Exploring Importance and Regulation of Autophagy in Cancer Stem Cells and Stem Cell-Based Therapies
Abstract
1. Introduction
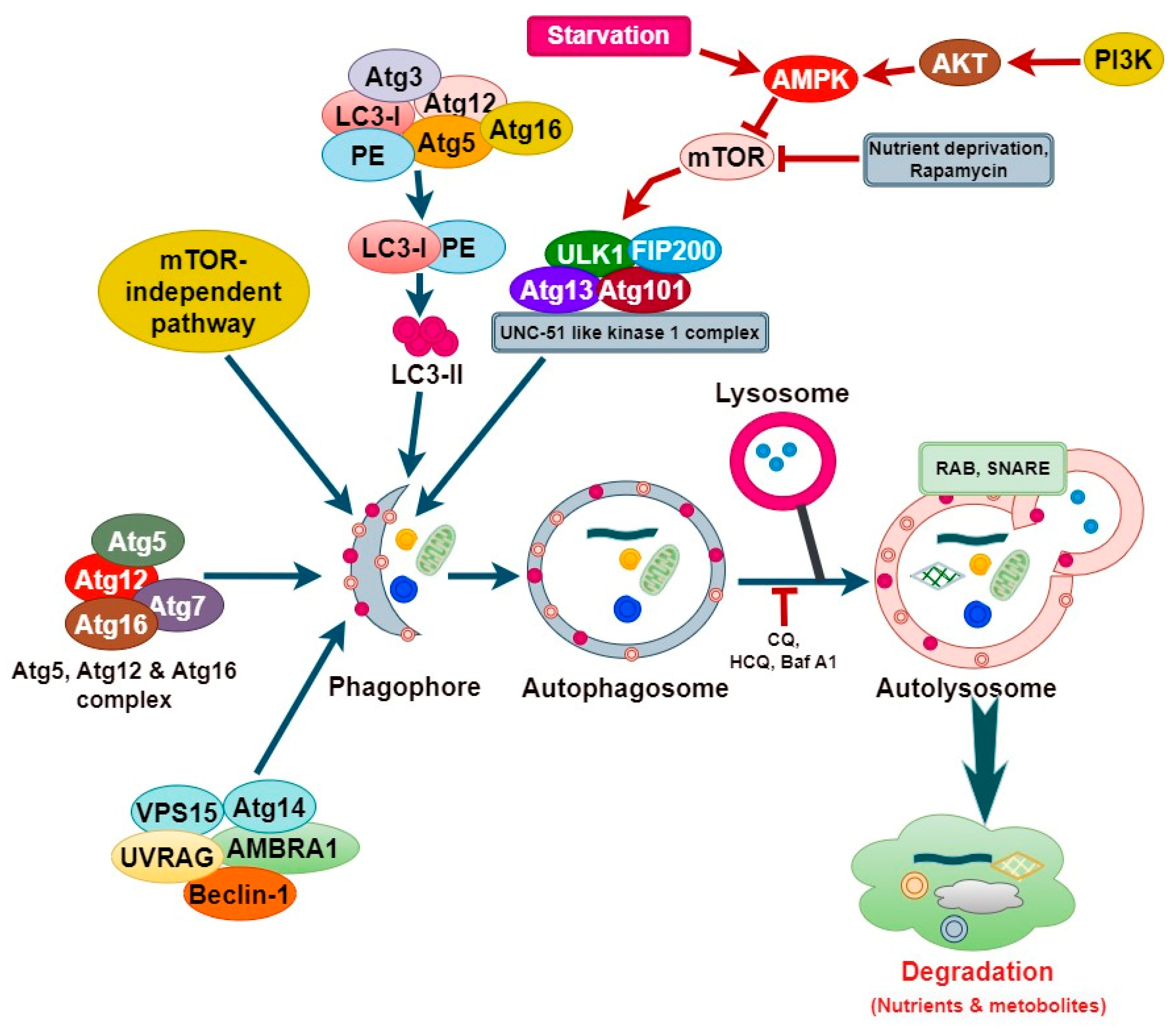
2. Molecular Mechanism of Autophagy
3. Formation and Biological Function of Cancer Stem Cells
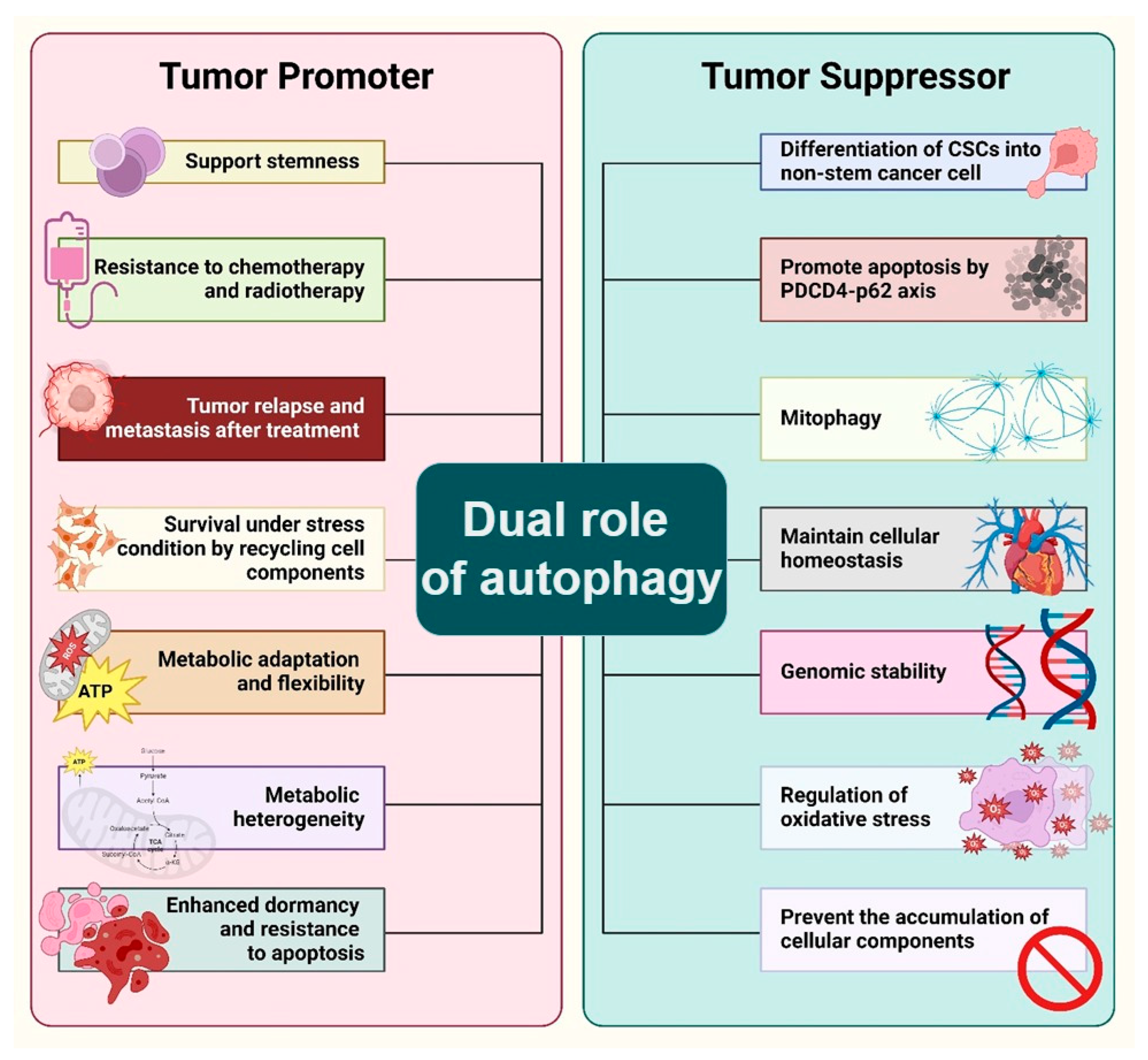
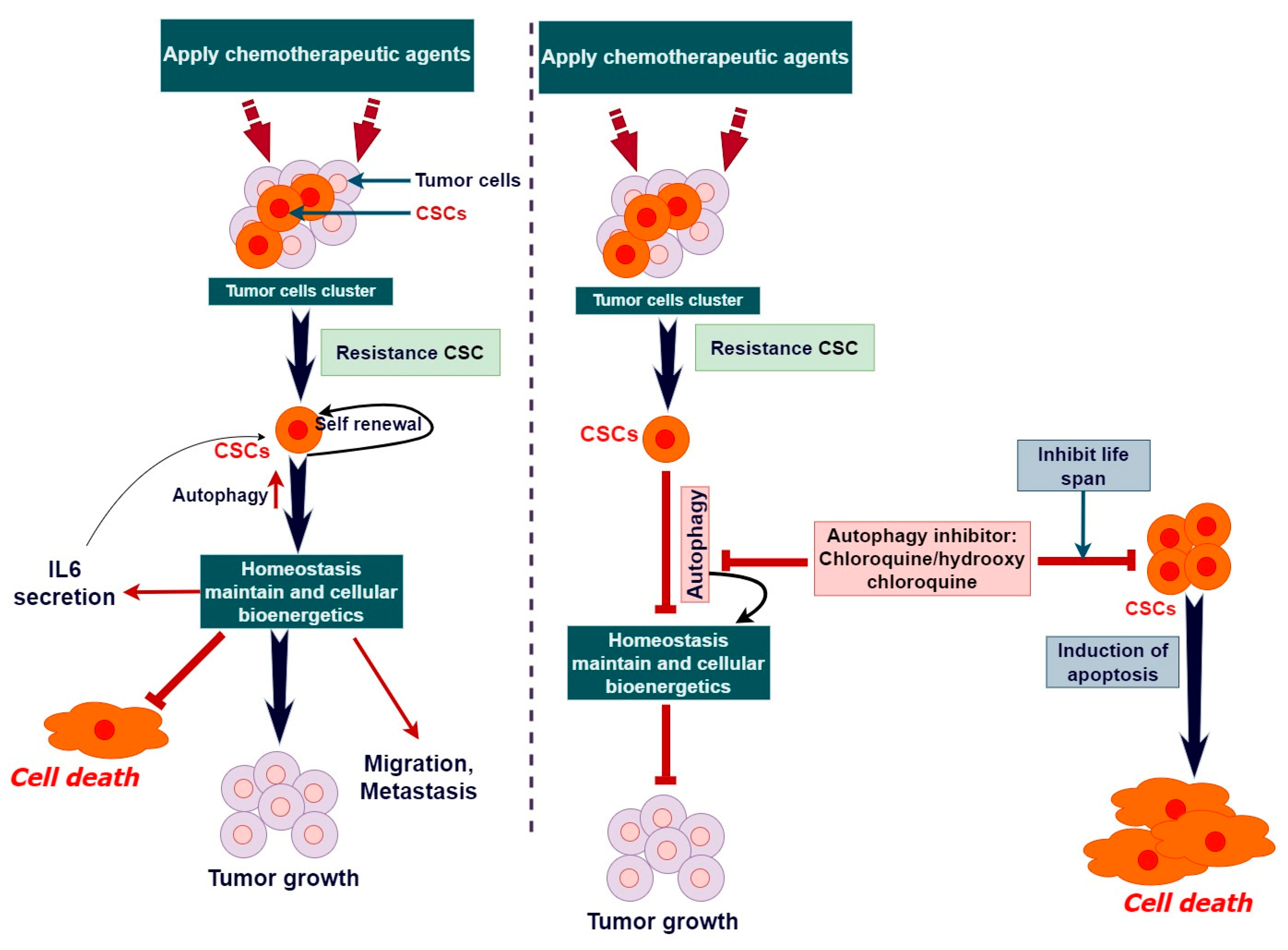
4. Dual Role of Autophagy in the Maintenance of Cancer Stem Cell
5. Cancer Stem Cell Model of Tissue and Organoid Regeneration
6. Therapeutic Role of Autophagy in Cancer Stem Cell and Stem Cell Therapy
7. Limitation and Challenges of Autophagy in Cancer Stem Cell and Stem Cell Therapy
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rahman, M.A.; Rahman, M.H.; Mamun-Or-Rashid, A.N.; Hwang, H.; Chung, S.; Kim, B.; Rhim, H. Autophagy modulation in aggresome formation: Emerging implications and treatments of Alzheimer’s disease. Biomedicines 2022, 10, 1027. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rahman, M.H.; Mamun-Or-Rashid, A.N.; Hwang, H.; Chung, S.; Kim, B.; Rhim, H. Molecular insights into therapeutic potential of autophagy modulation by natural products for cancer stem cells. Front. Cell Dev. Biol. 2020, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.W. The origins of human pluripotent stem cells: The road from a cancer to regenerative medicine. Vitr. Cell. Dev. Biol.-Anim. 2024, 60, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Prabhu, V.; Nehru, M. The stem cell niche: Key role on cell therapy in regenerative medicine. In Cancer Stem Cells and Signaling Pathways; Elsevier: Amsterdam, The Netherlands, 2024; pp. 265–275. [Google Scholar]
- Rahman, M.A.; Ahmed, K.R.; Haque, F.; Park, M.N.; Kim, B. Recent advances in cellular signaling interplay between redox metabolism and autophagy modulation in cancer: An overview of molecular mechanisms and therapeutic interventions. Antioxidants 2023, 12, 428. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Xia, Y.; Yin, Y.; Zhu, T.; Chen, F.; Hai, C. Autophagy, a double-edged sword for oral tissue regeneration. J. Adv. Res. 2023, 59, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Maleki, E.H.; Bahrami, A.R.; Matin, M.M. Cancer cell cycle heterogeneity as a critical determinant of therapeutic resistance. Genes. Dis. 2024, 11, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Nairuz, T.; Mahmud, Z.; Manik, R.K.; Kabir, Y. Cancer stem cells: An insight into the development of metastatic tumors and therapy resistance. Stem Cell Rev. Rep. 2023, 19, 1577–1595. [Google Scholar] [CrossRef]
- Ghosh, S.; Ts, D.P.; Chourasia, R.K.; Mahmood, A.A. Cancer Stem Cells: Cancer Stem Cells: Potential For Treatment. Int. J. Trends OncoSci. 2023, 72, 3411–3424. [Google Scholar] [CrossRef]
- Khan, A.Q.; Hasan, A.; Mir, S.S.; Rashid, K.; Uddin, S.; Stienhoff, M. Exploiting Transcription Factors to Target EMT and Cancer Stem Cells for Tumor Modulation and Therapy. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Strippoli, R.; Niayesh-Mehr, R.; Adelipour, M.; Khosravi, A.; Cordani, M.; Zarrabi, A.; Allameh, A. Contribution of Autophagy to Epithelial Mesenchymal Transition Induction during Cancer Progression. Cancers 2024, 16, 807. [Google Scholar] [CrossRef]
- Sharma, K.; Dey, S.; Karmakar, R.; Rengan, A.K. A comprehensive review of 3D cancer models for drug screening and translational research. Cancer Innov. 2024, 3, e102. [Google Scholar] [CrossRef]
- Lamichhane, A.; Tavana, H. Three-Dimensional Tumor Models to Study Cancer Stemness-Mediated Drug Resistance. Cell. Mol. Bioeng. 2024, 17, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.-L.; Jia, Y.-B.; Liu, X.-J.; Jia, Q.-L.; Guo, L.-K.; Wang, X.-X.; Yang, K.-M.; Wu, C.-H.; Liang, B.-B.; Ling, J.-H. Bibliometrics analysis based on the Web of Science: Current trends and perspective of gastric organoid during 2010–2023. World J. Gastroenterol. 2024, 30, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Srivastava, P.; Abbas, S.; Jegatheesan, J.; Ranjan, A.; Sharma, S.; Maurya, V.P.; Saxena, A.K.; Sharma, L.K. Emerging Role of Autophagy in Governing Cellular Dormancy, Metabolic Functions, and Therapeutic Responses of Cancer Stem Cells. Cells 2024, 13, 447. [Google Scholar] [CrossRef]
- Li, Y.-R.; Fang, Y.; Lyu, Z.; Zhu, Y.; Yang, L. Exploring the dynamic interplay between cancer stem cells and the tumor microenvironment: Implications for novel therapeutic strategies. J. Transl. Med. 2023, 21, 686. [Google Scholar] [CrossRef] [PubMed]
- Mia, M.A.R.; Dey, D.; Sakib, M.R.; Biswas, M.Y.; Prottay, A.A.S.; Paul, N.; Rimti, F.H.; Abdullah, Y.; Biswas, P.; Iftehimul, M.; et al. The efficacy of natural bioactive compounds against prostate cancer: Molecular targets and synergistic activities. Phytother. Res. 2023, 37, 5724–5754. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.H.; Hossain, M.S.; Biswas, P.; Islam, R.; Uddin, M.J.; Rahman, M.H.; Rhim, H. Molecular insights into the multifunctional role of natural compounds: Autophagy modulation and cancer prevention. Biomedicines 2020, 8, 517. [Google Scholar] [CrossRef]
- Eid, R.A.; Edeen, M.A.; Shedid, E.M.; Kamal, A.S.S.; Warda, M.M.; Mamdouh, F.; Khedr, S.A.; Soltan, M.A.; Jeon, H.W.; Zaki, M.S.A.; et al. Targeting cancer stem cells as the key driver of carcinogenesis and therapeutic resistance. Int. J. Mol. Sci. 2023, 24, 1786. [Google Scholar] [CrossRef] [PubMed]
- Doustmihan, A.; Fathi, M.; Mazloomi, M.; Salemi, A.; Hamblin, M.R.; Jahanban-Esfahlan, R. Molecular targets, therapeutic agents and multitasking nanoparticles to deal with cancer stem cells: A narrative review. J. Control. Release 2023, 363, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Vitto, V.A.M.; Bianchin, S.; Zolondick, A.A.; Pellielo, G.; Rimessi, A.; Chianese, D.; Yang, H.; Carbone, M.; Pinton, P.; Giorgi, C.; et al. Molecular Mechanisms of Autophagy in Cancer Development, Progression, and Therapy. Biomedicines 2022, 10, 1596. [Google Scholar] [CrossRef]
- Mazure, N.M.; Pouysségur, J. Hypoxia-induced autophagy: Cell death or cell survival? Curr. Opin. Cell. Biol. 2010, 22, 177–180. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Badadani, M. Autophagy Mechanism, Regulation, Functions, and Disorders. ISRN Cell Biol. 2012, 2012, 927064. [Google Scholar] [CrossRef]
- Polson, H.E.; de Lartigue, J.; Rigden, D.J.; Reedijk, M.; Urbé, S.; Clague, M.J.; Tooze, S.A. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy 2010, 6, 506–522. [Google Scholar] [CrossRef] [PubMed]
- Dooley, H.C.; Razi, M.; Polson, H.E.; Girardin, S.E.; Wilson, M.I.; Tooze, S.A. WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol. Cell 2014, 55, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Ktistakis, N.T.; Andrews, S.; Long, J. What is the advantage of a transient precursor in autophagosome biogenesis? Autophagy 2011, 7, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Debnath, J. Autophagy at the crossroads of catabolism and anabolism. Nat. Rev. Mol. Cell Biol. 2015, 16, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the Integrated Stress Response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Shen, H.-M.; Mizushima, N. At the end of the autophagic road: An emerging understanding of lysosomal functions in autophagy. Trends Biochem. Sci. 2014, 39, 61–71. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Baba, M.; Cao, Y.; Klionsky, D.J.; Ohashi, Y.; Munro, S.; Glick, M.E.B.S.; Geng, J.; Nair, U.; Yasumura-Yorimitsu, K.; et al. Self-interaction is critical for Atg9 transport and function at the phagophore assembly site during autophagy. Mol. Biol. Cell 2008, 19, 5506–5516. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.M.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells—Perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Bjerkvig, R.; Tysnes, B.B.; Aboody, K.S.; Najbauer, J.; Terzis, A.J. Opinion: The origin of the cancer stem cell: Current controversies and new insights. Nat. Rev. Cancer 2005, 5, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Matsui, W.; Huff, C.A.; Wang, Q.; Malehorn, M.T.; Barber, J.; Tanhehco, Y.; Smith, B.D.; Civin, C.I.; Jones, R.J. Characterization of clonogenic multiple myeloma cells. Blood 2004, 103, 2332. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Huntly, B.J.P.; Gilliland, D.G. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat. Rev. Cancer 2005, 5, 311–321. [Google Scholar] [CrossRef]
- Wang, J.; Wakeman, T.P.; Lathia, J.D.; Hjelmeland, A.B.; Wang, X.F.; White, R.R.; Rich, J.N.; Sullenger, B.A. Notch promotes radioresistance of glioma stem cells. Stem Cells 2010, 28, 17–28. [Google Scholar] [CrossRef]
- Kanwar, S.S.; Yu, Y.; Nautiyal, J.; Patel, B.B.; Majumdar, A.P. The Wnt/beta-catenin pathway regulates growth and maintenance of colonospheres. Mol. Cancer 2010, 9, 212. [Google Scholar] [CrossRef]
- Pardal, R.; Clarke, M.F.; Morrison, S.J. Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer 2003, 3, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-D.; Ma, D.-S.; Ma, C.-Y.; Bai, Y. Research progress on cardiac tissue construction of mesenchymal stem cells for myocardial infarction. Curr. Stem Cell Res. Ther. 2024, 19, 942–958. [Google Scholar] [CrossRef] [PubMed]
- Fatima, K.; Jan, S.; Malik, F.; Khan, S.U. Role of Cancer Stem Cells in Drug Resistance, in Drug Resistance in Cancer: Mechanisms and Strategies; Springer: New York, NY, USA, 2024; pp. 77–120. [Google Scholar]
- Mäbert, K.; Cojoc, M.; Peitzsch, C.; Kurth, I.; Souchelnytskyi, S.; Dubrovska, A. Cancer biomarker discovery: Current status and future perspectives. Int. J. Radiat. Biol. 2014, 90, 659–677. [Google Scholar] [CrossRef] [PubMed]
- Atashzar, M.R.; Baharlou, R.; Karami, J.; Abdollahi, H.; Rezaei, R.; Pourramezan, F.; Moghaddam, S.H.Z. Cancer stem cells: A review from origin to therapeutic implications. J. Cell. Physiol. 2020, 235, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Quintana, E.; Shackleton, M.; Foster, H.R.; Fullen, D.R.; Sabel, M.S.; Johnson, T.M.; Morrison, S.J. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell 2010, 18, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.C.; Shyh-Chang, N.; Yang, H.; Rai, A.; Umashankar, S.; Ma, S.; Soh, B.S.; Sun, L.L.; Tai, B.C.; Nga, M.E.; et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell 2012, 148, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, H.D.; Nakano, I.; Lazareff, J.A.; Masterman-Smith, M.; Geschwind, D.H.; Bronner-Fraser, M.; Kornblum, H.I. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. USA 2003, 100, 15178–15183. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, T.; Liu, A.Y.; Ouyang, G. Differentiation and transdifferentiation potentials of cancer stem cells. Oncotarget 2015, 6, 39550–39563. [Google Scholar] [CrossRef] [PubMed]
- Bussolati, B.; Bruno, S.; Grange, C.; Ferrando, U.; Camussi, G. Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB J. 2008, 22, 3696–3705. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A Perspective on Cancer Cell Metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Cioffi, M.; D’Alterio, C.; Camerlingo, R.; Tirino, V.; Consales, C.; Riccio, A.; Ierano, C.; Cecere, S.C.; Losito, N.S.; Greggi, S.; et al. Identification of a distinct population of CD133+CXCR4+ cancer stem cells in ovarian cancer. Sci. Rep. 2015, 5, 10357. [Google Scholar] [CrossRef]
- Liou, G.-Y. CD133 as a regulator of cancer metastasis through the cancer stem cells. Int. J. Biochem. Cell Biol. 2019, 106, 1–7. [Google Scholar] [CrossRef]
- Sampieri, K.; Fodde, R. Cancer stem cells and metastasis. Semin. Cancer Biol. 2012, 22, 187–193. [Google Scholar] [CrossRef]
- Collins, A.T.; Berry, P.A.; Hyde, C.; Stower, M.J.; Maitland, N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005, 65, 10946–10951. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Gupta, P.B.; Pastushenko, I.; Skibinski, A.; Blanpain, C.; Kuperwasser, C. Phenotypic Plasticity: Driver of Cancer Initiation, Progression, and Therapy Resistance. Cell Stem Cell 2019, 24, 65–78. [Google Scholar] [CrossRef]
- Yun, C.W.; Jeon, J.; Go, G.; Lee, J.H.; Lee, S.H. The dual role of autophagy in cancer development and a therapeutic strategy for cancer by targeting autophagy. Int. J. Mol. Sci. 2020, 22, 179. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef]
- Kimmelman, A.C.; White, E. Autophagy and Tumor Metabolism. Cell Metab. 2017, 25, 1037–1043. [Google Scholar] [CrossRef]
- Perrotta, C.; Cattaneo, M.G.; Molteni, R.; De Palma, C. Autophagy in the Regulation of Tissue Differentiation and Homeostasis. Front. Cell Dev. Biol. 2020, 8, 602901. [Google Scholar] [CrossRef]
- Flynn, A.L.B.; Calhoun, B.C.; Sharma, A.; Chang, J.C.; Almasan, A.; Schiemann, W.P. Autophagy inhibition elicits emergence from metastatic dormancy by inducing and stabilizing Pfkfb3 expression. Nat. Commun. 2019, 10, 3668. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-K.; Jeong, Y.-J.; Chang, Y.-C. PDCD4 inhibits lung tumorigenesis by the suppressing p62-Nrf2 signaling pathway and upregulating Keap1 expression. Am. J. Cancer Res. 2020, 10, 424–439. [Google Scholar]
- Gholamzad, A.; Khakpour, N.; Khosroshahi, E.M.; Asadi, S.; Koohpar, Z.K.; Matinahmadi, A.; Jebali, A.; Rashidi, M.; Hashemi, M.; Sadi, F.H.; et al. Cancer stem cells: The important role of CD markers, Signaling pathways, and MicroRNAs. Pathol.—Res. Pract. 2024, 256, 155227. [Google Scholar] [CrossRef]
- Naser, A.N.; Xing, T.; Tatum, R.; Lu, Q.; Boyer, P.J.; Chen, Y. Colonic crypt stem cell functions are controlled by tight junction protein claudin-7 through Notch/Hippo signaling. Ann. N. Y. Acad. Sci. 2024, 1535, 92–108. [Google Scholar] [CrossRef]
- Vara-Perez, M.; Felipe-Abrio, B.; Agostinis, P. Mitophagy in Cancer: A Tale of Adaptation. Cells 2019, 8, 493. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.-H.; Cao, Y.-T.; Zhang, L.-L.; Huang, F.; Yi, C. The Role of Mitochondrial Dynamics and Mitophagy in Carcinogenesis, Metastasis and Therapy. Front. Cell Dev. Biol. 2020, 8, 413. [Google Scholar] [CrossRef]
- Boya, P.; Codogno, P.; Rodriguez-Muela, N. Autophagy in stem cells: Repair, remodelling and metabolic reprogramming. Development 2018, 145, dev146506. [Google Scholar] [CrossRef]
- Prasad, C.P.; Gogia, A.; Batra, A. Essential role of aerobic glycolysis in epithelial-to-mesenchymal transition during carcinogenesis. Clin. Transl. Oncol. 2022, 24, 1844–1855. [Google Scholar] [CrossRef]
- Shen, Y.-A.; Chen, C.-C.; Chen, B.-J.; Wu, Y.-T.; Juan, J.-R.; Chen, L.-Y.; Teng, Y.-C.; Wei, Y.-H. Potential therapies targeting metabolic pathways in cancer stem cells. Cells 2021, 10, 1772. [Google Scholar] [CrossRef]
- Chae, Y.C.; Kim, J.H. Cancer stem cell metabolism: Target for cancer therapy. BMB Rep. 2018, 51, 319. [Google Scholar] [CrossRef]
- Galal, M.A.; Al-Rimawi, M.; Hajeer, A.; Dahman, H.; Alouch, S.; Aljada, A. Metformin: A Dual-Role Player in Cancer Treatment and Prevention. Int. J. Mol. Sci. 2024, 25, 4083. [Google Scholar] [CrossRef] [PubMed]
- Farnie, G.; Sotgia, F.; Lisanti, M.P. High mitochondrial mass identifies a sub-population of stem-like cancer cells that are chemo-resistant. Oncotarget 2015, 6, 30472–30486. [Google Scholar] [CrossRef] [PubMed]
- El Hout, M.; Cosialls, E.; Mehrpour, M.; Hamai, A. Crosstalk between autophagy and metabolic regulation of cancer stem cells. Mol. Cancer 2020, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Murthy, A. Targeting Autophagy to Treat Cancer: Challenges and Opportunities. Front. Pharmacol. 2020, 11, 590344. [Google Scholar] [CrossRef] [PubMed]
- Antoszczak, M. A comprehensive review of salinomycin derivatives as potent anticancer and anti-CSCs agents. Eur. J. Med. Chem. 2019, 166, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Jain, H.; Dhawan, P.; Rao, S.; Lalwani, N.; Shand, H. The Impediments of Cancer Stem Cells and An Exploration into the Nanomedical Solutions for Glioblastoma. Anti-Cancer Agents Med. Chem. 2023, 23, 368–382. [Google Scholar] [CrossRef]
- He, S.; Gou, X.; Zhang, S.; Zhang, X.; Huang, H.; Wang, W.; Yi, L.; Zhang, R.; Duan, Z.; Zhou, P.; et al. Nanodelivery Systems as a Novel Strategy to Overcome Treatment Failure of Cancer. Small Methods 2024, 8, e2301127. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.M.-T.; Chan, H.-Y.; Aziz, N.A.; Ramasamy, T.S.; Bong, J.-J.; Ch’ng, E.S.; Armon, S.; Peh, S.-C.; Teow, S.-Y. Interplay of autophagy and cancer stem cells in hepatocellular carcinoma. Mol. Biol. Rep. 2021, 48, 3695–3717. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. Characterization of miR-34 Effectors in Colorectal Cancer. Ph.D. Thesis, Ludwig-Maximilians-Universität München, München, Germany, 2024. [Google Scholar]
- Borah, A.; Raveendran, S.; Rochani, A.; Maekawa, T.; Kumar, D.S. Targeting self-renewal pathways in cancer stem cells: Clinical implications for cancer therapy. Oncogenesis 2015, 4, e177. [Google Scholar] [CrossRef]
- Kim, J.; Orkin, S.H. Embryonic stem cell-specific signatures in cancer: Insights into genomic regulatory networks and implications for medicine. Genome Med. 2011, 3, 75. [Google Scholar] [CrossRef]
- Chang, J.C. Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine 2016, 95 (Suppl. 1), S20–S25. [Google Scholar] [CrossRef]
- Qiu, H.; Fang, X.; Luo, Q.; Ouyang, G. Cancer stem cells: A potential target for cancer therapy. Cell. Mol. Life Sci. 2015, 72, 3411–3424. [Google Scholar] [CrossRef]
- Vidal, S.J.; Rodriguez-Bravo, V.; Galsky, M.; Cordon-Cardo, C.; Domingo-Domenech, J. Targeting cancer stem cells to suppress acquired chemotherapy resistance. Oncogene 2014, 33, 4451–4463. [Google Scholar] [CrossRef]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- De Sanctis, J.B.; Charris, J.; Blanco, Z.; Ramirez, H.; Martínez, G.P.; Mijares, M.R. Molecular mechanisms of chloroquine and hydroxychloroquine used in cancer therapy. Anti-Cancer Agents Med. Chem. Former. Curr. Med. Chem.-Anti-Cancer Agents 2023, 23, 1122–1144. [Google Scholar] [CrossRef]
- El-Hussein, A.; Manoto, S.L.; Ombinda-Lemboumba, S.; Alrowaili, Z.A.; Mthunzi-Kufa, P. A review of chemotherapy and photodynamic therapy for lung cancer treatment. Anti-Cancer Agents Med. Chem. Former. Curr. Med. Chem.-Anti-Cancer Agents 2021, 21, 149–161. [Google Scholar] [CrossRef]
- Bati, G.; Okvur, D.P. Invadopodia: Proteolytic feet of cancer cells. Turk. J. Biol. 2014, 38, 740–747. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Ho, W.J.; Pham, E.A.; Kim, J.W.; Ng, C.W.; Kim, J.H.; Kamei, D.T.; Wu, B.M. Incorporation of multicellular spheroids into 3-D polymeric scaffolds provides an improved tumor model for screening anticancer drugs. Cancer Sci. 2010, 101, 2637–2643. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.L.; Joshi, R.; Thakuri, P.S.; Tavana, H. Liquid-based three-dimensional tumor models for cancer research and drug discovery. Exp. Biol. Med. 2016, 241, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Suarez, C.; Bracarda, S.; Stadler, W.M.; Donskov, F.; Lee, J.L.; et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019, 393, 2404–2415. [Google Scholar] [CrossRef]
- Breslin, S.; O’driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Walter, S.; Walzl, A.; Kramer, N.; Unger, C.; Scherzer, M.; Unterleuthner, D.; Hengstschläger, M.; Krupitza, G.; Dolznig, H. Increased complexity in carcinomas: Analyzing and modeling the interaction of human cancer cells with their microenvironment. Semin. Cancer Biol. 2015, 35, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.J.; Zhou, Y.; Shi, X.X.; Kang, Y.J.; Lu, Z.S.; Li, Y.; Li, C.M.; Yu, L. Spontaneous formation of tumor spheroid on a hydrophilic filter paper for cancer stem cell enrichment. Colloids Surf. B Biointerfaces 2019, 174, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Tveit, K.M.; Endresen, L.; E Rugstad, H.; Fodstad, H.; Pihl, A. Comparison of two soft-agar methods for assaying chemosensitivity of human tumours in vitro: Malignant melanomas. Br. J. Cancer 1981, 44, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, H.; Maier, A.; Burger, A. Clonogenic assay with established human tumour xenografts: Correlation of in vitro to in vivo activity as a basis for anticancer drug discovery. Eur. J. Cancer 2004, 40, 802–820. [Google Scholar] [CrossRef] [PubMed]
- Sant, S.; Johnston, P.A. The production of 3D tumor spheroids for cancer drug discovery. Drug Discov. Today Technol. 2017, 23, 27–36. [Google Scholar] [CrossRef]
- Sutherland, R.M. Cell and Environment interactions in tumor microregions: The multicell spheroid model. Science 1988, 240, 177–184. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Weiswald, L.-B.; Bellet, D.; Dangles-Marie, V. Spherical cancer models in tumor biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Estrada, M.F.; Rebelo, S.P.; Abreu, S.; Silva, I.; Pinto, C.; Veloso, S.C.; Serra, A.T.; Boghaert, E.; Alves, P.M.; et al. Adaptable stirred-tank culture strategies for large scale production of multicellular spheroid-based tumor cell models. J. Biotechnol. 2016, 221, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Curcio, E.; Salerno, S.; Barbieri, G.; De Bartolo, L.; Drioli, E.; Bader, A. Mass transfer and metabolic reactions in hepatocyte spheroids cultured in rotating wall gas-permeable membrane system. Biomaterials 2007, 28, 5487–5497. [Google Scholar] [CrossRef] [PubMed]
- Walenta, S.; Doetsch, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Metabolic imaging in multicellular spheroids of oncogene-transfected fibroblasts. J. Histochem. Cytochem. 2000, 48, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Ruppen, J.; Cortes-Dericks, L.; Marconi, E.; Karoubi, G.; Schmid, R.A.; Peng, R.; Marti, T.M.; Guenat, O.T. A microfluidic platform for chemoresistive testing of multicellular pleural cancer spheroids. Lab. A Chip 2014, 14, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Perez, M.; Voytik-Harbin, S.L.; Rickus, J.L. Extracellular Matrix Properties Regulate the Migratory Response of Glioblastoma Stem Cells in Three-Dimensional Culture. Tissue Eng. Part. A 2015, 21, 2572–2582. [Google Scholar] [CrossRef]
- Raghavan, S.; Mehta, P.; Xie, Y.; Lei, Y.L.; Mehta, G. Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments. J. Immunother. Cancer 2019, 7, 190. [Google Scholar] [CrossRef]
- Reynolds, D.S.; Tevis, K.M.; Blessing, W.A.; Colson, Y.L.; Zaman, M.H.; Grinstaff, M.W. Breast Cancer Spheroids Reveal a Differential Cancer Stem Cell Response to Chemotherapeutic Treatment. Sci. Rep. 2017, 7, 10382. [Google Scholar] [CrossRef]
- Muenzner, J.K.; Kunze, P.; Lindner, P.; Polaschek, S.; Menke, K.; Eckstein, M.; Geppert, C.I.; Chanvorachote, P.; Baeuerle, T.; Hartmann, A.; et al. Generation and characterization of hepatocellular carcinoma cell lines with enhanced cancer stem cell potential. J. Cell. Mol. Med. 2018, 22, 6238–6248. [Google Scholar] [CrossRef]
- Martins-Neves, S.R.; Lopes, O.; Carmo, A.D.; A Paiva, A.; Simões, P.C.; Abrunhosa, A.J.; Gomes, C.M. Therapeutic implications of an enriched cancer stem-like cell population in a human osteosarcoma cell line. BMC Cancer 2012, 12, 139. [Google Scholar] [CrossRef]
- Herreros-Pomares, A.; De-Maya-Girones, J.D.; Calabuig-Farinas, S.; Lucas, R.; Martinez, A.; Pardo-Sanchez, J.M.; Alonso, S.; Blasco, A.; Guijarro, R.; Martorell, M.; et al. Lung tumorspheres reveal cancer stem cell-like properties and a score with prognostic impact in resected non-small-cell lung cancer. Cell Death Dis. 2019, 10, 660. [Google Scholar] [CrossRef]
- Restall, I.; Bozek, D.; Chesnelong, C.; Weiss, S. Live-Cell Imaging Assays to Study Glioblastoma Brain Tumor Stem Cell Migration and Invasion. J. Vis. Exp. 2018, 138, 58152. [Google Scholar]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 9. [Google Scholar] [CrossRef]
- Huang, Y.; Agrawal, B.; Sun, D.; Kuo, J.S.; Williams, J.C. Microfluidics-based devices: New tools for studying cancer and cancer stem cell migration. Biomicrofluidics 2011, 5, 013412. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Hassan, S.; Miri, A.K.; Maharjan, S.; Al-Kharboosh, R.; Quinones-Hinojosa, A.; Zhang, Y.S. Mimicking human pathophysiology in organ-on-chip devices. Adv. Biosyst. 2018, 2, 1800109. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Trujillo-de Santiago, G.T.; Flores-Garza, B.G.; Tavares-Negrete, J.A.; Lara-Mayorga, I.M.; González-Gamboa, I.; Zhang, Y.S.; Rojas-Martínez, A.; Ortiz-López, R.; Álvarez, M.M. The Tumor-on-Chip: Recent Advances in the Development of Microfluidic Systems to Recapitulate the Physiology of Solid Tumors. Materials 2019, 12, 2945. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.M.; Van Dyke, T.; Merlino, G.; Day, C.-P. Concepts in Cancer Modeling: A Brief History. Cancer Res. 2016, 76, 5921–5925. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E. Mechanisms of cancer cell invasion. Curr. Opin. Genet. Dev. 2005, 15, 87–96. [Google Scholar] [CrossRef]
- Franco, S.S.; Szczesna, K.; Iliou, M.S.; Al-Qahtani, M.; Mobasheri, A.; Kobolák, J.; Dinnyés, A. In vitro models of cancer stem cells and clinical applications. BMC Cancer 2016, 16, 23–49. [Google Scholar] [CrossRef]
- Allegra, A.; Alonci, A.; Penna, G.; Innao, V.; Gerace, D.; Rotondo, F.; Musolino, C. The cancer stem cell hypothesis: A guide to potential molecular targets. Cancer Investig. 2014, 32, 470–495. [Google Scholar] [CrossRef]
- Ciurea, M.E.; Georgescu, A.M.; Purcaru, S.O.; Artene, S.-A.; Emami, G.H.; Boldeanu, M.V.; Tache, D.E.; Dricu, A. cancer stem cells: Biological functions and therapeutically targeting. Int. J. Mol. Sci. 2014, 15, 8169–8185. [Google Scholar] [CrossRef]
- Vermeulen, L.; Felipe De Sousa, E.M.; Van Der Heijden, M.; Cameron, K.; De Jong, J.H.; Borovski, T.; Tuynman, J.B.; Todaro, M.; Merz, C.; Rodermond, H.; et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010, 12, 468–476. [Google Scholar] [CrossRef]
- van de Stolpe, A. On the origin and destination of cancer stem cells: A conceptual evaluation. Am. J. Cancer Res. 2013, 3, 107–116. [Google Scholar]
- Nazio, F.; Bordi, M.; Cianfanelli, V.; Locatelli, F.; Cecconi, F. Autophagy and cancer stem cells: Molecular mechanisms and therapeutic applications. Cell Death Differ. 2019, 26, 690–702. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, D.; Yu, J.; Dong, H.; Zhang, J.; Yang, S. Targeting autophagy in cancer stem cells as an anticancer therapy. Cancer Lett. 2017, 393, 33–39. [Google Scholar] [CrossRef]
- Wang, Q.; Bu, S.; Xin, D.; Li, B.; Wang, L.; Lai, D. Autophagy Is Indispensable for the Self-Renewal and Quiescence of Ovarian Cancer Spheroid Cells with Stem Cell-Like Properties. Oxidative Med. Cell. Longev. 2018, 2018, 7010472. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, S.; Chen, S.; Chen, J.; Wang, Z.; Wang, Y.; Zheng, H. SOX2 promotes chemoresistance, cancer stem cells properties, and epithelial-mesenchymal transition by β-catenin and Beclin1/autophagy signaling in colorectal cancer. Cell Death Dis. 2021, 12, 449. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Cho, H.-Y.; Jahanian, A.; Hofman, F.M.; Louie, S.G.; Schönthal, A.H.; Chen, T.C. Chloroquine enhances temozolomide cytotoxicity in malignant gliomas by blocking autophagy. Neurosurg. Focus. 2014, 37, E12. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Liu, Y.; Li, J.; Huang, J.H.; Hu, X.; Wu, E. Salinomycin as a potent anticancer stem cell agent: State of the art and future directions. Med. Res. Rev. 2022, 42, 1037–1063. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.-F.; Chen, M.-W.; Chen, K.-C.; Lou, P.-J.; Lin, S.Y.-F.; Hung, S.-C.; Hsiao, M.; Yao, C.-J.; Shieh, M.-J. Autophagy promotes resistance to photodynamic therapy-induced apoptosis selectively in colorectal cancer stem-like cells. Autophagy 2014, 10, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Uriho, A.; Tang, X.; Le, G.; Yang, S.; Harimana, Y.; Ishimwe, S.P.; Yiping, L.; Zhang, K.; Ma, S.; Muhoza, B. Effects of resveratrol on mitochondrial biogenesis and physiological diseases. Orient. Pharm. Exp. Med. 2021, 21, 1–14. [Google Scholar] [CrossRef]
- Xie, C.; Liang, C.; Wang, R.; Yi, K.; Zhou, X.; Li, X.; Chen, Y.; Miao, D.; Zhong, C.; Zhu, J. Resveratrol suppresses lung cancer by targeting cancer stem-like cells and regulating tumor microenvironment. J. Nutr. Biochem. 2023, 112, 109211. [Google Scholar] [CrossRef] [PubMed]
- Kreso, A.; van Galen, P.; Pedley, N.M.; Lima-Fernandes, E.; Frelin, C.; Davis, T.; Cao, L.; Baiazitov, R.; Du, W.; Sydorenko, N.; et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat. Med. 2014, 20, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Singh, S.K.; Saxena, A.K.; Tiwari, S.; Sharma, L.K.; Tiwari, M. Role of autophagy in regulation of glioma stem cells population during therapeutic stress. J. Stem Cells Regen. Med. 2020, 16, 80–89. [Google Scholar] [CrossRef]
- Chen, H.; Chan, D.C. Mitochondrial Dynamics in Regulating the Unique Phenotypes of Cancer and Stem Cells. Cell Metab. 2017, 26, 39–48. [Google Scholar] [CrossRef]
- Smith, A.G.; MacLeod, K.F. Autophagy, cancer stem cells and drug resistance. J. Pathol. 2019, 247, 708–718. [Google Scholar] [CrossRef]
- Li, D.; Peng, X.; He, G.; Liu, J.; Li, X.; Lin, W.; Fang, J.; Li, X.; Yang, S.; Yang, L.; et al. Crosstalk between autophagy and CSCs: Molecular mechanisms and translational implications. Cell Death Dis. 2023, 14, 409. [Google Scholar] [CrossRef]
- Bisht, S.; Nigam, M.; Kunjwal, S.S.; Sergey, P.; Mishra, A.P.; Sharifi-Rad, J. Cancer Stem Cells: From an Insight into the Basics to Recent Advances and Therapeutic Targeting. Stem Cells Int. 2022, 2022, 9653244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, F.; Wong, E.T.; Fonkem, E.; Hsieh, T.-C.; Wu, J.M.; Wu, E. Salinomycin: A Novel Anti-Cancer Agent with Known Anti-Coccidial Activities. Curr. Med. Chem. 2013, 20, 4095–4101. [Google Scholar] [CrossRef] [PubMed]
- Joshi, E.; Pandya, M.; Desai, U. Current Drugs and their Therapeutic Targets for Hypoxia-inducible Factors in Cancer. Curr. Protein Pept. Sci. 2023, 24, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Fath, M.K.; Ebrahimi, M.; Nourbakhsh, E.; Hazara, A.Z.; Mirzaei, A.; Shafieyari, S.; Salehi, A.; Hoseinzadeh, M.; Payandeh, Z.; Barati, G. PI3K/Akt/mTOR signaling pathway in cancer stem cells. Pathol.-Res. Pract. 2022, 237, 154010. [Google Scholar] [CrossRef]
- Herzog, A.E.; Warner, K.A.; Zhang, Z.; Bellile, E.; Bhagat, M.A.; Castilho, R.M.; Wolf, G.T.; Polverini, P.J.; Pearson, A.T.; Nör, J.E. The IL-6R and Bmi-1 axis controls self-renewal and chemoresistance of head and neck cancer stem cells. Cell Death Dis. 2021, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, M.Y.; Golmohammadi, M.; Yumashev, A.; Hjazi, A.; Toama, M.A.; AbdRabou, M.A.; Gehlot, A.; Alwaily, E.R.; Shirsalimi, N.; Yadav, P.K.; et al. Effects of metformin on cancers in experimental and clinical studies: Focusing on autophagy and AMPK/mTOR Signaling Pathways. Authorea Prepr. 2024. [Google Scholar] [CrossRef]
- Borlongan, M.C.; Saha, D.; Wang, H. Tumor Microenvironment: A Niche for Cancer Stem Cell Immunotherapy. Stem Cell Rev. Rep. 2023, 20, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Ajmeera, D.; Ajumeera, R. Drug repurposing: A novel strategy to target cancer stem cells and therapeutic resistance. Genes Dis. 2024, 11, 148–175. [Google Scholar] [CrossRef] [PubMed]
- Agalakova, N.I. Chloroquine and Chemotherapeutic Compounds in Experimental Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 945. [Google Scholar] [CrossRef]
- He, K.; Chen, M.; Liu, J.; Du, S.; Ren, C.; Zhang, J. Nanomedicine for cancer targeted therapy with autophagy regulation. Front. Immunol. 2024, 14, 1238827. [Google Scholar] [CrossRef]
- Li, Y.; Ma, R.; Hao, X. Therapeutic role of PTEN in tissue regeneration for management of neurological disorders: Stem cell behaviors to an in-depth review. Cell Death Dis. 2024, 15, 268. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Sun, M.; Liang, X.; Li, J.; Zhou, F.; Zhong, Z.; Zheng, Y. The Controversy, Challenges, and Potential Benefits of Putative Female Germline Stem Cells Research in Mammals. Stem Cells Int. 2015, 2016, 1728278. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-M.; Zhang, Q.; Zhou, R.-X.; Wu, Y.-L.; Li, Z.-X.; Zhang, D.-Y.; Yang, Y.-C.; Yang, R.-H.; Hu, Y.-J.; Xiong, K. Programmed cell death in stem cell-based therapy: Mechanisms and clinical applications. World J. Stem Cells 2021, 13, 386–415. [Google Scholar] [CrossRef] [PubMed]
- Balic, A.; Sørensen, M.D.; Trabulo, S.M.; Sainz, B., Jr.; Cioffi, M.; Vieira, C.R.; Miranda-Lorenzo, I.; Hidalgo, M.; Kleeff, J.; Erkan, M.; et al. Chloroquine Targets Pancreatic Cancer Stem Cells via Inhibition of CXCR4 and Hedgehog Signaling. Mol. Cancer Ther. 2014, 13, 1758–1771. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.A.; Iliopoulos, D.; Tsichlis, P.N.; Struhl, K. Metformin Selectively Targets Cancer Stem Cells, and Acts Together with Chemotherapy to Block Tumor Growth and Prolong Remission. Cancer Res. 2009, 69, 7507–7511. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.M.P.; de Sousa, R.W.R.; Ferreira, J.R.d.O.; Militão, G.C.G.; Bezerra, D.P. Chloroquine and hydroxychloroquine in antitumor therapies based on autophagy-related mechanisms. Pharmacol. Res. 2021, 168, 105582. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Cheng, X.; Du, R.; Xie, Y.; Zhang, Y. Advances in research on autophagy mechanisms in resistance to endometrial cancer treatment. Front. Oncol. 2024, 14, 1364070. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Elsaghir, A.; Sijercic, V.C.; Akhtar, M.S.; Gad, S.A.; Moses, A.; Zeleke, M.S.; Alanazi, F.E.; Ahmed, A.K.; Ramadan, Y.N. Mesenchymal stem cell therapy in diabetic foot ulcer: An updated comprehensive review. Health Sci. Rep. 2024, 7, e2036. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H. Biomarkers and targeted therapy for cancer stem cells. Trends Pharmacol. Sci. 2024, 45, 56–66. [Google Scholar] [CrossRef]
- Masoudi, M.; Moti, D.; Masoudi, R.; Auwal, A.; Hossain, M.M.; Pronoy, T.U.H.; Rashel, K.M.; Gopalan, V.; Islam, F. Metabolic adaptations in cancer stem cells: A key to therapy resistance. Biochim. et Biophys. Acta BBA-Mol. Basis Dis. 2024, 1870, 167164. [Google Scholar] [CrossRef]
- Pirmoradi, L.; Shojaei, S.; Ghavami, S.; Zarepour, A.; Zarrabi, A. Autophagy and Biomaterials: A Brief Overview of the Impact of Autophagy in Biomaterial Applications. Pharmaceutics 2023, 15, 2284. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Rayees, S.; Sharma, P.; Malik, F. Targeting redox regulation and autophagy systems in cancer stem cells. Clin. Exp. Med. 2022, 23, 1405–1423. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Wen, L. Development of Novel Therapeutic Strategies to Target Therapy Resistance and Cancer Stem Cells. Ph.D. Thesis, The University of Sydney, Camperdown, NSW, Australia, 2023. [Google Scholar]
- Kruyt, F.A. Cancer stem cells and cellular plasticity: A preface to the special issue “Advances in understanding cancer stem cell biology and perspectives for targeted therapy”. Biochem. Pharmacol. 2023, 214, 115670. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.; Gupta, G.; Bhat, A.A.; Almalki, W.H.; Alzarea, S.I.; Kazmi, I.; Saleem, S.; Khan, R.; Altwaijry, N.; Dureja, H.; et al. A review of Glycogen Synthase Kinase-3 (GSK3) inhibitors for cancers therapies. Int. J. Biol. Macromol. 2023, 253, 127375. [Google Scholar] [CrossRef] [PubMed]


| Aspect | Mechanism of Autophagy in CSCs | References |
|---|---|---|
| Therapeutic Role | Under stress, autophagy helps CSCs survive. Using autophagy to sensitize CSCs to conventional chemo and radiation can overcome treatment resistance. | [131] |
| CSCs Characteristics | CSCs have higher autophagy than non-stem cells, which helps them self-renew, initiate tumors, and resist therapy. | [143] |
| Chemoresistance | CSC viability and tumorigenicity can be reduced by autophagy inhibition. Blocking autophagy in ovarian CSCs enhances carboplatin therapy. The SOX2/β-catenin/Beclin1/autophagy pathway promotes colorectal cancer stemness and chemoresistance. | [144] |
| Drug Resistance | Activation of autophagy is associated with resistance to temozolomide and promotes the ability of cancer stem cells to change and adapt. | [145] |
| Therapeutic Strategies | Agents, such as salinomycin, chloroquine, nicardipine, and photodynamic treatment can sensitize CSCs to conventional chemotherapy by inhibiting autophagy. Resveratrol can slow lung cancer progression by targeting stem-like cells and altering the tumor microenvironment. | [146,147] |
| Pathway Inhibition | Drugs and chemicals that inhibit Akt, mTOR, and Hedgehog signaling pathways have been tested for their capacity to control CSC autophagy. Preclinical models indicate potential for CSC-specific protein inhibitors like BMI-1. | [148,149] |
| Challenges | Due to CSC heterogeneity and autophagy regulation complexity, developing effective treatments is tough. Therapy failure and disease recurrence occur because CSCs use autophagy to evade cell death from therapeutic interventions. | [150] |
| Immune Evasion | Autophagy, CSCs, and the tumor microenvironment interact to modulate immune responses and promote immune evasion in CSCs. | [151] |
| Combination Therapies | Combination medicines targeting autophagy and conventional cancer treatments may improve results and overcome therapeutic resistance in CSCs. More preclinical and clinical investigations are needed to confirm efficacy and safety. | [152] |
| Pharmacological Substances | In addition to typical cancer treatments, chloroquine and hydroxychloroquine have been explored to render CSCs more sensitive to cell death. | [153] |
| Genetic Interventions | Genetic therapies targeting autophagy regulators including ATG genes and mTOR signaling have demonstrated promising results in preclinical cancer models. | [154] |
| Regenerative Medicine | Regenerative medicine and cancer treatment depend on autophagy to control stem cell fate and function. It removes damaged organelles and protein aggregates to protect stem cells’ ability to replenish and differentiate. | [155,156] |
| Stem Cell Therapy | Autophagy increases stem cell survival and integration following transplantation, improving stem cell-based therapies. Research is needed to understand the complex interaction between autophagy, CSCs, and stem cell therapies. | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.A.; Apu, E.H.; Rakib-Uz-Zaman, S.M.; Chakraborti, S.; Bhajan, S.K.; Taleb, S.A.; Shaikh, M.H.; Jalouli, M.; Harrath, A.H.; Kim, B. Exploring Importance and Regulation of Autophagy in Cancer Stem Cells and Stem Cell-Based Therapies. Cells 2024, 13, 958. https://doi.org/10.3390/cells13110958
Rahman MA, Apu EH, Rakib-Uz-Zaman SM, Chakraborti S, Bhajan SK, Taleb SA, Shaikh MH, Jalouli M, Harrath AH, Kim B. Exploring Importance and Regulation of Autophagy in Cancer Stem Cells and Stem Cell-Based Therapies. Cells. 2024; 13(11):958. https://doi.org/10.3390/cells13110958
Chicago/Turabian StyleRahman, Md Ataur, Ehsanul Hoque Apu, S. M Rakib-Uz-Zaman, Somdeepa Chakraborti, Sujay Kumar Bhajan, Shakila Afroz Taleb, Mushfiq H. Shaikh, Maroua Jalouli, Abdel Halim Harrath, and Bonglee Kim. 2024. "Exploring Importance and Regulation of Autophagy in Cancer Stem Cells and Stem Cell-Based Therapies" Cells 13, no. 11: 958. https://doi.org/10.3390/cells13110958
APA StyleRahman, M. A., Apu, E. H., Rakib-Uz-Zaman, S. M., Chakraborti, S., Bhajan, S. K., Taleb, S. A., Shaikh, M. H., Jalouli, M., Harrath, A. H., & Kim, B. (2024). Exploring Importance and Regulation of Autophagy in Cancer Stem Cells and Stem Cell-Based Therapies. Cells, 13(11), 958. https://doi.org/10.3390/cells13110958











