The Regulation of the Disease-Causing Gene FXN
Abstract
1. Introduction
2. Transcriptional Regulation of the FXN Gene
3. FXN Gene Structure
4. FXN Transcript Isoforms
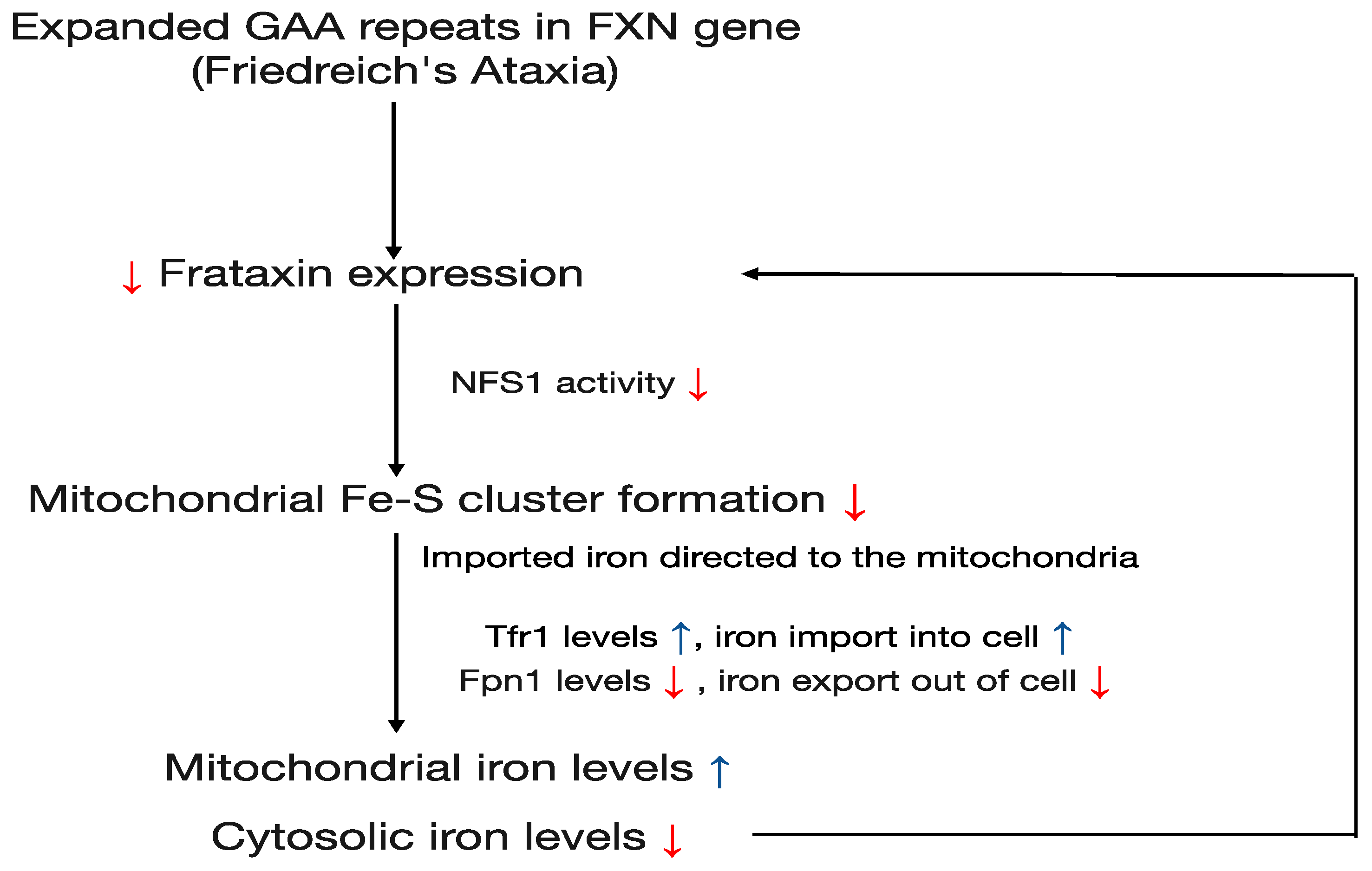
5. GAA Repeat Expansion
6. Transcription Factors
7. Iron
8. Post-Transcriptional Regulation of FXN Gene Expression
9. miRNAs
10. Post-Translational Regulation of Frataxin
11. Chaperones
12. Proteasome
13. Mitochondrial Proteases
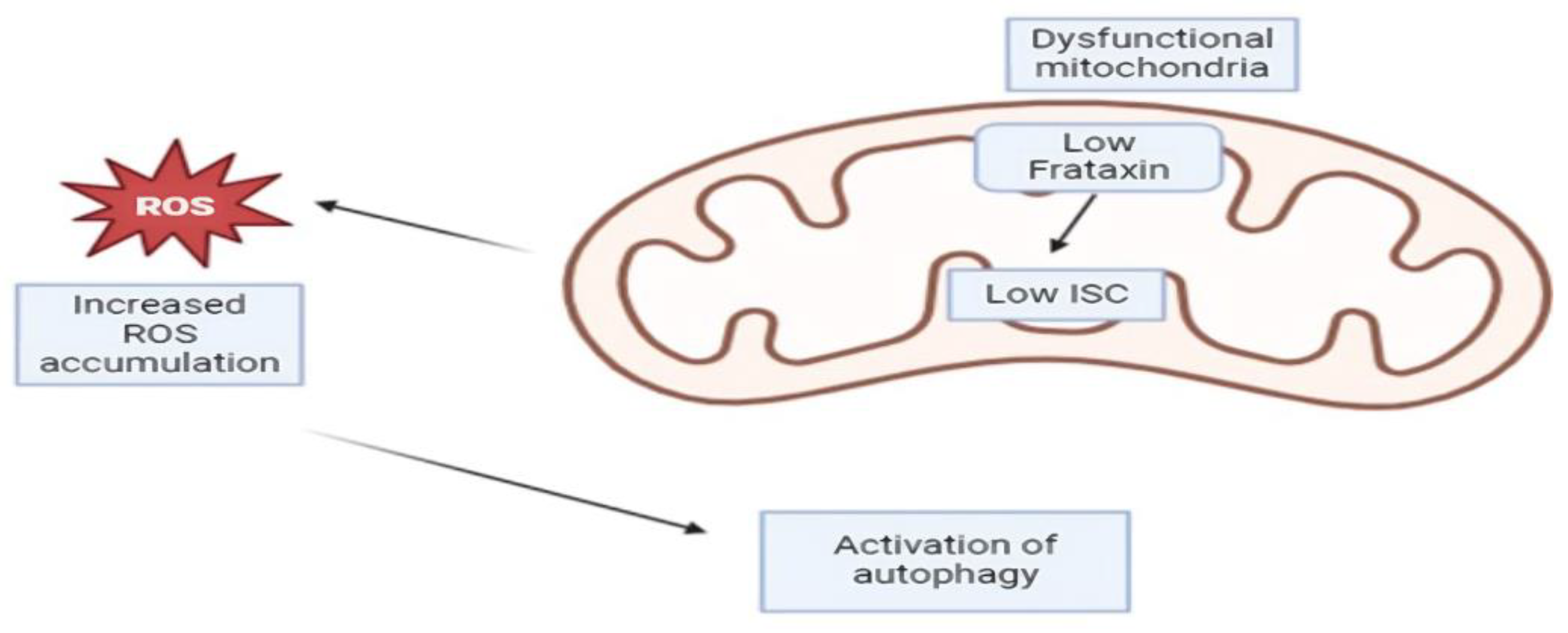
14. Autophagy
15. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Strawser, C.; Schadt, K.; Hauser, L.; McCormick, A.; Wells, M.; Larkindale, J.; Lin, H.; Lynch, D.R. Pharmacological therapeutics in Friedreich ataxia: The present state. Expert. Rev. Neurother. 2017, 17, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, A.H.; Becker, A.B.; Qian, J.; Gelman, B.B.; Mazurkiewicz, J.E. Friedreich ataxia: Developmental failure of the dorsal root entry zone. J. Neuropathol. Exp. Neurol. 2017, 76, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, A.H.; Becker, A.B.; Qian, J.; Feustel, P.J. Friedreich ataxia: Hypoplasia of spinal cord and dorsal root ganglia. J. Neuropathol. Exp. Neurol. 2017, 76, 101–108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koeppen, A.H.; Mazurkiewicz, J.E. Friedreich ataxia: Neuropathology revised. J. Neuropathol. Exp. Neurol. 2013, 72, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, M.H.; Boesch, S.; Nachbauer, W.; Mariotti, C.; Giunti, P. Clinical features of Friedreich’s ataxia: Classical and atypical phenotypes. J. Neurochem. 2013, 126 (Suppl. S1), 103–117. [Google Scholar] [CrossRef] [PubMed]
- Rummey, C.; Farmer, J.M.; Lynch, D.R. Predictors of loss of ambulation in Friedreich’s ataxia. EClinicalMedicine 2020, 18, 100213. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, V.; Montermini, L.; Moltò, M.D.; Pianese, L.; Cossée, M.; Cavalcanti, F.; Monros, E.; Rodius, F.; Duclos, F.; Monticelli, A.; et al. Friedreich’s ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 1996, 271, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Babcock, M.; Silva, D.; Oaks, R.; Davis-Kaplan, S.; Jiralerspong, S.; Montermini, L.; Pandolfo, M.; Kaplan, J. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science 1997, 276, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Foury, F.; Cazzalini, O. Deletion of the yeast homologue of the human gene associated with Friedreich’s ataxia elicits iron accumulation in mitochondria. FEBS Lett. 1997, 411, 373–377. [Google Scholar] [CrossRef]
- Rötig, A.; Lonlay, P.; Chretien, D.; Foury, F.; Koenig, M.; Sidi, D.; Munnich, A.; Rustin, P. Aconitase and mitochondrial iron–sulphur protein deficiency in Friedreich ataxia. Nat. Genet. 1997, 17, 215–217. [Google Scholar] [CrossRef]
- Stehling, O.; Elsässer, H.P.; Brückel, B.; Mühlenhoff, U.; Lill, R. Iron–sulfur protein maturation in human cells: Evidence for a function of frataxin. Hum. Mol. Genet. 2004, 13, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.; Mühlenhoff, U.; Lill, R. An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1. EMBO Rep. 2003, 4, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Lodi, R.; Cooper, J.M.; Bradley, J.L.; Manners, D.; Styles, P.; Taylor, D.J.; Schapira, A.H. Deficit of in vivo mitochondrial ATP production in patients with Friedreich ataxia. Proc. Natl. Acad. Sci. USA 1999, 96, 11492–11495. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.M.; Houshmand, M.; Hosseinkhani, S.; Nafissi, S.; Khatami, M. Complex I and ATP content deficiency in lymphocytes from Friedreich’s ataxia. Can. J. Neurol. Sci. 2009, 36, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.; Johnson, J.; Dong, Y.N.; Mercado-Ayon, E.; Warren, N.; Zhai, M.; McMillan, E.; Salovin, A.; Lin, H.; Lynch, D.R. Role of frataxin protein deficiency and metabolic dysfunction in Friedreich ataxia, an autosomal recessive mitochondrial disease. Neuronal Signal. 2018, 2, NS20180060. [Google Scholar] [CrossRef] [PubMed]
- Lazaropoulos, M.; Dong, Y.; Clark, E.; Greeley, N.R.; Seyer, L.A.; Brigatti, K.W.; Christie, C.; Perlman, S.L.; Wilmot, G.R.; Gomez, C.M.; et al. Frataxin levels in peripheral tissue in Friedreich ataxia. Ann. Clin. Transl. Neurol. 2015, 2, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.; Shaw, J.; Rowland, A.; Wallis, J.; South, S.; Nakamura, Y.; von Gabain, A.; Farrall, M.; Williamson, R. Mapping of mutation causing Friedreich’s ataxia to human chromosome 9. Nature 1988, 334, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Montermini, L.; Rodius, F.; Pianese, L.; Moltò, M.D.; Cossée, M.; Campuzano, V.; Cavalcanti, F.; Monticelli, A.; Palau, F.; Gyapay, G.; et al. The Friedreich ataxia critical region spans a 150-kb interval on chromosome 9q13. Am. J. Hum. Genet. 1995, 57, 1061–1067, Erratum in Am. J. Hum. Genet. 1995, 57, 1520. [Google Scholar] [PubMed]
- Greene, E.; Mahishi, L.; Entezam, A.; Kumari, D.; Usdin, K. Repeat-induced epigenetic changes in intron 1 of the frataxin gene and its consequences in Friedreich ataxia. Nucleic Acids Res. 2007, 35, 3383–3390. [Google Scholar] [CrossRef]
- Li, K.; Singh, A.; Crooks, D.R.; Dai, X.; Cong, Z.; Pan, L.; Ha, D.; Rouault, T.A. Expression of Human Frataxin Is Regulated by Transcription Factors SRF and TFAP2. PLoS ONE 2010, 5, e12286. [Google Scholar] [CrossRef]
- Fernández-Frías, I.; Pérez-Luz, S.; Díaz-Nido, J. Analysis of Putative Epigenetic Regulatory Elements in the FXN Genomic Locus. Int J Mol Sci. 2020, 21, 410. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Wang, J.; Gonzalez, T.J.; Asokan, A.; Napierala, J.S.; Napierala, M. Defining Transcription Regulatory Elements in the Human Frataxin Gene: Implications for Gene Therapy. Hum. Gene Ther. 2020, 31, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Cao, Y.; Dai, X.; Marelja, Z.; Zhou, D.; Mo, R.; Al-Mahdawi, S.; Pook, M.A.; Leimkühler, S.; Rouault, T.A.; et al. Novel frataxin isoforms may contribute to the pathological mechanism of Friedreich ataxia. PLoS ONE. 2012, 7, e47847. [Google Scholar] [CrossRef]
- Pianese, L.; Tammaro, A.; Turano, M.; De Biase, I.; Monticelli, A.; Cocozza, S. Identification of a novel transcript of X25, the human gene involved in Friedreich ataxia. Neurosci. Lett. 2002, 320, 137–140. [Google Scholar] [CrossRef]
- Abruzzo, P.M.; Marini, M.; Bolotta, A.; Malisardi, G.; Manfredini, S.; Ghezzo, A.; Pini, A.; Tasco, G.; Casadio, R. Frataxin mRNA isoforms in FRDA patients and normal subjects: Effect of tocotrienol supplementation. Biomed. Res. Int. 2013, 2013, 276808. [Google Scholar] [CrossRef] [PubMed]
- Rodden, L.N.; Gilliam, K.M.; Lam, C.; Rojsajjakul, T.; Mesaros, C.; Dionisi, C.; Pook, M.; Pandolfo, M.; Lynch, D.R.; Blair, I.A.; et al. DNA methylation in Friedreich ataxia silences expression of frataxin isoform E. Sci. Rep. 2022, 12, 5031. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Q.; Weng, L.; Hauser, L.A.; Strawser, C.J.; Mesaros, C.; Lynch, D.R.; Blair, I.A. Characterization of a new N-terminally acetylated extra-mitochondrial isoform of frataxin in human erythrocytes. Sci. Rep. 2018, 8, 17043. [Google Scholar] [CrossRef] [PubMed]
- Agro, M.; Diaz-Nido, J. Effect of mitochondrial and cytosolic FXN isoform expression on mitochondrial dynamics and metabolism. Int. J. Mol. Sci. 2020, 21, 8251. [Google Scholar] [CrossRef]
- Montermini, L.; Andermann, E.; Labuda, M.; Richter, A.; Pandolfo, M.; Cavalcanti, F.; Pianese, L.; Iodice, L.; Farina, G.; Monticelli, A.; et al. The Friedreich ataxia GAA triplet repeat: Premutation and normal alleles. Hum. Mol. Genet. 1997, 6, 1261–1266. [Google Scholar] [CrossRef]
- Pollard, L.M.; Sharma, R.; Gomez, M.; Shah, S.; Delatycki, M.B.; Pianese, L.; Monticelli, A.; Keats, B.J.B.; Bidichandani, S.I. Replication-mediated instability of the GAA triplet repeat mutation in Friedreich ataxia. Nucleic Acids Res. 2004, 32, 5962–5971. [Google Scholar] [CrossRef]
- Sharma, R.; Bhatti, S.; Gomez, M.; Clark, R.M.; Murray, C.; Ashizawa, T.; Bidichandani, S.I. The GAA triplet-repeat sequence in Friedreich ataxia shows a high level of somatic instability in vivo, with a significant predilection for large contractions. Hum. Mol. Genet. 2002, 11, 2175–2187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Long, A.; Napierala, J.S.; Polak, U.; Hauser, L.; Koeppen, A.H.; Lynch, D.R.; Napierala, M. Somatic instability of the expanded GAA repeats in Friedreich’s ataxia. PLoS ONE 2017, 12, e0189990. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.M.; De Biase, I.; Malykhina, A.P.; Al-Mahdawi, S.; Pook, M.; Bidichandani, S.I. The GAA triplet-repeat is unstable in the context of the human FXN locus and displays age-dependent expansions in cerebellum and DRG in a transgenic mouse model. Hum. Genet. 2007, 120, 633–640. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Biase, I.; Rasmussen, A.; Endres, D.; Al-Mahdawi, S.; Monticelli, A.; Cocozza, S.; Pook, M.; Bidichandani, S.I. Progressive GAA expansions in dorsal root ganglia of Friedreich’s ataxia patients. Ann. Neurol. 2007, 61, 55–60. [Google Scholar] [CrossRef] [PubMed]
- De Michele, G.; Cavalcanti, F.; Criscuolo, C.; Pianese, L.; Monticelli, A.; Filla, A.; Cocozza, S. Parental gender, age at birth and expansion length influence GAA repeat intergenerational instability in the X25 gene: Pedigree studies and analysis of sperm from patients with Friedreich’s ataxia. Hum. Mol. Genet. 1998, 7, 1901–1906. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delatycki, M.B.; Paris, D.; Gardner, R.J.; Forshaw, K.; Nicholson, G.A.; Nassif, N.; Williamson, R.; Forrest, S.M. Sperm DNA analysis in a Friedreich ataxia premutation carrier suggests both meiotic and mitotic expansion in the FRDA gene. J. Med. Genet. 1998, 35, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Pianese, L.; Cavalcanti, F.; De Michele, G.; Filla, A.; Campanella, G.; Calabrese, O.; Castaldo, I.; Monticelli, A.; Cocozza, S. The effect of parental gender on the GAA dynamic mutation in the FRDA gene. Am. J. Hum. Genet. 1997, 60, 460–463. [Google Scholar] [PubMed]
- Bidichandani, S.I.; Ashizawa, T.; Patel, P.I. The GAA triplet-repeat expansion in Friedreich ataxia interferes with transcription and may be associated with an unusual DNA structure. Am. J. Hum. Genet. 1998, 62, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, K.; Montermini, L.; Wells, R.D.; Pandolfo, M. Inhibitory effects of expanded GAA.TTC triplet repeats from intron I of the Friedreich ataxia gene on transcription and replication in vivo. J. Biol. Chem. 1998, 273, 14588–14595. [Google Scholar] [CrossRef]
- Ohshima, K.; Sakamoto, N.; Labuda, M.; Poirier, J.; Moseley, M.L.; Montermini, L.; Ranum, L.P.; Wells, R.D.; Pandolfo, M. A nonpathogenic GAAGGA repeat in the Friedreich gene: Implications for pathogenesis. Neurology 1999, 53, 1854–1857. [Google Scholar] [CrossRef]
- Sakamoto, N.; Larson, J.E.; Iyer, R.R.; Montermini, L.; Pandolfo, M.; Wells, R.D. GGA*TCC-interrupted triplets in long GAA*TTC repeats inhibit the formation of triplex and sticky DNA structures, alleviate transcription inhibition, and reduce genetic instabilities. J. Biol. Chem. 2001, 276, 27178–27187. [Google Scholar] [CrossRef] [PubMed]
- Nethisinghe, S.; Kesavan, M.; Ging, H.; Labrum, R.; Polke, J.M.; Islam, S.; Garcia-Moreno, H.; Callaghan, M.F.; Cavalcanti, F.; Pook, M.A.; et al. Interruptions of the FXN GAA Repeat Tract Delay the Age at Onset of Friedreich’s Ataxia in a Location Dependent Manner. Int. J. Mol. Sci. 2021, 22, 7507. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahdawi, S.; Ging, H.; Bayot, A.; Cavalcanti, F.; La Cognata, V.; Cavallaro, S.; Giunti, P.; Pook, M.A. Large Interruptions of GAA Repeat Expansion Mutations in Friedreich Ataxia Are Very Rare. Front. Cell. Neurosci. 2018, 12, 443. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Brown, J.M.; Buckle, V.J.; Wade-Martins, R.; Lufino, M.M. Expanded GAA repeats impair FXN gene expression and reposition the FXN locus to the nuclear lamina in single cells. Hum. Mol. Genet. 2015, 24, 3457–3471. [Google Scholar] [CrossRef] [PubMed]
- Groh, M.; Lufino, M.M.; Wade-Martins, R.; Gromak, N. R-loops associated with triplet repeat expansions promote gene silencing in Friedreich ataxia and fragile X syndrome. PLoS Genet. 2014, 10, e1004318. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Ohshima, K.; Montermini, L.; Pandolfo, M.; Wells, R.D. Sticky DNA, a self-associated complex formed at long GAA*TTC repeats in intron 1 of the frataxin gene, inhibits transcription. J. Biol. Chem. 2001, 276, 27171–27177. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.D. DNA triplexes and Friedreich ataxia. FASEB J. 2008, 22, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Saveliev, A.; Everett, C.; Sharpe, T.; Webster, Z.; Festenstein, R. DNA triplet repeats mediate heterochromatin-protein-1-sensitive variegated gene silencing. Nature 2003, 422, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Sandi, C.; Sandi, M.; Anjomani Virmouni, S.; Al-Mahdawi, S.; Pook, M.A. Epigenetic-based therapies for Friedreich ataxia. Front Genet. 2014, 5, 165. [Google Scholar] [CrossRef]
- Herman, D.; Jenssen, K.; Burnett, R.; Soragni, E.; Perlman, S.L.; Gottesfeld, J.M. Histone deacetylase inhibitors reverse gene silencing in Friedreich’s ataxia. Nat. Chem. Biol. 2006, 2, 551–558. [Google Scholar] [CrossRef]
- Al-Mahdawi, S.; Pinto, R.M.; Ismail, O.; Varshney, D.; Lymperi, S.; Sandi, C.; Trabzuni, D.; Pook, M. The Friedreich ataxia GAA repeat expansion mutation induces comparable epigenetic changes in human and transgenic mouse brain and heart tissues. Hum. Mol. Genet. 2008, 17, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.K.; Torres, R.; Yandim, C.; Law, P.P.; Khadayate, S.; Mauri, M.; Grosan, C.; Chapman-Rothe, N.; Giunti, P.; Pook, M.; et al. Heterochromatinization induced by GAA-repeat hyperexpansion in Friedreich’s ataxia can be reduced upon HDAC inhibition by vitamin B3. Hum. Mol. Genet. 2013, 22, 2662–2675. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Y.; Polak, U.; Lin, K.; Shen, J.; Farmer, J.; Seyer, L.; Bhalla, A.D.; Rozwadowska, N.; Lynch, D.R.; et al. Expanded GAA repeats impede transcription elongation through the FXN gene and induce transcriptional silencing that is restricted to the FXN locus. Hum. Mol. Genet. 2015, 24, 6932–6943. [Google Scholar]
- Punga, T.; Bühler, M. Long intronic GAA repeats causing Friedreich ataxia impede transcription elongation. EMBO Mol. Med. 2010, 2, 120–129. [Google Scholar] [CrossRef]
- Kumari, D.; Biacsi, R.E.; Usdin, K. Repeat expansion affects both transcription initiation and elongation in friedreich ataxia cells. J. Biol. Chem. 2011, 286, 4209–4215. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Wang, J.; Zhang, S.; Giles, K.; Prakash, T.P.; Rigo, F.; Napierala, J.S.; Napierala, M. Premature transcription termination at the expanded GAA repeats and aberrant alternative polyadenylation contributes to the Frataxin transcriptional deficit in Friedreich’s ataxia. Hum. Mol. Genet. 2022, 31, 3539–3557. [Google Scholar] [CrossRef]
- Rocca, C.J.; Rainaldi, J.N.; Sharma, J.; Shi, Y.; Haquang, J.H.; Luebeck, J.; Mali, P.; Cherqui, S. CRISPR-Cas9 Gene Editing of Hematopoietic Stem Cells from Patients with Friedreich’s Ataxia. Mol. Ther. Methods Clin. Dev. 2020, 17, 1026–1036. [Google Scholar] [CrossRef]
- Mishra, P.; Sivakumar, A.; Johnson, A.; Pernaci, C.; Warden, A.S.; El-Hachem, L.R.; Hansen, E.; Badell-Grau, R.A.; Khare, V.; Ramirez, G.; et al. Gene editing improves endoplasmic reticulum-mitochondrial contacts and unfolded protein response in Friedreich’s ataxia iPSC-derived neurons. Front. Pharmacol. 2024, 15, 1323491. [Google Scholar] [CrossRef] [PubMed]
- Mazzara, P.G.; Muggeo, S.; Luoni, M.; Massimino, L.; Zaghi, M.; Valverde, P.T.; Brusco, S.; Marzi, M.J.; Palma, C.; Colasante, G.; et al. Frataxin gene editing rescues Friedreich’s ataxia pathology in dorsal root ganglia organoid-derived sensory neurons. Nat. Commun. 2020, 11, 4178. [Google Scholar] [CrossRef]
- Li, Y.; Polak, U.; Bhalla, A.D.; Rozwadowska, N.; Butler, J.S.; Lynch, D.R.; Dent, S.Y.R.; Napierala, M. Excision of Expanded GAA Repeats Alleviates the Molecular Phenotype of Friedreich’s Ataxia. Mol. Ther. 2015, 23, 1055–1065. [Google Scholar] [CrossRef]
- Shore, P.; Sharrocks, A.D. The MADS-box family of transcription factors. Eur. J. Biochem. 1995, 229, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dalton, S.; Marais, R.; Wynne, J.; Treisman, R. Isolation and characterization of SRF accessory proteins. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1993, 340, 325–332. [Google Scholar] [PubMed]
- Eckert, D.; Buhl, S.; Weber, S.; Jäger, R.; Schorle, H. The AP-2 family of transcription factors. Genome Biol. 2005, 6, 246. [Google Scholar] [CrossRef] [PubMed]
- Petryniak, B.; Staudt, L.M.; Postema, C.E.; McCormack, W.T.; Thompson, C.B. Characterization of chicken octamer-binding proteins demonstrates that POU domain-containing homeobox transcription factors have been highly conserved during vertebrate evolution. Proc. Natl. Acad. Sci. USA 1990, 87, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Stollar, E.; Chang, J.; Grossmann, J.G.; O’Brien, R.; Ladbury, J.; Carpenter, B.; Roberts, S.; Luisi, B. Expression of the Oct-1 transcription factor and characterization of its interactions with the Bob1 coactivator. Biochemistry 2001, 40, 6580–6588. [Google Scholar] [CrossRef] [PubMed]
- Puspasari, N.; Rowley, S.M.; Gordon, L.; Lockhart, P.J.; Ioannou, P.A.; Delatycki, M.B.; Sarsero, J.P. Long range regulation of human FXN gene expression. PLoS ONE 2011, 6, e22001. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, R.; Lan, N.N.; Tai, T.T.; Adachi, Y.; Kawazoe, A.; Mu, A.; Taketani, S. p53 directly regulates the transcription of the human frataxin gene and its lack of regulation in tumor cells decreases the utilization of mitochondrial iron. Gene 2014, 551, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Sawamoto, M.; Imai, T.; Umeda, M.; Fukuda, K.; Kataoka, T.; Taketani, S. The p53-dependent expression of frataxin controls 5-aminolevulinic acid-induced accumulation of protoporphyrin IX and photo-damage in cancerous cells. Photochem. Photobiol. 2013, 89, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Helma, R.; Bažantová, P.; Petr, M.; Adámik, M.; Renčiuk, D.; Tichý, V.; Pastuchová, A.; Soldánová, Z.; Pečinka, P.; Bowater, R.P.; et al. p53 Binds Preferentially to Non-B DNA Structures Formed by the Pyrimidine-Rich Strands of GAA.TTC Trinucleotide Repeats Associated with Friedreich’s Ataxia. Molecules 2019, 24, 2078. [Google Scholar] [CrossRef]
- Cherif, K.; Gérard, C.; Rousseau, J.; Ouellet, D.L.; Chapdelaine, P.; Tremblay, J.P. Increased Frataxin Expression Induced in Friedreich Ataxia Cells by Platinum TALE-VP64s or Platinum TALE-SunTag. Mol. Ther. Nucleic Acids. 2018, 12, 19–32. [Google Scholar] [CrossRef]
- Sanjana, N.E.; Cong, L.; Zhou, Y.; Cunniff, M.M.; Feng, G.; Zhang, F. A transcription activator-like effector toolbox for genome engineering. Nat. Protoc. 2012, 7, 171–192. [Google Scholar] [CrossRef] [PubMed]
- Castro, I.H.; Pignataro, M.F.; Sewell, K.E.; Espeche, L.D.; Herrera, M.G.; Noguera, M.E.; Dain, L.; Nadra, A.D.; Aran, M.; Smal, C.; et al. Frataxin Structure and Function. Subcell. Biochem. 2019, 93, 393–438. [Google Scholar] [PubMed]
- Zhang, W.; Xu, L.; Zhao, H.; Li, K. Mammalian mitochondrial iron-sulfur cluster biogenesis and transfer and related human diseases. Biophys. Rep. 2021, 7, 127–141. [Google Scholar] [PubMed]
- Bridwell-Rabb, J.; Fox, N.G.; Tsai, C.L.; Winn, A.M.; Barondeau, D.P. Human frataxin activates Fe-S cluster biosynthesis by facilitating sulfur transfer chemistry. Biochemistry 2014, 53, 4904–4913. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.L.; Becker, E.M.; Whitnall, M.; Suryo Rahmanto, Y.; Ponka, P.; Richardson, D.R. Elucidation of the mechanism of mitochondrial iron loading in Friedreich’s ataxia by analysis of a mouse mutant. Proc. Natl. Acad. Sci. USA 2009, 106, 16381–16386. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.B. Iron dysregulation in Friedreich ataxia. Semin. Pediatr. Neurol. 2006, 13, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Besse, E.K.; Ha, D.; Kovtunovych, G.; Rouault, T.A. Iron-dependent regulation of frataxin expression: Implications for treatment of Friedreich ataxia. Hum. Mol. Genet. 2008, 17, 2265–2273. [Google Scholar] [CrossRef] [PubMed]
- Petit, F.; Drecourt, A.; Dussiot, M.; Zangarelli, C.; Hermine, O.; Munnich, A.; Rötig, A. Defective palmitoylation of transferrin receptor triggers iron overload in Friedreich ataxia fibroblasts. Blood 2021, 137, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.R.; Huang, M.L.; Whitnall, M.; Becker, E.M.; Ponka, P.; Suryo Rahmanto, Y. The ins and outs of mitochondrial iron-loading: The metabolic defect in Friedreich’s ataxia. J. Mol. Med. 2010, 88, 323–329. [Google Scholar] [CrossRef]
- Kakhlon, O.; Manning, H.; Breuer, W.; Melamed-Book, N.; Lu, C.; Cortopassi, G.; Munnich, A.; loav Cabantchik, Z. Cell functions impaired by frataxin deficiency are restored by drug-mediated iron relocation. Blood 2008, 112, 5219–5227. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lau, Y.M.; Ng, K.M.; Lai, W.H.; Ho, S.L.; Tse, H.F.; Siu, C.W.; Ho, P.W. Efficient attenuation of Friedreich’s ataxia (FRDA) cardiomyopathy by modulation of iron homeostasis-human induced pluripotent stem cell (hiPSC) as a drug screening platform for FRDA. Int. J. Cardiol. 2016, 203, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, M.; Arpa, J.; Delatycki, M.B.; Le Quan Sang, K.H.; Mariotti, C.; Munnich, A.; Sanz-Galego, I.; Tai, G.; Tarnopolsky, M.A.; Taroni, F. Deferiprone in Friedreich ataxia: A 6-month randomized controlled trial. Ann. Neurol. 2014, 76, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef] [PubMed]
- Place, R.F.; Li, L.C.; Pookot, D.; Noonan, E.J.; Dahiya, R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc. Natl. Acad. Sci. USA 2008, 105, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Misiorek, J.O.; Schreiber, A.M.; Urbanek-Trzeciak, M.O.; Jazurek-Ciesiołka, M.; Hauser, L.A.; Lynch, D.R.; Napierala, J.S.; Napierala, M. A Comprehensive Transcriptome Analysis Identifies FXN and BDNF as Novel Targets of miRNAs in Friedreich’s Ataxia Patients. Mol. Neurobiol. 2020, 57, 2639–2653. [Google Scholar] [CrossRef] [PubMed]
- Mahishi, L.H.; Hart, R.P.; Lynch, D.R.; Ratan, R.R. miR-886-3p levels are elevated in Friedreich ataxia. J. Neurosci. 2012, 32, 9369–9373. [Google Scholar] [CrossRef] [PubMed]
- Seco-Cervera, M.; González-Rodríguez, D.; Ibáñez-Cabellos, J.S.; Peiró-Chova, L.; González-Cabo, P.; García-López, E.; Vílchez, J.J.; Sanz-Gallego, I.; Pallardó, F.V.; García-Giménez, J.L. Circulating miR-323-3p is a biomarker for cardiomyopathy and an indicator of phenotypic variability in Friedreich’s ataxia patients. Sci. Rep. 2017, 7, 5237. [Google Scholar] [CrossRef] [PubMed]
- Quesada, M.P.; Jones, J.; Rodríguez-Lozano, F.J.; Moraleda, J.M.; Martinez, S. Novel aberrant genetic and epigenetic events in Friedreich’s ataxia. Exp. Cell Res. 2015, 335, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Katsu-Jiménez, Y.; Loría, F.; Corona, J.C.; Díaz-Nido, J. Gene transfer of brain-derived neurotrophic factor (BDNF) prevents neurodegeneration triggered by FXN deficiency. Mol. Ther. 2016, 24, 877–889. [Google Scholar] [CrossRef]
- Cavadini, P.; Adamec, J.; Taroni, F.; O Gakh, O.; Isaya, G. Two-step processing of human frataxin by mitochondrial processing peptidase. Precursor and intermediate forms are cleaved at different rates. J. Biol. Chem. 2000, 275, 41469–41475. [Google Scholar] [CrossRef]
- Ran, Q.; Wadhwa, R.; Kawai, R.; Kaul, S.C.; Sifers, R.N.; Bick, R.J.; Smith, J.R.; Pereira-Smith, O.M. Extramitochondrial localization of mortalin/mthsp70/PBP74/GRP75. Biochem. Biophys. Res. Commun. 2000, 275, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Londono, C.; Osorio, C.; Gama, V.; Alzate, O. Mortalin, apoptosis, and neurodegeneration. Biomolecules 2012, 2, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Voisine, C.; Cheng, Y.C.; Ohlson, M.; Schilke, B.; Hoff, K.; Beinert, H.; Marszalek, J.; Craig, E.A. Jac1, a mitochondrial J-type chaperone, is involved in the biogenesis of Fe/S clusters in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2001, 98, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Frederick, R.O.; Kim, J.H.; Reinen, N.M.; Tonelli, M.; Markley, J.L. Human mitochondrial chaperone (mtHSP70) and cysteine desulfurase (NFS1) bind preferentially to the disordered conformation, whereas co-chaperone (HSC20) binds to the structured conformation of the iron–sulfur cluster scaffold protein (ISCU). J. Biol. Chem. 2013, 288, 28755–28770. [Google Scholar] [CrossRef] [PubMed]
- Burbulla, L.F.; Fitzgerald, J.C.; Stegen, K.; Westermeier, J.; Thost, A.K.; Kato, H.; Mokranjac, D.; Sauerwald, J.; Martins, L.M.; Woitalla, D.; et al. Mitochondrial proteolytic stress induced by loss of mortalin function is rescued by Parkin and PINK1. Cell Death Dis. 2014, 5, e1180. [Google Scholar] [CrossRef] [PubMed]
- Geissler, A.; Rassow, J.; Pfanner, N.; Voos, W. Mitochondrial import driving forces: Enhanced trapping by matrix Hsp70 stimulates translocation and reduces the membrane potential dependence of loosely folded preproteins. Mol. Cell. Biol. 2001, 21, 7097–7104. [Google Scholar] [CrossRef] [PubMed]
- Horst, M.; Oppliger, W.; Rospert, S.; Schönfeld, H.J.; Schatz, G.; Azem, A. Sequential action of two hsp70 complexes during protein import into mitochondria. EMBO J. 1997, 16, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; D’Silva, P.; Walter, W.; Marszalek, J.; Craig, E.A. Regulated cycling of mitochondrial Hsp70 at the protein import channel. Science 2003, 300, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Napoli, E.; Cortopassi, G. Mitochondrial frataxin interacts with ISD11 of the NFS1/ISCU complex and multiple mitochondrial chaperones. Hum. Mol. Genet. 2012, 21, 1457–1469. [Google Scholar] [CrossRef]
- Knight, S.A.; Sepuri, N.B.; Pain, D.; Dancis, A. Mt-Hsp70 homolog, Ssc2p, required for maturation of yeast frataxin and mitochondrial iron homeostasis. J. Biol. Chem. 1998, 273, 18389–18393. [Google Scholar] [CrossRef]
- Voisine, C.; Schilke, B.; Ohlson, M.; Beinert, H.; Marszalek, J.; Craig, E.A. Role of the mitochondrial Hsp70s, Ssc1 and Ssq1, in the maturation of Yfh1. Mol. Cell. Biol. 2000, 20, 3677–3684. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dong, Y.N.; McMillan, E.; Clark, E.M.; Lin, H.; Lynch, D.R. GRP75 overexpression rescues frataxin deficiency and mitochondrial phenotypes in Friedreich ataxia cellular models. Hum. Mol. Genet. 2019, 28, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, J.P.; Langer, T.; Davis, T.A.; Wiedmann, M. Control of folding and membrane translocation by binding of the chaperone DnaJ to nascent polypeptides. Proc. Natl. Acad. Sci. USA 1993, 90, 10216–10220. [Google Scholar] [CrossRef] [PubMed]
- Silver, P.A.; Way, J.C. Eukaryotic DnaJ homologs and the specificity of Hsp70 activity. Cell 1993, 74, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Syken, J.; De-Medina, T.; Münger, K. TID1, a human homolog of the Drosophila tumor suppressor l(2)tid, encodes two mitochondrial modulators of apoptosis with opposing functions. Proc. Natl. Acad. Sci. USA 1999, 96, 8499–8504. [Google Scholar] [CrossRef] [PubMed]
- Trentin, G.A.; Yin, X.; Tahir, S.; Lhotak, S.; Farhang-Fallah, J.; Li, Y.; Rozakis-Adcock, M. A mouse homologue of the Drosophila tumor suppressor l(2)tid gene defines a novel Ras GTPase-activating protein (RasGAP)-binding protein. J. Biol. Chem. 2001, 276, 13087–13095. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Daniels, C.K.; Cao, S. Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacol. Ther. 2012, 136, 354–374. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Cenciarelli, C.; Shao, Z.; Vidal, M.; Parks, W.P.; Pagano, M.; Cheng-Mayer, C. Human T cell leukemia virus type 1 Tax associates with a molecular chaperone complex containing hTid-1 and Hsp70. Curr. Biol. 2001, 11, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Syken, J.; Macian, F.; Agarwal, S.; Rao, A.; Münger, K. TID1, a mammalian homologue of the drosophila tumor suppressor lethal(2) tumorous imaginal discs, regulates activation-induced cell death in Th2 cells. Oncogene 2003, 22, 4636–4641. [Google Scholar] [CrossRef]
- Tarunina, M.; Alger, L.; Chu, G.; Munger, K.; Gudkov, A.; Jat, P.S. Functional genetic screen for genes involved in senescence: Role of Tid1, a homologue of the Drosophila tumor suppressor l(2)tid, in senescence and cell survival. Mol. Cell Biol. 2004, 24, 10792–10801. [Google Scholar] [CrossRef]
- Lo, J.F.; Hayashi, M.; Woo-Kim, S.; Tian, B.; Huang, J.F.; Fearns, C.; Takayama, S.; Zapata, J.M.; Yang, Y.; Lee, J.D. Tid1, a cochaperone of the heat shock 70 protein and the mammalian counterpart of the Drosophila tumor suppressor l(2)tid, is critical for early embryonic development and cell survival. Mol. Cell Biol. 2004, 24, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chiou, S.H.; Huang, C.Y.; Jan, C.I.; Lin, S.C.; Hu, W.Y.; Chou, S.H.; Liu, C.J.; Lo, J.F. Tid1 functions as a tumour suppressor in head and neck squamous cell carcinoma. J. Pathol. 2009, 219, 347–355. [Google Scholar] [CrossRef]
- Dong, Y.N.; Ngaba, L.V.; An, J.; Adeshina, M.W.; Warren, N.; Wong, J.; Lynch, D.R. A peptide derived from TID1S rescues frataxin deficiency and mitochondrial defects in FRDA cellular models. Front. Pharmacol. 2024, 15, 1352311. [Google Scholar] [CrossRef] [PubMed]
- Rufini, A.; Fortuni, S.; Arcuri, G.; Condò, I.; Serio, D.; Incani, O.; Malisan, F.; Ventura, N.; Testi, R. Preventing the ubiquitin–proteasome-dependent degradation of frataxin, the protein defective in Friedreich’s ataxia. Hum. Mol. Genet. 2011, 20, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Benini, M.; Fortuni, S.; Condò, I.; Alfedi, G.; Malisan, F.; Toschi, N.; Serio, D.; Massaro, D.S.; Arcuri, G.; Testi, R.; et al. E3 Ligase RNF126 Directly Ubiquitinates Frataxin, Promoting Its Degradation: Identification of a Potential Therapeutic Target for Friedreich Ataxia. Cell Rep. 2017, 18, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Rufini, A.; Cavallo, F.; Condò, I.; Fortuni, S.; De Martino, G.; Incani, O.; Di Venere, A.; Benini, M.; Massaro, D.S.; Arcuri, G.; et al. Highly specific ubiquitin-competing molecules effectively promote frataxin accumulation and partially rescue the aconitase defect in Friedreich ataxia cells. Neurobiol. Dis. 2015, 75, 91–99. [Google Scholar] [CrossRef]
- Cherubini, F.; Serio, D.; Guccini, I.; Fortuni, S.; Arcuri, G.; Condò, I.; Rufini, A.; Moiz, S.; Camerini, S.; Crescenzi, M.; et al. Src inhibitors modulate frataxin protein levels. Hum. Mol. Genet. 2015, 24, 4296–4305. [Google Scholar] [CrossRef]
- Feng, Y.; Nouri, K.; Schimmer, A.D. Mitochondrial ATP-Dependent Proteases-Biological Function and Potential Anti-Cancer Targets. Cancers 2021, 13, 2020. [Google Scholar] [CrossRef]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From bi+ogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284. [Google Scholar] [CrossRef]
- Nabhan, J.F.; Gooch, R.L.; Chekler, E.L.; Pierce, B.; Bulawa, C.E. Perturbation of cellular proteostasis networks identifies pathways that modulate precursor and intermediate but not mature levels of frataxin. Sci. Rep. 2015, 5, 18251. [Google Scholar] [CrossRef]
- Hamon, M.P.; Bulteau, A.L.; Friguet, B. Mitochondrial proteases and protein quality control in ageing and longevity. Ageing Res. Rev. 2015, 23, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Bayot, A.; Gareil, M.; Rogowska-Wrzesinska, A.; Roepstorff, P.; Friguet, B.; Bulteau, A.L. Identification of novel oxidized protein substrates and physiological partners of the mitochondrial ATP-dependent Lon-like protease Pim1. J. Biol. Chem. 2010, 285, 11445–11457. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.M.; Chen, O.S.; Li, L.; Kaplan, J.; Bhuiyan, S.A.; Natarajan, S.K.; Bard, M.; Cox, J.E. Altered sterol metabolism in budding yeast affects mitochondrial iron-sulfur (Fe-S) cluster synthesis. J. Biol. Chem. 2018, 293, 10782–10795. [Google Scholar] [CrossRef]
- Hackett, P.T.; Jia, X.; Li, L.; Ward, D.M. Posttranslational regulation of mitochondrial frataxin and identification of compounds that increase frataxin levels in Friedreich’s ataxia. J. Biol. Chem. 2022, 298, 101982. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.R.; Kilonsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Ebato, C.; Uchida, T.; Arakawa, M.; Komatsu, M.; Ueno, T.; Komiya, K.; Azuma, K.; Hirose, T.; Tanaka, K.; Kominami, E.; et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metabolism. 2008, 8, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.L.-H.; Sivagurunathan, S.; Ting, S.; Jansson, P.J.; Austin, C.J.D.; Kelly, M.; Semsarian, C.; Zhang, D.; Richardson, D.R. Molecular and functional alterations in a mouse cardiac model of Friedreich ataxia: Activation of the integrated stress response, eIF2alpha phosphorylation, and the induction of downstream targets. Am. J. Pathol. 2013, 183, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, A.; Maglioni, S.; Palikaras, K.; Shaik, A.; Strappazzon, F.; Brinkmann, V.; Torgovnick, A.; Castelein, N.; De Henau, S.; Braeckman, B.P.; et al. Iron-Starvation-Induced Mitophagy Mediates Lifespan Extension upon Mitochondrial Stress in C. elegans. Curr. Biol. 2015, 25, 1810–1822. [Google Scholar] [CrossRef]
- Schiavi, A.; Torgovnick, A.; Kell, A.; Megalou, E.; Castelein, N.; Guccini, I.; Marzocchella, L.; Gelino, S.; Hansen, M.; Malisan, F.; et al. Autophagy induction extends lifespan and reduces lipid content in response to frataxin silencing in C. elegans. Exp. Gerontol. 2013, 48, 191–201. [Google Scholar] [CrossRef]
| FXN Gene Regulation Factors | Treatment Options |
|---|---|
| GAA repeat expansion | CRISPR-Cas9- or zinc finger nuclease-mediated removal of GAA repeat expansion |
| Transcription factors | Transcription Activator-Like Effectors (TALE) (TALE-VP64s) |
| Iron | Iron chelator-Deferiprone |
| miRNAs | Anti-miRNA oligonucleotide targeting miRNA-224-5 or miRNA-886-3p |
| Chaperones | GRP75 overexpression, TID1S448-453 peptide |
| Proteasome | Ubiquitin-competing molecules and Src tyrosine kinase inhibitor |
| Mitochondrial proteases | Inhibitors of PITRM1 and Lon1 protease |
| Autophagy | ULK1 inhibitor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.N.; Mercado-Ayón, E.; Coulman, J.; Flatley, L.; Ngaba, L.V.; Adeshina, M.W.; Lynch, D.R. The Regulation of the Disease-Causing Gene FXN. Cells 2024, 13, 1040. https://doi.org/10.3390/cells13121040
Dong YN, Mercado-Ayón E, Coulman J, Flatley L, Ngaba LV, Adeshina MW, Lynch DR. The Regulation of the Disease-Causing Gene FXN. Cells. 2024; 13(12):1040. https://doi.org/10.3390/cells13121040
Chicago/Turabian StyleDong, Yi Na, Elizabeth Mercado-Ayón, Jennifer Coulman, Liam Flatley, Lucie Vanessa Ngaba, Miniat W. Adeshina, and David R. Lynch. 2024. "The Regulation of the Disease-Causing Gene FXN" Cells 13, no. 12: 1040. https://doi.org/10.3390/cells13121040
APA StyleDong, Y. N., Mercado-Ayón, E., Coulman, J., Flatley, L., Ngaba, L. V., Adeshina, M. W., & Lynch, D. R. (2024). The Regulation of the Disease-Causing Gene FXN. Cells, 13(12), 1040. https://doi.org/10.3390/cells13121040





