Role of 4-Thiazolidinone–Pyrazoline/Indoline Hybrids Les-4369 and Les-3467 in BJ and A549 Cell Lines
Abstract
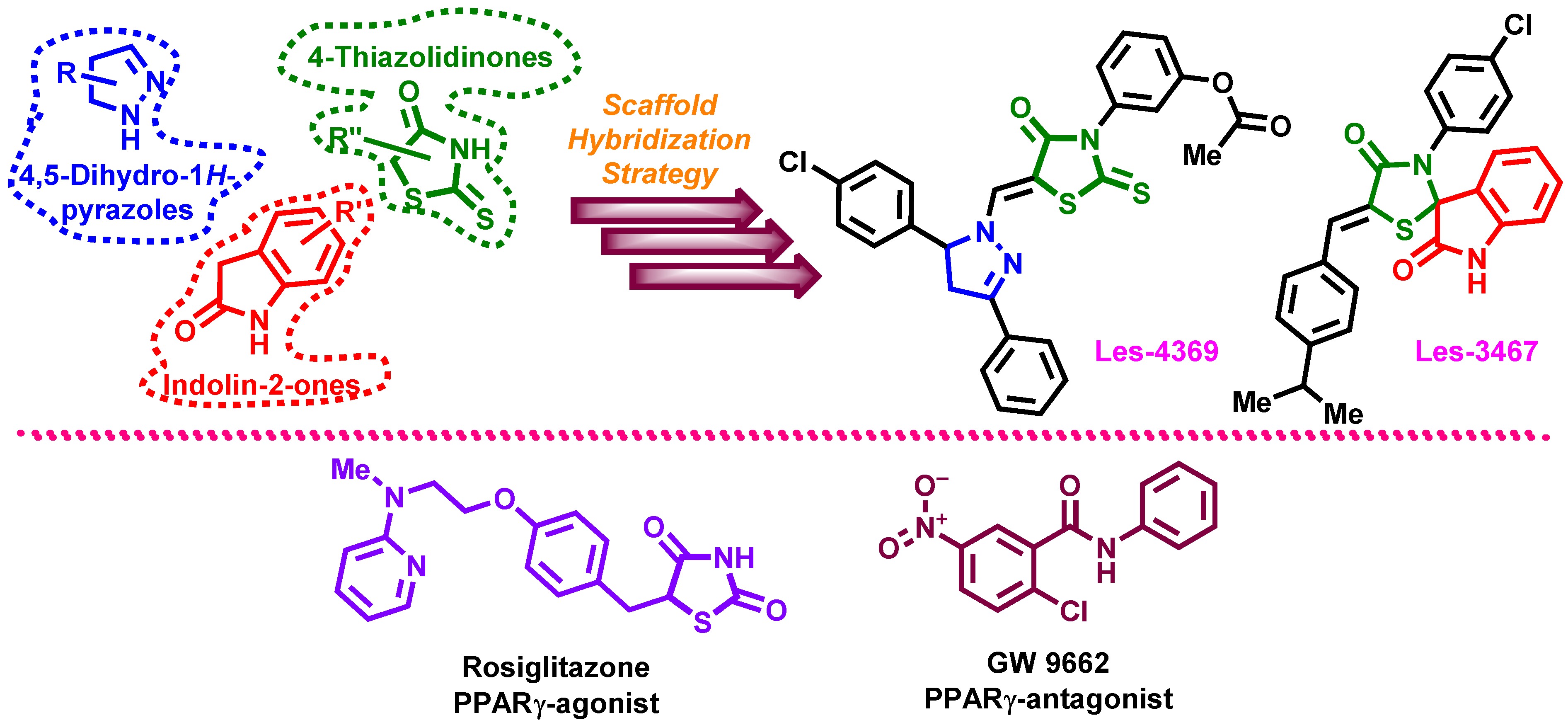
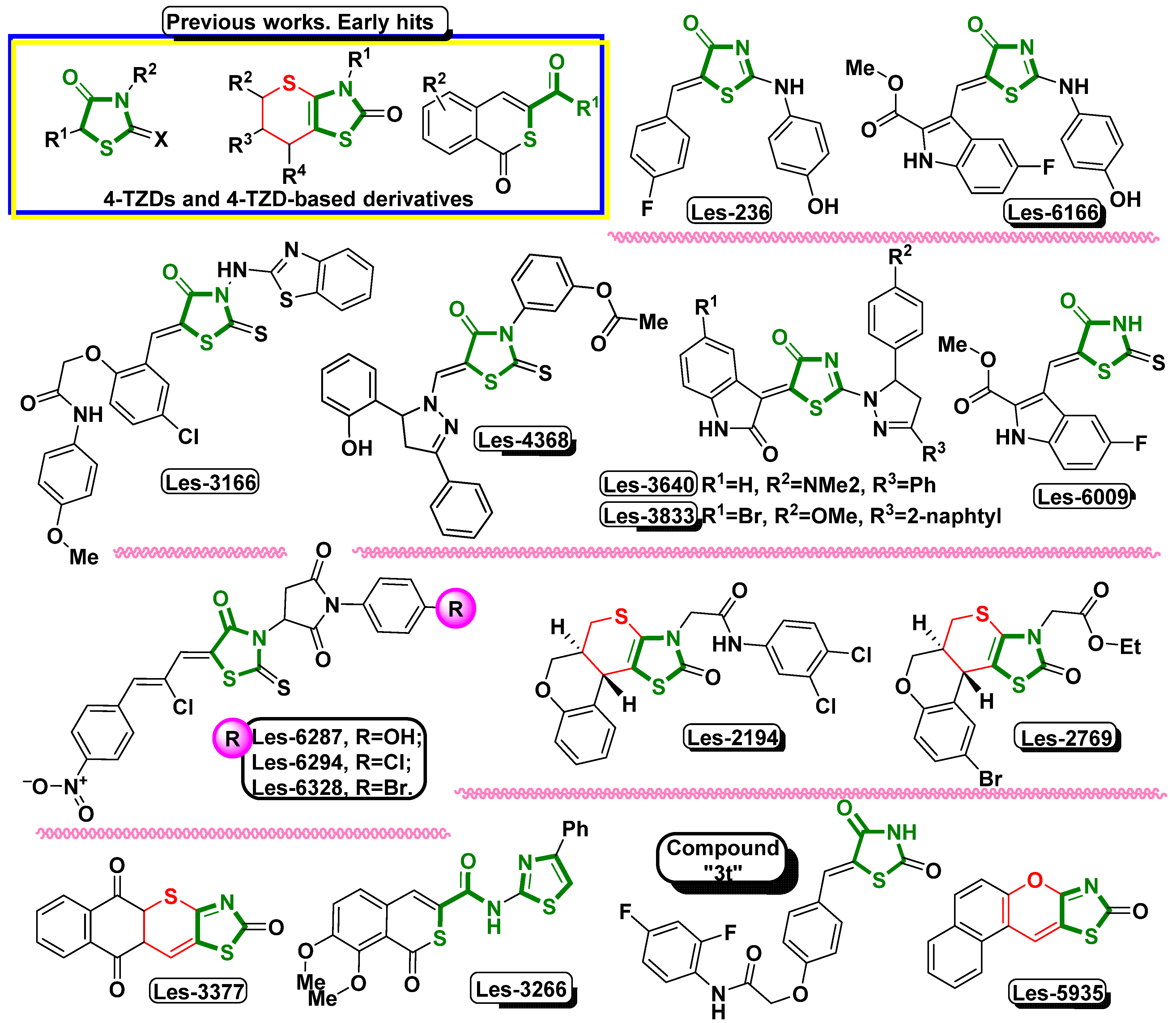
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Intracellular ROS Level
2.4. Resazurin-Reduction-Based Cell Viability Assay
2.5. LDH Cytotoxicity Assay
2.6. Caspase-3 Activity Assay
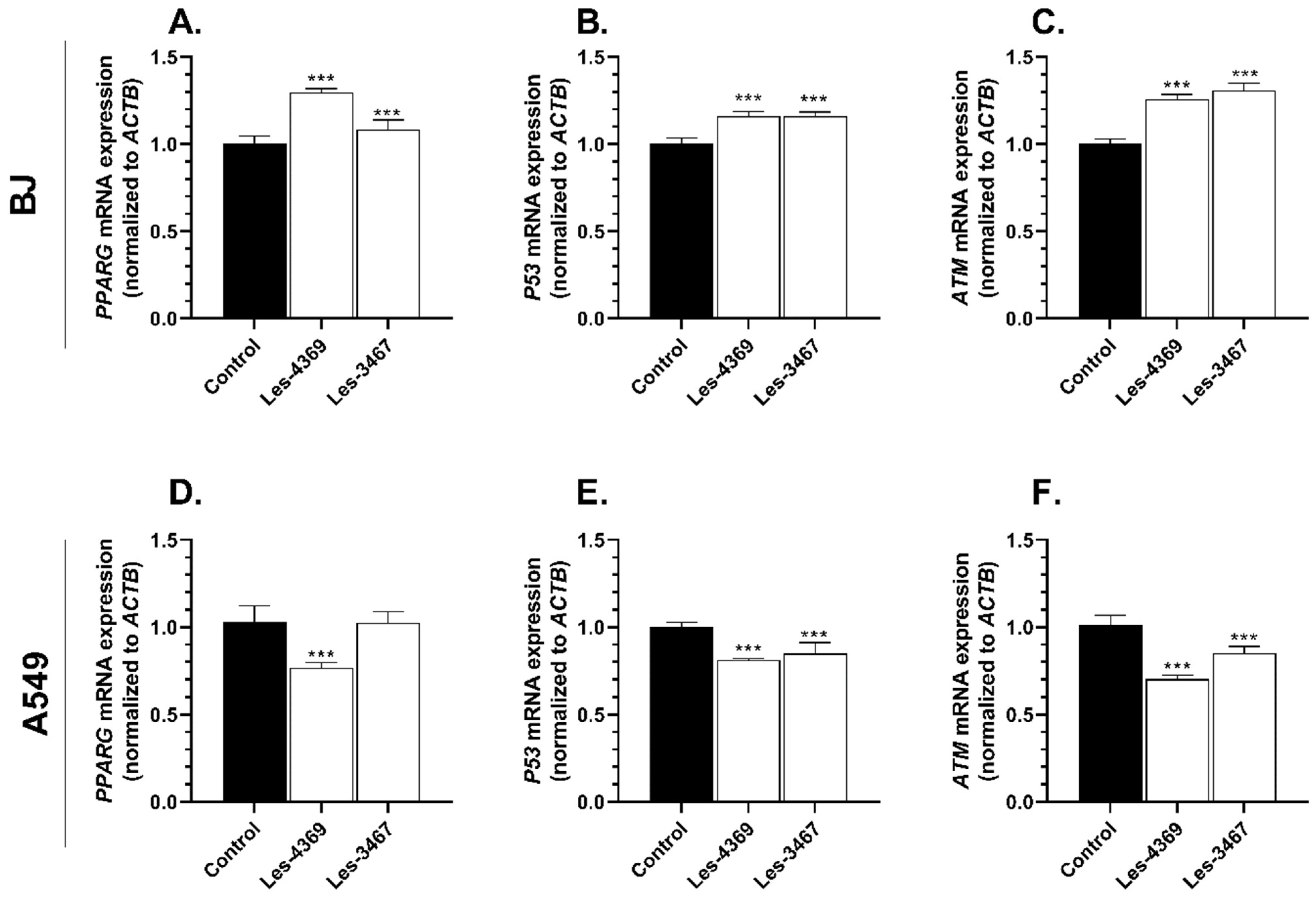
2.7. Real-Time PCR Analysis of PPARγ, ATM, and P53 mRNA Expression
2.8. Hoechst 33342- and Calcein–AM-Based Staining
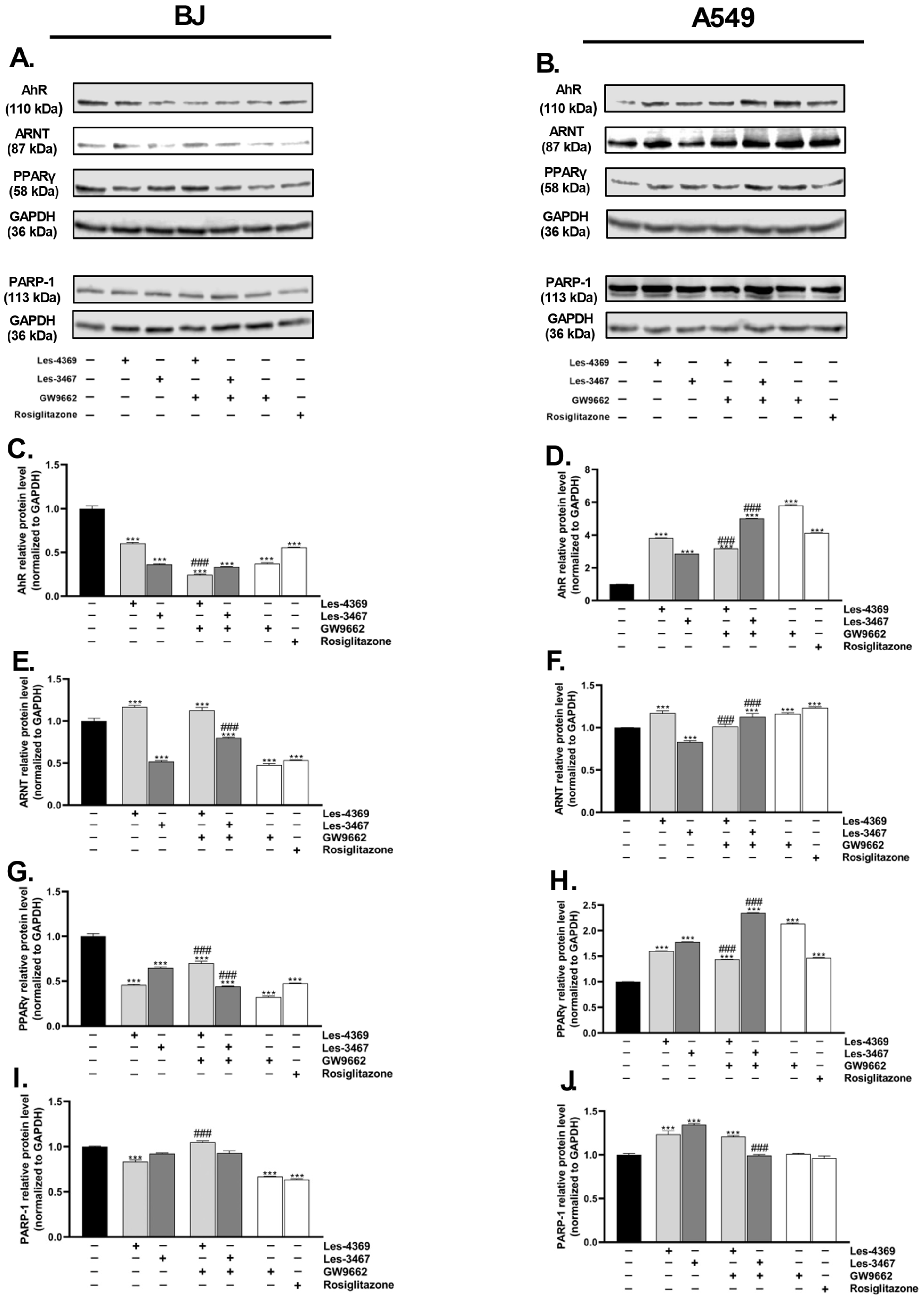
2.9. Western Blot
2.10. Statistical Analysis
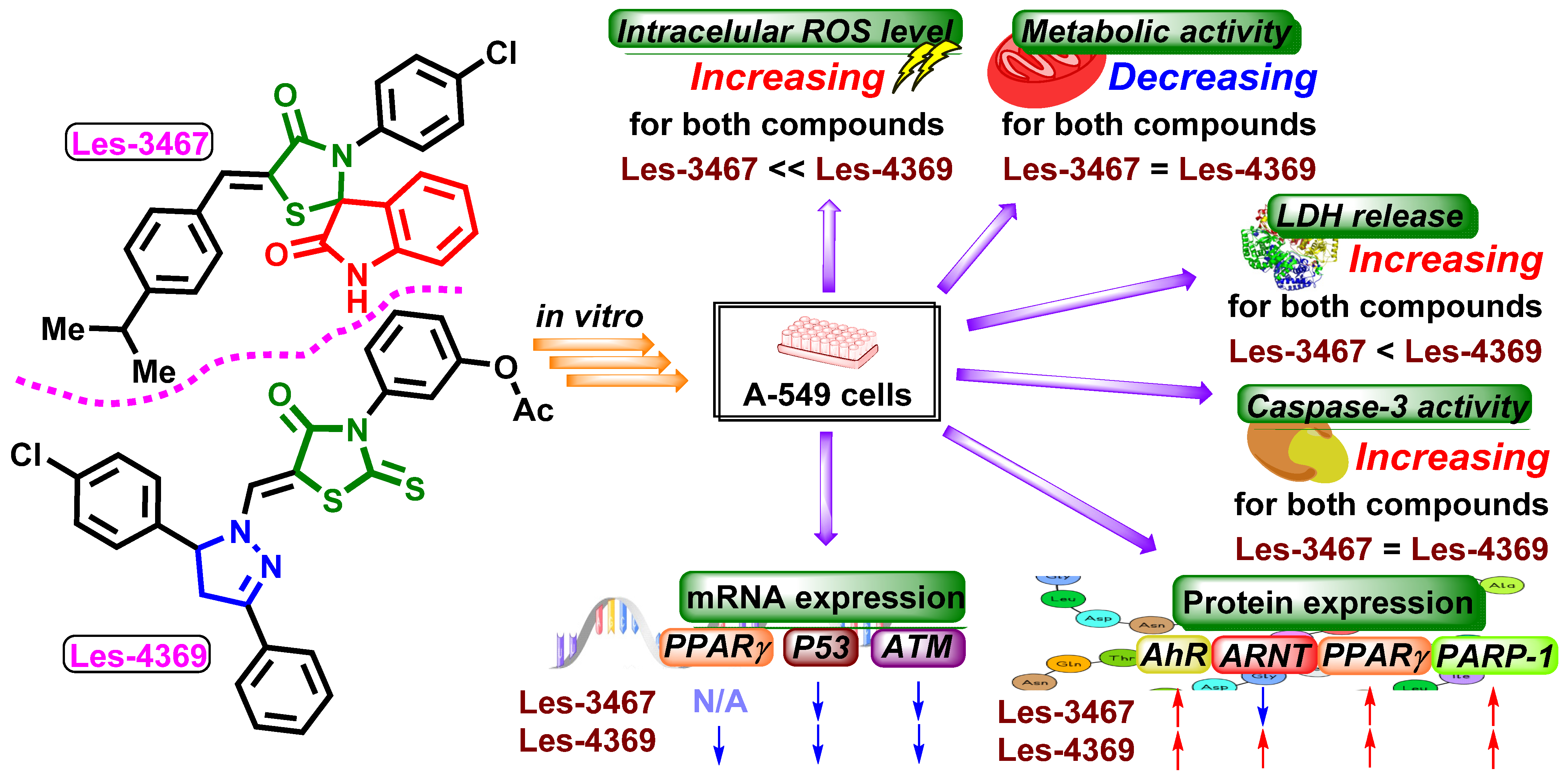
3. Results and Discussion
3.1. ROS Production
3.2. Resazurin Reduction Assay
3.3. LDH Release Assay
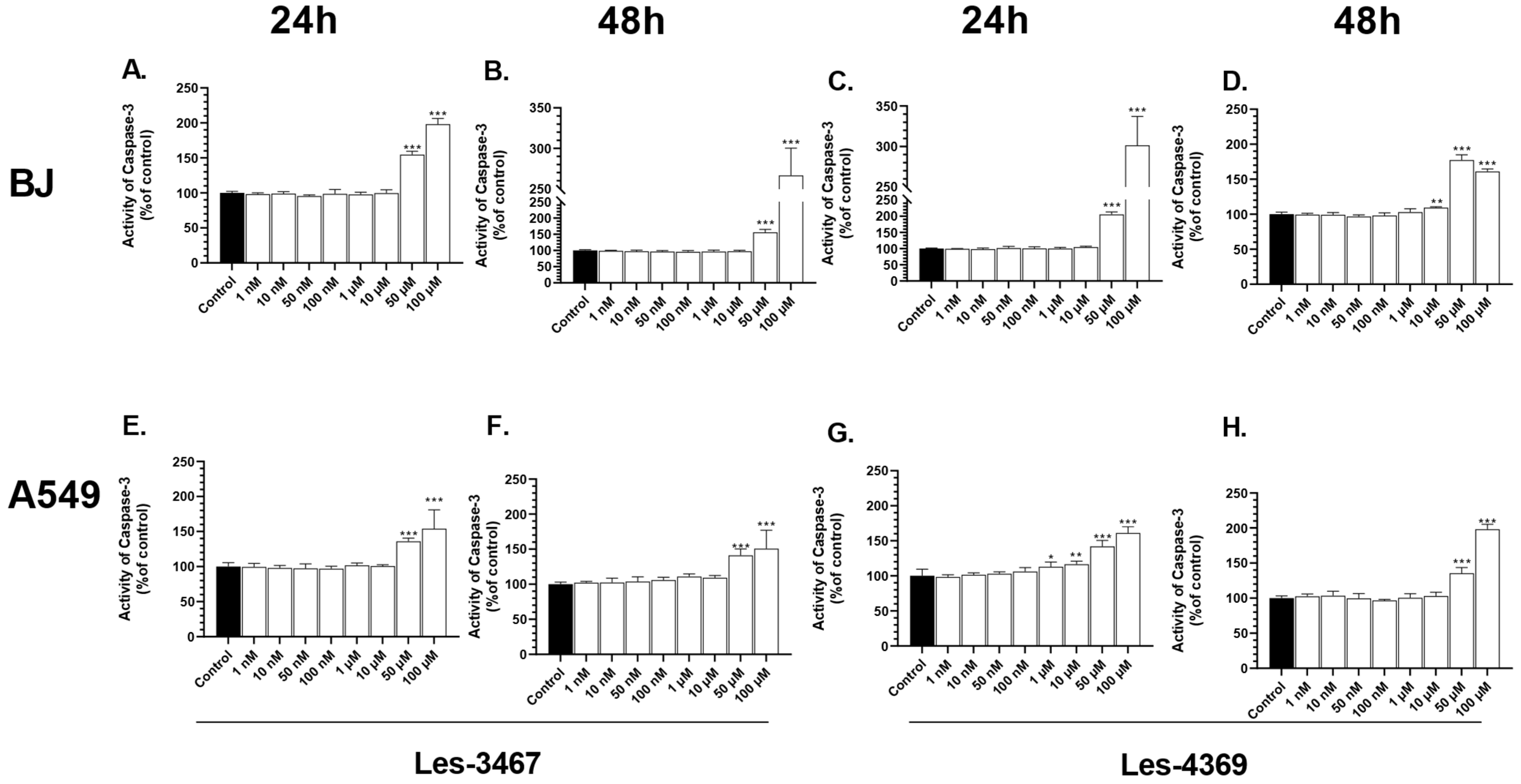
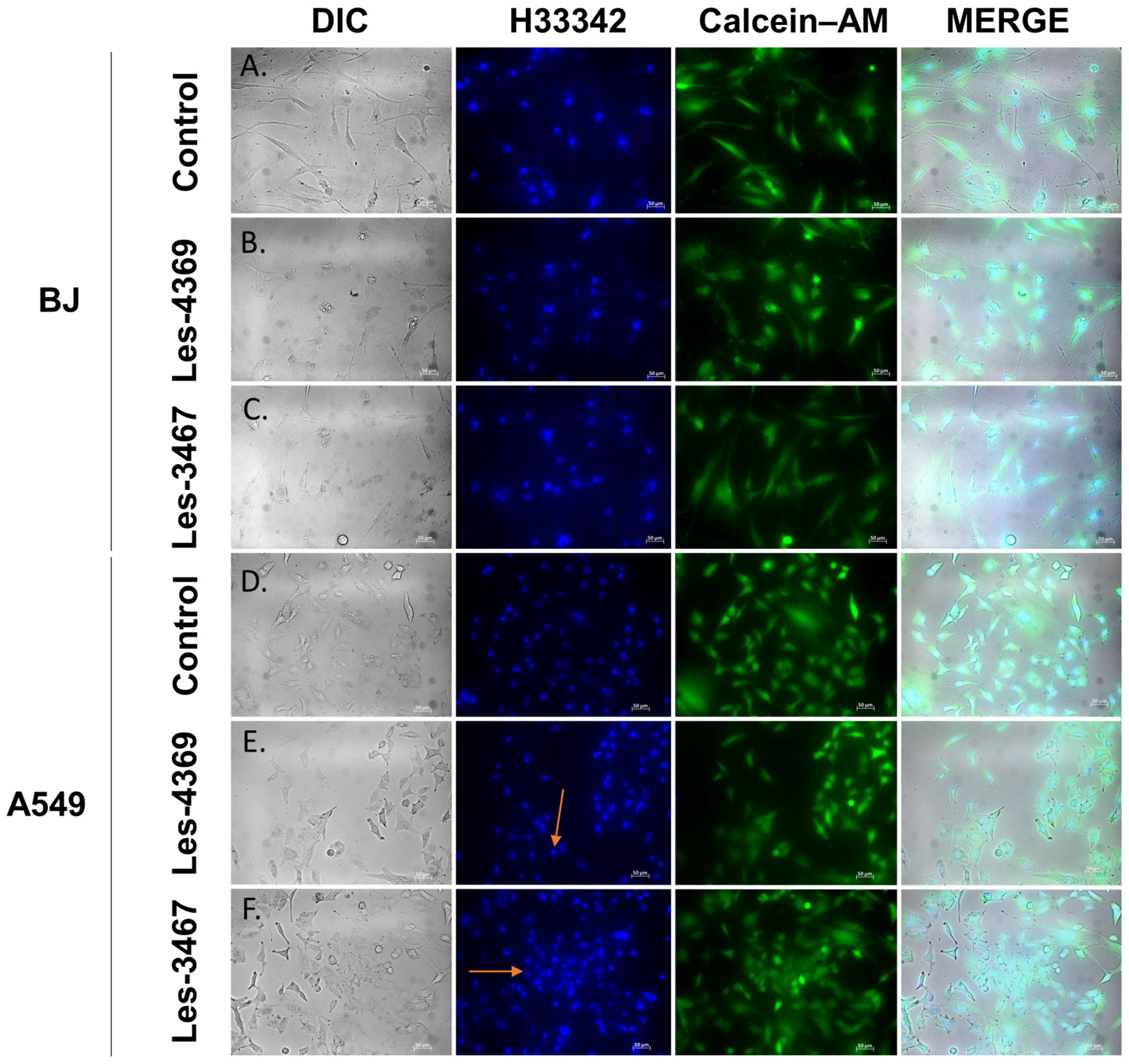
3.4. Caspase-3 Activity Assay and Fluorescence Microscope Analysis
3.5. Gene and Protein Expression Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- McLaughlin, J.; Berkman, J.; Nana-Sinkam, P. Targeted Therapies in Non-Small Cell Lung Cancer: Present and Future. Fac. Rev. 2023, 12, 22. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA. Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Zou, K.; Sun, P.; Huang, H.; Zhuo, H.; Qie, R.; Xie, Y.; Luo, J.; Li, N.; Li, J.; He, J.; et al. Etiology of Lung Cancer: Evidence from Epidemiologic Studies. J. Natl. Cancer Cent. 2022, 2, 216–225. [Google Scholar] [CrossRef]
- Tripathi, A.C.; Gupta, S.J.; Fatima, G.N.; Sonar, P.K.; Verma, A.; Saraf, S.K. 4-Thiazolidinones: The Advances Continue. Eur. J. Med. Chem. 2014, 72, 52–77. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. 5-Ene-4-Thiazolidinones—An Efficient Tool in Medicinal Chemistry. Eur. J. Med. Chem. 2017, 140, 542–594. [Google Scholar] [CrossRef]
- Finiuk, N.; Boiko, N.; Klyuchivska, O.; Kobylinska, L.; Kril, I.; Zimenkovsky, B.; Lesyk, R.; Stoika, R. 4-Thiazolidinone Derivative Les-3833 Effectively Inhibits Viability of Human Melanoma Cells through Activating Apoptotic Mechanisms. Croat. Med. J. 2017, 58, 129–139. [Google Scholar] [CrossRef]
- Dewar, B.J.; Gardner, O.S.; Chen, C.S.; Earp, H.S.; Samet, J.M.; Graves, L.M. Capacitative Calcium Entry Contributes to the Differential Transactivation of the Epidermal Growth Factor Receptor in Response to Thiazolidinediones. Mol. Pharmacol. 2007, 72, 1146–1156. [Google Scholar] [CrossRef]
- Gardner, O.S.; Shiau, C.W.; Chen, C.S.; Graves, L.M. Peroxisome Proliferator-Activated Receptor γ-Independent Activation of P38 MAPK by Thiazolidinediones Involves Calcium/Calmodulin-Dependent Protein Kinase II and Protein Kinase R: Correlation with Endoplasmic Reticulum Stress. J. Biol. Chem. 2005, 280, 10109–10118. [Google Scholar] [CrossRef] [PubMed]
- Gardner, O.S.; Dewar, B.J.; Earp, H.S.; Samet, J.M.; Graves, L.M. Dependence of Peroxisome Proliferator-Activated Receptor Ligand-Induced Mitogen-Activated Protein Kinase Signaling on Epidermal Growth Factor Receptor Transactivation. J. Biol. Chem. 2003, 278, 46261–46269. [Google Scholar] [CrossRef] [PubMed]
- Lennon, A.M.; Ramaugé, M.; Dessouroux, A.; Pierre, M. MAP Kinase Cascades Are Activated in Astrocytes and Preadipocytes by 15-Deoxy-Delta(12-14)-Prostaglandin J(2) and the Thiazolidinedione Ciglitazone through Peroxisome Proliferator Activator Receptor Gamma-Independent Mechanisms Involving Reactive Oxygenat. J. Biol. Chem. 2002, 277, 29681–29685. [Google Scholar] [CrossRef]
- Huang, W.C.; Chio, C.C.; Chi, K.H.; Wu, H.M.; Lin, W.W. Superoxide Anion-Dependent Raf/MEK/ERK Activation by Peroxisome Proliferator Activated Receptor γ Agonists 15-Deoxy-Δ12,14-Prostaglandin J2, Ciglitazone, and GW1929. Exp. Cell Res. 2002, 277, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Kulp, S.K.; Chen, C.S. Energy Restriction as an Antitumor Target of Thiazolidinediones. J. Biol. Chem. 2010, 285, 9780–9791. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Skóra, B.; Kryshchyshyn-Dylevych, A.; Kaminskyy, D.; Khyluk, D.; Lesyk, R. 4-Thiazolidinone-Based Derivatives Rosiglitazone and Pioglitazone Affect the Expression of Antioxidant Enzymes in Different Human Cell Lines. Biomed. Pharmacother. 2021, 139, 111684. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Leja, M.L.; Kaminskyy, D.V.; Binduga, U.E.; Pinyazhko, O.R.; Lesyk, R.B.; Gmiński, J. Study of Novel Anticancer 4-Thiazolidinone Derivatives. Chem. Biol. Interact. 2017, 262, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.S.; Vora, D.K.; Ramaa, C.S. Thiazolidine-2,4-Diones: Progress towards Multifarious Applications. Bioorg. Med. Chem. 2013, 21, 1599–1620. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ortiz, J.M.; Tranque, P.; Burgos, M.; Vaquero, C.F.; Llopis, J. Glitazones Induce Astroglioma Cell Death by Releasing Reactive Oxygen Species from Mitochondria: Modulation of Cytotoxicity by Nitric Oxide. Mol. Pharmacol. 2007, 72, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Cerquetti, L.; Sampaoli, C.; Amendola, D.; Bucci, B.; Masuelli, L.; Marchese, R.; Misiti, S.; De Venanzi, A.; Poggi, M.; Toscano, V.; et al. Rosiglitazone Induces Autophagy in H295R and Cell Cycle Deregulation in SW13 Adrenocortical Cancer Cells. Exp. Cell Res. 2011, 317, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.T.; Lakshmi, S.P.; Reddy, R.C. PPAR γ as a Novel Therapeutic Target in Lung Cancer. PPAR Res. 2016, 2016, 8972570. [Google Scholar] [CrossRef]
- Villapol, S. Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell. Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef]
- Chi, T.; Wang, M.; Wang, X.; Yang, K.; Xie, F.; Liao, Z.; Wei, P. PPAR-γ Modulators as Current and Potential Cancer Treatments. Front. Oncol. 2021, 11, 737776. [Google Scholar] [CrossRef]
- Aki, T.; Uemura, K. Cell Death and Survival Pathways Involving ATM Protein Kinase. Genes 2021, 12, 1581. [Google Scholar] [CrossRef]
- Ozaki, T.; Nakagawara, A. Role of P53 in Cell Death and Human Cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef]
- Biirkle, A. PARP and the Release of Apoptosis-Inducing Factor from Mitochondria. In Poly(ADP-Ribosyl)ation; Springer: Boston, MA, USA, 2006; ISBN 0387333711. [Google Scholar]
- Gobeil, S.; Boucher, C.C.; Nadeau, D.; Poirier, G.G. Characterization of the Necrotic Cleavage of Poly (ADP-Ribose) Polymerase (PARP-1): Implication of Lysosomal Proteases. Cell Death Differ. 2001, 8, 588–594. [Google Scholar] [CrossRef]
- Li, R.; Luo, R.; Luo, Y.; Hou, Y.; Wang, J.; Zhang, Q.; Chen, X.; Hu, L.; Zhou, J. Biological Function, Mediate Cell Death Pathway and Their Potential Regulated Mechanisms for Post-Mortem Muscle Tenderization of PARP1: A Review. Front. Nutr. 2022, 9, 1093939. [Google Scholar] [CrossRef]
- Paris, A.; Tardif, N.; Galibert, M.D.; Corre, S. AhR and Cancer: From Gene Profiling to Targeted Therapy. Int. J. Mol. Sci. 2021, 22, 752. [Google Scholar] [CrossRef]
- Grishanova, A.Y.; Perepechaeva, M.L. Aryl Hydrocarbon Receptor in Oxidative Stress as a Double Agent and Its Biological and Therapeutic Significance. Int. J. Mol. Sci. 2022, 23, 6719. [Google Scholar] [CrossRef]
- Dietrich, C. Antioxidant Functions of the Aryl Hydrocarbon Receptor. Stem Cells Int. 2016, 2016, 7943495. [Google Scholar] [CrossRef]
- Barouki, R.; Morel, Y. Repression of Cytochrome P450 1A1 Gene Expression by Oxidative Stress: Mechanisms and Biological Implications. Biochem. Pharmacol. 2001, 61, 511–516. [Google Scholar] [CrossRef]
- Zhu, K.; Meng, Q.; Zhang, Z.; Yi, T.; He, Y.; Zheng, J.; Lei, W. Aryl Hydrocarbon Receptor Pathway: Role, Regulation and Intervention in Atherosclerosis Therapy (Review). Mol. Med. Rep. 2019, 20, 4763–4773. [Google Scholar] [CrossRef]
- Skóra, B.; Matuszewska, P.; Masicz, M.; Sikora, K.; Słomczewska, M.; Sołtysek, P.; Szychowski, K.A. Crosstalk between the Aryl Hydrocarbon Receptor (AhR) and the Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) as a Key Factor in the Metabolism of Silver Nanoparticles in Neuroblastoma (SH-SY5Y) Cells in vitro. Toxicol. Appl. Pharmacol. 2023, 458, 116339. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skóra, B. Involvement of the Aryl Hydrocarbon Receptor (AhR) in the Mechanism of Action of Elastin-Derived Peptide (VGVAPG) and Its Impact on Neurosteroidogenesis. Neurochem. Int. 2023, 171, 105615. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skóra, B.; Wójtowicz, A.K. Triclosan Affects the Expression of Nitric Oxide Synthases (NOSs), Peroxisome Proliferator-Activated Receptor Gamma (PPARγ), and Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-ΚB) in Mouse Neocortical Neurons in Vitro. Toxicol. Vitr. 2021, 73, 105143. [Google Scholar] [CrossRef]
- Wójtowicz, A.K.; Szychowski, K.A.; Wnuk, A.; Kajta, M. Dibutyl Phthalate (DBP)-Induced Apoptosis and Neurotoxicity Are Mediated via the Aryl Hydrocarbon Receptor (AhR) but Not by Estrogen Receptor Alpha (ERα), Estrogen Receptor Beta (ERβ), or Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) in Mouse C. Neurotox. Res. 2017, 31, 77–89. [Google Scholar] [CrossRef]
- Shan, J.; Ma, X.F.; Wu, M.Y.; Lin, Y.J.; Wang, Y.; Wang, R.; Li, H.M.; Wu, Z.L.; Xu, H.M. Preliminary Study on the Role of Aryl Hydrocarbon Receptor in the Neurotoxicity of Three Typical Bisphenol Compounds (BPA, BPS and TBBPA) at Environmentally Relevant Concentrations to Adult Zebrafish (Danio Rerio). Heliyon 2023, 9, e16649. [Google Scholar] [CrossRef]
- Tang, T.; Lin, X.; Yang, H.; Zhou, L.C.; Wang, Z.; Shan, G.; Guo, Z.M. Overexpression of Antioxidant Enzymes Upregulates Aryl Hydrocarbon Receptor Expression via Increased Sp1 DNA-Binding Activity. Free Radic. Biol. Med. 2010, 49, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Bar, M.; Skóra, B.; Tabęcka-Łonczyńska, A.; Holota, S.; Khyluk, D.; Roman, O.; Lesyk, R.; Szychowski, K.A. New 4-Thiazolidinone-Based Molecules Les-2769 and Les-3266 as Possible PPARγ Modulators. Bioorg. Chem. 2022, 128, 106075. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Leja, M.L.; Kaminskyy, D.V.; Kryshchyshyn, A.P.; Binduga, U.E.; Pinyazhko, O.R.; Lesyk, R.B.; Tobiasz, J.; Gmiński, J. Anticancer Properties of 4-Thiazolidinone Derivatives Depend on Peroxisome Proliferator-Activated Receptor Gamma (PPARγ). Eur. J. Med. Chem. 2017, 141, 162–168. [Google Scholar] [CrossRef]
- Skóra, B.; Lewińska, A.; Kryshchyshyn-Dylevych, A.; Kaminskyy, D.; Lesyk, R.; Szychowski, K.A. Evaluation of Anticancer and Antibacterial Activity of Four 4-Thiazolidinone-Based Derivatives. Molecules 2022, 27, 894. [Google Scholar] [CrossRef]
- Senkiv, J.; Finiuk, N.; Kaminskyy, D.; Havrylyuk, D.; Wojtyra, M.; Kril, I.; Gzella, A.; Stoika, R.; Lesyk, R. 5-Ene-4-Thiazolidinones Induce Apoptosis in Mammalian Leukemia Cells. Eur. J. Med. Chem. 2016, 117, 33–46. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Khyluk, D.; Vasylenko, O.; Zaprutko, L.; Lesyk, R. A Facile Synthesis and Anticancer Activity Evaluation of Spiro[Thiazolidinone-Isatin] Conjugates. Sci. Pharm. 2011, 79, 763–777. [Google Scholar] [CrossRef]
- Kaja, S.; Payne, A.J.; Naumchuk, Y.; Koulen, P. Quantification of Lactate Dehydrogenase for Cell Viability Testing Using Cell Lines and Primary Cultured Astrocytes. Curr. Protoc. Toxicol. 2017, 72, 2.26.1–2.26.10. [Google Scholar] [CrossRef]
- Maier, J.K.X.; Lahoua, Z.; Gendron, N.H.; Fetni, R.; Johnston, A.; Davoodi, J.; Rasper, D.; Roy, S.; Slack, R.S.; Nicholson, D.W.; et al. The Neuronal Apoptosis Inhibitory Protein Is a Direct Inhibitor of Caspases 3 and 7. J. Neurosci. 2002, 22, 2035–2043. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skóra, B.; Kryshchyshyn-Dylevych, A.; Kaminskyy, D.; Tobiasz, J.; Lesyk, R.B.; Gmiński, J. 4-Thiazolidinone-Based Derivatives Do Not Affect Differentiation of Mouse Embryo Fibroblasts (3T3-L1 Cell Line) into Adipocytes. Chem. Biol. Interact. 2021, 345, 109538. [Google Scholar] [CrossRef]
- Abd Al Moaty, M.N.; El Ashry, E.S.H.; Awad, L.F.; Mostafa, A.; Abu-Serie, M.M.; Teleb, M. Harnessing ROS-Induced Oxidative Stress for Halting Colorectal Cancer via Thiazolidinedione-Based SOD Inhibitors. ACS Omega 2022, 7, 21267–21279. [Google Scholar] [CrossRef]
- Lee, A. Measuring Cell-Viability By Resazurin (Alamarblue®) Assay Using Photopette®. Life Sci. 2017, 1–3. [Google Scholar]
- Xian, D.; Lai, R.; Song, J.; Xiong, X.; Zhong, J. Emerging Perspective: Role of Increased ROS and Redox Imbalance in Skin Carcinogenesis. Oxid. Med. Cell. Longev. 2019, 2019, 8127362. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef]
- Finiuk, N.; Kaleniuk, E.; Holota, S.; Stoika, R.; Lesyk, R.; Szychowski, K.A. Pyrrolidinedione-Thiazolidinone Hybrid Molecules with Potent Cytotoxic Effect in Squamous Cell Carcinoma SCC-15 Cells. Bioorg. Med. Chem. 2023, 92, 117442. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, 465–468. [Google Scholar] [CrossRef]
- Uchide, N.; Ohyama, K.; Bessho, T.; Toyoda, H. Lactate Dehydrogenase Leakage as a Marker for Apoptotic Cell Degradation Induced by Influenza Virus Infection in Human Fetal Membrane Cells. Intervirology 2009, 52, 164–173. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Kaminskyy, D.V.; Leja, M.L.; Kryshchyshyn, A.P.; Lesyk, R.B.; Tobiasz, J.; Wnuk, M.; Pomianek, T.; Gmiński, J. Anticancer Properties of 5Z-(4-Fluorobenzylidene)-2-(4-Hydroxyphenylamino)-Thiazol-4-One. Sci. Rep. 2019, 9, 10609. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; Moravec, R.A. Use of Multiple Assay Endpoints to Investigate the Effects of Incubation Time, Dose of Toxin, and Planting Density in Cell-Based Cytotoxicity Assays. Assay Drug Dev. Technol. 2004, 2, 51–62. [Google Scholar] [CrossRef]
- Nichani, K.; Li, J.; Suzuki, M.; Houston, J.P. Evaluation of Caspase-3 Activity During Apoptosis with Fluorescence Lifetime-Based Cytometry Measurements and Phasor Analyses. Cytom. Part A 2020, 97, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, S.R.; Wilski, N.A.; Aplin, A.E. Fueling the Fire: Inflammatory Forms of Cell Death and Implications for Cancer Immunotherapy. Cancer Discov. 2021, 11, 266–281. [Google Scholar] [CrossRef]
- Eskandari, E.; Eaves, C.J. Paradoxical Roles of Caspase-3 in Regulating Cell Survival, Proliferation, and Tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Mor, G.; Alvero, A.B. Apoptosis and Cancer: Methods and Protocols, 2nd ed.; Humana: New York, NY, USA, 2014; Volume 1219, pp. 1–206. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Waterhouse, N.J. Analyzing Cell Death by Nuclear Staining with Hoechst 33342. Cold Spring Harb. Protoc. 2016, 2016, 778–781. [Google Scholar] [CrossRef]
- Santavanond, J.P.; Rutter, S.F.; Atkin-Smith, G.K.; Poon, I.K.H. Apoptotic Bodies: Mechanism of Formation, Isolation and Functional Relevance. Subcell. Biochem. 2021, 97, 61–88. [Google Scholar] [CrossRef]
- Shen, Y.; White, E. P53-Dependent Apoptosis Pathways. Adv. Cancer Res. 2001, 82, 55–84. [Google Scholar] [CrossRef]
- Yadav, D.K.; Rai, R.; Kumar, N.; Singh, S.; Misra, S.; Sharma, P.; Shaw, P.; Pérez-Sánchez, H.; Mancera, R.L.; Choi, E.H.; et al. New Arylated Benzo[h]Quinolines Induce Anti-Cancer Activity by Oxidative Stress-Mediated DNA Damage. Sci. Rep. 2016, 6, 38128. [Google Scholar] [CrossRef]
- Dong, J.R.; Chang, W.W.; Chen, S.M. Nerolidol Inhibits Proliferation of Leiomyoma Cells via Reactive Oxygen Species-Induced DNA Damage and Downregulation of the ATM/Akt Pathway. Phytochemistry 2021, 191, 112901. [Google Scholar] [CrossRef]
- Paterniti, I.; Scuderi, S.A.; Casili, G.; Lanza, M.; Mare, M.; Giuffrida, R.; Colarossi, C.; Portelli, M.; Cuzzocrea, S.; Esposito, E. Poly (Adp-Ribose) Polymerase Inhibitor, Abt888, Improved Cisplatin Effect in Human Oral Cell Carcinoma. Biomedicines 2021, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Khordadmehr, M.; Shahbazi, R.; Baradaran, B.; Sadreddini, S.; Shanehbandi, D.; Hajiasgharzadeh, K. Restoring of MiR-193a-5p Sensitizes Breast Cancer Cells to Paclitaxel through P53 Pathway. Adv. Pharm. Bull. 2020, 10, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Blanquicett, C.; Roman, J.; Hart, C.M. Thiazolidinediones as Anti-Cancer Agents. Cancer Ther. 2008, 6, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on MRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Gu, C.; Gonzalez, J.; Zhang, T.; Kamel-Reid, S.; Wells, R.A. The Aryl Hydrocarbon Receptor Nuclear Translocator (ARNT) Modulates the Antioxidant Response in AML Cells. Leuk. Res. 2013, 37, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Skóra, B.; Kryshchyshyn-Dylevych, A.; Kaminskyy, D.; Rybczyńska-Tkaczyk, K.; Lesyk, R.; Gmiński, J. Induction of Cyp450 Enzymes by 4-Thiazolidinone-Based Derivatives in 3T3-L1 Cells in Vitro. Naunyn. Schmiedebergs. Arch. Pharmacol. 2021, 394, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Shrivastava, N.; Husain, A. Synthesis and sar strategy of thiazolidinedione: A novel approach for cancer treatment. J. Chil. Chem. Soc. 2020, 65, 4817–4832. [Google Scholar] [CrossRef]
- Vasaturo, M.; Fiengo, L.; De Tommasi, N.; Sabatino, L.; Ziccardi, P.; Colantuoni, V.; Bruno, M.; Cerchia, C.; Novellino, E.; Lupo, A.; et al. A Compound-Based Proteomic Approach Discloses 15-Ketoatractyligenin Methyl Ester as a New PPARγ Partial Agonist with Anti-Proliferative Ability. Sci. Rep. 2017, 7, 41273. [Google Scholar] [CrossRef]
- Malaviya, A.; Sylvester, P.W. Mechanisms Mediating the Effects of γ-Tocotrienol When Used in Combination with PPAR γ Agonists or Antagonists on MCF-7 and MDA-MB-231 Breast Cancer Cells. Int. J. Breast Cancer 2013, 2013, 101705. [Google Scholar] [CrossRef]
- Girnun, G.D.; Naseri, E.; Vafai, S.B.; Qu, L.; Szwaya, J.D.; Bronson, R.; Alberta, J.A.; Spiegelman, B.M. Synergy between PPARγ Ligands and Platinum-Based Drugs in Cancer. Cancer Cell 2007, 11, 395–406. [Google Scholar] [CrossRef]
- Colin-Cassin, C.; Yao, X.; Cerella, C.; Chbicheb, S.; Kuntz, S.; Mazerbourg, S.; Boisbrun, M.; Chapleur, Y.; Diederich, M.; Flament, S.; et al. PPARγ-Inactive Δ2-Troglitazone Independently Triggers ER Stress and Apoptosis in Breast Cancer Cells. Mol. Carcinog. 2015, 54, 393–404. [Google Scholar] [CrossRef]
- Joshi, H.; Patil, V.; Tilekar, K.; Upadhyay, N.; Gota, V.; Ramaa, C.S. Benzylidene Thiazolidinediones: Synthesis, in Vitro Investigations of Antiproliferative Mechanisms and in Vivo Efficacy Determination in Combination with Imatinib. Bioorg. Med. Chem. Lett. 2020, 30, 127561. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosińska, K.; Skóra, B.; Holota, S.; Shepeta, Y.; Tabęcka-Łonczyńska, A.; Lesyk, R.; Szychowski, K.A. Role of 4-Thiazolidinone–Pyrazoline/Indoline Hybrids Les-4369 and Les-3467 in BJ and A549 Cell Lines. Cells 2024, 13, 1007. https://doi.org/10.3390/cells13121007
Kosińska K, Skóra B, Holota S, Shepeta Y, Tabęcka-Łonczyńska A, Lesyk R, Szychowski KA. Role of 4-Thiazolidinone–Pyrazoline/Indoline Hybrids Les-4369 and Les-3467 in BJ and A549 Cell Lines. Cells. 2024; 13(12):1007. https://doi.org/10.3390/cells13121007
Chicago/Turabian StyleKosińska, Karolina, Bartosz Skóra, Serhii Holota, Yulia Shepeta, Anna Tabęcka-Łonczyńska, Roman Lesyk, and Konrad A. Szychowski. 2024. "Role of 4-Thiazolidinone–Pyrazoline/Indoline Hybrids Les-4369 and Les-3467 in BJ and A549 Cell Lines" Cells 13, no. 12: 1007. https://doi.org/10.3390/cells13121007
APA StyleKosińska, K., Skóra, B., Holota, S., Shepeta, Y., Tabęcka-Łonczyńska, A., Lesyk, R., & Szychowski, K. A. (2024). Role of 4-Thiazolidinone–Pyrazoline/Indoline Hybrids Les-4369 and Les-3467 in BJ and A549 Cell Lines. Cells, 13(12), 1007. https://doi.org/10.3390/cells13121007









