Abstract
Matrin-3 (MATR3) was initially discovered as a component of the nuclear matrix about thirty years ago. Since then, accumulating studies have provided evidence that MATR3 not only plays a structural role in the nucleus, but that it is also an active protein involved in regulating gene expression at multiple levels, including chromatin organization, DNA transcription, RNA metabolism, and protein translation in the nucleus and cytoplasm. Furthermore, MATR3 may play a critical role in various cellular processes, including DNA damage response, cell proliferation, differentiation, and survival. In addition to the revelation of its biological role, recent studies have reported MATR3’s involvement in the context of various diseases, including neurodegenerative and neurodevelopmental diseases, as well as cancer. Moreover, sequencing studies of patients revealed a handful of disease-associated mutations in MATR3 linked to amyotrophic lateral sclerosis (ALS), which further elevated the gene’s importance as a topic of study. In this review, we synthesize the current knowledge regarding the diverse functions of MATR3 in DNA- and RNA-related processes, as well as its involvement in various diseases, with a particular emphasis on ALS.
1. Introduction
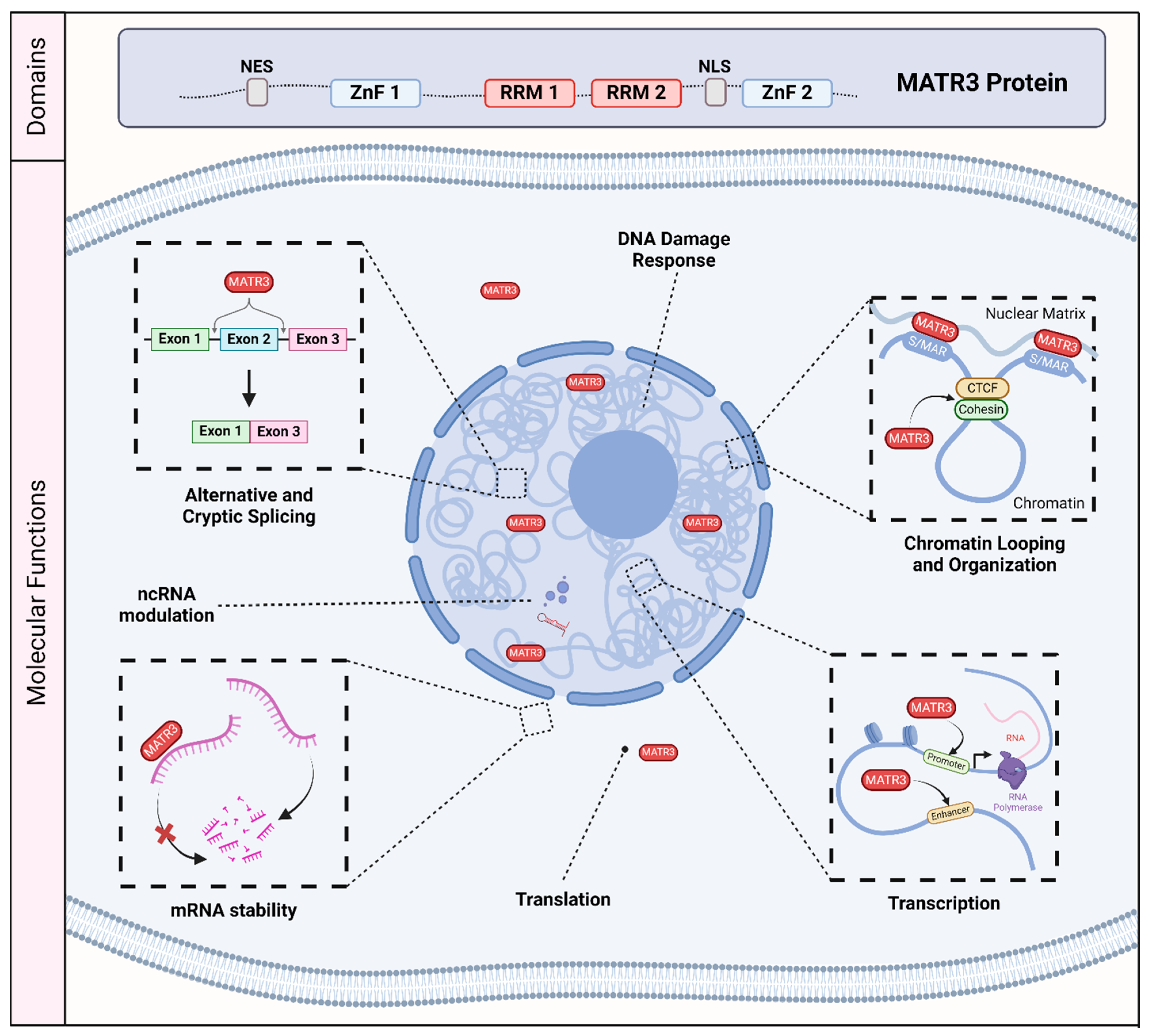
Matrin-3 (MATR3) was initially discovered as one of the major components of the inner nuclear matrix [1,2]. It has also been independently discovered as an A/T-rich DNA-binding nuclear scaffold protein initially named P130, and as a hypo-phosphorylated variant named P123, but was later renamed as MATR3 [3,4,5,6,7]. MATR3 is primarily localized to the nucleus, consistent with its primary sequence containing a bipartite nuclear localization signal (NLS) [7,8]. It also encodes a nuclear export signal (NES) [7] which may suggest that MATR3 may also play a role in the cytoplasm. In addition, MATR3 has two C2H2 zinc finger (ZnF) domains which allow for binding to DNA, and two RNA recognition motifs (RRM) which allow for binding to RNA [7] (Figure 1).
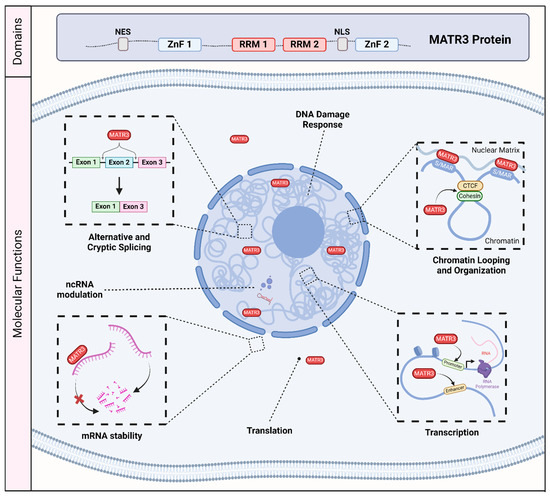
Figure 1.
The molecular and biological roles of MATR3. MATR3 contains a nuclear export signal (NES), a nuclear localization signal (NLS), as well as two zinc finger (ZnF) domains and two RNA recognition motifs (RRM). As a nucleic acid binding protein, MATR3 plays a role in various process associated with DNA and RNA, including the DNA damage response, chromatin looping and organization, transcription, translation, mRNA stability, modulation of noncoding RNA (ncRNA), and RNA splicing. Scaffold/matrix attachment region, S/MAR; CCCTC-binding factor, CTCF.
As MATR3 was initially described as a nuclear matrix protein, MATR3 may play a structural or scaffolding role. However, accumulating studies since its discovery have demonstrated its role in various molecular and cellular processes involving DNA and RNA, including chromatin organization [9,10,11], DNA transcription and repair [6,12,13,14], and RNA splicing [15,16,17,18,19] (Figure 1). Recent studies have investigated its biological function in various cell types, including induced pluripotent stem cells, neuronal stem cells, differentiated neurons, and muscle cells, expanding our knowledge of the roles that MATR3 plays in modulating cellular health and function [10,11,12,20,21,22,23]. In addition, there have been an increasing number of studies implicating MATR3 in a variety of diseases. Most notably, missense mutations in MATR3 have been linked to amyotrophic lateral sclerosis (ALS), a fatal neurodegenerative disease caused by the loss of motor neurons [24], as well as in neurodevelopmental diseases [16,25]. Furthermore, recent studies have revealed that MATR3 may play a critical role in other diseases, including cancer [26,27,28,29,30,31].
Here, we summarize the reported roles that MATR3 plays in both DNA- and RNA-associated processes. In addition, we discuss the role of MATR3 in the modulation of cellular health and function. Lastly, we touch upon the involvement of MATR3 in the context of disease, with a particular emphasis on ALS.
2. MATR3’s Role in DNA-Related Processes
Accumulating studies have shown that MATR3 plays a role in a variety of functions related to DNA (Figure 1). MATR3 contains two C2H2-type ZnF domains that work cooperatively to bind DNA. Deletion of one ZnF domain reduces DNA binding, while deletion of both ZnF domains completely abolishes this capability [7]. Early studies have demonstrated that MATR3 recognizes and binds to repetitive, adenine/thymine (A/T)-rich DNA fragments, and that the DNA binding ability of MATR3 depends on its serine/threonine phosphorylation and the degree of the DNA bending [3,4,5]. MATR3 has also been demonstrated to bind to DNA fragments corresponding to the A/T-rich scaffold or matrix attachment region (S/MAR) [6], a chromosomal region that anchors chromatin to the nuclear matrix [32]. Additionally, MATR3 has been shown to associate with other S/MAR binding proteins, such as scaffold attachment factor A (SAFA) and scaffold attachment factor B (SAFB) [33]. Furthermore, immunostaining studies showed that MATR3 appears as a higher-order network-like structure that diffusely labels the nucleoplasm [9,33,34]. These studies provided clues that MATR3 may play a role in organizing chromatin structure.
MATR3’s role in chromatin organization is further supported by protein–protein interaction studies showing that MATR3 interacts with a variety of chromatin remodelling factors as well as chromatin architectural proteins, such as CCCTC-binding factor (CTCF) and members of the cohesin complex [10,33,35] (Table 1). Moreover, loss of MATR3 led to a global reorganization of chromatin architecture in both mouse erythroleukemia (MEL) and mouse embryonic stem (mES) cells [10]. Furthermore, MATR3 loss in MEL cells decreased the chromatin occupancy of CTCF and the cohesin complex, which impaired proper chromatin interaction and expression of CTCF/cohesin-regulated genes, suggesting that MATR3 stabilizes the binding of CTCF/cohesin to chromatin, thereby maintaining the chromatin structure [10]. MATR3 loss in a mouse myoblast cell line similarly resulted in alterations to chromatin accessibility, chromatin loop domain interactions, occupancy of the CTCF and cohesin complex, and Yin Yang 1 (YY1)-mediated enhancer-promoter loop formation [11]. Another recent study has also proposed a role for MATR3 in the 3D organization of chromatin through association with antisense LINE1 [9]. X-chromosome inactivation (XCI) is an example of an extreme modification of chromatin composition/organization. Curiously, MATR3 has been recently linked to the maintenance of the inactive X-chromosome (Xi) [36,37]. Specifically, MATR3, in conjunction with other RBPs, has been shown to interact with Xist, a long noncoding RNA that mediates XCI. Loss of this interaction led to Xist dispersal and reactivation of the genes on the Xi. Taken together, it is interesting to note that despite its ability to bind DNA, MATR3’s role in chromatin organization does not seem to rely on its DNA-binding function but more on its ability to interact with other proteins and/or with RNA. More studies are needed to fully elucidate if MATR3’s DNA-binding capability is indeed dispensable in its ability to modify chromatin organization.
Aside from its association with S/MAR [6,7], a chromosomal region that is implicated in transcription regulation [38], and its proximity to functional genomic areas associated with transcription [34], MATR3 has also been proposed to play a role in transcription regulation. Notably, an early work on MATR3 function provided evidence that MATR3 is involved in transcription [6]. Later studies refined the role of MATR3 in transcription and demonstrated that MATR3 binds to enhancer regions, in part due to its interaction with the transcription factor PIT1, and regulates the expression of PIT1-target genes [13]. MATR3 also has been shown to bind to the promoter regions of OCT4 and YTHDF1, which are genes involved in the maintenance of stem cell pluripotency, as well as to the promoter of other genes involved in embryonic development and stemness [12]. Moreover, MATR3 has also been shown to interact with RNA Polymerase II [39,40] which adds another layer to MATR3’s role in transcription regulation.
Another process that MATR3 has been implicated in is the DNA damage response (DDR), a complex network of signaling pathways that senses and responds to DNA damage. Proteomic interaction studies have shown that MATR3 interacts with proteins associated with the DDR or DNA repair, including the DNA-PK holoenzyme subunits Ku70 and Ku80, H2AX, RAD50, and RUVBL1/2 [33,39,41,42] (Table 1). Moreover, MATR3 has been found to be phosphorylated by DDR-associated kinases ATM [20,41,43] and Chk1 [44], and it has been found that the levels of ATM-phosphorylated MATR3 increased after DNA damage [41]. MATR3 has also been shown to interact with and modify the retention times of SFPQ and NONO [41], which have been previously implicated in DDR [45]. Another possible contributory role of MATR3 in the DDR is its ability to modulate the levels of other factors that may also play a role in DDR. Indeed, MATR3 depletion resulted in a decrease in the mRNA and protein levels of RAD51, a key player in DNA repair via homologous recombination (HR) [14]. More strikingly, MATR3 depletion led to a decrease in the formation of DNA damage-induced RAD51 foci and negatively impacted DNA repair by HR [14]. MATR3 has also been shown to regulate the levels of RAD17 and UHRF2 [16,46], which have both been shown to have roles in DDR [47,48,49,50].

Table 1.
MATR3 interacts with noncoding RNA as well as a variety of proteins involved in chromatin organization, nucleic acid metabolism, and protein translation.
Table 1.
MATR3 interacts with noncoding RNA as well as a variety of proteins involved in chromatin organization, nucleic acid metabolism, and protein translation.
| Interactor | Ref | Interactor | Ref | Interactor | Ref |
|---|---|---|---|---|---|
| AGO1 | [51] | RPL10A | [12,52] | RPS12 | [12] |
| AGO2 | [51] | RPL11 | [12] | RPS13 | [52,53] |
| ALYREF | [53] | RPL13/A | [52] | RPS14 | [12] |
| BAZ1A | [33] | RPL14 | [52] | RPS15A | [33] |
| CHD3 | [33] | RPL15 | [52] | RPS16 | [12] |
| CTCF | [10] | RPL17 | [12] | RPS18 | [12,53] |
| DDX17 | [54] | RPL18 | [12,52] | RPS2 | [52] |
| DUX4 | [55] | RPL18A | [12,33] | RPS23 | [12] |
| eEF1a1 | [12] | RPL19 | [12] | RPS27 | [12] |
| EFTU | [12] | RPL22 | [12] | RPS3 | [12] |
| EIF3C | [52] | RPL23 | [12] | RPS3A | [12,52] |
| EIF3CL | [12] | RPL23A | [12] | RPS4X | [12,52] |
| EIF3D | [12] | RPL26L1 | [52] | RPS6 | [52] |
| EIF3F | [12] | RPL27 | [12,52,53] | RPS7 | [12] |
| EIF4A1 | [12] | RPL28 | [12,52] | RPS8 | [12] |
| EIF4A3 | [12] | RPL3 | [52] | RPS9 | [12,52] |
| ESCO2 | [52,53] | RPL30 | [12] | RRBP1 | [52] |
| EXOSC3 | [54] | RPL32 | [12] | RRP12 | [53] |
| GARS | [12] | RPL36 | [12] | RSL1D1 | [52] |
| H2AX | [42] | RPL38 | [12] | RUVBL1/2 | [39] |
| Ku70, Ku80 | [41] | RPL4 | [52] | SAFB | [33,52] |
| Neat1 | [21] | RPL5 | [12] | SARNP | [53] |
| NONO | [12,41] | RPL6 | [12,52] | SFPQ | [39,41,53] |
| PINCR | [56] | RPL7 | [12,52] | SMARCA4 | [33] |
| PIT1 | [13] | RPL7A | [52] | SMC3 | [10] |
| POLR2A | [40] | RPL8 | [52] | SYDC | [12] |
| pre-miR-138-2 | [57] | RPL9 | [12] | SYIC | [12] |
| PTBP | [12,15,39,53] | RPLP0 | [53] | SYLC | [12] |
| RAD21 | [10] | RPLP2 | [12] | Xist | [36,37] |
| RAD50 | [39] | RPS11 | [52,53] | ZAP (ZC3HAV1) | [52,54] |
| RPL10 | [12,33,52] |
3. MATR3’s Role in RNA-Related Processes
MATR3 contains two RRM domains that can independently bind RNA [7], and the solution structures of both RRM domains have been previously resolved [58]. Deletion of both RRMs abolishes the RNA-binding capability of MATR3 [7]. Indeed, accumulating studies have demonstrated that MATR3 plays a role in RNA-related processes (Figure 1). Specifically, MATR3 has been shown to bind to and regulate mRNA stability [12,14,46,59]. MATR3 also plays a role in the nuclear retention of hyper-edited RNA [60], as well as in mRNA export through its interaction with the Transcription and Export (TREX) complex [53]. In addition, MATR3 has also been shown to bind to critical myogenic transcripts and regulate their RNA processing [21].
MATR3 has also been shown to interact with noncoding RNA, such as long noncoding RNA (lncRNA) and micro-RNA (miRNA). For instance, MATR3 binds to the lncRNA Neat1, and depletion of MATR3 increases Neat1 levels by increasing its stability [21], suggesting a possible role of MATR3 in regulating Neat1 lncRNA decay. Another lncRNA that has been known to bind with MATR3 is PINCR, which is strongly induced by DNA damage and regulated by p53 [56]. MATR3 has been shown to be important for the localization of the MATR3-PINCR-p53 complex to the enhancers of select p53/PINCR-regulated genes and plays a key role in modulating the expression of these genes after DNA damage. Additionally, MATR3 has also been shown to bind with pre-miR-138-2, hindering it from being further processed into the mature form [57]. Lastly, MATR3 has also been identified as an interactor of the Argonaute complexes, suggesting a possible role in miRNA-induced silencing [51].
Another process that MATR3 regulates is RNA splicing. Alternative splicing (AS) is a mechanism that enhances transcriptomic and proteomic diversity from a limited number of protein coding genes through the use of differing combinations of splice sites. Many reports have shown that MATR3 interacts with a variety of proteins that are known to function in splicing regulation [15,33,46,61]. Several studies have shown that the major role of MATR3 is a repressor of exon inclusion [15,16,17,19]. MATR3’s splicing function is dependent on its RRM domains, while the ZnF domains are largely dispensable [15]. Specifically, MATR3 binding is enriched in intronic pyrimidine-rich sequences around or within the repressed exons [15,17]. Moreover, several studies have demonstrated that MATR3 works together with PTBP to regulate RNA splicing, but it may also act independently [15,17,18].
In addition to alternative splicing, MATR3 has also been linked to non-canonical splicing. Introns of the eukaryotic genome often contains sequences that resemble splice sites or polyadenylation (poly(A)) sites, often referred to as “cryptic” sites [62]. Recognition of these cryptic sequences by the splicing machinery may lead to non-canonical splicing that may then result in the production of an aberrant transcript and/or protein. One form of non-canonical splicing is the inclusion of previously unannotated exons, also commonly referred to as cryptic exons (CEs), which often contain premature termination codons (PTCs) that can target a CE-containing transcript to nonsense-mediated decay (NMD), thereby decreasing its transcript levels. A known source of these cryptic splice or poly(A) sites are LINE elements, the most abundant transposable elements in the human genome [18,62]. Similar to its functionality in AS, MATR3 has been shown to repress the recognition of cryptic splice sites, thus repressing the inclusion of CEs [18]. This function is mediated through the binding of MATR3 in the deep intronic regions where these cryptic sites are located. Another study recently examined global MATR3-dependent cryptic splicing events (CSEs) within functional genes [16]. Conforming to previous findings [15], the RRM domains, particularly RRM2, are required for mediating the CE repression function of MATR3 [16].
Recent studies have also reported that MATR3 plays a role in the regulation of global protein synthesis. Although MATR3 is predominantly nuclear in localization, the presence of both a nuclear localization signal and a nuclear export signal may suggest that it may have roles in both the nucleus and the cytoplasm [63]. Indeed, a recent study identified the protein interactome of MATR3 in human induced pluripotent stem cells (hiPSCs) and revealed that MATR3 associates with members of the translational machinery [12] (Table 1). Other interaction proteomic analyses of MATR3 interactors revealed that MATR3 interacts with various ribosomal proteins [33,52,53] (Table 1). Additionally, MATR3 has been found to be partially cytoplasmic in localization in hiPSCs with a portion of cytoplasmic MATR3 (about 20%) colocalizing with members of the eIF3 complex (eIF3A and eIF3C) [12], a protein complex important for the initiation of protein synthesis [64]. Notably, depletion of MATR3 decreased the translational efficiency of pluripotency regulating genes including NANOG, LIN28A, and SOX2 in hiPSCs [12]. Of note, an early study of MATR3 localization has also described a population of MATR3 that is detectable in the cytoplasmic, microsomal, and polysomal fractions of rat liver and Ac2F hepatoma cells [7].
4. Regulation of MATR3 Degradation
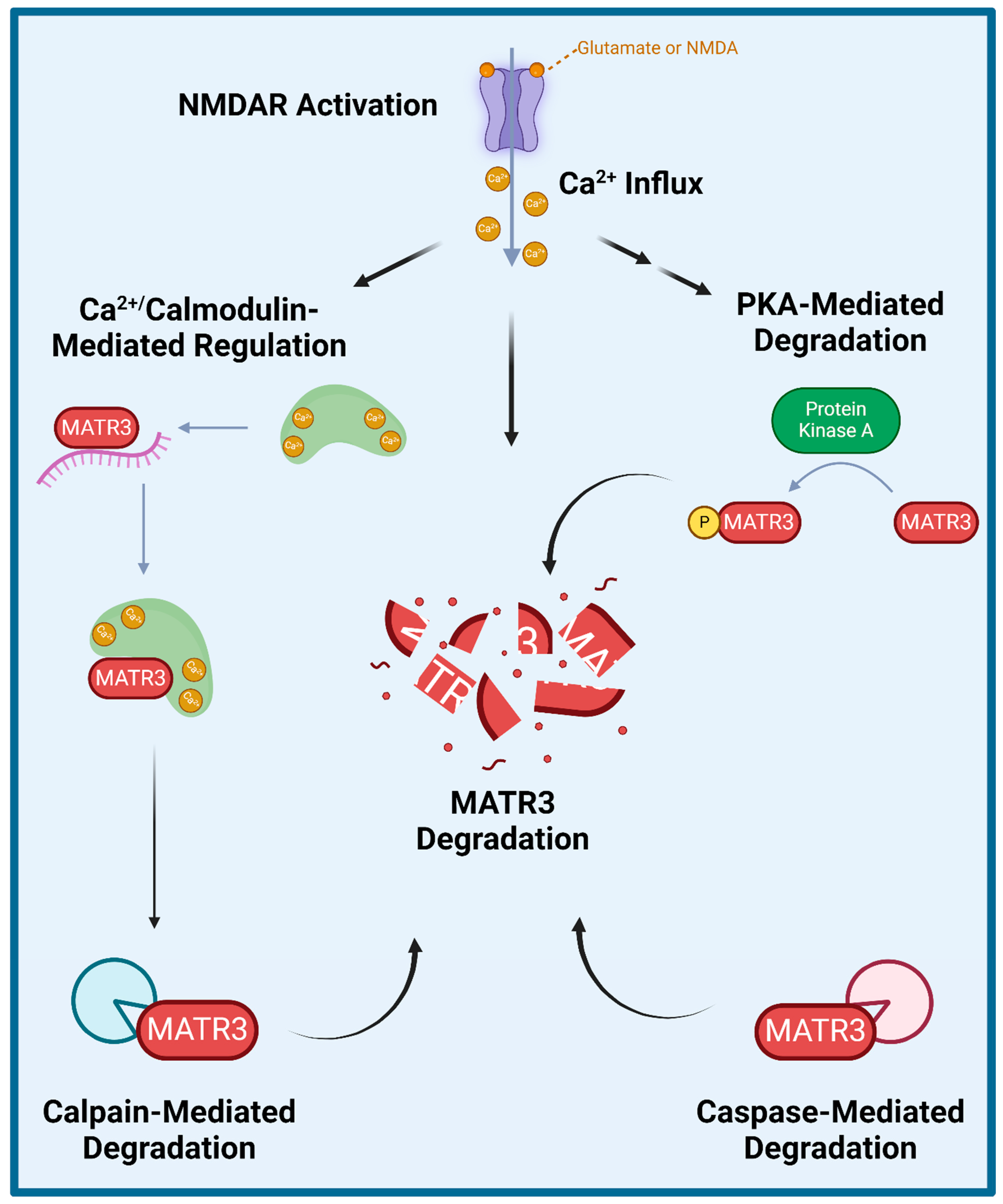
Several studies have explored how wildtype MATR3 is regulated and degraded (Figure 2). Specifically, MATR3 has been shown to be phosphorylated by protein kinase A (PKA) and then subsequently degraded in rat cerebellar granule neurons exposed to N-methyl-D-aspartate (NMDA) [65]. Furthermore, inhibition of NMDA receptors (NMDAR) or of PKA activity prevented NMDA-induced phosphorylation and the subsequent degradation of MATR3, suggesting that the NMDAR-PKA signaling is important for MATR3 degradation. Additionally, a few studies have shown that MATR3 degradation could be mediated by caspases and/or calpains. A previous study demonstrated that MATR3 is a Ca2+-dependent calmodulin-binding protein harboring a caspase cleavage site and that it could be cleaved by caspases-3, -5, -6, -7, -8, and -10 [66]. Another study also demonstrated that in addition to multiple caspases, MATR3 can also be cleaved by calpains 1 and 2 [67]. Complementarily, caspase inhibition has been shown to increase MATR3 levels, likely through the prevention of its caspase-mediated degradation [68]. Lastly, a recent study reported similar findings in that MATR3 abundance is regulated in an NMDAR-, Ca2+- and calpain-dependent manner in rat primary cortical neurons [69]. Intriguingly, this study showed that PKA inhibitor H-89 did not significantly prevent NMDA-mediated MATR3 degradation in cortical neurons [69], suggesting that MATR3 degradation may not be dependent on PKA phosphorylation, which is in contrast to the previous findings in cerebellar granule neurons [65]. This study also showed that shortly upon activation of glutamatergic signalling or after Ca2+ influx, calmodulin binds to MATR3 and impairs MATR3 RNA-binding capability [69]. These studies suggest that NMDAR-signalling and the resulting Ca2+ influx leads to the activation of calmodulin, which allows it to bind to MATR3 and displace MATR3-bound RNA, leaving MATR3 susceptible to degradation by calpains.
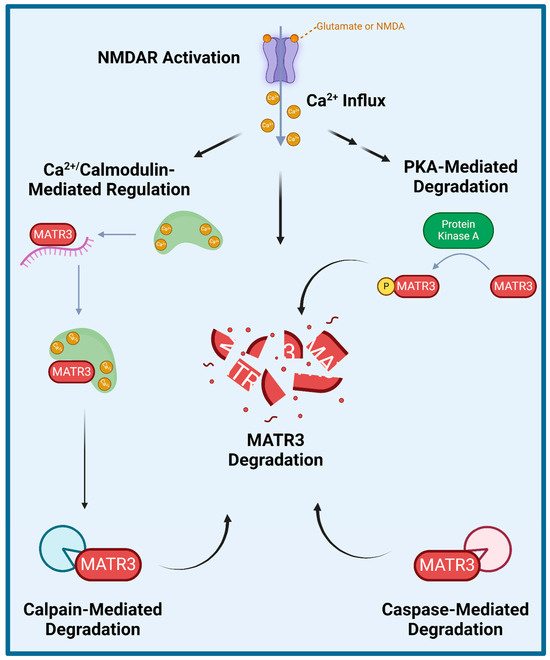
Figure 2.
Proposed mechanisms of MATR3 degradation. Several studies have shown that MATR3 can be degraded through a variety of mechanisms including enzymatic cleavage mediated by calpains and a variety of caspases, and following activation of N-methyl D-aspartate (NMDA) receptor (NMDAR) or phosphorylation catalyzed by protein kinase A (PKA).
5. MATR3’s Role in the Regulation of Development and Differentiation
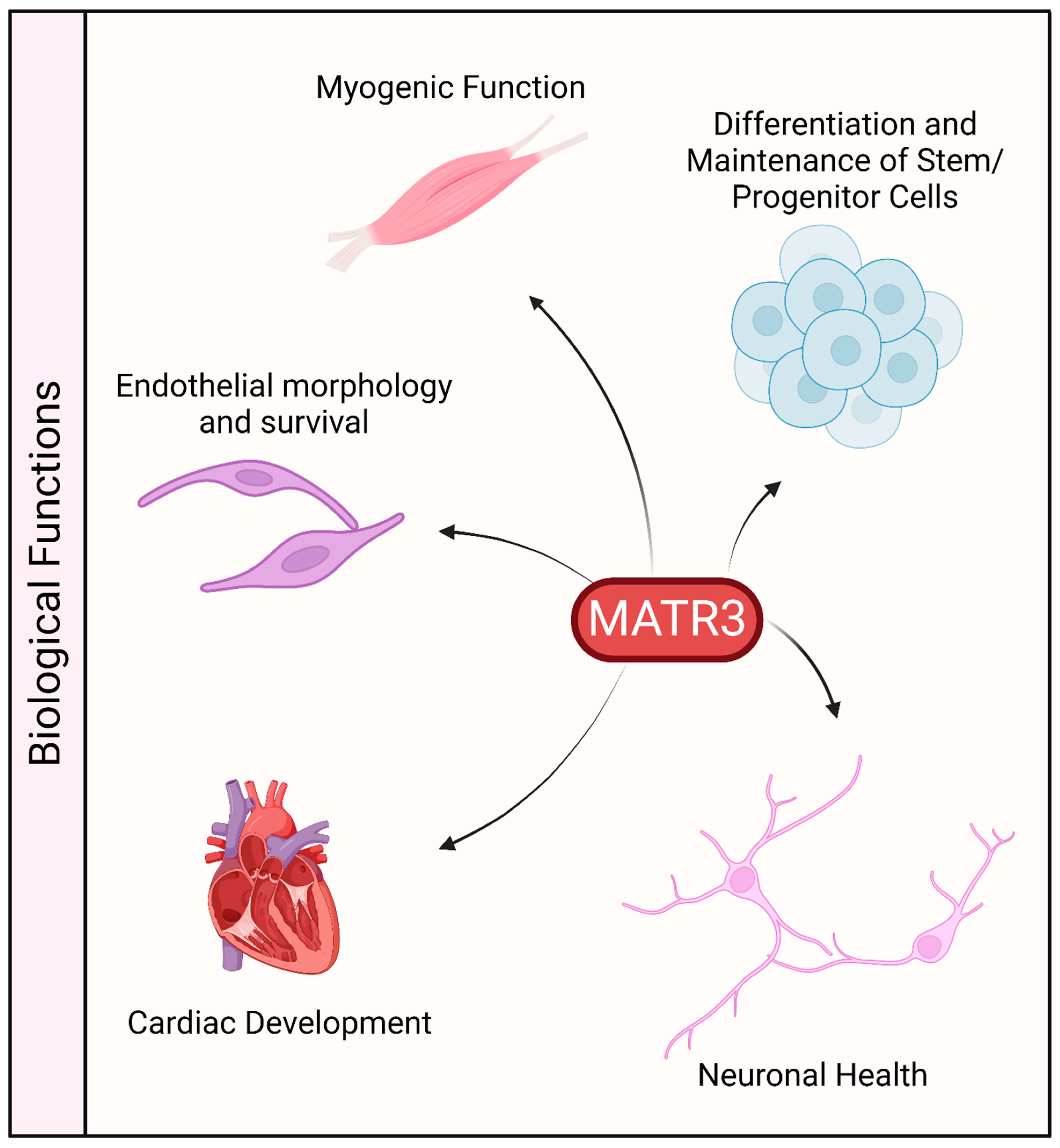
The protein sequence of MATR3 is highly conserved in vertebrates. It is ubiquitously expressed in various tissues, including the central nervous system, muscles, and various organs, which may suggest that MATR3 may play an important role in these organs (Figure 3). Indeed, constitutive deletion of Matr3 in mice is perinatally lethal [70], suggesting that MATR3 may play an important role in development. Likewise, homozygous disruption of Matr3 3’ UTR in mice, through gene trapping (Matr3Gt-ex13 mice) is early embryonically lethal [71]. Notably, Matr3Gt-ex13 heterozygotes are viable but exhibit incompletely penetrant cardiac defects, suggesting a possible role of MATR3 in cardiac development.
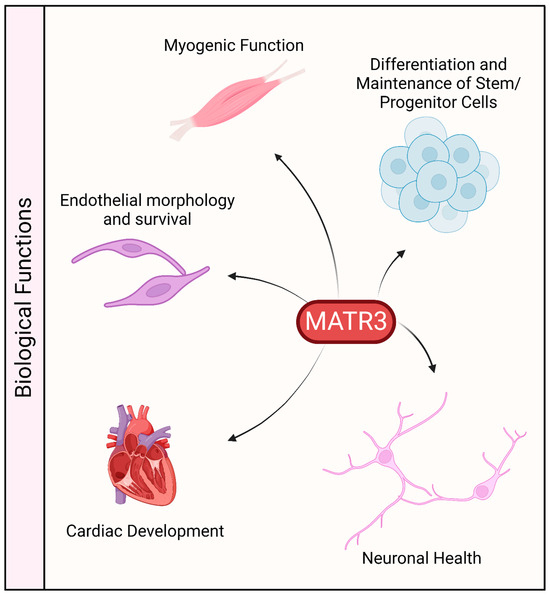
Figure 3.
Biological functions associated with MATR3. MATR3 has been shown to play a role in maintaining or modulating the function and/or development of a variety of cell types.
MATR3 has also been recently shown to be involved in the maintenance of pluripotency in hiPSCs [12]. Stable depletion of MATR3 resulted in reduced growth and colony formation capability in suboptimal single-cell growth conditions. The downregulation of MATR3 also led to a decrease in the levels of pluripotency factors, including OCT4, NANOG, KLF4, and YTHDF1. Notably, overexpression of MATR3 in MATR3-depleted hiPSCs rescued the reduced growth phenotype and the reduction in pluripotency factors suggesting that these phenotypes are indeed mediated by the loss of MATR3. Furthermore, neurons differentiated from MATR3-depleted hiPSCs displayed a reduction in neurite length and arborization, suggesting that in addition to maintaining stemness, MATR3 also plays a role in terminal neuronal maturation.
In addition to hiPSCs, MATR3 has also been reported to affect the proliferation and/or differentiation of other stem cells or precursor cells. Knockdown of MATR3 in neural stem cells (NSCs) decreased cell proliferation and neurosphere formation and also induced the differentiation of these stem cells, as observed through the extension of neurites [20]. Additionally, in utero knockdown of MATR3 in mouse hippocampal regions where NSCs reside resulted in the failure of MATR3-depleted cells to migrate to the cortical plate and differentiate into neurons, resulting in the disorganization of this region. Similarly, depletion of MATR3 in murine primary myoblasts also decreased proliferation and impaired proper differentiation [21]. Depletion of MATR3 in the mouse myoblast cell line C2C12 similarly led to an impairment in muscle differentiation [11]. Lastly, knockout of Matr3 in murine erythroid cells and mouse embryonic stem cells resulted in phenotypes indicative of a state of accelerated differentiation [10].
MATR3 was also shown to be important for cell survival and growth in other cells. Depletion of MATR3 reduced the proliferation and altered the morphology of endothelial cells [23]. Furthermore, prolonged depletion of MATR3 led to a decrease in cell viability and an increase in cell death. In primary cortical neurons, depletion of MATR3 is similarly toxic [22]. Lastly, a recent study has demonstrated that MATR3 regulates mitotic spindle dynamics and cell proliferation by controlling the alternative splicing of CDC14B in colorectal cancer cells [19].
6. MATR3 in the Context of Disease
6.1. Matrin-3 Mutations in Amyotrophic Lateral Sclerosis
Mutations in MATR3 have been linked to amyotrophic lateral sclerosis (ALS), a devastating adult-onset neurodegenerative disease that is characterized by the loss of motor neurons, leading to muscle atrophy, paralysis, and death [24,72] (Table 2). Intriguingly, ALS-associated missense mutations in MATR3 cluster outside of its functional domains [24,73,74,75,76,77,78], particularly in the intrinsically disordered regions (IDRs) (Figure 4). Of these MATR3 mutations, the most common mutation is the Serine-85 to Cysteine (S85C) mutation located in the N-terminal IDR of MATR3. The S85C mutation was initially linked to another adult-onset disorder, namely vocal cord and pharyngeal weakness with distal myopathy (VCPDM) [79,80], but was later reclassified as slowly progressive ALS due to the presence of neurogenic features involving motor neuron symptoms [24]. Notably, MATR3 S85C has also been associated to VCPDM in several additional families with or without similar neurogenic features [81,82,83,84,85,86,87], suggesting that this mutation may cause a spectrum of phenotypes ranging from pure myopathy to motor neuron disease. Similarly, mice that harbor the S85C mutation in the mouse Matr3 gene also show robust phenotypes, including motor function defects, muscle atrophy, and early death [70], suggesting that the S85C mutation is a genetic determinant of myopathy and ALS. Conversely, the Phe115Cys (F115C) mutation in MATR3 has been initially linked to familial ALS [24], but resequencing on the same ALS family revealed an intronic mutation in another ALS-linked gene KIF5A [88], suggesting that the F115C mutation may not be causative of ALS. This finding was supported by the lack of pathogenicity of the F115C mutation in a knock-in mouse model [89]. The Pro154Ser (P154S) mutation is another ALS-linked mutation in MATR3, initially found in a sporadic ALS patient [24]. Previous studies in various cell lines have shown that expression of MATR3 P154S leads to alterations in nuclear mRNA export [53], impairment in MATR3 condensate dynamics in yeast [90], and increased toxicity compared to MATR3 WT in rat primary cortical neurons [22]. Although these studies suggest that the P154S mutation may impact MATR3 function and cause toxicity, the MATR3 P154S knock-in mice did not display any overt phenotypes, suggesting that the P154S mutation may not induce pathogenicity [91]. In addition, the Thr622Ala (T622A) mutation was another mutation in MATR3 that was identified in familial ALS [24]. Similar to the P154S mutant, expression of MATR3 T622A resulted in an impairment in MATR3 condensate dynamics in yeast [90] and increased toxicity relative to MATR3 WT [22]. Notably, although global mRNA export is not affected by the expression of MATR3 T622A, defects in the mRNA export of other ALS-linked genes TDP43 and FUS were observed in MATR3 T622A-expressing cells [53].
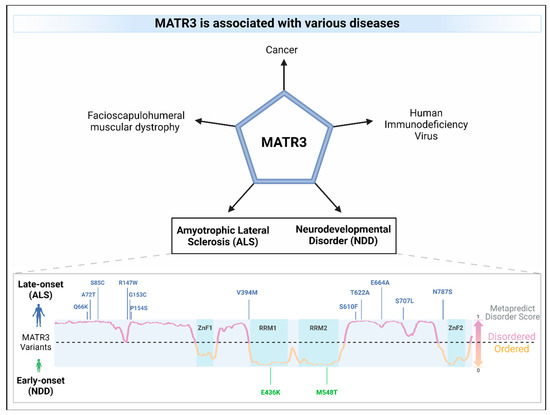
Figure 4.
MATR3 is associated with various diseases. Missense mutations in MATR3 have been linked to amyotrophic lateral sclerosis (ALS; mutations in blue) as well as to neurodevelopmental disorders (NDD; mutations in green). ALS-associated mutations are located in the intrinsically disordered regions of MATR3, as predicted using the Metapredict v2 webserver (metapredict.net) [92] while missense mutations in the ordered regions were identified in NDDs. A residue having a Metapredict disorder score above 0.5 indicates that it is likely to be in an intrinsically disordered region (lilac), whereas a score below 0.5 indicates that it is likely to be in an ordered region (orange). The domains of MATR3 were indicated (zinc finger, ZnF; RNA recognition motif, RRM) and colored in turquoise.
Figure 4.
MATR3 is associated with various diseases. Missense mutations in MATR3 have been linked to amyotrophic lateral sclerosis (ALS; mutations in blue) as well as to neurodevelopmental disorders (NDD; mutations in green). ALS-associated mutations are located in the intrinsically disordered regions of MATR3, as predicted using the Metapredict v2 webserver (metapredict.net) [92] while missense mutations in the ordered regions were identified in NDDs. A residue having a Metapredict disorder score above 0.5 indicates that it is likely to be in an intrinsically disordered region (lilac), whereas a score below 0.5 indicates that it is likely to be in an ordered region (orange). The domains of MATR3 were indicated (zinc finger, ZnF; RNA recognition motif, RRM) and colored in turquoise.
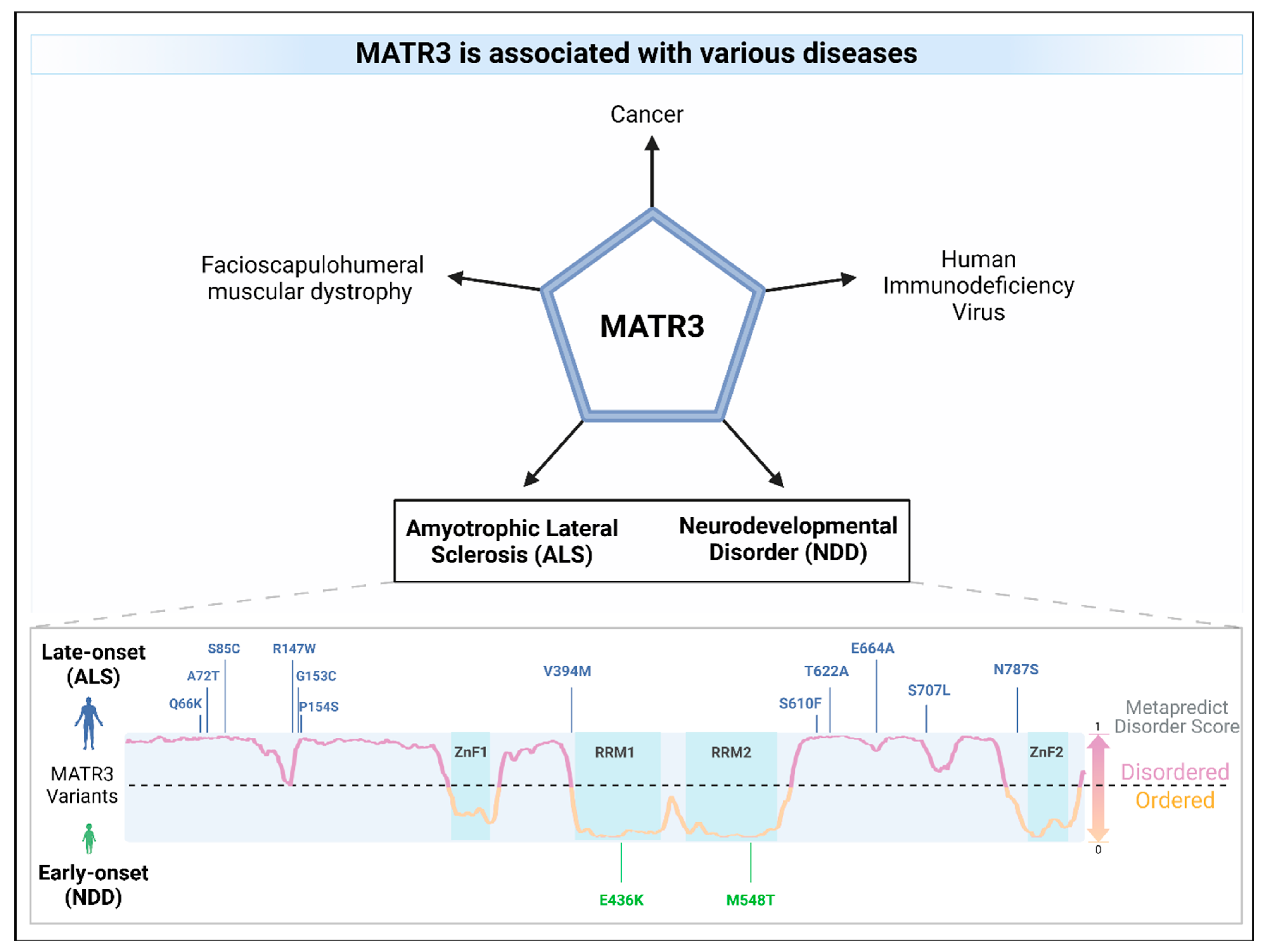

Table 2.
Combined annotation-dependent depletion (CADD) score of disease-associated missense mutations in MATR3.
Table 2.
Combined annotation-dependent depletion (CADD) score of disease-associated missense mutations in MATR3.
| Associated Disease * | HGVS.c (NM_199189.3) | HGVS.p (NP_954659.1) | CADD GRCh37-v1.7 | CADD GRCh38-v1.7 | Ref | ||
|---|---|---|---|---|---|---|---|
| Position (GRCh37) | CADD Score (PHRED) | Position (GRCh38) | CADD Score (PHRED) | ||||
| ALS | c.151C>T | p.(Arg51Cys) | 138643255 | 35 | 139307566 | 34 | [72] |
| ALS | c.182G>A | p.(Ser61Asn) | 138643286 | 33 | 139307597 | 33 | [72] |
| ALS | c.196C>A | p.(Gln66Lys) | 138643300 | 34 | 139307611 | 29.9 | [77] |
| ALS | c.214G>A | p.(Ala72Thr) | 138643318 | 31 | 139307629 | 29.4 | [73] |
| ALS | c.254C>G | p.(Ser85Cys) | 138643358 | 35 | 139307669 | 35 | [24] |
| ALS | c.296C>G | p.(Ser99Cys) | 138643400 | 35 | 139307711 | 35 | [72] |
| ALS | c.393C>A | p.(Asp131Glu) | 138643497 | 27 | 139307808 | 32 | [72] |
| ALS | c.439A>T | p.(Arg147Trp) | 138643543 | 35 | 139307854 | 35 | [74] |
| ALS | c.457G>T | p.(Gly153Cys) | 138643561 | 35 | 139307872 | 35 | [77] |
| ALS | c.460C>T | p.(Pro154Ser) | 138643564 | 27.8 | 139307875 | 25.1 | [24] |
| ALS | c.545G>A | p.(Arg182Lys) | 138643649 | 34 | 139307960 | 34 | [72] |
| ALS | c.561T>G | p.(Asp187Glu) | 138643665 | 28.6 | 139307976 | 32 | [72] |
| ALS | c.883A>G | p.(Ile295Val) | 138643987 | 34 | 139308298 | 34 | [72] |
| ALS | c.926A>G | p.(His309Arg) | 138650377 | 28.2 | 139314688 | 28.8 | [72] |
| ALS | c.949C>T | p.(Arg317Cys) | 138650400 | 34 | 139314711 | 34 | [72] |
| ALS | c.998A>G | p.(Asn333Ser) | 138651409 | 19.48 | 139315720 | 18.05 | [72] |
| ALS | c.1102C>A | p.(Pro368Thr) | 138651850 | 24.7 | 139316161 | 24.2 | [72] |
| ALS | c.1175G>T | p.(Gly392Val) | 138652787 | 25.2 | 139317098 | 25.6 | [72] |
| ALS | c.1180G>A | p.(Val394Met) | 138652792 | 22.8 | 139317103 | 22.5 | [75] |
| ALS | c.1282C>A | p.(His428Asn) | 138653384 | 28.2 | 139317695 | 25.1 | [72] |
| NDD | c.1306G>A | p.(Glu436Lys) | 138653408 | 28.7 | 139317719 | 31 | [25] |
| NDD | c.1643T>C | p.(Met548Thr) | 138657627 | 25.5 | 139321938 | 25.7 | [16] |
| ALS | c.1786T>A | p.(Ser596Thr) | 138658294 | 20.2 | 139322605 | 18.97 | [72] |
| ALS | c.1829C>T | p.(Ser610Phe) | 138658337 | 28.6 | 139322648 | 27 | [76] |
| ALS | c.1837G>C | p.(Asp613His) | 138658345 | 26.6 | 139322656 | 25.8 | [72] |
| ALS | c.1864A>G | p.(Thr622Ala) | 138658372 | 0.189 | 139322683 | 1.475 | [24] |
| ALS | c.1867G>A | p.(Glu623Lys) | 138658375 | 22.3 | 139322686 | 22.8 | [72] |
| ALS | c.1879C>G | p.(Gln627Glu) | 138658387 | 20.6 | 139322698 | 18.76 | [72] |
| ALS | c.1921G>C | p.(Asp641His) | 138658429 | 23.3 | 139322740 | 23 | [72] |
| ALS | c.1948A>C | p.(Met650Leu) | 138658456 | 18.49 | 139322767 | 17.49 | [72] |
| ALS | c.1991A>C | p.(Glu664Ala) | 138658499 | 24.3 | 139322810 | 23.6 | [77] |
| ALS | c.2062G>T | p.(Ala688Ser) | 138658570 | 7.085 | 139322881 | 8.125 | [72] |
| ALS | c.2075A>G | p.(Lys692Arg) | 138658583 | 22.3 | 139322894 | 23.4 | [72] |
| ALS | c.2120C>T | p.(Ser707Leu) | 138658628 | 22.2 | 139322939 | 18.97 | [77] |
| ALS | c.2135A>G | p.(Lys712Arg) | 138658643 | 22.1 | 139322954 | 24 | [72] |
| ALS | c.2203A>G | p.(Ile735Val) | 138661183 | 18.45 | 139325494 | 18.66 | [72] |
| ALS | c.2219A>G | p.(Asn740Ser) | 138661199 | 18.61 | 139325510 | 16.56 | [72] |
| ALS | c.2234C>T | p.(Ala745Val) | 138661214 | 21.8 | 139325525 | 21 | [72] |
| ALS | c.2251G>A | p.(Ala751Thr) | 138661231 | 19.59 | 139325542 | 17.87 | [72] |
| ALS | c.2275A>G | p.(Ser759Gly) | 138661255 | 22.3 | 139325566 | 21.8 | [72] |
| ALS | c.2360A>G | p.(Asn787Ser) | 138661340 | 21.3 | 139325651 | 20.7 | [77] |
| ALS | c.2504A>G | p.(Asn835Ser) | 138665044 | 17.22 | 139329355 | 17.24 | [72] |
Missense mutations in red were obtained from the previous literature, whereas missense mutations in black were obtained from the Project MinE data browser (AFcontrols = 0). CADD scores, which indicate the predicted deleteriousness of each mutation, were obtained using CADD v1.7 [93]. * Amyotrophic lateral sclerosis, ALS; neurodevelopmental diseases, NDD.
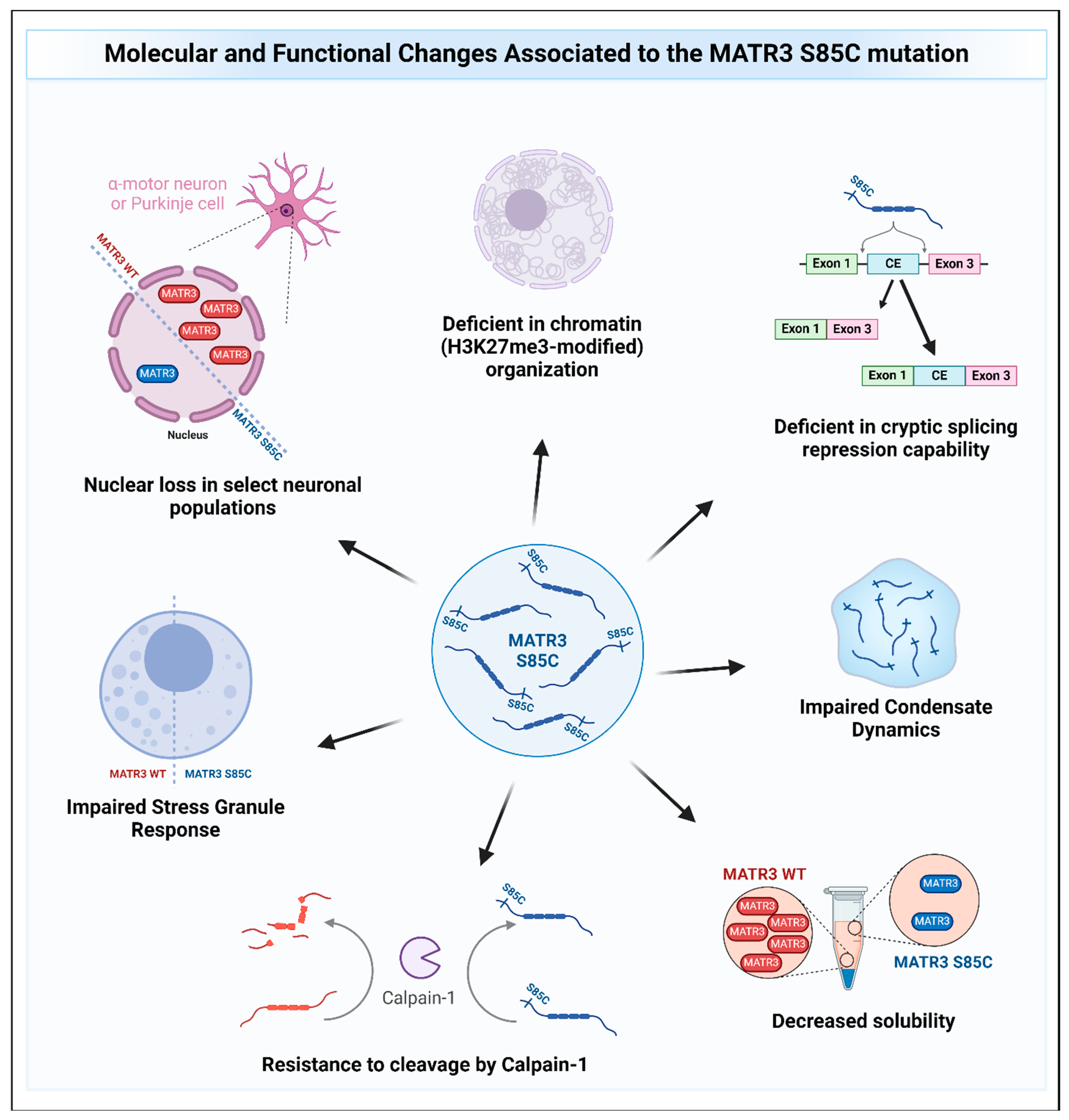
As accumulating genetic studies corroborate that the S85C mutation in MATR3 is pathogenic, various animal models were used to mimic the genetic condition and address the mechanism by which the S85C mutation causes the disease. Several mouse models of MATR3 S85C has been generated. Constitutive global overexpression of MATR3 S85C in mice resulted in myopathic phenotypes, spinal motor neuron degeneration, increased glial markers suggestive of neuroinflammation in the spinal cord, impairment in weight gain, motor function defects, and decreased lifespan [94]. However, the phenotypic comparisons were made with non-transgenic control and not with transgenic MATR3 WT mice, which complicates the interpretation of the resulting phenotypes as either stemming from the S85C mutation or an artifact of MATR3 overexpression. To counter this, a knock-in mouse model that harbors the S85C mutation in the endogenous mouse Matr3 locus was generated [70]. Homozygous MATR3 S85C knock-in mice (Matr3S85C/S85C) recapitulate key features of early-stage ALS, including an impairment in weight gain and a progressive and age-dependent deficit in motor function, including impairment in motor coordination and gait, as well as muscle weakness. Neuropathological analyses at the disease end stage revealed a striking loss of Purkinje cells in the cerebellum and defects in the neuromuscular junction (NMJ). Interestingly, these ALS mice show a profound loss of MATR3 staining in a subset of neurons affected in ALS, such as in Purkinje cells and alpha motor neurons, but not in gamma motor neurons, which are typically spared in ALS. The relevance of this presymptomatic MATR3 loss in these neurons is still unclear and requires further study; however, the depletion of MATR3 has been previously shown to be toxic to cortical neurons in vitro [22], suggesting that MATR3 loss may contribute to Purkinje cell loss and motor function defects. Further characterization of MATR3 S85C knock-in mice extended the population of neurons that display MATR3 loss to include the alpha motor neurons and interneurons in the cervical and thoracic spinal cord, and a subset of upper motor neurons and hippocampal CA1 neurons in the brain [95], without being associated with cell body loss. These results suggest that the S85C mutation affects selective neuronal populations in other regions of the brain in addition to motor-controlling neurons, which requires further investigation.
Additional studies support the hypothesis that the S85C mutation leads to partial loss of MATR3 function. Characterization of MATR3 S85C from multiple models consistently show that the S85C mutant is less soluble compared to wild type (WT) MATR3 [16,22,24,80,96,97], suggesting that the S85C mutation reduces the levels of functional MATR3. However, a concomitant increase in the insoluble levels of S85C was observed compared to the wildtype MATR3, suggesting that the S85C mutation may change the biochemical properties of the MATR3 protein, which may cause toxicity. Additionally, the S85C mutation has been found to impair the CE repression capability of MATR3, further supporting the idea that the S85C mutation is a partial loss-of-function mutation [16].
Other recent studies have also provided evidence on how MATR3 S85C may cause disease (Figure 5). Indeed, studies have shown that MATR3 S85C causes toxicity in various model systems, including human and mouse cell lines, primary neurons, yeast, and Drosophila [9,22,90,96,97,98]. In a motor neuron-like NSC-34 cell line, the S85C mutation was shown to alter the protein–protein interactions and/or colocalization of MATR3 with members of the TREX complex, leading to defects in nuclear mRNA export [53]. However, another study conducted on HEK293 cells reported that the protein interactome of MATR3 S85C is similar to that of the WT protein and did not identify MATR3 interaction with TREX complex members, suggesting cell-type specific MATR3 interactions [52]. In addition, MATR3 S85C was recently shown to be resistant to degradation by calpain-1, an enzyme previously reported to degrade MATR3 WT [67,69], suggesting a possible gain-of-function mechanism for this mutation [69]. Moreover, it has been shown that MATR3 S85C was not sufficient to rescue the redistribution of H3K27me3-modified chromatin upon depletion of MATR3 in mouse neuroblast cells and that the S85C mutant protein was less dynamic compared to the wildtype MATR3 protein [9], suggesting that dysregulation of chromatin organization and defects in protein dynamics may contribute to the mutation’s disease-causing potential. In a yeast model, the S85C mutation was shown to also impair condensate dynamics [90]. Intriguingly, it was previously shown that a fragment of wildtype MATR3 that contains the N-terminal IDR can phase-separate when targeted to the nucleus [99]. Conforming to the previous two findings, introduction of the S85C mutation into this phase-separating fragment similarly impaired droplet formation [99]. These studies suggest that the S85C mutation seems to affect the phase separation capability of MATR3, but how this impairment in phase separation mediates toxicity is not yet known. Stress granule formation upon treatment of the stressor was also shown to be impaired in S85C fibroblasts, evidenced by a reduction in the formation of stress granules (SGs) within cells [98]. Furthermore, findings from two independent groups have shown that expressing MATR3 in the motor neurons or muscles of flies led to an impairment in motor function and shortened lifespan with mutant MATR3 S85C being more toxic compared to MATR3 WT [96,97]. Candidate genetic screens from both groups identified that knockdown of axonal transport genes enhances toxicity [97], while knockdown of the Drosophila homolog of HNRNPM suppressed the toxicity of MATR3 S85C but not MATR3 WT [96]. How the S85C mutation affects MATR3 function and causes toxicity is beginning to be unraveled, and further studies will help accelerate our understanding of the disease and to develop treatments for patients.
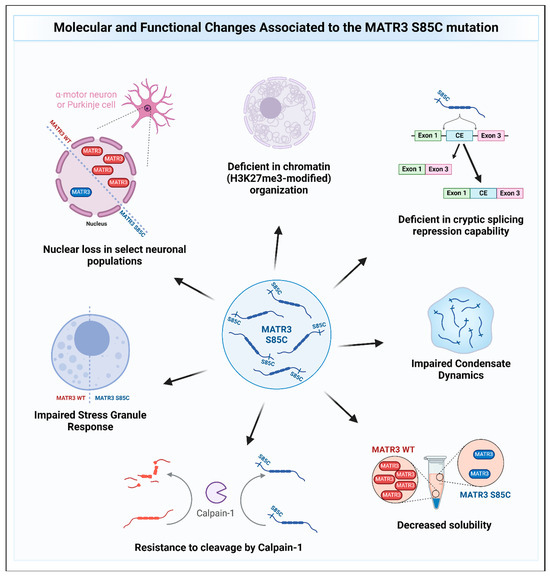
Figure 5.
The molecular and functional changes associated with the MATR3 S85C mutation. Pathological changes to MATR3 function and properties due to the missense S85C mutation include, but are not limited to, deficiencies in its normal function and impairment of its biochemical properties.
6.2. Matrin-3 Mutations in Neurodevelopmental Diseases
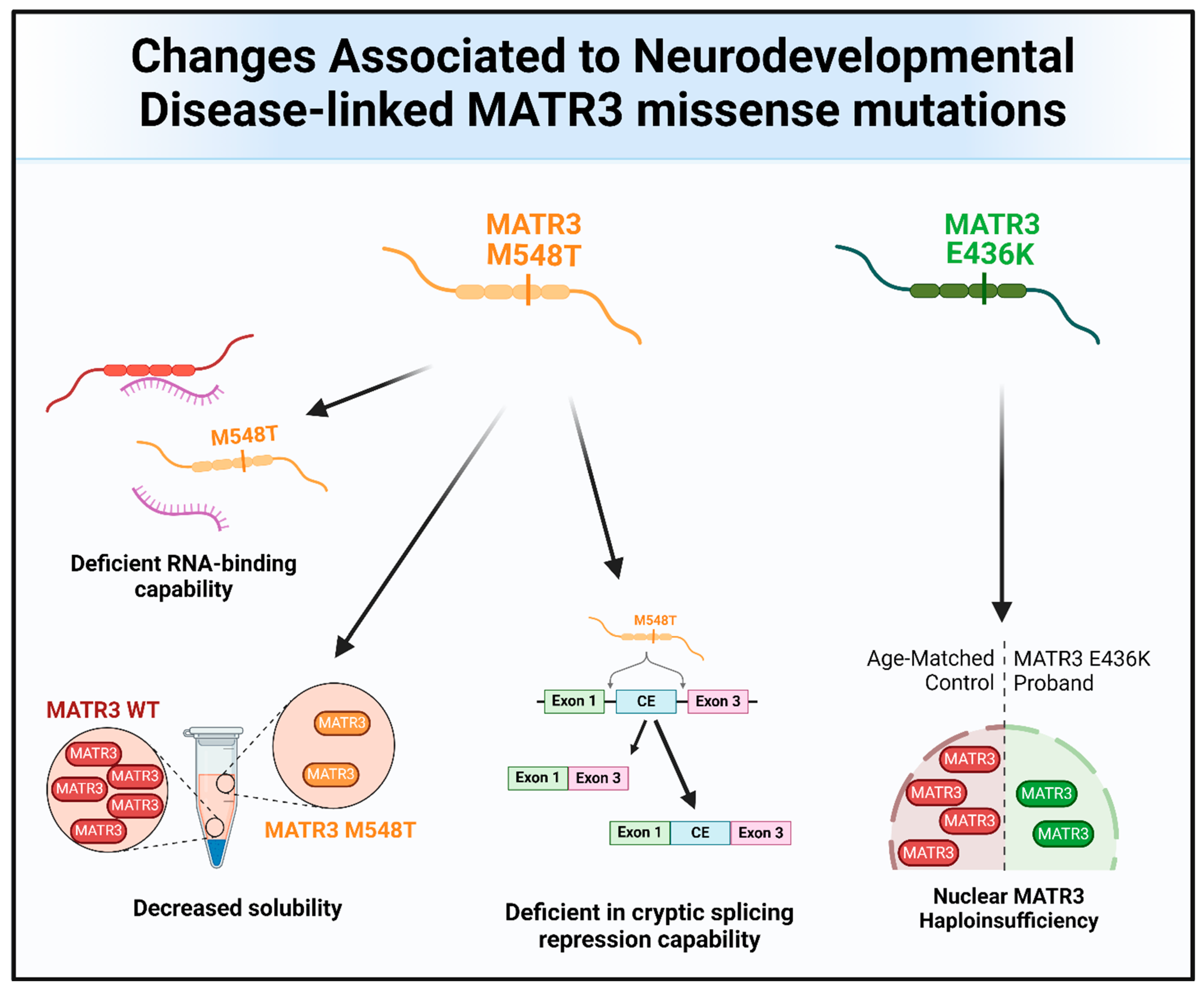
Missense mutations in MATR3 have also been linked to neurodevelopmental diseases (Figure 4 and Figure 6). Intriguingly, two reported neurodevelopmental disease-associated MATR3 variants contain mutations in the ordered domains of MATR3, particularly in the RRM domains. The E436K mutation, located in the RRM1 domain of MATR3, has been reported to lead to haploinsufficiency with reduced MATR3 levels [25]. This patient displayed developmental disability, muscular hypotonia, and early-onset neurodegeneration. Another patient with a MATR3 M548T mutation, located in the RRM2 domain, was recently reported [16]. Similar to the patient with the E436K mutation, the patient with the M548T mutation was reported to have motor phenotypes and intellectual disability. Characterization of the MATR3 M548T variant revealed that it is deficient in RNA binding and CE repression capability when compared to MATR3 WT, suggesting that this mutation impairs RRM2 function. However, it is still unknown whether the E436K variant in the RRM1 is similarly deficient in RNA binding and CE repression capabilities. Comparing the phenotypic trajectory and age of onset of the two neurodevelopmental disease-associated MATR3 variants to the ALS-associated MATR3 S85C variant, it seems to suggest that mutations in the ordered domains of MATR3 may be linked to more severe phenotypes earlier in life. How these disease-associated variants in MATR3 lead to different disease onset and progression is still currently unclear but, nonetheless, it is an interesting conundrum that requires further research.
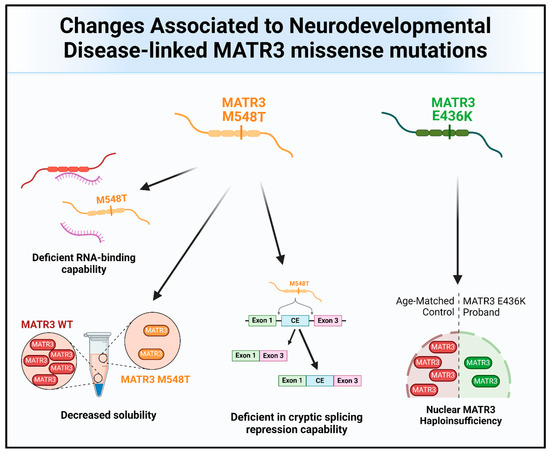
Figure 6.
The molecular and functional changes associated with the MATR3 neurodevelopmental disease-associated mutations. Pathological changes to MATR3 function and properties due to the neurodevelopmental disease-associated missense mutation include, but are not limited to, deficiencies in its normal function and impairment of its biochemical properties.
6.3. Matrin-3 Dysregulation in Other Diseases
Dysregulation in MATR3 expression has been linked to other diseases, including cancer (Figure 4). Elevated levels of MATR3 are associated with lower overall survival, advanced clinical stage, and/or higher tumor grade in patients with neuroblastoma [100] or hepatocellular carcinoma (HCC) [26]. Downregulation of MATR3 in HCC cell lines resulted in decreased cell proliferation and increased cell cycle arrest, suggesting that MATR3 promotes cell proliferation in these cell lines. Similarly, knockdown of MATR3 decreased cell proliferation and/or increased apoptosis in oral squamous cell carcinoma cells [27], malignant melanoma cells [28], and colorectal cancer cells [19]. Moreover, depletion of MATR3 in a xenograft mouse model significantly reduced tumoral volume and size compared to control, supporting an oncogenic role for MATR3 [26,28]. On the other hand, there are also a few studies reporting an opposite role for MATR3, suggesting its tumor-suppressor-like function. In triple-negative breast cancer (TNBC), high levels of MATR3 promoted apoptosis and inhibited epithelial-mesenchymal transition, migration, and invasion of the cancer cells [29]. Additionally, high MATR3 levels are associated with better prognosis in breast cancer patients. Similarly, high MATR3 protein expression was also associated with higher overall survival in patients with non-small cell lung cancer [30] or clear-cell renal cell carcinoma [31]. Taken together, these studies demonstrate that MATR3 dysregulation is associated with cancer, but it may play a different role depending on the cancer type. More studies are required to examine why MATR3 plays a differential role in these cancer types as well as to pinpoint the exact mechanisms as to how MATR3 contributes to cancer disease progression.
Beyond cancer, MATR3 has also been reported to have ties to other diseases, such as facioscapulohumeral muscular dystrophy (FSHD). FSHD is a neuromuscular disorder caused by the de-repression of the transcription factor DUX4, whose target genes are known to be toxic to muscle cells [55]. In FSHD muscle cells, MATR3 has been shown to bind to the DUX4 DNA-binding domain and inhibit DUX4-mediated gene expression, thereby reducing apoptosis and improving myogenic defects [55]. Intriguingly, these effects seem to be mediated by the N-terminal IDR of MATR3 as it is both necessary and sufficient for the inhibition of the DUX4-mediated phenotypes.
Lastly, the role of MATR3 has also been studied in the regulation of human immunodeficiency virus (HIV) gene expression. Several studies have demonstrated that MATR3 is a cofactor and modulator of Rev, an HIV-1 encoded protein that functions in the nuclear export of viral mRNA [101,102,103]. Furthermore, depletion of MATR3 inhibited HIV-1 replication [103]. Conversely, overexpression of MATR3 augmented HIV-1 replication. However, MATR3’s effect on HIV-1 viral replication may be mediated by its ability to modulate the inhibition of HIV-1 through ZAP, a host antiviral protein, through its interaction with ZAP and the ZAP degradation complex [54].
7. Conclusions
Since the discovery of MATR3 as a nuclear matrix protein about 30 years ago, our understanding of its roles has greatly expanded. We now know that MATR3 is not merely a scaffolding protein; it has also been found to play a pleiotropic role at multiple levels during gene expression, contributing to chromatin organization, transcription, RNA metabolism, and translation by collaborating with various protein partners. Additionally, accumulating evidence also revealed that MATR3 may play a critical role in the DNA damage response. In terms of its cellular function, MATR3 plays an important role in cell proliferation, differentiation, and survival. More studies are required to reveal how MATR3 works together with its interactors to regulate gene expression in a specific cellular context. After the identification of disease-associated mutations, MATR3 has become a more attractive target for further study. We now have some clues as to how the ALS-linked S85C mutation alters MATR3 function and causes disease; however, additional studies are required to understand how these disease-associated mutations alter MATR3’s structure, properties, and function, and how this mutant protein leads to cellular dysfunction and cause disease. Recent studies have also revealed MATR3’s involvement in other diseases, including cancer, further underscoring the importance in studying the role of MATR3 in various cellular and disease contexts. Determining the role of MATR3 would help identify potential therapeutic targets for various diseases, from neurodegeneration to cancer, and enable development of therapeutic strategies.
Author Contributions
J.R.S. and J.P. wrote the draft and edited the manuscript. J.R.S. designed and generated the tables and figures using BioRender.com. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Natural Sciences and Engineering Research Council of Canada (NSERC, DGECR-2018-00306), Canadian Institutes of Health Research (CIHR, 202104PJT-462444-NSB-CEAB-275899), ALS Association Prevention Program and the Canada Research Chairs Program (CRC-2021-00063). J.R.S was supported by a Catalyzing the Talent Pipeline Scholarship from the David Dime Family Catalyst Initiative in Molecular Genetics at the University of Toronto.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nakayasu, H.; Berezney, R. Nuclear Matrins: Identification of the Major Nuclear Matrix Proteins. Proc. Natl. Acad. Sci. USA 1991, 88, 10312–10316. [Google Scholar] [CrossRef] [PubMed]
- Belgrader, P.; Dey, R.; Berezney, R. Molecular Cloning of Matrin 3. A 125-Kilodalton Protein of the Nuclear Matrix Contains an Extensive Acidic Domain. J. Biol. Chem. 1991, 266, 9893–9899. [Google Scholar] [CrossRef] [PubMed]
- Hibino, Y.; Nakamura, K.; Asano, S.; Sugano, N. Affinity of a Highly Repetitive Bent DNA for Nuclear Scaffold Proteins from Rat Liver. Biochem. Biophys. Res. Commun. 1992, 184, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Hibino, Y.; Nakamura, K.; Tsukada, S.; Sugano, N. Purification and Characterization of Nuclear Scaffold Proteins Which Bind to a Highly Repetitive Bent DNA from Rat Liver. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1993, 1174, 162–170. [Google Scholar] [CrossRef]
- Hibino, Y.; Ohzeki, H.; Hirose, N.; Sugano, N. Involvement of Phosphorylation in Binding of Nuclear Scaffold Proteins from Rat Liver to a Highly Repetitive DNA Component. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1998, 1396, 88–96. [Google Scholar] [CrossRef]
- Hibino, Y.; Ohzeki, H.; Sugano, N.; Hiraga, K. Transcription Modulation by a Rat Nuclear Scaffold Protein, P130, and a Rat Highly Repetitive DNA Component or Various Types of Animal and Plant Matrix or Scaffold Attachment Regions. Biochem. Biophys. Res. Commun. 2000, 279, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Hibino, Y.; Usui, T.; Morita, Y.; Hirose, N.; Okazaki, M.; Sugano, N.; Hiraga, K. Molecular Properties and Intracellular Localization of Rat Liver Nuclear Scaffold Protein P130. Biochim. Biophys. Acta 2006, 1759, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Hisada-Ishii, S.; Ebihara, M.; Kobayashi, N.; Kitagawa, Y. Bipartite Nuclear Localization Signal of Matrin 3 Is Essential for Vertebrate Cells. Biochem. Biophys. Res. Commun. 2007, 354, 72–76. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Gao, Z.; Ma, X.; Wang, Q.; Xu, X.; Cai, X.; Zhang, Y.; Zhang, Z.; Wei, G.; et al. MATR3 -antisense LINE1 RNA Meshwork Scaffolds Higher-order Chromatin Organization. EMBO Rep. 2023, 24, e57550. [Google Scholar] [CrossRef]
- Cha, H.J.; Uyan, Ö.; Kai, Y.; Liu, T.; Zhu, Q.; Tothova, Z.; Botten, G.A.; Xu, J.; Yuan, G.-C.; Dekker, J.; et al. Inner Nuclear Protein Matrin-3 Coordinates Cell Differentiation by Stabilizing Chromatin Architecture. Nat. Commun. 2021, 12, 6241. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, Q.; Kai, Y.; Bingham, T.; Wang, S.; Cha, H.J.; Mehta, S.; Schlaeger, T.M.; Yuan, G.-C.; Orkin, S.H. Matrin3 Mediates Differentiation through Stabilizing Chromatin Loop-Domain Interactions and YY1 Mediated Enhancer-Promoter Interactions. Nat. Commun. 2024, 15, 1274. [Google Scholar] [CrossRef] [PubMed]
- Pollini, D.; Loffredo, R.; Maniscalco, F.; Cardano, M.; Micaelli, M.; Bonomo, I.; Licata, N.V.; Peroni, D.; Tomaszewska, W.; Rossi, A.; et al. Multilayer and MATR3-Dependent Regulation of mRNAs Maintains Pluripotency in Human Induced Pluripotent Stem Cells. iScience 2021, 24, 102197. [Google Scholar] [CrossRef]
- Skowronska-Krawczyk, D.; Ma, Q.; Schwartz, M.; Scully, K.; Li, W.; Liu, Z.; Taylor, H.; Tollkuhn, J.; Ohgi, K.A.; Notani, D.; et al. Required Enhancer-Matrin-3 Network Interactions for a Homeodomain Transcription Program. Nature 2014, 514, 257–261. [Google Scholar] [CrossRef]
- Shi, L.; Sun, J.; Kinomura, A.; Fukuto, A.; Horikoshi, Y.; Tashiro, S. Matrin3 Promotes Homologous Recombinational Repair by Regulation of RAD51. J. Biochem. 2019, 166, 343–351. [Google Scholar] [CrossRef]
- Coelho, M.B.; Attig, J.; Bellora, N.; König, J.; Hallegger, M.; Kayikci, M.; Eyras, E.; Ule, J.; Smith, C.W. Nuclear Matrix Protein Matrin3 Regulates Alternative Splicing and Forms Overlapping Regulatory Networks with PTB. EMBO J. 2015, 34, 653–668. [Google Scholar] [CrossRef]
- Khan, M.; Chen, X.X.L.; Dias, M.; Santos, J.R.; Kour, S.; You, J.; Van Bruggen, R.; Youssef, M.M.M.; Wan, Y.; Liu, Z.; et al. MATR3 Pathogenic Variants Differentially Impair Its Cryptic Splicing Repression Function. FEBS Lett. 2024, 598, 415–436. [Google Scholar] [CrossRef] [PubMed]
- Uemura, Y.; Oshima, T.; Yamamoto, M.; Reyes, C.J.; Costa Cruz, P.H.; Shibuya, T.; Kawahara, Y. Matrin3 Binds Directly to Intronic Pyrimidine-Rich Sequences and Controls Alternative Splicing. Genes Cells 2017, 22, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Attig, J.; Agostini, F.; Gooding, C.; Chakrabarti, A.M.; Singh, A.; Haberman, N.; Zagalak, J.A.; Emmett, W.; Smith, C.W.J.; Luscombe, N.M.; et al. Heteromeric RNP Assembly at LINEs Controls Lineage-Specific RNA Processing. Cell 2018, 174, 1067–1081.e17. [Google Scholar] [CrossRef]
- Muys, B.R.; Shrestha, R.L.; Anastasakis, D.G.; Pongor, L.; Li, X.L.; Grammatikakis, I.; Polash, A.; Chari, R.; Gorospe, M.; Harris, C.C.; et al. Matrin3 Regulates Mitotic Spindle Dynamics by Controlling Alternative Splicing of CDC14B. Cell Rep. 2023, 42, 112260. [Google Scholar] [CrossRef]
- Niimori-Kita, K.; Tamamaki, N.; Koizumi, D.; Niimori, D. Matrin-3 Is Essential for Fibroblast Growth Factor 2-Dependent Maintenance of Neural Stem Cells. Sci. Rep. 2018, 8, 13412. [Google Scholar] [CrossRef]
- Banerjee, A.; Vest, K.E.; Pavlath, G.K.; Corbett, A.H. Nuclear Poly(A) Binding Protein 1 (PABPN1) and Matrin3 Interact in Muscle Cells and Regulate RNA Processing. Nucleic Acids Res. 2017, 45, 10706–10725. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.M.; Miguez, R.A.; Li, X.; Ho, Y.-S.; Feldman, E.L.; Barmada, S.J. Matrin 3-Dependent Neurotoxicity Is Modified by Nucleic Acid Binding and Nucleocytoplasmic Localization. eLife 2018, 7, e35977. [Google Scholar] [CrossRef] [PubMed]
- Przygodzka, P.; Boncela, J.; Cierniewski, C.S. Matrin 3 as a Key Regulator of Endothelial Cell Survival. Exp. Cell Res. 2011, 317, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.O.; Pioro, E.P.; Boehringer, A.; Chia, R.; Feit, H.; Renton, A.E.; Pliner, H.A.; Abramzon, Y.; Marangi, G.; Winborn, B.J.; et al. Mutations in the Matrin 3 Gene Cause Familial Amyotrophic Lateral Sclerosis. Nat. Neurosci. 2014, 17, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Zech, M.; Seibt, A.; Zumbaum, B.; Klee, D.; Meitinger, T.; Winkelmann, J.; Mayatepek, E.; Wagner, M.; Distelmaier, F. MATR3 Haploinsufficiency and Early-Onset Neurodegeneration. Brain 2021, 144, e72. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Jamal, M.; Zeng, X.; Lei, Y.; Xiao, D.; Wei, Z.; Zhang, C.; Zhang, X.; Pan, S.; Ding, Q.; et al. Matrin-3 Acts as a Potential Biomarker and Promotes Hepatocellular Carcinoma Progression by Interacting with Cell Cycle-Regulating Genes. Cell Cycle 2024, 23, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Nho, S.; Yoon, G.; Seo, J.; Oh, H.; Cho, S.; Kim, H.; Choi, H.; Shim, J.; Chae, J. Licochalcone-H Induces the Apoptosis of Human Oral Squamous Cell Carcinoma Cells via Regulation of Matrin-3. Oncol. Rep. 2018, 41, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, H.; Fukushima, S.; Kimura, T.; Okada, E.; Ishibashi, T.; Mizuhashi, S.; Kanemaru, H.; Kajihara, I.; Makino, K.; Miyashita, A.; et al. Matrin-3 Plays an Important Role in Cell Cycle and Apoptosis for Survival in Malignant Melanoma. J. Dermatol. Sci. 2020, 100, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, S.J.; Kwon, Y.; Ma, L.; Kim, J. Tumor Suppressive Function of Matrin 3 in the Basal-like Breast Cancer. Biol. Res. 2020, 53, 42. [Google Scholar] [CrossRef]
- Durślewicz, J.; Klimaszewska-Wiśniewska, A.; Jóźwicki, J.; Antosik, P.; Kozerawski, K.; Grzanka, D.; Braun, M. Prognostic Significance of MATR3 in Stage I and II Non-Small Cell Lung Cancer Patients. J. Cancer Res. Clin. Oncol. 2022, 148, 3313–3322. [Google Scholar] [CrossRef]
- Durślewicz, J.; Klimaszewska-Wiśniewska, A.; Antosik, P.; Grzanka, D. Low Expression of MATR3 Is Associated with Poor Survival in Clear Cell Renal Cell Carcinoma. Biomedicines 2023, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Narwade, N.; Patel, S.; Alam, A.; Chattopadhyay, S.; Mittal, S.; Kulkarni, A. Mapping of Scaffold/Matrix Attachment Regions in Human Genome: A Data Mining Exercise. Nucleic Acids Res. 2019, 47, 7247–7261. [Google Scholar] [CrossRef] [PubMed]
- Zeitz, M.J.; Malyavantham, K.S.; Seifert, B.; Berezney, R. Matrin 3: Chromosomal Distribution and Protein Interactions. J. Cell. Biochem. 2009, 108, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Malyavantham, K.S.; Bhattacharya, S.; Barbeitos, M.; Mukherjee, L.; Xu, J.; Fackelmayer, F.O.; Berezney, R. Identifying Functional Neighborhoods within the Cell Nucleus: Proximity Analysis of Early S-phase Replicating Chromatin Domains to Sites of Transcription, RNA Polymerase II, HP1γ, Matrin 3 and SAF-A. J. Cell. Biochem. 2008, 105, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Fujii, H. Direct Identification of Insulator Components by Insertional Chromatin Immunoprecipitation. PLoS ONE 2011, 6, e26109. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Zhang, Q.C.; da Rocha, S.T.; Flynn, R.A.; Bharadwaj, M.; Calabrese, J.M.; Magnuson, T.; Heard, E.; Chang, H.Y. Systematic Discovery of Xist RNA Binding Proteins. Cell 2015, 161, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Pandya-Jones, A.; Markaki, Y.; Serizay, J.; Chitiashvili, T.; Mancia Leon, W.R.; Damianov, A.; Chronis, C.; Papp, B.; Chen, C.-K.; McKee, R.; et al. A Protein Assembly Mediates Xist Localization and Gene Silencing. Nature 2020, 587, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Martens, J.H.A.; Verlaan, M.; Kalkhoven, E.; Dorsman, J.C.; Zantema, A. Scaffold/Matrix Attachment Region Elements Interact with a P300—Scaffold Attachment Factor A Complex and Are Bound by Acetylated Nucleosomes. Mol. Cell. Biol. 2002, 22, 2598–2606. [Google Scholar] [CrossRef] [PubMed]
- Chi, B.; O’Connell, J.D.; Yamazaki, T.; Gangopadhyay, J.; Gygi, S.P.; Reed, R. Interactome Analyses Revealed That the U1 snRNP Machinery Overlaps Extensively with the RNAP II Machinery and Contains Multiple ALS/SMA-Causative Proteins. Sci. Rep. 2018, 8, 8755. [Google Scholar] [CrossRef]
- Chi, B.; O’Connell, J.D.; Iocolano, A.D.; Coady, J.A.; Yu, Y.; Gangopadhyay, J.; Gygi, S.P.; Reed, R. The Neurodegenerative Diseases ALS and SMA Are Linked at the Molecular Level via the ASC-1 Complex. Nucleic Acids Res. 2018, 46, 11939–11951. [Google Scholar] [CrossRef]
- Salton, M.; Lerenthal, Y.; Wang, S.-Y.; Chen, D.J.; Shiloh, Y. Involvement of Matrin 3 and SFPQ/NONO in the DNA Damage Response. Cell Cycle 2010, 9, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zou, P.; Yao, J.; Yun, D.; Bao, H.; Du, R.; Long, J.; Chen, X. Proteomic Dissection of Cell Type-Specific H2AX-Interacting Protein Complex Associated with Hepatocellular Carcinoma. J. Proteome Res. 2010, 9, 1402–1415. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Ballif, B.A.; Smogorzewska, A.; McDonald, E.R.; Hurov, K.E.; Luo, J.; Bakalarski, C.E.; Zhao, Z.; Solimini, N.; Lerenthal, Y.; et al. ATM and ATR Substrate Analysis Reveals Extensive Protein Networks Responsive to DNA Damage. Science 2007, 316, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Blasius, M.; Forment, J.V.; Thakkar, N.; Wagner, S.A.; Choudhary, C.; Jackson, S.P. A Phospho-Proteomic Screen Identifies Substrates of the Checkpoint Kinase Chk1. Genome Biol. 2011, 12, R78. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, L.; Li, Z.; Li, S.; Dynan, W.S. SFPQ•NONO and XLF Function Separately and Together to Promote DNA Double-Strand Break Repair via Canonical Nonhomologous End Joining. Nucleic Acids Res. 2017, 45, 1848–1859. [Google Scholar] [CrossRef] [PubMed]
- Salton, M.; Elkon, R.; Borodina, T.; Davydov, A.; Yaspo, M.L.; Halperin, E.; Shiloh, Y. Matrin 3 Binds and Stabilizes mRNA. PLoS ONE 2011, 6, e23882. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Cui, S.; Bian, C.; Yu, X. Uhrf2 Is Important for DNA Damage Response in Vascular Smooth Muscle Cells. Biochem. Biophys. Res. Commun. 2013, 441, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Hanaki, S.; Habara, M.; Shimada, M. UV-induced Activation of ATR Is Mediated by UHRF2. Genes. Cells 2021, 26, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, X.; Zeng, S.; Zhang, T.; Cheng, F.; Chen, R.; Duan, C. UHRF2 Promotes DNA Damage Response by Decreasing P21 via RING Finger Domain. Biotechnol. Lett. 2018, 40, 1181–1188. [Google Scholar] [CrossRef]
- Wang, Q.; Goldstein, M.; Alexander, P.; Wakeman, T.P.; Sun, T.; Feng, J.; Lou, Z.; Kastan, M.B.; Wang, X.-F. Rad17 Recruits the MRE11-RAD50-NBS1 Complex to Regulate the Cellular Response to DNA Double-Strand Breaks. EMBO J. 2014, 33, 862–877. [Google Scholar] [CrossRef]
- Höck, J.; Weinmann, L.; Ender, C.; Rüdel, S.; Kremmer, E.; Raabe, M.; Urlaub, H.; Meister, G. Proteomic and Functional Analysis of Argonaute-containing mRNA–Protein Complexes in Human Cells. EMBO Rep. 2007, 8, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Iradi, M.C.G.; Triplett, J.C.; Thomas, J.D.; Davila, R.; Crown, A.M.; Brown, H.; Lewis, J.; Swanson, M.S.; Xu, G.; Rodriguez-Lebron, E.; et al. Characterization of Gene Regulation and Protein Interaction Networks for Matrin 3 Encoding Mutations Linked to Amyotrophic Lateral Sclerosis and Myopathy. Sci. Rep. 2018, 8, 4049. [Google Scholar] [CrossRef] [PubMed]
- Boehringer, A.; Garcia-Mansfield, K.; Singh, G.; Bakkar, N.; Pirrotte, P.; Bowser, R. ALS Associated Mutations in Matrin 3 Alter Protein-Protein Interactions and Impede mRNA Nuclear Export. Sci. Rep. 2017, 7, 14529. [Google Scholar] [CrossRef] [PubMed]
- Erazo, A.; Goff, S.P. Nuclear Matrix Protein Matrin 3 Is a Regulator of ZAP-Mediated Retroviral Restriction. Retrovirology 2015, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Runfola, V.; Giambruno, R.; Caronni, C.; Pannese, M.; Andolfo, A.; Gabellini, D. MATR3 Is an Endogenous Inhibitor of DUX4 in FSHD Muscular Dystrophy. Cell Rep. 2023, 42, 113120. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Gryder, B.; Woods, W.S.; Subramanian, M.; Jones, M.F.; Li, X.L.; Jenkins, L.M.; Shabalina, S.A.; Mo, M.; Dasso, M.; et al. Prosurvival Long Noncoding RNA PINCR Regulates a Subset of P53 Targets in Human Colorectal Cancer Cells by Binding to Matrin 3. eLife 2017, 6, e23244. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.; Treiber, T.; Meister, G.; Schratt, G. The Nuclear Matrix Protein Matr3 Regulates Processing of the Synaptic microRNA-138-5p. Neurobiol. Learn. Mem. 2019, 159, 36–45. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Kuwasako, K.; Takizawa, M.; Takahashi, M.; Tsuda, K.; Nagata, T.; Watanabe, S.; Tanaka, A.; Kobayashi, N.; Kigawa, T.; et al. 1H, 13C and 15N Resonance Assignments and Solution Structures of the Two RRM Domains of Matrin-3. Biomol. NMR Assign. 2022, 16, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Luo, E.-C.; Nathanson, J.L.; Tan, F.E.; Schwartz, J.L.; Schmok, J.C.; Shankar, A.; Markmiller, S.; Yee, B.A.; Sathe, S.; Pratt, G.A.; et al. Large-Scale Tethered Function Assays Identify Factors That Regulate mRNA Stability and Translation. Nat. Struct. Mol. Biol. 2020, 27, 989–1000. [Google Scholar] [CrossRef]
- Zhang, Z.; Carmichael, G.G. The Fate of dsRNA in the Nucleus: A P54(Nrb)-Containing Complex Mediates the Nuclear Retention of Promiscuously A-to-I Edited RNAs. Cell 2001, 106, 465–475. [Google Scholar] [CrossRef]
- Damianov, A.; Ying, Y.; Lin, C.-H.; Lee, J.-A.; Tran, D.; Vashisht, A.A.; Bahrami-Samani, E.; Xing, Y.; Martin, K.C.; Wohlschlegel, J.A.; et al. Rbfox Proteins Regulate Splicing as Part of a Large Multiprotein Complex LASR. Cell 2016, 165, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.R.; Blazquez, L.; Ule, J. Lessons from Non-Canonical Splicing. Nat. Rev. Genet. 2016, 17, 407–421. [Google Scholar] [CrossRef]
- Gama-Carvalho, M.; Carmo-Fonseca, M. The Rules and Roles of Nucleocytoplasmic Shuttling Proteins. FEBS Lett. 2001, 498, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, A.G. eIF3: A Versatile Scaffold for Translation Initiation Complexes. Trends Biochem. Sci. 2006, 31, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Sánchez-Pérez, A.M.; Montoliu, C.; Berezney, R.; Malyavantham, K.; Costa, L.G.; Calvete, J.J.; Felipo, V. Activation of NMDA Receptors Induces Protein Kinase A-mediated Phosphorylation and Degradation of Matrin 3. Blocking These Effects Prevents NMDA-induced Neuronal Death. J. Neurochem. 2005, 94, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Alexander Valencia, C.; Ju, W.; Liu, R. Matrin 3 Is a Ca2+/Calmodulin-Binding Protein Cleaved by Caspases. Biochem. Biophys. Res. Commun. 2007, 361, 281–286. [Google Scholar] [CrossRef] [PubMed]
- De Marco, G.; Lomartire, A.; Manera, U.; Canosa, A.; Grassano, M.; Casale, F.; Fuda, G.; Salamone, P.; Rinaudo, M.T.; Colombatto, S.; et al. Effects of Intracellular Calcium Accumulation on Proteins Encoded by the Major Genes Underlying Amyotrophic Lateral Sclerosis. Sci. Rep. 2022, 12, 395. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.; Van Loveren, H. Matrin 3 Co-Immunoprecipitates with the Heat Shock Proteins Glucose-Regulated Protein 78 (GRP78), GRP75 and Glutathione S-Transferase π Isoform 2 (GSTπ2) in Thymoma Cells. Biochimie 2014, 101, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.M.; Wu, J.J.; Gillies, C.A.; Doctrove, Q.A.; Li, X.; Huang, H.; Tank, E.H.M.; Shakkottai, V.G.; Barmada, S. Neuronal Activity Regulates Matrin 3 Abundance and Function in a Calcium-Dependent Manner through Calpain-Mediated Cleavage and Calmodulin Binding. Proc. Natl. Acad. Sci. USA 2023, 120, e2206217120. [Google Scholar] [CrossRef]
- Kao, C.S.; Van Bruggen, R.; Kim, J.R.; Chen, X.X.L.; Chan, C.; Lee, J.; Cho, W.I.; Zhao, M.; Arndt, C.; Maksimovic, K.; et al. Selective Neuronal Degeneration in MATR3 S85C Knock-in Mouse Model of Early-Stage ALS. Nat. Commun. 2020, 11, 5304. [Google Scholar] [CrossRef]
- Quintero-Rivera, F.; Xi, Q.J.; Keppler-Noreuil, K.M.; Lee, J.H.; Higgins, A.W.; Anchan, R.M.; Roberts, A.E.; Seong, I.S.; Fan, X.; Lage, K.; et al. MATR3 Disruption in Human and Mouse Associated with Bicuspid Aortic Valve, Aortic Coarctation and Patent Ductus Arteriosus. Hum. Mol. Genet. 2015, 24, 2375–2389. [Google Scholar] [CrossRef] [PubMed]
- Project MinE ALS Sequencing Consortium Project MinE: Study Design and Pilot Analyses of a Large-Scale Whole-Genome Sequencing Study in Amyotrophic Lateral Sclerosis. Eur. J. Hum. Genet. 2018, 26, 1537–1546. [CrossRef] [PubMed]
- Lin, K.P.; Tsai, P.C.; Liao, Y.C.; Chen, W.T.; Tsai, C.P.; Soong, B.W.; Lee, Y.C. Mutational Analysis of MATR3 in Taiwanese Patients with Amyotrophic Lateral Sclerosis. Neurobiol. Aging 2015, 36, 2005.e1–2005.e4. [Google Scholar] [CrossRef] [PubMed]
- Origone, P.; Verdiani, S.; Bandettini Di Poggio, M.; Zuccarino, R.; Vignolo, M.; Caponnetto, C.; Mandich, P. A Novel Arg147Trp MATR3 Missense Mutation in a Slowly Progressive ALS Italian Patient. Amyotroph Lateral Scler Front. Degener 2015, 16, 530–531. [Google Scholar] [CrossRef] [PubMed]
- Leblond, C.S.; Gan-Or, Z.; Spiegelman, D.; Laurent, S.B.; Szuto, A.; Hodgkinson, A.; Dionne-Laporte, A.; Provencher, P.; Carvalho, M.; Orru, S.; et al. Replication Study of MATR3 in Familial and Sporadic Amyotrophic Lateral Sclerosis. Neurobiol. Aging 2016, 37, 209.e17–209.e21. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, J.; Tang, L.; Zhang, N.; Fan, D. MATR3 Mutation Analysis in a Chinese Cohort with Sporadic Amyotrophic Lateral Sclerosis. Neurobiol Aging 2016, 38, 218.e3–218.e4. [Google Scholar] [CrossRef] [PubMed]
- Marangi, G.; Lattante, S.; Doronzio, P.N.; Conte, A.; Tasca, G.; Monforte, M.; Patanella, A.K.; Bisogni, G.; Meleo, E.; La Spada, S.; et al. Matrin 3 Variants Are Frequent in Italian ALS Patients. Neurobiol. Aging 2017, 49, 218.e1–218.e7. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.; Zucchi, E.; Martinelli, I.; Gianferrari, G.; Simonini, C.; Amedei, A.; Niccolai, E.; Gellera, C.; Pensato, V.; Mandrioli, J. Duplication of Exons 15 and 16 in Matrin-3: A Phenotype Bridging Amyotrophic Lateral Sclerosis and Immune-Mediated Disorders. Neurol. Sci. 2022, 43, 1419–1421. [Google Scholar] [CrossRef] [PubMed]
- Feit, H.; Silbergleit, A.; Schneider, L.B.; Gutierrez, J.A.; Fitoussi, R.P.; Réyès, C.; Rouleau, G.A.; Brais, B.; Jackson, C.E.; Beckmann, J.S.; et al. Vocal Cord and Pharyngeal Weakness with Autosomal Dominant Distal Myopathy: Clinical Description and Gene Localization to 5q31. Am. J. Hum. Genet. 1998, 63, 1732–1742. [Google Scholar] [CrossRef]
- Senderek, J.; Garvey, S.M.; Krieger, M.; Guergueltcheva, V.; Urtizberea, A.; Roos, A.; Elbracht, M.; Stendel, C.; Tournev, I.; Mihailova, V.; et al. Autosomal-Dominant Distal Myopathy Associated with a Recurrent Missense Mutation in the Gene Encoding the Nuclear Matrix Protein, Matrin 3. Am. J. Hum. Genet. 2009, 84, 511–518. [Google Scholar] [CrossRef]
- Müller, T.J.; Kraya, T.; Stoltenburg-Didinger, G.; Hanisch, F.; Kornhuber, M.; Stoevesandt, D.; Senderek, J.; Weis, J.; Baum, P.; Deschauer, M.; et al. Phenotype of Matrin-3–Related Distal Myopathy in 16 G Erman Patients. Ann. Neurol. 2014, 76, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Mori, A.; Nishida, Y.; Kurisaki, R.; Tawara, N.; Nishikami, T.; Misumi, Y.; Ueyama, H.; Imamura, S.; Higuchi, Y.; et al. Clinicopathological Features of the First Asian Family Having Vocal Cord and Pharyngeal Weakness with Distal Myopathy Due to a MATR3 Mutation. Neuropathol. Appl. Neurobio 2015, 41, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Kraya, T.; Schmidt, B.; MüLLER, T.; Hanisch, F. Impairment of Respiratory Function in Late-Onset Distal Myopathy Due to MATR3 Mutation: Short Reports. Muscle Nerve 2015, 51, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Palmio, J.; Evilä, A.; Bashir, A.; Norwood, F.; Viitaniemi, K.; Vihola, A.; Huovinen, S.; Straub, V.; Hackman, P.; Hirano, M.; et al. Re-Evaluation of the Phenotype Caused by the Common MATR3 p.Ser85Cys Mutation in a New Family. J. Neurol. Neurosurg. Psychiatry 2016, 87, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Laforêt, P.; Malfatti, E.; Metay, C.; Jobic, V.; Carlier, R. Clinical and Histopathological Characterization of the First French Case of MATR3 -Related Distal Myopathy. Neuromuscul. Disord. 2017, 27, S139. [Google Scholar] [CrossRef]
- Cavalli, M.; Cardani, R.; Renna, L.V.; Toffetti, M.; Villa, L.; Meola, G. First Family of MATR3-Related Distal Myopathy From Italy: The Role of Muscle Biopsy in the Diagnosis and Characterization of a Still Poorly Understood Disease. Front. Neurol. 2021, 12, 715386. [Google Scholar] [CrossRef] [PubMed]
- Manini, A.; Velardo, D.; Ciscato, P.; Cinnante, C.; Moggio, M.; Comi, G.; Corti, S.; Ronchi, D. Expanding the Phenotypic Spectrum of Vocal Cord and Pharyngeal Weakness with Distal Myopathy Due to the p.S85C MATR3 Mutation. Neurol. Genet. 2022, 8, e200006. [Google Scholar] [CrossRef] [PubMed]
- Saez-Atienzar, S.; Dalgard, C.L.; Ding, J.; Chiò, A.; Alba, C.; Hupalo, D.N.; Wilkerson, M.D.; Bowser, R.; Pioro, E.P.; Bedlack, R.; et al. Identification of a Pathogenic Intronic KIF5A Mutation in an ALS-FTD Kindred. Neurology 2020, 95, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, R.; Maksimovic, K.; You, J.; Tran, D.D.; Lee, H.J.; Khan, M.; Kao, C.S.; Kim, J.R.; Cho, W.; Chen, X.X.L.; et al. MATR3 F115C Knock-in Mice Do Not Exhibit Motor Defects or Neuropathological Features of ALS. Biochem. Biophys. Res. Commun. 2021, 568, 48–54. [Google Scholar] [CrossRef]
- Sprunger, M.L.; Lee, K.; Sohn, B.S.; Jackrel, M.E. Molecular Determinants and Modifiers of Matrin-3 Toxicity, Condensate Dynamics, and Droplet Morphology. iScience 2022, 25, 103900. [Google Scholar] [CrossRef]
- Dominick, M.; Houchins, N.; Venugopal, V.; Zuberi, A.R.; Lutz, C.M.; Meechooveet, B.; Van Keuren-Jensen, K.; Bowser, R.; Medina, D.X. MATR3 P154S Knock-in Mice Do Not Exhibit Motor, Muscle or Neuropathologic Features of ALS. Biochem. Biophys. Res. Commun. 2023, 645, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Emenecker, R.J.; Griffith, D.; Holehouse, A.S. Metapredict: A Fast, Accurate, and Easy-to-Use Predictor of Consensus Disorder and Structure. Biophys. J. 2021, 120, 4312–4319. [Google Scholar] [CrossRef] [PubMed]
- Schubach, M.; Maass, T.; Nazaretyan, L.; Röner, S.; Kircher, M. CADD v1.7: Using Protein Language Models, Regulatory CNNs and Other Nucleotide-Level Scores to Improve Genome-Wide Variant Predictions. Nucleic Acids Res. 2024, 52, D1143–D1154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yamashita, S.; Hara, K.; Doki, T.; Tawara, N.; Ikeda, T.; Misumi, Y.; Zhang, Z.; Matsuo, Y.; Nagai, M.; et al. A Mutant MATR3 Mouse Model to Explain Multisystem Proteinopathy. J. Pathol. 2019, 249, 182–192. [Google Scholar] [CrossRef]
- You, J.; Maksimovic, K.; Lee, J.; Khan, M.; Masuda, R.; Park, J. Selective Loss of MATR3 in Spinal Interneurons, Upper Motor Neurons and Hippocampal CA1 Neurons in a MATR3 S85C Knock-In Mouse Model of Amyotrophic Lateral Sclerosis. Biology 2022, 11, 298. [Google Scholar] [CrossRef]
- Ramesh, N.; Kour, S.; Anderson, E.N.; Rajasundaram, D.; Pandey, U.B. RNA-Recognition Motif in Matrin-3 Mediates Neurodegeneration through Interaction with hnRNPM. Acta Neuropathol. Commun. 2020, 8, 138. [Google Scholar] [CrossRef]
- Zhao, M.; Kao, C.S.; Arndt, C.; Tran, D.D.; Cho, W.I.; Maksimovic, K.; Chen, X.X.L.; Khan, M.; Zhu, H.; Qiao, J.; et al. Knockdown of Genes Involved in Axonal Transport Enhances the Toxicity of Human Neuromuscular Disease-Linked MATR3 Mutations in Drosophila. FEBS Lett. 2020, 594, 2800–2818. [Google Scholar] [CrossRef]
- Mensch, A.; Meinhardt, B.; Bley, N.; Hüttelmaier, S.; Schneider, I.; Stoltenburg-Didinger, G.; Kraya, T.; Müller, T.; Zierz, S. The p.S85C-Mutation in MATR3 Impairs Stress Granule Formation in Matrin-3 Myopathy. Exp. Neurol. 2018, 306, 222–231. [Google Scholar] [CrossRef]
- Gallego-Iradi, M.C.; Strunk, H.; Crown, A.M.; Davila, R.; Brown, H.; Rodriguez-Lebron, E.; Borchelt, D.R. N-Terminal Sequences in Matrin 3 Mediate Phase Separation into Droplet-like Structures That Recruit TDP43 Variants Lacking RNA Binding Elements. Lab. Investig. 2019, 99, 1030–1040. [Google Scholar] [CrossRef]
- Yang, T.-W.; Sahu, D.; Chang, Y.-W.; Hsu, C.-L.; Hsieh, C.-H.; Huang, H.-C.; Juan, H.-F. RNA-Binding Proteomics Reveals MATR3 Interacting with lncRNA SNHG1 to Enhance Neuroblastoma Progression. J. Proteome Res. 2018, 18, 406–416. [Google Scholar] [CrossRef]
- Kula, A.; Guerra, J.; Knezevich, A.; Kleva, D.; Myers, M.P.; Marcello, A. Characterization of the HIV-1 RNA Associated Proteome Identifies Matrin 3 as a Nuclear Cofactor of Rev Function. Retrovirology 2011, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Yedavalli, V.S.; Jeang, K.-T. Matrin 3 Is a Co-Factor for HIV-1 Rev in Regulating Post-Transcriptional Viral Gene Expression. Retrovirology 2011, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Sarracino, A.; Gharu, L.; Kula, A.; Pasternak, A.O.; Avettand-Fenoel, V.; Rouzioux, C.; Bardina, M.; De Wit, S.; Benkirane, M.; Berkhout, B.; et al. Posttranscriptional Regulation of HIV-1 Gene Expression during Replication and Reactivation from Latency by Nuclear Matrix Protein MATR3. mBio 2018, 9, e02158-18. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).