons in Subjects with Bipolar Disorder and Schizophrenia. Biol. Psychiatry 2018, 84, 787–796. [Google Scholar] [CrossRef]
- Zhang, Y.; Sloan, S.A.; Clarke, L.E.; Caneda, C.; Plaza, C.A.; Blumenthal, P.D.; Vogel, H.; Steinberg, G.K.; Edwards, M.S.; Li, G.; et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016, 89, 37–53. [Google Scholar] [CrossRef]
- Sardi, S.P.; Murtie, J.; Koirala, S.; Patten, B.A.; Corfas, G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell 2006, 127, 185–197. [Google Scholar] [CrossRef]
- Naka, H.; Nakamura, S.; Shimazaki, T.; Okano, H. Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nat. Neurosci. 2008, 11, 1014–1023. [Google Scholar] [CrossRef]
- Darmanis, S.; Sloan, S.A.; Zhang, Y.; Enge, M.; Caneda, C.; Shuer, L.M.; Hayden Gephart, M.G.; Barres, B.A.; Quake, S.R. A survey of human brain transcriptome diversity at the single cell level. Proc. Natl. Acad. Sci. USA 2015, 112, 7285–7290. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhang, S.; Fan, X.; Wu, Q.; Yan, L.; Dong, J.; Zhang, H.; Li, L.; Sun, L.; Pan, N.; et al. A single-cell RNA-seq survey of the developmental landscape of the human prefrontal cortex. Nature 2018, 555, 524–528. [Google Scholar] [CrossRef]
- Rowitch, D.H.; Kriegstein, A.R. Developmental genetics of vertebrate glial-cell specification. Nature 2010, 468, 214–222. [Google Scholar] [CrossRef]
- Noctor, S.C.; Flint, A.C.; Weissman, T.A.; Dammerman, R.S.; Kriegstein, A.R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 2001, 409, 714–720. [Google Scholar] [CrossRef]
- Miyata, T.; Kawaguchi, A.; Okano, H.; Ogawa, M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 2001, 31, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Kornack, D.R.; Rakic, P. Radial and horizontal deployment of clonally related cells in the primate neocortex: Relationship to distinct mitotic lineages. Neuron 1995, 15, 311–321. [Google Scholar] [CrossRef]
- Noctor, S.C.; Martinez-Cerdeno, V.; Ivic, L.; Kriegstein, A.R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004, 7, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Kawaguchi, A.; Saito, K.; Kawano, M.; Muto, T.; Ogawa, M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 2004, 131, 3133–3145. [Google Scholar] [CrossRef]
- Hartfuss, E.; Galli, R.; Heins, N.; Gotz, M. Characterization of CNS precursor subtypes and radial glia. Dev. Biol. 2001, 229, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.A.; Murphy, B.; Danzer, S.C.; Kuan, C.Y. Developmental and post-injury cortical gliogenesis: A genetic fate-mapping study with Nestin-CreER mice. Glia 2009, 57, 1115–1129. [Google Scholar] [CrossRef]
- Schmechel, D.E.; Rakic, P. A Golgi study of radial glial cells in developing monkey telencephalon: Morphogenesis and transformation into astrocytes. Anat. Embryol. 1979, 156, 115–152. [Google Scholar] [CrossRef] [PubMed]
- Holst, C.B.; Brochner, C.B.; Vitting-Seerup, K.; Mollgard, K. Astrogliogenesis in human fetal brain: Complex spatiotemporal immunoreactivity patterns of GFAP, S100, AQP4 and YKL-40. J. Anat. 2019, 235, 590–615. [Google Scholar] [CrossRef]
- Marin-Padilla, M. Prenatal development of fibrous (white matter), protoplasmic (gray matter), and layer I astrocytes in the human cerebral cortex: A Golgi study. J. Comp. Neurol. 1995, 357, 554–572. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, H.J.; Gadisseux, J.F.; Evrard, P. Topographical and cytological evolution of the glial phase during prenatal development of the human brain: Histochemical and electron microscopic study. J. Neuropathol. Exp. Neurol. 1988, 47, 166–188. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, C.S.; Amici, M.; Bortolotto, Z.A.; Doherty, A.; Csaba, Z.; Fafouri, A.; Dournaud, P.; Gressens, P.; Collingridge, G.L.; Peineau, S. The role of JAK-STAT signaling within the CNS. JAK-STAT 2013, 2, e22925. [Google Scholar] [CrossRef] [PubMed]
- Barnabe-Heider, F.; Wasylnka, J.A.; Fernandes, K.J.; Porsche, C.; Sendtner, M.; Kaplan, D.R.; Miller, F.D. Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron 2005, 48, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Koblar, S.A.; Turnley, A.M.; Classon, B.J.; Reid, K.L.; Ware, C.B.; Cheema, S.S.; Murphy, M.; Bartlett, P.F. Neural precursor differentiation into astrocytes requires signaling through the leukemia inhibitory factor receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 3178–3181. [Google Scholar] [CrossRef]
- Bonni, A.; Sun, Y.; Nadal-Vicens, M.; Bhatt, A.; Frank, D.A.; Rozovsky, I.; Stahl, N.; Yancopoulos, G.D.; Greenberg, M.E. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science 1997, 278, 477–483. [Google Scholar] [CrossRef]
- Molne, M.; Studer, L.; Tabar, V.; Ting, Y.T.; Eiden, M.V.; McKay, R.D. Early cortical precursors do not undergo LIF-mediated astrocytic differentiation. J. Neurosci. Res. 2000, 59, 301–311. [Google Scholar] [CrossRef]
- Sun, Y.; Nadal-Vicens, M.; Misono, S.; Lin, M.Z.; Zubiaga, A.; Hua, X.; Fan, G.; Greenberg, M.E. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 2001, 104, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Yanagisawa, M.; Arakawa, H.; Kimura, N.; Hisatsune, T.; Kawabata, M.; Miyazono, K.; Taga, T. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 1999, 284, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Rajan, P.; Panchision, D.M.; Newell, L.F.; McKay, R.D. BMPs signal alternately through a SMAD or FRAP-STAT pathway to regulate fate choice in CNS stem cells. J. Cell Biol. 2003, 161, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.E.; Mehler, M.F.; Mabie, P.C.; Zang, Z.; Santschi, L.; Kessler, J.A. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron 1996, 17, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Gomes, W.A.; Mehler, M.F.; Kessler, J.A. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev. Biol. 2003, 255, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Perez, S.E.; Qiao, Z.; Verdi, J.M.; Hicks, C.; Weinmaster, G.; Anderson, D.J. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 2000, 101, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Deneen, B.; Ho, R.; Lukaszewicz, A.; Hochstim, C.J.; Gronostajski, R.M.; Anderson, D.J. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron 2006, 52, 953–968. [Google Scholar] [CrossRef] [PubMed]
- Namihira, M.; Kohyama, J.; Semi, K.; Sanosaka, T.; Deneen, B.; Taga, T.; Nakashima, K. Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Dev. Cell 2009, 16, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Kornblum, H.I.; Hussain, R.; Wiesen, J.; Miettinen, P.; Zurcher, S.D.; Chow, K.; Derynck, R.; Werb, Z. Abnormal astrocyte development and neuronal death in mice lacking the epidermal growth factor receptor. J. Neurosci. Res. 1998, 53, 697–717. [Google Scholar] [CrossRef]
- Mujtaba, T.; Piper, D.R.; Kalyani, A.; Groves, A.K.; Lucero, M.T.; Rao, M.S. Lineage-restricted neural precursors can be isolated from both the mouse neural tube and cultured ES cells. Dev. Biol. 1999, 214, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Dinh Duong, T.A.; Hoshiba, Y.; Saito, K.; Kawasaki, K.; Ichikawa, Y.; Matsumoto, N.; Shinmyo, Y.; Kawasaki, H. FGF Signaling Directs the Cell Fate Switch from Neurons to Astrocytes in the Developing Mouse Cerebral Cortex. J. Neurosci. 2019, 39, 6081–6094. [Google Scholar] [CrossRef] [PubMed]
- Shinmyo, Y.; Saito, K.; Hamabe-Horiike, T.; Kameya, N.; Ando, A.; Kawasaki, K.; Duong, T.A.D.; Sakashita, M.; Roboon, J.; Hattori, T.; et al. Localized astrogenesis regulates gyrification of the cerebral cortex. Sci. Adv. 2022, 8, eabi5209. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Li, H.; Fuentealba, L.C.; Molofsky, A.V.; Taveira-Marques, R.; Zhuang, H.; Tenney, A.; Murnen, A.T.; Fancy, S.P.; Merkle, F.; et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 2012, 337, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, O.A.; Bartels, T.; Holmqvist, S.; Kleshchevnikov, V.; Martirosyan, A.; Polioudakis, D.; Ben Haim, L.; Young, A.M.H.; Batiuk, M.Y.; Prakash, K.; et al. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat. Neurosci. 2020, 23, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Diaz-Castro, B.; Shigetomi, E.; Monte, E.; Octeau, J.C.; Yu, X.; Cohn, W.; Rajendran, P.S.; Vondriska, T.M.; Whitelegge, J.P.; et al. Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 2017, 95, 531–549.e539. [Google Scholar] [CrossRef] [PubMed]
- Tcw, J.; Wang, M.; Pimenova, A.A.; Bowles, K.R.; Hartley, B.J.; Lacin, E.; Machlovi, S.I.; Abdelaal, R.; Karch, C.M.; Phatnani, H.; et al. An Efficient Platform for Astrocyte Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Rep. 2017, 9, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Vadodaria, K.C.; Jaeger, B.N.; Mei, A.; Lefcochilos-Fogelquist, S.; Mendes, A.P.D.; Erikson, G.; Shokhirev, M.; Randolph-Moore, L.; Fredlender, C.; et al. Differentiation of Inflammation-Responsive Astrocytes from Glial Progenitors Generated from Human Induced Pluripotent Stem Cells. Stem Cell Rep. 2017, 8, 1757–1769. [Google Scholar] [CrossRef]
- Krencik, R.; Zhang, S.C. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nat. Protoc. 2011, 6, 1710–1717. [Google Scholar] [CrossRef]
- Jovanovic, V.M.; Weber, C.; Slamecka, J.; Ryu, S.; Chu, P.H.; Sen, C.; Inman, J.; De Sousa, J.F.; Barnaeva, E.; Hirst, M.; et al. A defined roadmap of radial glia and astrocyte differentiation from human pluripotent stem cells. Stem Cell Rep. 2023, 18, 1701–1720. [Google Scholar] [CrossRef]
- Tchieu, J.; Calder, E.L.; Guttikonda, S.R.; Gutzwiller, E.M.; Aromolaran, K.A.; Steinbeck, J.A.; Goldstein, P.A.; Studer, L. NFIA is a gliogenic switch enabling rapid derivation of functional human astrocytes from pluripotent stem cells. Nat. Biotechnol. 2019, 37, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Canals, I.; Ginisty, A.; Quist, E.; Timmerman, R.; Fritze, J.; Miskinyte, G.; Monni, E.; Hansen, M.G.; Hidalgo, I.; Bryder, D.; et al. Rapid and efficient induction of functional astrocytes from human pluripotent stem cells. Nat. Methods 2018, 15, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tao, Y.; Bradley, R.; Du, Z.; Tao, Y.; Kong, L.; Dong, Y.; Jones, J.; Yan, Y.; Harder, C.R.K.; et al. Fast Generation of Functional Subtype Astrocytes from Human Pluripotent Stem Cells. Stem Cell Rep. 2018, 11, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Berryer, M.H.; Tegtmeyer, M.; Binan, L.; Valakh, V.; Nathanson, A.; Trendafilova, D.; Crouse, E.; Klein, J.A.; Meyer, D.; Pietilainen, O.; et al. Robust induction of functional astrocytes using NGN2 expression in human pluripotent stem cells. iScience 2023, 26, 106995. [Google Scholar] [CrossRef] [PubMed]
- Krencik, R.; Weick, J.P.; Liu, Y.; Zhang, Z.J.; Zhang, S.C. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat. Biotechnol. 2011, 29, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Sloan, S.A.; Darmanis, S.; Huber, N.; Khan, T.A.; Birey, F.; Caneda, C.; Reimer, R.; Quake, S.R.; Barres, B.A.; Pasca, S.P. Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron 2017, 95, 779–790.e776. [Google Scholar] [CrossRef] [PubMed]
- Barbar, L.; Jain, T.; Zimmer, M.; Kruglikov, I.; Sadick, J.S.; Wang, M.; Kalpana, K.; Rose, I.V.L.; Burstein, S.R.; Rusielewicz, T.; et al. CD49f Is a Novel Marker of Functional and Reactive Human iPSC-Derived Astrocytes. Neuron 2020, 107, 436–453.e412. [Google Scholar] [CrossRef] [PubMed]
- Rapino, F.; Natoli, T.; Limone, F.; O’Connor, E.; Blank, J.; Tegtmeyer, M.; Chen, W.; Norabuena, E.; Narula, J.; Hazelbaker, D.; et al. Small-molecule screen reveals pathways that regulate C4 secretion in stem cell-derived astrocytes. Stem Cell Rep. 2023, 18, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.P.; Yang, N.; Vierbuchen, T.; Ostermeier, A.; Fuentes, D.R.; Yang, T.Q.; Citri, A.; Sebastiano, V.; Marro, S.; Sudhof, T.C.; et al. Induction of human neuronal cells by defined transcription factors. Nature 2011, 476, 220–223. [Google Scholar] [CrossRef]
- Ieda, M.; Fu, J.D.; Delgado-Olguin, P.; Vedantham, V.; Hayashi, Y.; Bruneau, B.G.; Srivastava, D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010, 142, 375–386. [Google Scholar] [CrossRef]
- Shen, C.N.; Slack, J.M.; Tosh, D. Molecular basis of transdifferentiation of pancreas to liver. Nat. Cell Biol. 2000, 2, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Ye, M.; Feng, R.; Graf, T. Stepwise reprogramming of B cells into macrophages. Cell 2004, 117, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Caiazzo, M.; Giannelli, S.; Valente, P.; Lignani, G.; Carissimo, A.; Sessa, A.; Colasante, G.; Bartolomeo, R.; Massimino, L.; Ferroni, S.; et al. Direct conversion of fibroblasts into functional astrocytes by defined transcription factors. Stem Cell Rep. 2015, 4, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, M.L.; Zang, T.; Zhang, C.L. Direct Reprogramming Rather than iPSC-Based Reprogramming Maintains Aging Hallmarks in Human Motor Neurons. Front. Mol. Neurosci. 2017, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Sosunov, A.A.; McKhann, G.M., 2nd; Goldman, J.E. The origin of Rosenthal fibers and their contributions to astrocyte pathology in Alexander disease. Acta Neuropathol. Commun. 2017, 5, 27. [Google Scholar] [CrossRef]
- Li, L.; Tian, E.; Chen, X.; Chao, J.; Klein, J.; Qu, Q.; Sun, G.; Sun, G.; Huang, Y.; Warden, C.D.; et al. GFAP Mutations in Astrocytes Impair Oligodendrocyte Progenitor Proliferation and Myelination in an hiPSC Model of Alexander Disease. Cell Stem Cell 2018, 23, 239–251.e236. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Funayama, M.; Miyake, M.; Tsukita, K.; Era, T.; Osaka, H.; Ayaki, T.; Takahashi, R.; Inoue, H. Modeling Alexander disease with patient iPSCs reveals cellular and molecular pathology of astrocytes. Acta Neuropathol. Commun. 2016, 4, 69. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Huang, B.S.; Notaras, M.J.; Lodhi, A.; Barrio-Alonso, E.; Lituma, P.J.; Wolujewicz, P.; Witztum, J.; Longo, F.; Chen, M.; et al. Astrocytes derived from ASD individuals alter behavior and destabilize neuronal activity through aberrant Ca2+ signaling. Mol. Psychiatry 2022, 27, 2470–2484. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.B.; Freitas, B.C.; Pignatari, G.C.; Fernandes, I.R.; Sebat, J.; Muotri, A.R.; Beltrao-Braga, P.C.B. Modeling the Interplay between Neurons and Astrocytes in Autism Using Human Induced Pluripotent Stem Cells. Biol. Psychiatry 2018, 83, 569–578. [Google Scholar] [CrossRef]
- Jones, V.C.; Atkinson-Dell, R.; Verkhratsky, A.; Mohamet, L. Aberrant iPSC-derived human astrocytes in Alzheimer’s disease. Cell Death Dis. 2017, 8, e2696. [Google Scholar] [CrossRef]
- Hiatt, J.; Cavero, D.A.; McGregor, M.J.; Zheng, W.; Budzik, J.M.; Roth, T.L.; Haas, K.M.; Wu, D.; Rathore, U.; Meyer-Franke, A.; et al. Efficient generation of isogenic primary human myeloid cells using CRISPR-Cas9 ribonucleoproteins. Cell Rep. 2021, 35, 109105. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.A.; Stein, J.L.; Krostag, A.R.; Nelson, A.M.; Marken, J.S.; Menon, V.; May, R.C.; Yao, Z.; Kaykas, A.; Geschwind, D.H.; et al. Genome engineering of isogenic human ES cells to model autism disorders. Nucleic Acids Res. 2015, 43, e65. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.Y.; Hu, S.; Lan, F.; Lee, A.S.; Huber, B.; Lisowski, L.; Liang, P.; Huang, M.; de Almeida, P.E.; et al. Genome editing of human embryonic stem cells and induced pluripotent stem cells with zinc finger nucleases for cellular imaging. Circ. Res. 2012, 111, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Seo, J.; Gao, F.; Feldman, H.M.; Wen, H.L.; Penney, J.; Cam, H.P.; Gjoneska, E.; Raja, W.K.; Cheng, J.; et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 2018, 98, 1294. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, M.; Petersen, A.J.; Naumenko, N.; Puttonen, K.; Lehtonen, S.; Gubert Olive, M.; Shakirzyanova, A.; Leskela, S.; Sarajarvi, T.; Viitanen, M.; et al. PSEN1 Mutant iPSC-Derived Model Reveals Severe Astrocyte Pathology in Alzheimer’s Disease. Stem Cell Rep. 2017, 9, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- de Rus Jacquet, A.; Alpaugh, M.; Denis, H.L.; Tancredi, J.L.; Boutin, M.; Decaestecker, J.; Beauparlant, C.; Herrmann, L.; Saint-Pierre, M.; Parent, M.; et al. The contribution of inflammatory astrocytes to BBB impairments in a brain-chip model of Parkinson’s disease. Nat. Commun. 2023, 14, 3651. [Google Scholar] [CrossRef] [PubMed]
- Szebenyi, K.; Wenger, L.M.D.; Sun, Y.; Dunn, A.W.E.; Limegrover, C.A.; Gibbons, G.M.; Conci, E.; Paulsen, O.; Mierau, S.B.; Balmus, G.; et al. Human ALS/FTD brain organoid slice cultures display distinct early astrocyte and targetable neuronal pathology. Nat. Neurosci. 2021, 24, 1542–1554. [Google Scholar] [CrossRef] [PubMed]
- Birger, A.; Ben-Dor, I.; Ottolenghi, M.; Turetsky, T.; Gil, Y.; Sweetat, S.; Perez, L.; Belzer, V.; Casden, N.; Steiner, D.; et al. Human iPSC-derived astrocytes from ALS patients with mutated C9ORF72 show increased oxidative stress and neurotoxicity. EBioMedicine 2019, 50, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Serio, A.; Bilican, B.; Barmada, S.J.; Ando, D.M.; Zhao, C.; Siller, R.; Burr, K.; Haghi, G.; Story, D.; Nishimura, A.L.; et al. Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proc. Natl. Acad. Sci. USA 2013, 110, 4697–4702. [Google Scholar] [CrossRef]
- Tyzack, G.E.; Hall, C.E.; Sibley, C.R.; Cymes, T.; Forostyak, S.; Carlino, G.; Meyer, I.F.; Schiavo, G.; Zhang, S.C.; Gibbons, G.M.; et al. A neuroprotective astrocyte state is induced by neuronal signal EphB1 but fails in ALS models. Nat. Commun. 2017, 8, 1164. [Google Scholar] [CrossRef]
- Baloh, R.H.; Johnson, J.P.; Avalos, P.; Allred, P.; Svendsen, S.; Gowing, G.; Roxas, K.; Wu, A.; Donahue, B.; Osborne, S.; et al. Transplantation of human neural progenitor cells secreting GDNF into the spinal cord of patients with ALS: A phase 1/2a trial. Nat. Med. 2022, 28, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Ponath, G.; Lincoln, M.R.; Levine-Ritterman, M.; Park, C.; Dahlawi, S.; Mubarak, M.; Sumida, T.; Airas, L.; Zhang, S.; Isitan, C.; et al. Enhanced astrocyte responses are driven by a genetic risk allele associated with multiple sclerosis. Nat. Commun. 2018, 9, 5337. [Google Scholar] [CrossRef] [PubMed]
- Perriot, S.; Mathias, A.; Perriard, G.; Canales, M.; Jonkmans, N.; Merienne, N.; Meunier, C.; El Kassar, L.; Perrier, A.L.; Laplaud, D.A.; et al. Human Induced Pluripotent Stem Cell-Derived Astrocytes Are Differentially Activated by Multiple Sclerosis-Associated Cytokines. Stem Cell Rep. 2018, 11, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Akkouh, I.A.; Vandenberghe, M.; Osete, J.R.; Hughes, T.; Heine, V.; Smeland, O.B.; Glover, J.C.; Andreassen, O.A.; Djurovic, S. A human iPSC-astroglia neurodevelopmental model reveals divergent transcriptomic patterns in schizophrenia. Transl. Psychiatry 2021, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Windrem, M.S.; Osipovitch, M.; Liu, Z.; Bates, J.; Chandler-Militello, D.; Zou, L.; Munir, J.; Schanz, S.; McCoy, K.; Miller, R.H.; et al. Human iPSC Glial Mouse Chimeras Reveal Glial Contributions to Schizophrenia. Cell Stem Cell 2017, 21, 195–208.e196. [Google Scholar] [CrossRef] [PubMed]
- Aijaz, A.; Li, M.; Smith, D.; Khong, D.; LeBlon, C.; Fenton, O.S.; Olabisi, R.M.; Libutti, S.; Tischfield, J.; Maus, M.V.; et al. Biomanufacturing for clinically advanced cell therapies. Nat. Biomed. Eng. 2018, 2, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Terstegge, S.; Laufenberg, I.; Pochert, J.; Schenk, S.; Itskovitz-Eldor, J.; Endl, E.; Brustle, O. Automated maintenance of embryonic stem cell cultures. Biotechnol. Bioeng. 2007, 96, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Conway, M.K.; Gerger, M.J.; Balay, E.E.; O’Connell, R.; Hanson, S.; Daily, N.J.; Wakatsuki, T. Scalable 96-well Plate Based iPSC Culture and Production Using a Robotic Liquid Handling System. J. Vis. Exp. 2015, 99, e52755. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J.; Anderson, D.; Chandra, A.; Smith, N.M.; Young, L.E.; Williams, D.; Denning, C. Automated, scalable culture of human embryonic stem cells in feeder-free conditions. Biotechnol. Bioeng. 2009, 102, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Paull, D.; Sevilla, A.; Zhou, H.; Hahn, A.K.; Kim, H.; Napolitano, C.; Tsankov, A.; Shang, L.; Krumholz, K.; Jagadeesan, P.; et al. Automated, high-throughput derivation, characterization and differentiation of induced pluripotent stem cells. Nat. Methods 2015, 12, 885–892. [Google Scholar] [CrossRef]
- Konagaya, S.; Ando, T.; Yamauchi, T.; Suemori, H.; Iwata, H. Long-term maintenance of human induced pluripotent stem cells by automated cell culture system. Sci. Rep. 2015, 5, 16647. [Google Scholar] [CrossRef] [PubMed]
- Crombie, D.E.; Daniszewski, M.; Liang, H.H.; Kulkarni, T.; Li, F.; Lidgerwood, G.E.; Conquest, A.; Hernandez, D.; Hung, S.S.; Gill, K.P.; et al. Development of a Modular Automated System for Maintenance and Differentiation of Adherent Human Pluripotent Stem Cells. SLAS Discov. 2017, 22, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Hussain, W.; Moens, N.; Veraitch, F.S.; Hernandez, D.; Mason, C.; Lye, G.J. Reproducible culture and differentiation of mouse embryonic stem cells using an automated microwell platform. Biochem. Eng. J. 2013, 77, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Vanmarcke, G.; Sai-Hong Chui, J.; Cooreman, A.; De Vos, K.; Cleuren, L.; Van Rossom, R.; Garcia-Llorens, G.; Izuel Idoype, T.; Boon, R.; Kumar Gautam, M.; et al. Automated Generation of hiPSC-Derived Hepatic Progeny by Cost-Efficient Compounds. Stem Cells 2023, 41, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Tristan, C.A.; Ormanoglu, P.; Slamecka, J.; Malley, C.; Chu, P.H.; Jovanovic, V.M.; Gedik, Y.; Jethmalani, Y.; Bonney, C.; Barnaeva, E.; et al. Robotic high-throughput biomanufacturing and functional differentiation of human pluripotent stem cells. Stem Cell Rep. 2021, 16, 3076–3092. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Jovanovic, V.M.; Tristan, C.A.; Weber, C.; Chu, P.H.; Inman, J.; Ryu, S.; Jethmalani, Y.; Ferreira de Sousa, J.; Ormanoglu, P.; et al. Scalable generation of sensory neurons from human pluripotent stem cells. Stem Cell Rep. 2023, 18, 1030–1047. [Google Scholar] [CrossRef] [PubMed]
- Noel, G.; Stevenson, S.; Moukhles, H. A high throughput screen identifies chemical modulators of the laminin-induced clustering of dystroglycan and aquaporin-4 in primary astrocytes. PLoS ONE 2011, 6, e17559. [Google Scholar] [CrossRef] [PubMed]
- James, L.R.; Andrews, S.; Walker, S.; de Sousa, P.R.; Ray, A.; Russell, N.A.; Bellamy, T.C. High-throughput analysis of calcium signalling kinetics in astrocytes stimulated with different neurotransmitters. PLoS ONE 2011, 6, e26889. [Google Scholar] [CrossRef] [PubMed]
- Berryer, M.H.; Rizki, G.; Nathanson, A.; Klein, J.A.; Trendafilova, D.; Susco, S.G.; Lam, D.; Messana, A.; Holton, K.M.; Karhohs, K.W.; et al. High-content synaptic phenotyping in human cellular models reveals a role for BET proteins in synapse assembly. Elife 2023, 12, e80168. [Google Scholar] [CrossRef]
- Badia-Soteras, A.; de Vries, J.; Dykstra, W.; Broersen, L.M.; Verkuyl, J.M.; Smit, A.B.; Verheijen, M.H.G. High-Throughput Analysis of Astrocyte Cultures Shows Prevention of Reactive Astrogliosis by the Multi-Nutrient Combination Fortasyn Connect. Cells 2022, 11, 1428. [Google Scholar] [CrossRef]
- Guerreiro, S.; Maciel, P. Transition from Animal-Based to Human Induced Pluripotent Stem Cells (iPSCs)-Based Models of Neurodevelopmental Disorders: Opportunities and Challenges. Cells 2023, 12, 538. [Google Scholar] [CrossRef] [PubMed]
- Zushin, P.H.; Mukherjee, S.; Wu, J.C. FDA Modernization Act 2.0: Transitioning beyond animal models with human cells, organoids, and AI/ML-based approaches. J. Clin. Investig. 2023, 133, e175824. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Vartiainen, N.E.; Ying, W.; Chan, P.H.; Koistinaho, J.; Swanson, R.A. Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. J. Neurochem. 2001, 77, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Thorne, N.; Malik, N.; Shah, S.; Zhao, J.; Class, B.; Aguisanda, F.; Southall, N.; Xia, M.; McKew, J.C.; Rao, M.; et al. High-Throughput Phenotypic Screening of Human Astrocytes to Identify Compounds That Protect Against Oxidative Stress. Stem Cells Transl. Med. 2016, 5, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Bassil, R.; Shields, K.; Granger, K.; Zein, I.; Ng, S.; Chih, B. Improved modeling of human AD with an automated culturing platform for iPSC neurons, astrocytes and microglia. Nat. Commun. 2021, 12, 5220. [Google Scholar] [CrossRef]
- De Leo, J.A.; Tawfik, V.L.; LaCroix-Fralish, M.L. The tetrapartite synapse: Path to CNS sensitization and chronic pain. Pain 2006, 122, 17–21. [Google Scholar] [CrossRef]

| Reference | Time to Reach Functional Astrocyte | Culture Method | Differentiating Compounds | Scalability |
|---|---|---|---|---|
| Krencik et al. (2011) [75] | 180 days | 2D culture—exogenous induction | Expanded with EGF, FGF2. Differentiated with CNTF | 1 hPSC: 2.8 × 1012 astrocytes, cryopreservable |
| Tcw et al. (2017) [67] | 30 days | 2D culture—exogenous induction | Expanded with FGF2. Differentiated with AM ScienCell (FBS) | Expandable, cryopreservable |
| Sloan et al. (2017) [76] | 250–590 days | 3D culture—human cerebral cortical spheroids | hSC Neural Induction: FGF2, compound-C, SB-431542, EGF, BDNF, NTS | |
| Santos et al. (2017) [68] | ~56 days | 2D culture—exogenous induction | Expanded and differentiated with AM ScienCell (FBS), Noggin, PDGF-AA, FGF2, EGF, FBS, LIF | Expandable, cryopreservable |
| Li et al. (2018) [73] | 52 days | 2D culture—DOX inducible transcription factors | Induced transcription factors: NFIA, SOX9. Exogenous Factors: heparin, FGF2, EGF, BMP4 and CNTF | |
| Canals et al. (2018) [72] | 14–21 days | 2D culture—DOX inducible transcription factors | Induced transcription factors: SOX9, NF1B. Exogenous factors: FBS, FGF, HB-EGF, CNTF, BMP4 | |
| Tchieu et al. (2019) [71] | 60 days | 2D culture—DOX inducible transcription factor | Induced transcription factor: NFIA. Exogenous factors: FGF2, EGF, HB-EGF | |
| Jovanovic et al. (2023) [70] | 50 days | 2D culture—exogenous induction | Expanded and differentiated with LDN-193189, Jagged-1, DIL-1, Onc-M, PDGF-AA, LIF, CNTF, 1% lipid suppl, hNRG1, forskolin, T3, PMA, Ascorbic Acid | CompacT SelecT robotic cell culture platform: 500 million astrocytes, cryopreservable |
| Reference | Immature or Mature-like | Multi-Omics Datasets | Functional Assays | Co-Culture | ||||
|---|---|---|---|---|---|---|---|---|
| Glutamate Uptake | Phagocytotic Activity | Calcium Transients | Inflammatory Response | Glycogen Storage | ||||
| Krencik et al. (2011) [75] | Immature | Patch clamp electrophysiology | Calcium indicator Fluo-4 | Neuronal co-culture with derived astrocytes increased astrocytic maturity and neuronal synapses | ||||
| Tcw et al. (2017) [67] | Immature | RNA-seq | pHrodo red conjugated-myelin and zymosan bioparticles | Calcium indicator Fluo-4AM | Multi-Analyte ELISArray after Abeta42 treatment | Derived-astrocyte co-culture increased the phagocytic activity of BV2 microglia | ||
| Sloan et al. (2017) [76] | Mature | scRNA-seq | Radioactive glutamate (L-[2,3,4-3 H] glutamate) | pHrodo red-labeled mouse synaptosomes | Neuronal co-culture with immature and mature astrocytes displayed similar synaptogenic abilities. Mature astrocytes increased Ca+ activity significantly more than immature ones | |||
| Santos et al. (2017) [68] | Immature | RNA-seq | Radioactive [3H] glutamate | Calcium indicator Fluo-4 | Flow cytometry-based approach and RNA-seq | Neuronal co-culture with inflammatory cytokine stimulated derived-astrocytes resulted in significant decrease in neuronal survival and dendritic length | ||
| Li et al. (2018) [73] | Immature | RNA-seq, DNA methylation | Glutamate assay kit | Calcium indicator Fluo-4 | iAstro co-culture resulted in greater iN neurite growth, length, and branching | |||
| Canals et al. (2018) [72] | Mature | Colorimetric glutamate assay kit | Calcium indicator Fluo-4 | RT-qPCR | Basic Fuchsin staining | Co-culture with iAs resulted in greater iN synapse formation | ||
| Tchieu et al. (2019) [71] | Immature | RNA-seq, DNA methylation, ATAC-seq | Calcium indicator Fura-2 | C3 ELISA Kit, RT-qPCR | Induced-astrocyte co-culture resulted in neuronal protection from excitotoxicity, structural and functional synapses, and decreased resting membrane potential | |||
| Jovanovic et al. (2023) [70] | Immature | scRNA-seq, DNA methylation, ATAC-seq | Colorimetric glutamate assay kit | FLIPR Penta high-content calcium imager | C3 ELISA Kit | Periodic acid–Schiff staining | Derived-astrocyte co-culture with neurons resulted in greater neurite length, greater electrical activity, and greater SYP vesicle protein expression as well as greater astrocytic maturation markers | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanovic, V.M.; Mesch, K.T.; Tristan, C.A. hPSC-Derived Astrocytes at the Forefront of Translational Applications in Neurological Disorders. Cells 2024, 13, 903. https://doi.org/10.3390/cells13110903
Jovanovic VM, Mesch KT, Tristan CA. hPSC-Derived Astrocytes at the Forefront of Translational Applications in Neurological Disorders. Cells. 2024; 13(11):903. https://doi.org/10.3390/cells13110903
Chicago/Turabian StyleJovanovic, Vukasin M., Kendall T. Mesch, and Carlos A. Tristan. 2024. "hPSC-Derived Astrocytes at the Forefront of Translational Applications in Neurological Disorders" Cells 13, no. 11: 903. https://doi.org/10.3390/cells13110903
APA StyleJovanovic, V. M., Mesch, K. T., & Tristan, C. A. (2024). hPSC-Derived Astrocytes at the Forefront of Translational Applications in Neurological Disorders. Cells, 13(11), 903. https://doi.org/10.3390/cells13110903








