The Immune Checkpoint Protein PD-L1 Regulates Ciliogenesis and Hedgehog Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Plasmids
2.3. Antibodies and Reagents
2.4. Cell Immunofluorescence Staining
2.5. Western Blot Analysis and Immunoprecipitation
2.6. RNA Interference
2.7. Real-Time Quantitative Reverse Transcription PCR (qRT-PCR)
2.8. Microscopy and Imaging
2.9. Statistics
3. Results
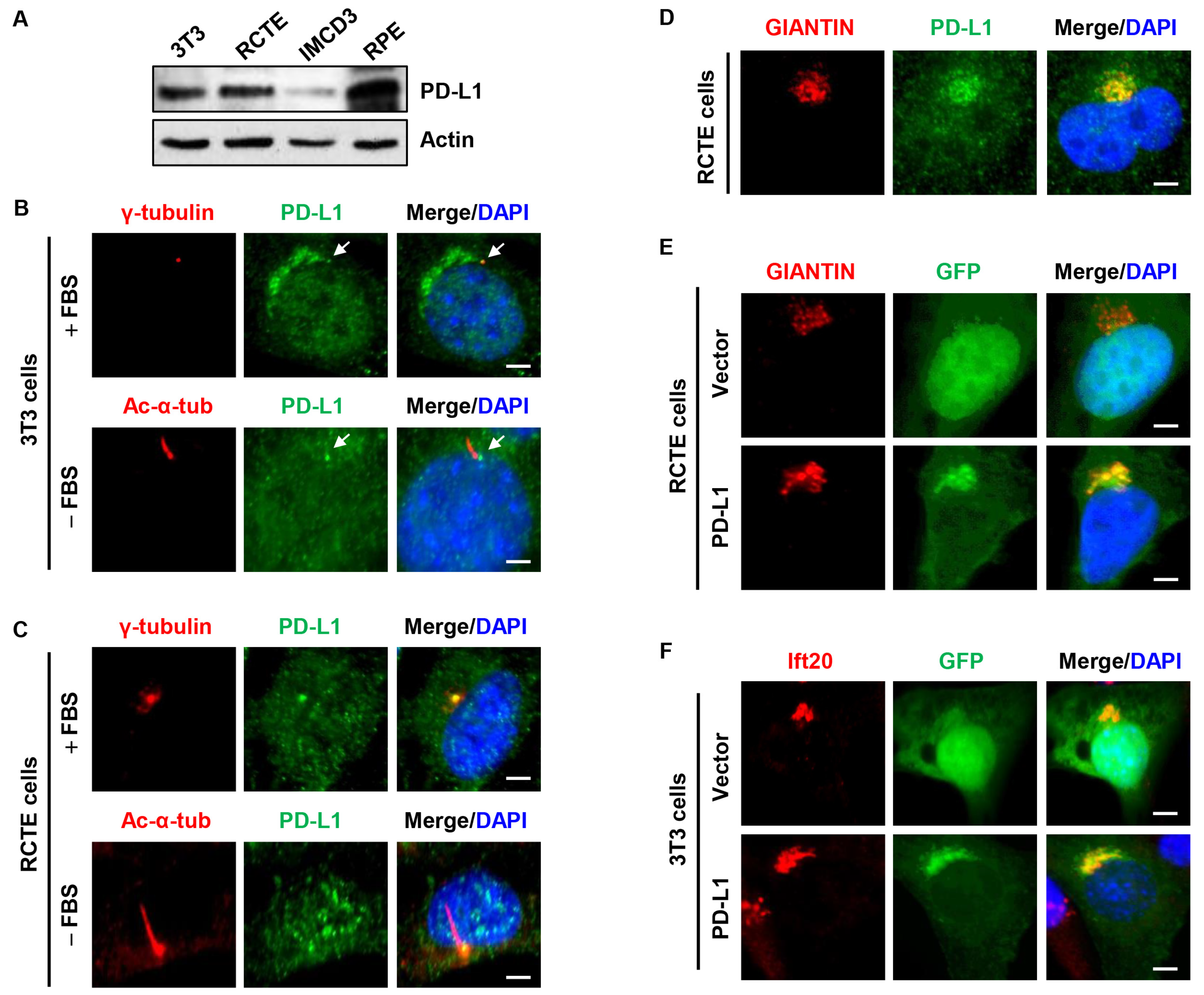
3.1. PD-L1 Is Localized to the Centrosome/Basal Body and the Golgi Complex
3.2. PD-L1 Segregates with the Spindle Poles and Affects Mitosis and Cytokinesis
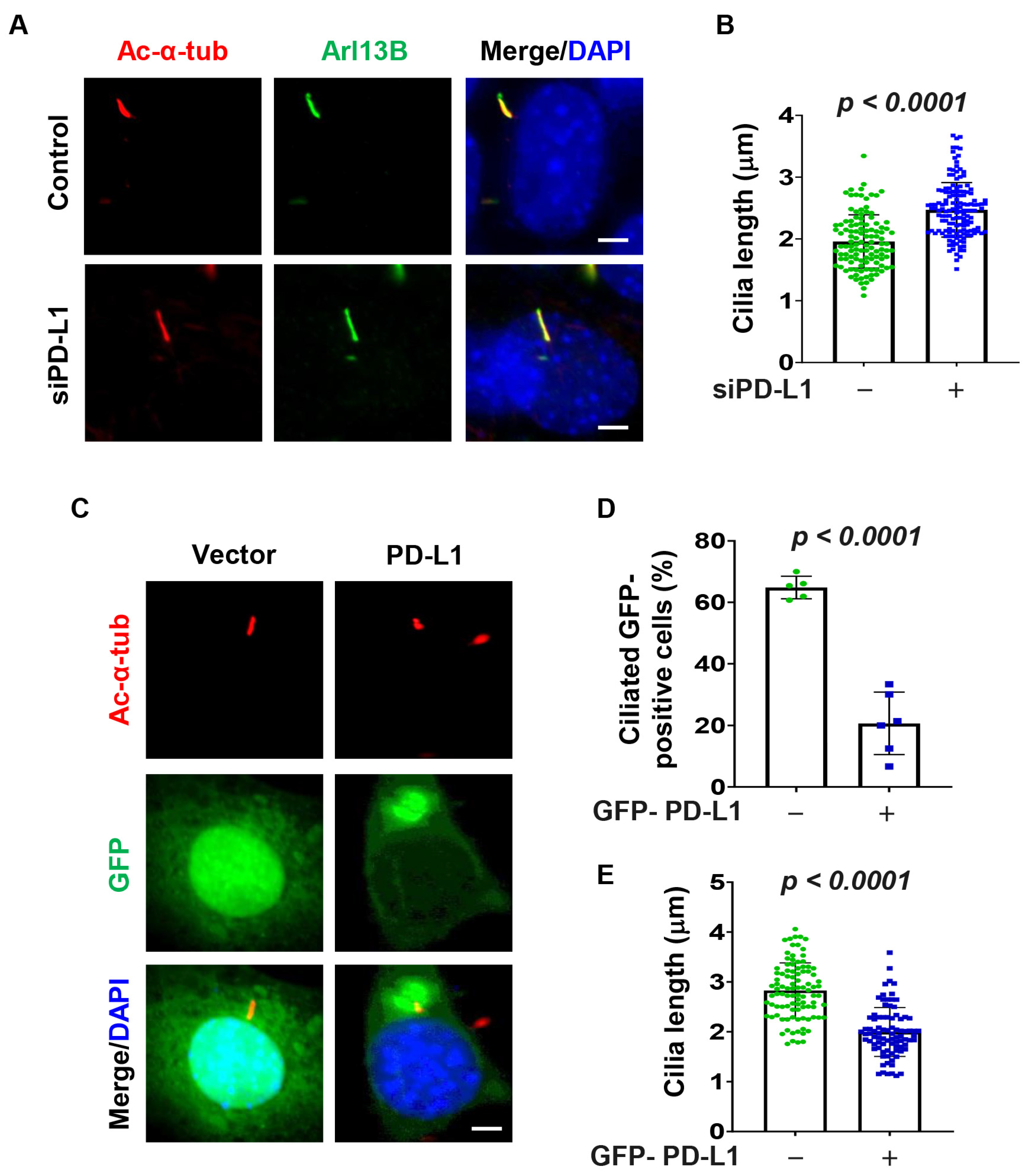
3.3. PD-L1 Regulates Ciliogenesis
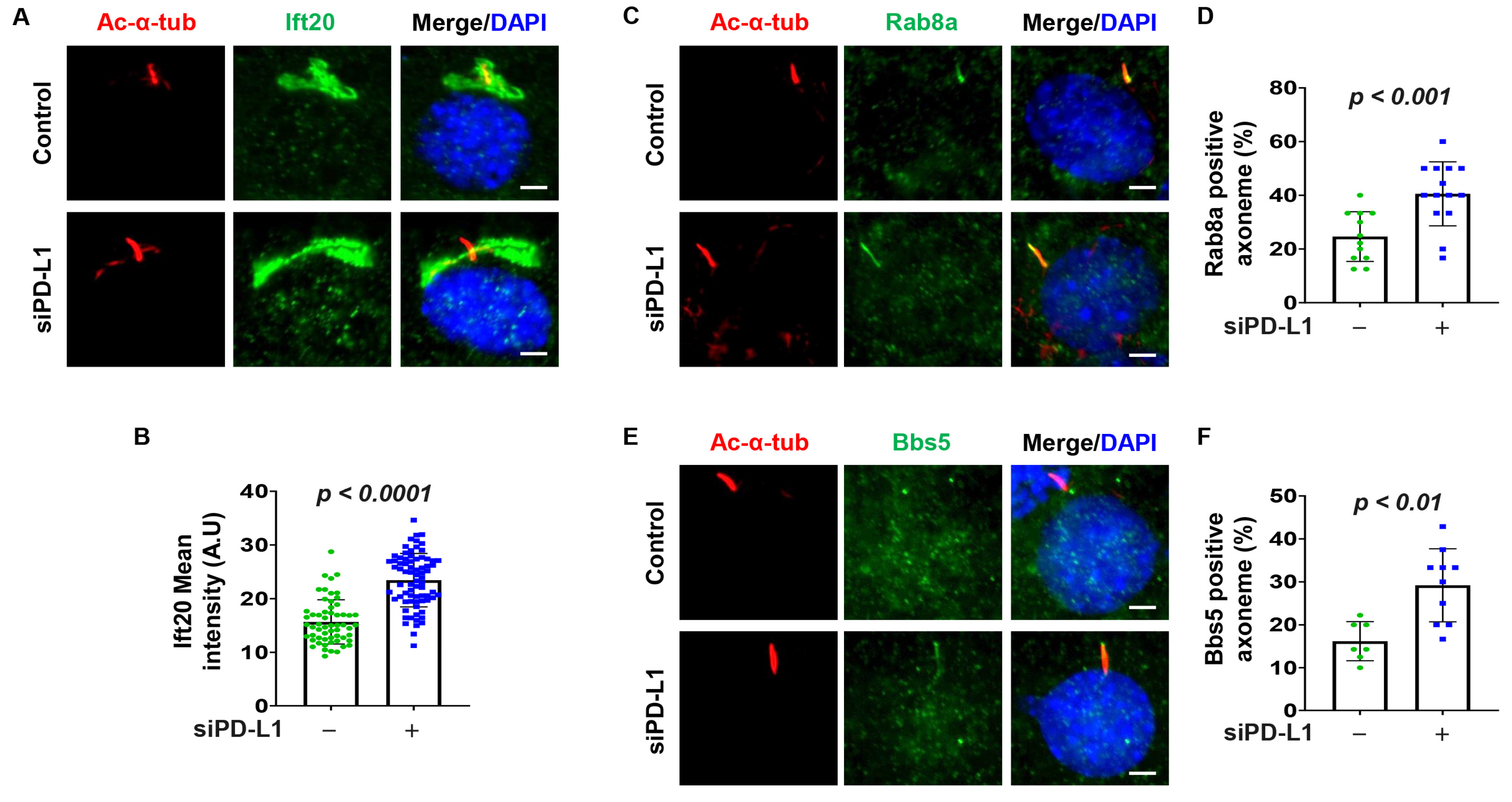
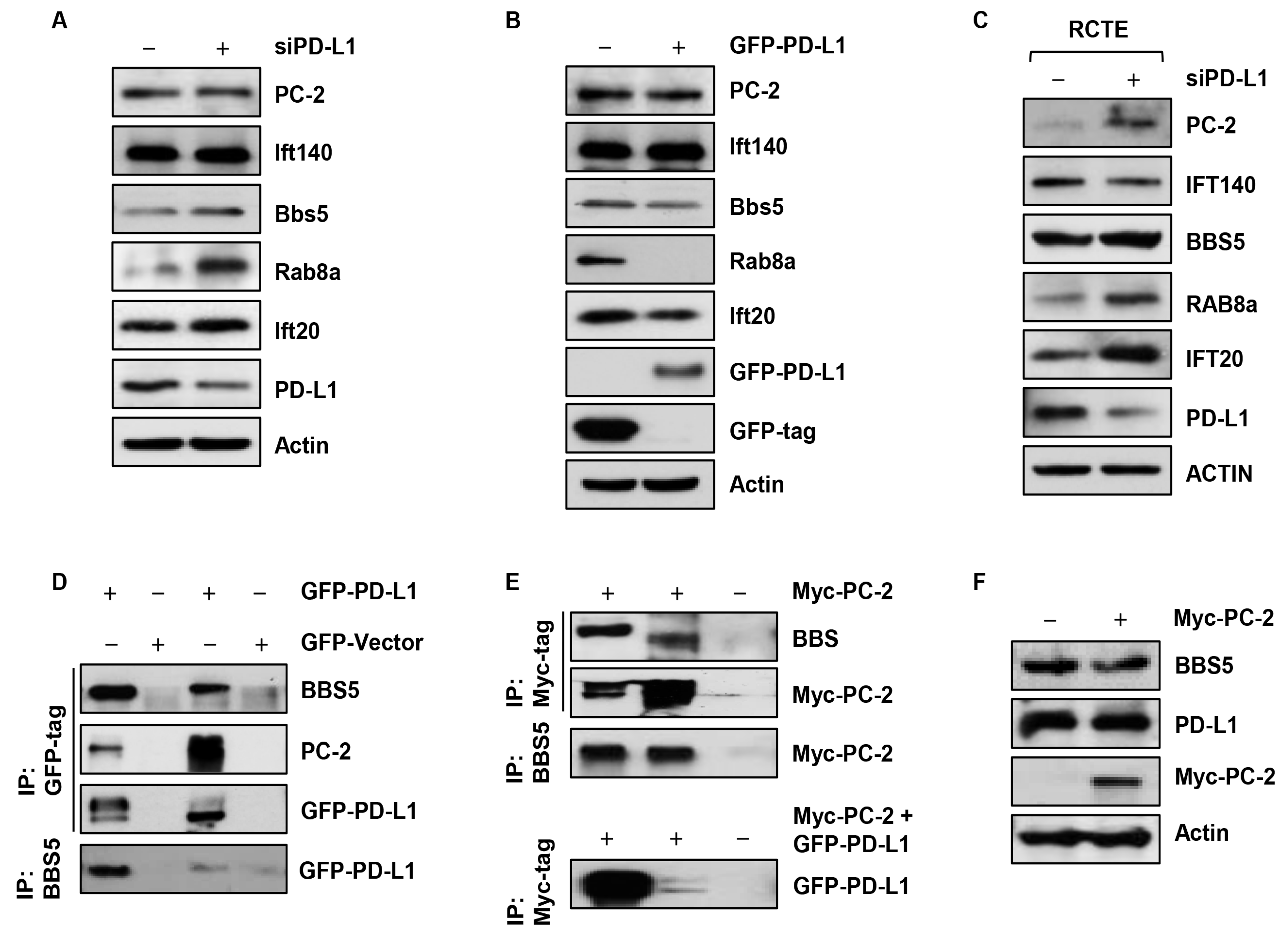
3.4. Depletion of PD-L1 Increases Golgi Accumulation of Ift20, and Ciliary Rab8a and BBS5
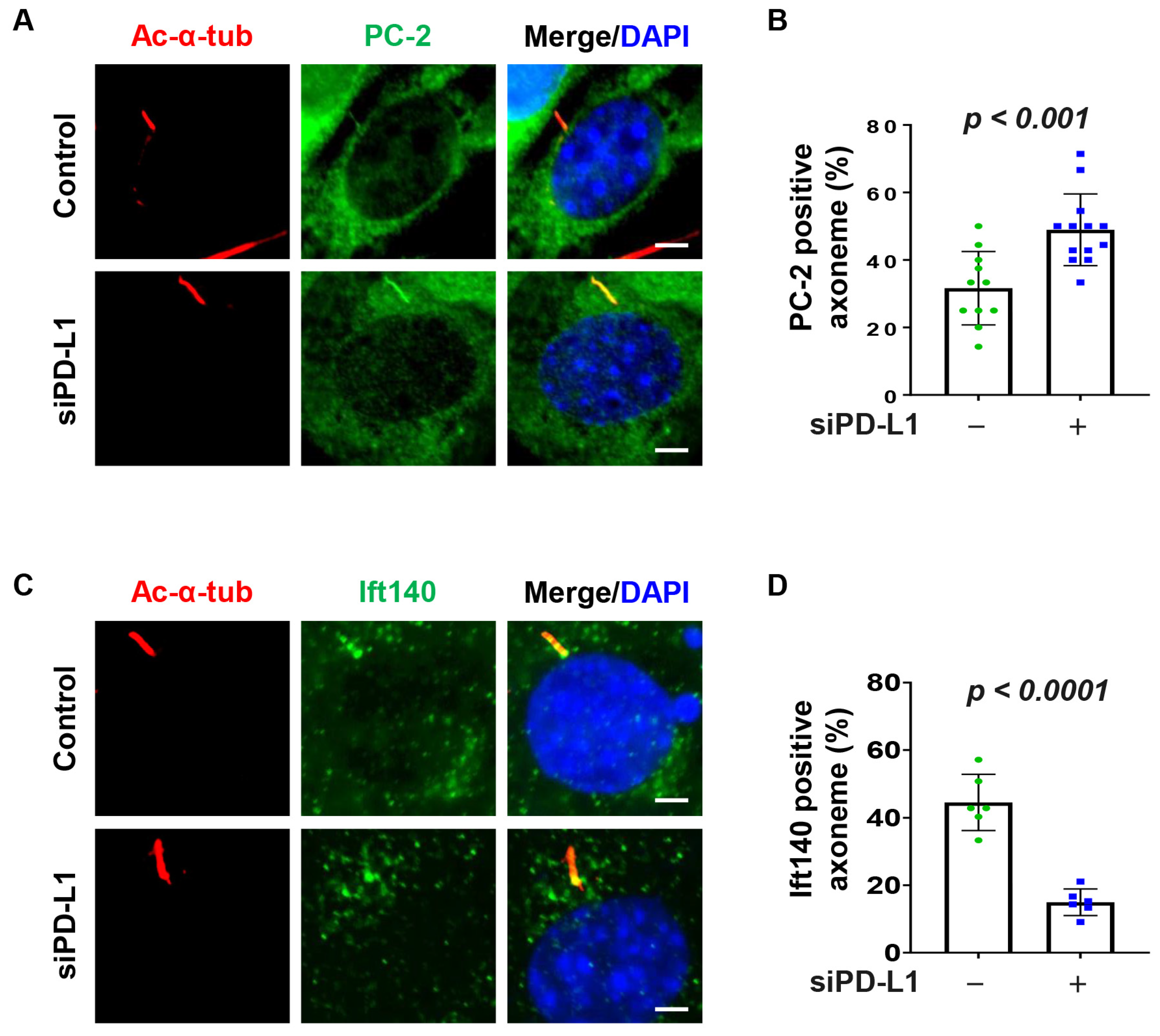
3.5. Depletion of PD-L1 Increases Ciliary PC-2
3.6. PD-L1 Regulates PC-2 Ciliary Localization in a BBS5 Independent Manner
3.7. PD-L1 Forms a Complex with BBS5 and PC-2
3.8. PD-L1 Regulates the Activation of Hh Signaling Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anvarian, Z.; Mykytyn, K.; Mukhopadhyay, S.; Pedersen, L.B.; Christensen, S.T. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 2019, 15, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Adamiok-Ostrowska, A.; Piekielko-Witkowska, A. Ciliary Genes in Renal Cystic Diseases. Cells 2020, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Goetz, S.C.; Liem, K.F., Jr.; Anderson, K.V. The spinocerebellar ataxia-associated gene Tau tubulin kinase 2 controls the initiation of ciliogenesis. Cell 2012, 151, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.M.; Leaper, M.J.; Bayliss, R. The primary cilium Guardian of organ development and homeostasis. Organogenesis 2014, 10, 62–68. [Google Scholar] [CrossRef]
- Karalis, V.; Donovan, K.E.; Sahin, M. Primary Cilia Dysfunction in Neurodevelopmental Disorders beyond Ciliopathies. J. Dev. Biol. 2022, 10, 54. [Google Scholar] [CrossRef]
- Focsa, I.O.; Budisteanu, M.; Balgradean, M. Clinical and genetic heterogeneity of primary ciliopathies (Review). Int. J. Mol. Med. 2021, 48, 176. [Google Scholar] [CrossRef]
- Kathem, S.H.; Mohieldin, A.M.; Nauli, S.M. The Roles of Primary cilia in Polycystic Kidney Disease. AIMS Mol. Sci. 2014, 1, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Marshall, W.F. Intraflagellar Transport and Ciliary Dynamics. Cold Spring Harb. Perspect. Biol. 2017, 9, a021998. [Google Scholar] [CrossRef]
- Liu, P.W.; Lechtreck, K.F. The Bardet-Biedl syndrome protein complex is an adapter expanding the cargo range of intraflagellar transport trains for ciliary export. Proc. Natl. Acad. Sci. USA 2018, 115, E934–E943. [Google Scholar] [CrossRef]
- Ye, F.; Nager, A.R.; Nachury, M.V. BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. J. Cell Biol. 2018, 217, 1847–1868. [Google Scholar] [CrossRef]
- Wingfield, J.L.; Lechtreck, K.F.; Lorentzen, E. Trafficking of ciliary membrane proteins by the intraflagellar transport/BBSome machinery. Essays Biochem. 2018, 62, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalo, F.R.; Reiter, J.F. Open Sesame: How Transition Fibers and the Transition Zone Control Ciliary Composition. Cold Spring Harb. Perspect. Biol. 2017, 9, a028134. [Google Scholar] [CrossRef] [PubMed]
- Bangs, F.; Anderson, K.V. Primary Cilia and Mammalian Hedgehog Signaling. Cold Spring Harb. Perspect. Biol. 2017, 9, a028175. [Google Scholar] [CrossRef] [PubMed]
- Gigante, E.D.; Caspary, T. Signaling in the primary cilium through the lens of the Hedgehog pathway. Wiley Interdiscip. Rev. Dev. Biol. 2020, 9, e377. [Google Scholar] [CrossRef] [PubMed]
- Liem, K.F.; Ashe, A.; He, M.; Satir, P.; Moran, J.; Beier, D.; Wicking, C.; Anderson, K.V. The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking. J. Cell Biol. 2012, 197, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, Y.I.; Lin, C.W.; Chuang, P.T. Hedgehog signaling from the primary cilium to the nucleus: An emerging picture of ciliary localization, trafficking and transduction. Curr. Opin. Genet. Dev. 2013, 23, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Seo, S.; Bugge, K.; Stone, E.M.; Sheffield, V.C. BBS proteins interact genetically with the IFT pathway to influence SHH-related phenotypes. Hum. Mol. Genet. 2012, 21, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Blacque, O.E.; Scheidel, N.; Kuhns, S. Rab GTPases in cilium formation and function. Small GTPases 2018, 9, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Huang, K.Y. Transport of Ciliary Membrane Proteins. Front. Cell Dev. Biol. 2020, 7, 381. [Google Scholar] [CrossRef]
- Masson, J.; El Ghouzzi, V. Golgi Dysfunctions in Ciliopathies. Cells 2022, 11, 2773. [Google Scholar] [CrossRef] [PubMed]
- Joo, K.; Kim, C.G.; Lee, M.S.; Moon, H.Y.; Lee, S.H.; Kim, M.J.; Kweon, H.S.; Park, W.Y.; Kim, C.H.; Gleeson, J.G.; et al. CCDC41 is required for ciliary vesicle docking to the mother centriole. Proc. Natl. Acad. Sci. USA 2013, 110, 5987–5992. [Google Scholar] [CrossRef] [PubMed]
- Douanne, T.; Stinchcombe, J.C.; Griffiths, G.M. Teasing out function from morphology: Similarities between primary cilia and immune synapses. J. Cell Biol. 2021, 220, e202102089. [Google Scholar] [CrossRef]
- Finetti, F.; Paccani, S.R.; Riparbelli, M.G.; Giacomello, E.; Perinetti, G.; Pazour, G.J.; Rosenbaum, J.L.; Baldari, C.T. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat. Cell Biol. 2009, 11, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Chong, W.M.; Wang, W.J.; Mazo, G.; Tanos, B.; Chen, Z.M.; Tran, T.M.N.; Chen, Y.D.; Weng, R.R.; Huang, C.E.; et al. Super-resolution architecture of mammalian centriole distal appendages reveals distinct blade and matrix functional components. Nat. Commun. 2018, 9, 2023. [Google Scholar] [CrossRef]
- Stinchcombe, J.C.; Randzavola, L.O.; Angus, K.L.; Mantell, J.M.; Verkade, P.; Griffiths, G.M. Mother Centriole Distal Appendages Mediate Centrosome Docking at the Immunological Synapse and Reveal Mechanistic Parallels with Ciliogenesis. Curr. Biol. 2015, 25, 3239–3244. [Google Scholar] [CrossRef]
- Cassioli, C.; Onnis, A.; Finetti, F.; Capitani, N.; Brunetti, J.; Compeer, E.B.; Niederlova, V.; Stepanek, O.; Dustin, M.L.; Baldari, C.T. The Bardet-Biedl syndrome complex component BBS1 controls T cell polarity during immune synapse assembly. J. Cell Sci. 2021, 134, jcs258462. [Google Scholar] [CrossRef] [PubMed]
- Follit, J.A.; Tuft, R.A.; Fogarty, K.E.; Pazour, G.J. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell 2006, 17, 3781–3792. [Google Scholar] [CrossRef]
- Vivar, O.I.; Masi, G.; Carpier, J.M.; Magalhaes, J.G.; Galgano, D.; Pazour, G.J.; Amigorena, S.; Hivroz, C.; Baldari, C.T. IFT20 controls LAT recruitment to the immune synapse and T-cell activation in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 386–391. [Google Scholar] [CrossRef]
- He, H.T.; Marguet, D. T-cell antigen receptor triggering and lipid rafts: A matter of space and time scales-Talking Point on the involvement of lipid rafts in T-cell activation. Embo Rep. 2008, 9, 525–530. [Google Scholar] [CrossRef]
- de la Roche, M.; Ritter, A.T.; Angus, K.L.; Dinsmore, C.; Earnshaw, C.H.; Reiter, J.F.; Griffiths, G.M. Hedgehog Signaling Controls T Cell Killing at the Immunological Synapse. Science 2013, 342, 1247–1250. [Google Scholar] [CrossRef]
- Garcia, G.; Raleigh, D.R.; Reiter, J.F. How the Ciliary Membrane Is Organized Inside-Out to Communicate Outside-In. Curr. Biol. 2018, 28, R421–R434. [Google Scholar] [CrossRef] [PubMed]
- Cassioli, C.; Baldari, C.T. A Ciliary View of the Immunological Synapse. Cells 2019, 8, 789. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Abiko, K.; Hamanishi, J.; Matsumura, N.; Mandai, M. Dynamic host immunity and PD-L1/PD-1 blockade efficacy: Developments after “IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer”. Br. J. Cancer 2023, 128, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Dilek, N.; Poirier, N.; Hulin, P.; Coulon, F.; Mary, C.; Ville, S.; Vie, H.; Clémenceau, B.; Blancho, G.; Vanhove, B. Targeting CD28, CTLA-4 and PD-L1 Costimulation Differentially Controls Immune Synapses and Function of Human Regulatory and Conventional T-Cells. PLoS ONE 2013, 8, e83139. [Google Scholar] [CrossRef] [PubMed]
- Zinselmeyer, B.H.; Heydari, S.; Sacristán, C.; Nayak, D.; Cammer, M.; Herz, J.; Cheng, X.X.; Davis, S.J.; Dustin, M.L.; McGavern, D.B. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J. Exp. Med. 2013, 210, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.Y.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.W.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Wang, H.; Zhao, Q.; Xia, Y.; Hu, X.Y.; Guo, J.M. PD-L1 induces epithelial-to-mesenchymal transition via activating SREBP-1c in renal cell carcinoma. Med. Oncol. 2015, 32, 212. [Google Scholar] [CrossRef] [PubMed]
- Minin, A.A. Dispersal of Golgi apparatus in nocodazole-treated fibroblasts is a kinesin-driven process. J. Cell Sci. 1997, 110, 2495–2505. [Google Scholar] [CrossRef]
- Storrie, B.; Yang, W. Dynamics of the interphase mammalian Golgi complex as revealed through drugs producing reversible Golgi disassembly. Biochim. Biophys. Acta 1998, 1404, 127–137. [Google Scholar] [CrossRef]
- Westlake, C.J.; Baye, L.M.; Nachury, M.V.; Wright, K.J.; Ervin, K.E.; Phu, L.; Chalouni, C.; Beck, J.S.; Kirkpatrick, D.S.; Slusarski, D.C.; et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc. Natl. Acad. Sci. USA 2011, 108, 2759–2764. [Google Scholar] [CrossRef] [PubMed]
- Schmid, F.M.; Schou, K.B.; Vilhelm, M.J.; Holm, M.S.; Breslin, L.; Farinelli, P.; Larsen, L.A.; Andersen, J.S.; Pedersen, L.B.; Christensen, S.T. IFT20 modulates ciliary PDGFRα signaling by regulating the stability of Cbl E3 ubiquitin ligases. J. Cell Biol. 2018, 217, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.W.; Zhang, Y.X.; Wei, Q.; Huang, Y.; Li, Y.; Ling, K.; Hu, J.H. BBS4 and BBS5 show functional redundancy in the BBSome to regulate the degradative sorting of ciliary sensory receptors. Sci. Rep. 2015, 5, 11855. [Google Scholar] [CrossRef] [PubMed]
- Goetz, S.C.; Anderson, K.V. The primary cilium: A signalling centre during vertebrate development. Nat. Rev. Genet. 2010, 11, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Wheway, G.; Nazlamova, L.; Hancock, J.T. Signaling through the Primary Cilium. Front. Cell Dev. Biol. 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Mack, G.J.; Ou, Y.C.; Rattner, J.B. Integrating centrosome structure with protein composition and function in animal cells. Microsc. Res. Tech. 2000, 49, 409–419. [Google Scholar] [CrossRef]
- Zimmerman, W.; Doxsey, S.J. Construction of centrosomes and spindle poles by molecular motor-driven assembly of protein particles. Traffic 2000, 1, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jin, J.; Wang, Y.; Fang, L.; Min, L.; Wang, X.; Ding, L.; Weng, L.; Xiao, T.; Zhou, T.; et al. PD-L1 regulates genomic stability via interaction with cohesin-SA1 in the nucleus. Signal Transduct. Target. Ther. 2021, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Xu, H.X.; Yao, Q.; Li, W.Z.; Huang, Q.; Outeda, P.; Cebotaru, V.; Chiaravalli, M.; Boletta, A.; Piontek, K.; et al. Ciliary membrane proteins traffic through the Golgi via a Rabep1/GGA1/Arl3-dependent mechanism. Nat. Commun. 2014, 5, 5482. [Google Scholar] [CrossRef]
- Nishita, M.; Park, S.Y.; Nishio, T.; Kamizaki, K.; Wang, Z.C.; Tamada, K.; Takumi, T.; Hashimoto, R.; Otani, H.; Pazour, G.J.; et al. Ror2 signaling regulates Golgi structure and transport through IFT20 for tumor invasiveness. Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef]
- Ma, M.; Gallagher, A.R.; Somlo, S. Ciliary Mechanisms of Cyst Formation in Polycystic Kidney Disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a028209. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.J.; Wu, Z.X.; Wang, J.H.; Luo, G.W.; Lin, H.Y.; Fan, Y.; Zhou, C.C. Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies. Signal Transduct. Target. Ther. 2023, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, D.; Anderson, K.V. Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. USA 2005, 102, 11325–11330. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.M.; Wang, B.L.; Niswander, L.A. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 2005, 132, 3103–3111. [Google Scholar] [CrossRef]
- Parra, E.R.; Villalobos, P.; Rodriguez-Canales, J. The Multiple Faces of Programmed Cell Death Ligand 1 Expression in Malignant and Nonmalignant Cells. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 287–294. [Google Scholar] [CrossRef]
- Gou, Q.; Dong, C.; Xu, H.; Khan, B.; Jin, J.; Liu, Q.; Shi, J.; Hou, Y. PD-L1 degradation pathway and immunotherapy for cancer. Cell Death Dis. 2020, 11, 955. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agborbesong, E.; Li, X. The Immune Checkpoint Protein PD-L1 Regulates Ciliogenesis and Hedgehog Signaling. Cells 2024, 13, 1003. https://doi.org/10.3390/cells13121003
Agborbesong E, Li X. The Immune Checkpoint Protein PD-L1 Regulates Ciliogenesis and Hedgehog Signaling. Cells. 2024; 13(12):1003. https://doi.org/10.3390/cells13121003
Chicago/Turabian StyleAgborbesong, Ewud, and Xiaogang Li. 2024. "The Immune Checkpoint Protein PD-L1 Regulates Ciliogenesis and Hedgehog Signaling" Cells 13, no. 12: 1003. https://doi.org/10.3390/cells13121003



