Social Isolation Induces Changes in the Monoaminergic Signalling in the Rat Medial Prefrontal Cortex
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Behavioural Tests
2.2.1. Isolation of Animals and Study Design
2.2.2. Sociability and Social Novelty Discrimination
2.2.3. Social-Interaction Test
2.2.4. Male-Intruder Test
2.2.5. Open-Field Test
2.2.6. Elevated-Plus-Maze Test
2.2.7. Forced-Swim Test
2.3. Microdissection of Medial Prefrontal Cortex
2.4. RNA Sequencing
2.5. Bioinformatics
2.6. Quantitative RT-PCR
2.7. Htr2c Antagonist Treatment
2.8. Statistical Analysis
3. Results
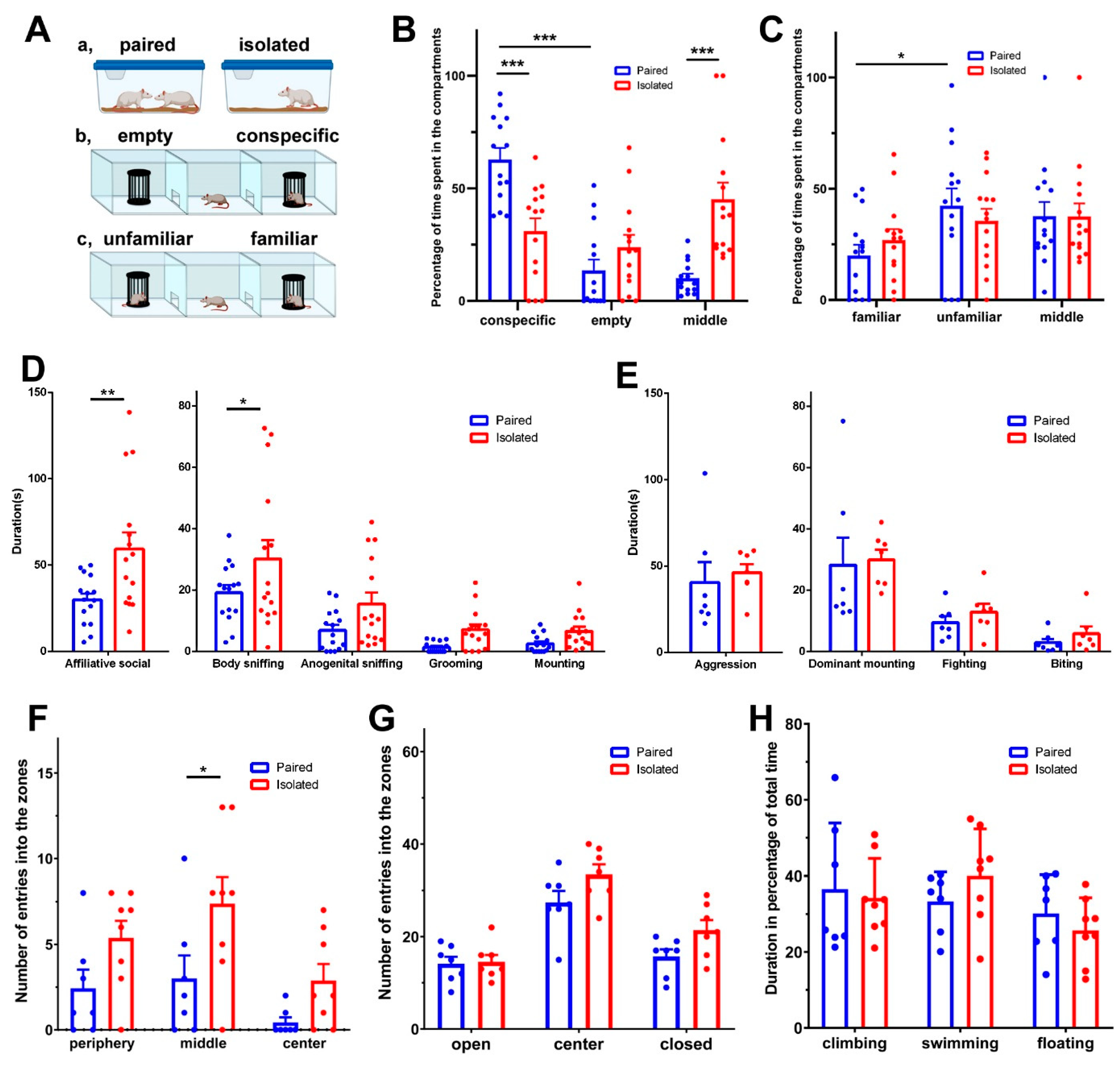
3.1. Behavioural Effects of Isolation
3.1.1. Sociability
3.1.2. Social Preference
3.1.3. Affiliative Interactions
3.1.4. Aggressive Behaviour
3.1.5. Locomotor Activity
3.1.6. Anxiety-like Behaviour
3.1.7. Depression-like Behaviour
3.2. Altered Gene Expression in the mPFC Due to Social Isolation
3.2.1. RNA-Seq
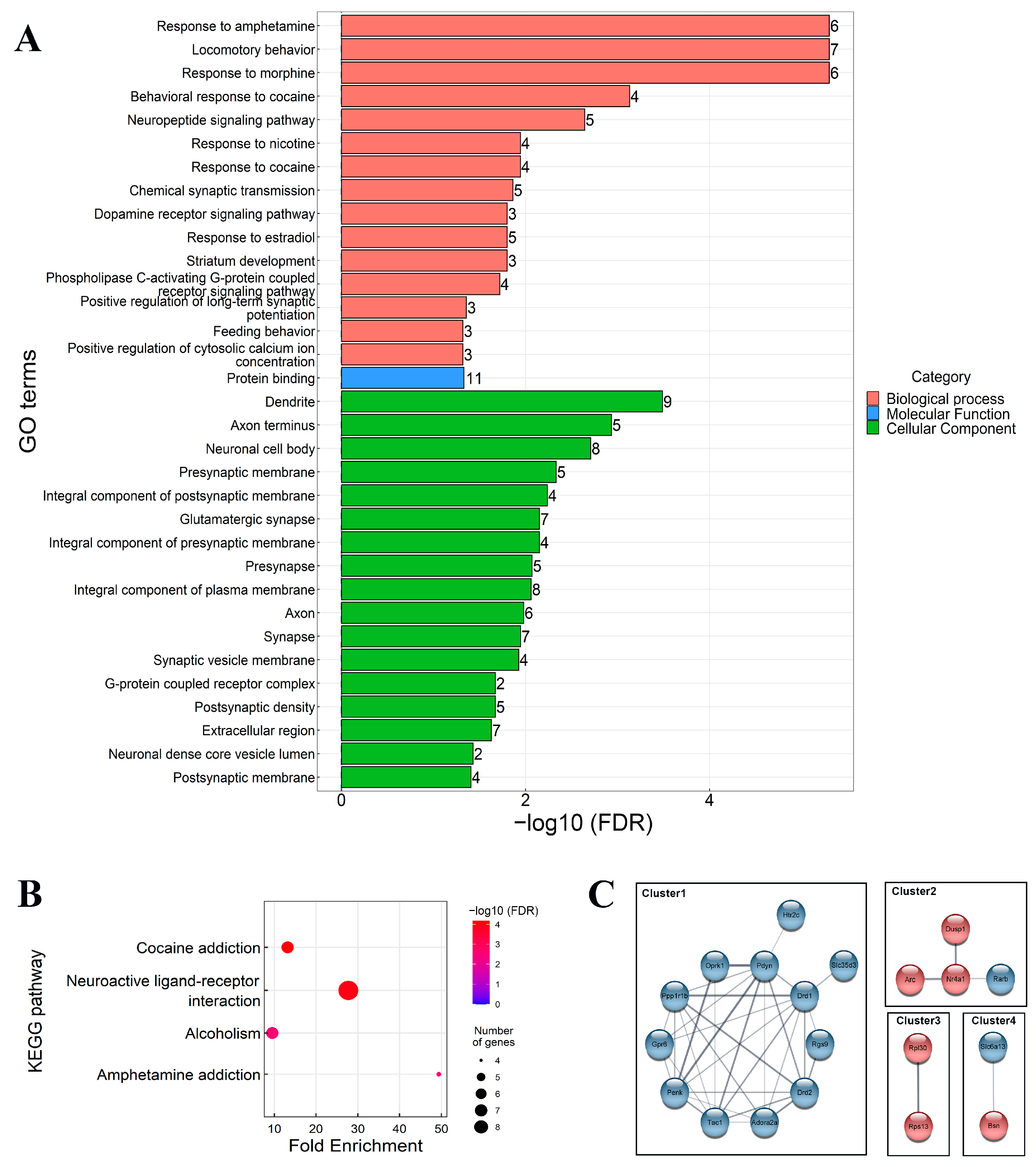
3.2.2. Bioinformatics Analysis of DEGs
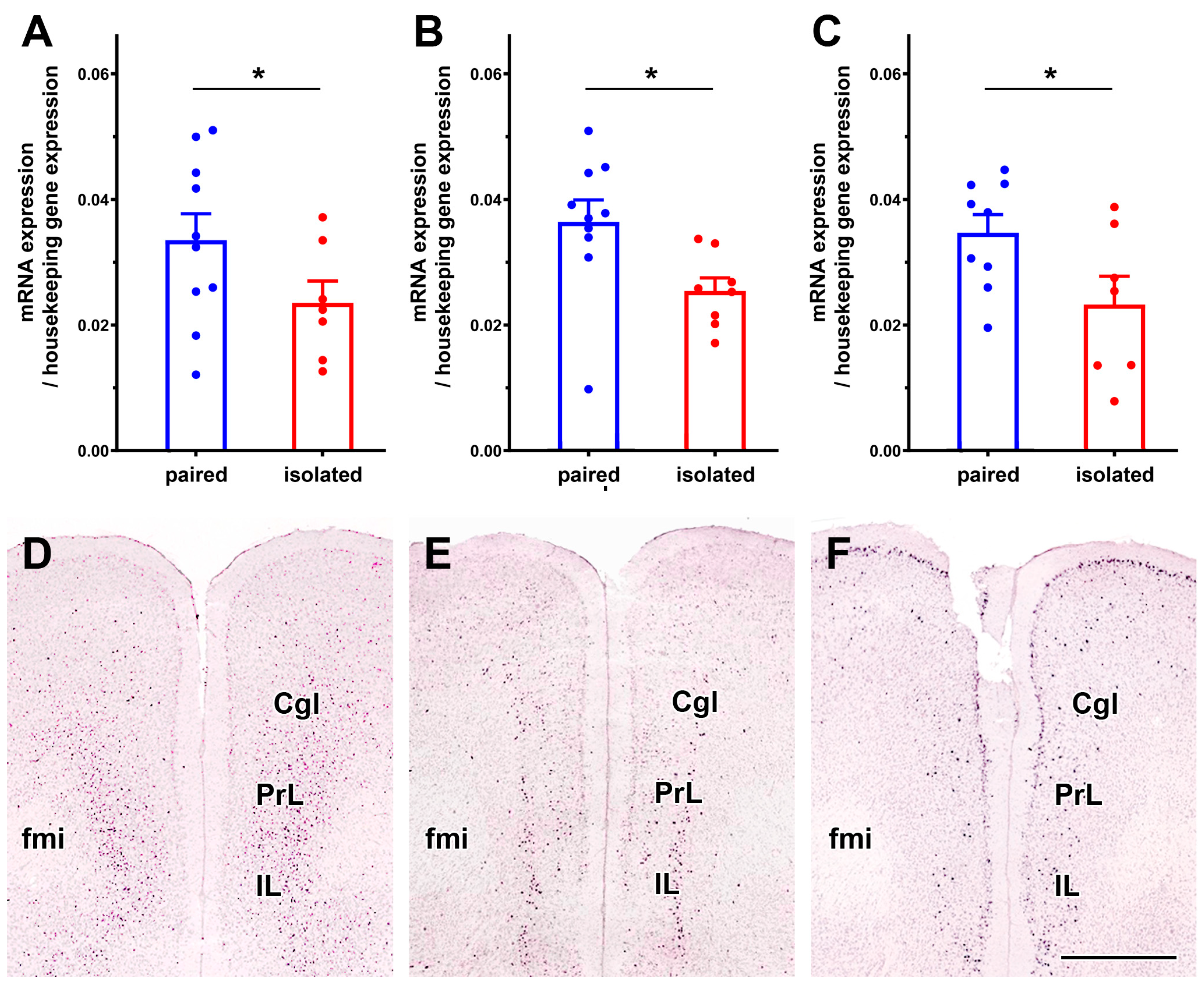
3.2.3. Validation of Selected Genes
3.3. Behavioural Analysis after Htr2c Antagonist Treatment
3.3.1. Alterations in Social Behaviours
3.3.2. Locomotion, Anxiety- and Depression-like Behaviour after Htr2c Antagonist Treatment
4. Discussion
4.1. Behavioural Effects of Social Isolation
4.2. Social Isolation-Induced Gene Expressional Alterations
4.3. The Role of Serotonin 2C Receptor in Social Novelty Discrimination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karelina, K.; DeVries, A.C. Modeling social influences on human health. Psychosom. Med. 2011, 73, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Alberts, S.C. Social influences on survival and reproduction: Insights from a long-term study of wild baboons. J. Anim. Ecol. 2019, 88, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. The surprising empathic abilities of rodents. Trends Cogn. Sci. 2012, 16, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Akyazi, I.; Eraslan, E. Transmission of stress between cagemates: A study in rats. Physiol. Behav. 2014, 123, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, F.; Khan, M.I.; Zubair, M.; Dehpour, A.R. Neurobiology and consequences of social isolation stress in animal model-A comprehensive review. Biomed. Pharmacother. 2018, 105, 1205–1222. [Google Scholar] [CrossRef] [PubMed]
- Jabarin, R.; Netser, S.; Wagner, S. Beyond the three-chamber test: Toward a multimodal and objective assessment of social behavior in rodents. Mol. Autism. 2022, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Schweinfurth, M.K. The social life of Norway rats (Rattus norvegicus). eLife 2020, 9, e54020. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami Bartal, I.; Rodgers, D.A.; Bernardez Sarria, M.S.; Decety, J.; Mason, P. Pro-social behavior in rats is modulated by social experience. Elife 2014, 3, e01385. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Z.; Wang, B.; Wang, Y.; Ding, W. Activation of D1R signaling in the medial prefrontal cortex rescues maternal separation-induced behavioral deficits through restoration of excitatory neurotransmission. Behav. Brain Res. 2023, 441, 114287. [Google Scholar] [CrossRef]
- Sun, L.; Min, L.; Li, M.; Shao, F. Juvenile social isolation leads to schizophrenia-like behaviors via excess lactate production by astrocytes. Brain Res. Bull. 2021, 174, 240–249. [Google Scholar] [CrossRef]
- Lopez, K.; Baker, M.R.; Toth, M. Single cell transcriptomic representation of social dominance in prefrontal cortex and the influence of preweaning maternal and postweaning social environment. Sci. Rep. 2024, 14, 2206. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.E.M.; Neumann, I.D.; de Jong, T.R. Post-weaning social isolation exacerbates aggression in both sexes and affects the vasopressin and oxytocin system in a sex-specific manner. Neuropharmacology 2019, 156, 107504. [Google Scholar] [CrossRef] [PubMed]
- Biro, L.; Toth, M.; Sipos, E.; Bruzsik, B.; Tulogdi, A.; Bendahan, S.; Sandi, C.; Haller, J. Structural and functional alterations in the prefrontal cortex after post-weaning social isolation: Relationship with species-typical and deviant aggression. Brain Struct. Funct. 2017, 222, 1861–1875. [Google Scholar] [CrossRef] [PubMed]
- Bilecki, W.; Latusz, J.; Gawlinska, K.; Chmelova, M.; Mackowiak, M. Prenatal MAM treatment altered fear conditioning following social isolation: Relevance to schizophrenia. Behav. Brain Res. 2021, 406, 113231. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.M.; Zhou, X.; Cunningham, A.M.; Lipschultz, A.P.; Ramakrishnan, A.; Cates, H.M.; Bagot, R.C.; Shen, L.; Zhang, B.; Nestler, E.J. Sex-Specific Transcriptional Changes in Response to Adolescent Social Stress in the Brain’s Reward Circuitry. Biol. Psychiatry 2022, 91, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Fone, K.C.; Porkess, M.V. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2008, 32, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- Lukkes, J.L.; Mokin, M.V.; Scholl, J.L.; Forster, G.L. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm. Behav. 2009, 55, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Lukkes, J.L.; Watt, M.J.; Lowry, C.A.; Forster, G.L. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front. Behav. Neurosci. 2009, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.A.; Printz, D.; Ledoux, M.; McEwen, B.S. Isolation stress increases tyrosine hydroxylase mRNA in the locus coeruleus and midbrain and decreases proenkephalin mRNA in the striatum and nucleus accumbens. Brain Res. Mol. Brain Res. 1991, 11, 301–308. [Google Scholar] [CrossRef]
- Vitale, E.M.; Smith, A.S. Neurobiology of Loneliness, Isolation, and Loss: Integrating Human and Animal Perspectives. Front. Behav. Neurosci. 2022, 16, 846315. [Google Scholar] [CrossRef]
- Filipovic, D.; Novak, B.; Xiao, J.; Yan, Y.; Yeoh, K.; Turck, C.W. Chronic Fluoxetine Treatment of Socially Isolated Rats Modulates Prefrontal Cortex Proteome. Neuroscience 2022, 501, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, J.R.; Fatma, M.; Loh, Y.E.; Traore, S.R.; Stefan, T.; Chen, T.H.; Nestler, E.J.; Labonte, B. Shared Transcriptional Signatures in Major Depressive Disorder and Mouse Chronic Stress Models. Biol Psychiatry 2020, 88, 159–168. [Google Scholar] [CrossRef]
- Wallace, D.L.; Han, M.H.; Graham, D.L.; Green, T.A.; Vialou, V.; Iniguez, S.D.; Cao, J.L.; Kirk, A.; Chakravarty, S.; Kumar, A.; et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat. Neurosci. 2009, 12, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, L.; Mastorci, F.; Graiani, G.; Razzoli, M.; Trombini, M.; Pico-Alfonso, M.A.; Arban, R.; Grippo, A.J.; Quaini, F.; Sgoifo, A. Social defeat and isolation induce clear signs of a depression-like state, but modest cardiac alterations in wild-type rats. Physiol. Behav. 2012, 106, 142–150. [Google Scholar] [CrossRef]
- Guarnera, J.; Yuen, E.; Macpherson, H. The Impact of Loneliness and Social Isolation on Cognitive Aging: A Narrative Review. J. Alzheimers Dis. Rep. 2023, 7, 699–714. [Google Scholar] [CrossRef]
- Song, M.K.; Lee, J.H.; Kim, Y.J. Effect of chronic handling and social isolation on emotion and cognition in adolescent rats. Physiol. Behav. 2021, 237, 113440. [Google Scholar] [CrossRef]
- Krupina, N.A.; Shirenova, S.D.; Khlebnikova, N.N. Prolonged Social Isolation, Started Early in Life, Impairs Cognitive Abilities in Rats Depending on Sex. Brain Sci. 2020, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Duerler, P.; Vollenweider, F.X.; Preller, K.H. A neurobiological perspective on social influence: Serotonin and social adaptation. J. Neurochem. 2022, 162, 60–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Chen, A.; Li, Y.; Xing, X.; Lu, H. Medial prefrontal cortex in neurological diseases. Physiol. Genom. 2019, 51, 432–442. [Google Scholar] [CrossRef]
- Park, G.; Ryu, C.; Kim, S.; Jeong, S.J.; Koo, J.W.; Lee, Y.S.; Kim, S.J. Social isolation impairs the prefrontal-nucleus accumbens circuit subserving social recognition in mice. Cell. Rep. 2021, 35, 109104. [Google Scholar] [CrossRef]
- Biro, L.; Miskolczi, C.; Szebik, H.; Bruzsik, B.; Varga, Z.K.; Szente, L.; Toth, M.; Halasz, J.; Mikics, E. Post-weaning social isolation in male mice leads to abnormal aggression and disrupted network organization in the prefrontal cortex: Contribution of parvalbumin interneurons with or without perineuronal nets. Neurobiol. Stress 2023, 25, 100546. [Google Scholar] [CrossRef] [PubMed]
- Weiss, I.C.; Pryce, C.R.; Jongen-Relo, A.L.; Nanz-Bahr, N.I.; Feldon, J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav. Brain Res. 2004, 152, 279–295. [Google Scholar] [CrossRef]
- Bittar, T.P.; Labonte, B. Functional Contribution of the Medial Prefrontal Circuitry in Major Depressive Disorder and Stress-Induced Depressive-Like Behaviors. Front. Behav. Neurosci. 2021, 15, 699592. [Google Scholar] [CrossRef]
- Begni, V.; Sanson, A.; Pfeiffer, N.; Brandwein, C.; Inta, D.; Talbot, S.R.; Riva, M.A.; Gass, P.; Mallien, A.S. Social isolation in rats: Effects on animal welfare and molecular markers for neuroplasticity. PLoS ONE 2020, 15, e0240439. [Google Scholar] [CrossRef] [PubMed]
- Chocyk, A.; Bobula, B.; Dudys, D.; Przyborowska, A.; Majcher-Maslanka, I.; Hess, G.; Wedzony, K. Early-life stress affects the structural and functional plasticity of the medial prefrontal cortex in adolescent rats. Eur. J. Neurosci. 2013, 38, 2089–2107. [Google Scholar] [CrossRef]
- Hall, F.S. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit. Rev. Neurobiol. 1998, 12, 129–162. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H. Ethological approach to social isolation effects in behavioral studies of laboratory rodents. Behav. Brain Res. 2018, 341, 98–108. [Google Scholar] [CrossRef]
- Liu, D.; Rahman, M.; Johnson, A.; Tsutsui-Kimura, I.; Pena, N.; Talay, M.; Logeman, B.L.; Finkbeiner, S.; Choi, S.; Capo-Battaglia, A.; et al. A Hypothalamic Circuit Underlying the Dynamic Control of Social Homeostasis. bioRxiv 2023, preprint. [Google Scholar] [CrossRef]
- Lee, C.R.; Chen, A.; Tye, K.M. The neural circuitry of social homeostasis: Consequences of acute versus chronic social isolation. Cell 2021, 184, 2794–2795. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Cacioppo, S.; Capitanio, J.P.; Cole, S.W. The neuroendocrinology of social isolation. Annu. Rev. Psychol. 2015, 66, 733–767. [Google Scholar] [CrossRef]
- Gunaydin, L.A.; Grosenick, L.; Finkelstein, J.C.; Kauvar, I.V.; Fenno, L.E.; Adhikari, A.; Lammel, S.; Mirzabekov, J.J.; Airan, R.D.; Zalocusky, K.A.; et al. Natural neural projection dynamics underlying social behavior. Cell 2014, 157, 1535–1551. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.N.; Wagner, S. The role of the prefrontal cortex in social interactions of animal models and the implications for autism spectrum disorder. Front. Psychiatry 2023, 14, 1205199. [Google Scholar] [CrossRef] [PubMed]
- Atmore, K.H.; Stein, D.J.; Harvey, B.H.; Russell, V.A.; Howells, F.M. Differential effects of social isolation rearing on glutamate- and GABA-stimulated noradrenaline release in the rat prefrontal cortex and hippocampus. Eur. Neuropsychopharmacol. 2020, 36, 111–120. [Google Scholar] [CrossRef] [PubMed]
- van Kerkhof, L.W.; Damsteegt, R.; Trezza, V.; Voorn, P.; Vanderschuren, L.J. Social play behavior in adolescent rats is mediated by functional activity in medial prefrontal cortex and striatum. Neuropsychopharmacology 2013, 38, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Gandy, H.M.; Hollis, F.; Hernandez, C.M.; McQuail, J.A. Aging or chronic stress impairs working memory and modulates GABA and glutamate gene expression in prelimbic cortex. Front. Aging Neurosci. 2023, 15, 1306496. [Google Scholar] [CrossRef]
- Vanderschuren, L.J.; Achterberg, E.J.; Trezza, V. The neurobiology of social play and its rewarding value in rats. Neurosci. Biobehav. Rev. 2016, 70, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, J.T.; Hawkley, L.C.; Norman, G.J.; Berntson, G.G. Social isolation. Ann. N. Y. Acad. Sci. 2011, 1231, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Usui, N.; Ono, Y.; Aramaki, R.; Berto, S.; Konopka, G.; Matsuzaki, H.; Shimada, S. Early Life Stress Alters Gene Expression and Cytoarchitecture in the Prefrontal Cortex Leading to Social Impairment and Increased Anxiety. Front. Genet. 2021, 12, 754198. [Google Scholar] [CrossRef]
- Murphy, K.J.; Ter Horst, J.P.; Cassidy, A.W.; DeSouza, I.E.; Morgunova, M.; Li, C.; Connole, L.M.; O’Sullivan, N.C.; Loscher, J.S.; Brady, A.T.; et al. Temporal dysregulation of cortical gene expression in the isolation reared Wistar rat. J. Neurochem. 2010, 113, 601–614. [Google Scholar] [CrossRef]
- Filipovic, D.; Novak, B.; Xiao, J.; Yan, Y.; Bernardi, R.E.; Turck, C.W. Chronic fluoxetine treatment in socially-isolated rats modulates the prefrontal cortex synaptoproteome. J. Proteomics 2023, 282, 104925. [Google Scholar] [CrossRef]
- George, J.M.; Bell, Z.W.; Condliffe, D.; Dohrer, K.; Abaurrea, T.; Spencer, K.; Leitao, A.; Gahr, M.; Hurd, P.J.; Clayton, D.F. Acute social isolation alters neurogenomic state in songbird forebrain. Proc. Natl. Acad. Sci. USA 2020, 117, 23311–23316. [Google Scholar] [CrossRef] [PubMed]
- Lavenda-Grosberg, D.; Lalzar, M.; Leser, N.; Yaseen, A.; Malik, A.; Maroun, M.; Barki-Harrington, L.; Wagner, S. Acute social isolation and regrouping cause short- and long-term molecular changes in the rat medial amygdala. Mol. Psychiatry 2022, 27, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Guardiola-Lemaitre, B.; De Bodinat, C.; Delagrange, P.; Millan, M.J.; Munoz, C.; Mocaer, E. Agomelatine: Mechanism of action and pharmacological profile in relation to antidepressant properties. Br. J. Pharmacol. 2014, 171, 3604–3619. [Google Scholar] [CrossRef] [PubMed]
- Péter, A. Solomon Coder: A Simple Solution for Behavior Coding. v 16.06.26. 2015. 2016. Available online: http://solomoncoder.com (accessed on 22 March 2022).
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 2007. [Google Scholar]
- Rein, B.; Ma, K.; Yan, Z. A standardized social preference protocol for measuring social deficits in mouse models of autism. Nat. Protoc. 2020, 15, 3464–3477. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Leko, A.H.; Cservenak, M.; Szabo, E.R.; Hanics, J.; Alpar, A.; Dobolyi, A. Insulin-like growth factor 1 and its binding protein-3 are regulators of lactation and maternal responsiveness. Sci. Rep. 2017, 7, 3396. [Google Scholar] [CrossRef]
- Csikos, V.; Olah, S.; Dora, F.; Arrasz, N.; Cservenak, M.; Dobolyi, A. Microglia depletion prevents lactation by inhibition of prolactin secretion. iScience 2023, 26, 106264. [Google Scholar] [CrossRef] [PubMed]
- Lein, E.S.; Hawrylycz, M.J.; Ao, N.; Ayres, M.; Bensinger, A.; Bernard, A.; Boe, A.F.; Boguski, M.S.; Brockway, K.S.; Byrnes, E.J.; et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007, 445, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Neuwirth, L.S.; Verrengia, M.T.; Harikinish-Murrary, Z.I.; Orens, J.E.; Lopez, O.E. Under or Absent Reporting of Light Stimuli in Testing of Anxiety-Like Behaviors in Rodents: The Need for Standardization. Front. Mol. Neurosci. 2022, 15, 912146. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Osako, Y.; Takahashi, K.; Hidaka, C.; Tomita, K.; Yuri, K. Effects of post-weaning social isolation on social behaviors and oxytocinergic activity in male and female rats. Heliyon 2019, 5, e01646. [Google Scholar] [CrossRef] [PubMed]
- Kercmar, J.; Budefeld, T.; Grgurevic, N.; Tobet, S.A.; Majdic, G. Adolescent social isolation changes social recognition in adult mice. Behav. Brain Res. 2011, 216, 647–651. [Google Scholar] [CrossRef]
- Shahar-Gold, H.; Gur, R.; Wagner, S. Rapid and reversible impairments of short- and long-term social recognition memory are caused by acute isolation of adult rats via distinct mechanisms. PLoS ONE 2013, 8, e65085. [Google Scholar] [CrossRef] [PubMed]
- Leser, N.; Wagner, S. The effects of acute social isolation on long-term social recognition memory. Neurobiol. Learn. Mem. 2015, 124, 97–103. [Google Scholar] [CrossRef]
- Fukumitsu, K.; Kaneko, M.; Maruyama, T.; Yoshihara, C.; Huang, A.J.; McHugh, T.J.; Itohara, S.; Tanaka, M.; Kuroda, K.O. Amylin-Calcitonin receptor signaling in the medial preoptic area mediates affiliative social behaviors in female mice. Nat. Commun. 2022, 13, 709. [Google Scholar] [CrossRef] [PubMed]
- Niesink, R.J.; van Ree, J.M. Short-term isolation increases social interactions of male rats: A parametric analysis. Physiol. Behav. 1982, 29, 819–825. [Google Scholar] [CrossRef]
- Matthews, G.A.; Tye, K.M. Neural mechanisms of social homeostasis. Ann. N. Y. Acad. Sci. 2019, 1457, 5–25. [Google Scholar] [CrossRef]
- Ma, Y.K.; Zeng, P.Y.; Chu, Y.H.; Lee, C.L.; Cheng, C.C.; Chen, C.H.; Su, Y.S.; Lin, K.T.; Kuo, T.H. Lack of social touch alters anxiety-like and social behaviors in male mice. Stress 2022, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.L.; Ferreira, V.M.; Carobrez Ade, P.; Morato, G.S. Individual housing from rearing modifies the performance of young rats on the elevated plus-maze apparatus. Physiol. Behav. 1996, 60, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H.; Stinus, L.; Le Moal, M.; Cador, M. Social deprivation enhances the vulnerability of male Wistar rats to stressor- and amphetamine-induced behavioral sensitization. Psychopharmacology 1995, 117, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Voikar, V.; Polus, A.; Vasar, E.; Rauvala, H. Long-term individual housing in C57BL/6J and DBA/2 mice: Assessment of behavioral consequences. Genes Brain Behav. 2005, 4, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Bourke, C.H.; Neigh, G.N. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm. Behav. 2011, 60, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Beery, A.K.; Kaufer, D. Stress, social behavior, and resilience: Insights from rodents. Neurobiol. Stress 2015, 1, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.B.; Silva, B.A.; Perova, Z.; Marrone, L.; Masferrer, M.E.; Zhan, Y.; Kaplan, A.; Greetham, L.; Verrechia, V.; Halman, A.; et al. Prefrontal cortical control of a brainstem social behavior circuit. Nat. Neurosci. 2017, 20, 260–270. [Google Scholar] [CrossRef]
- Grossmann, T. The role of medial prefrontal cortex in early social cognition. Front. Hum. Neurosci. 2013, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Marta Perez-Rando, R.G. The Medial Prefrontal Cortex (mPFC) and Addictions; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Medendorp, W.E.; Petersen, E.D.; Pal, A.; Wagner, L.M.; Myers, A.R.; Hochgeschwender, U.; Jenrow, K.A. Altered Behavior in Mice Socially Isolated During Adolescence Corresponds with Immature Dendritic Spine Morphology and Impaired Plasticity in the Prefrontal Cortex. Front. Behav. Neurosci. 2018, 12, 87. [Google Scholar] [CrossRef]
- Hosseinbor, M.; Yassini Ardekani, S.M.; Bakhshani, S.; Bakhshani, S. Emotional and social loneliness in individuals with and without substance dependence disorder. Int. J. High Risk Behav. Addict. 2014, 3, e22688. [Google Scholar] [CrossRef]
- Brandt, L.; Liu, S.; Heim, C.; Heinz, A. The effects of social isolation stress and discrimination on mental health. Transl. Psychiatry 2022, 12, 398. [Google Scholar] [CrossRef] [PubMed]
- Euston, D.R.; Gruber, A.J.; McNaughton, B.L. The role of medial prefrontal cortex in memory and decision making. Neuron 2012, 76, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Santana, N.; Artigas, F. Laminar and Cellular Distribution of Monoamine Receptors in Rat Medial Prefrontal Cortex. Front. Neuroanat. 2017, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Capuzzo, G.; Floresco, S.B. Prelimbic and Infralimbic Prefrontal Regulation of Active and Inhibitory Avoidance and Reward-Seeking. J. Neurosci. 2020, 40, 4773–4787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, K.; Bao, J.; Wu, H. Oxytocin enhances the triangular association among behavior, resting-state, and task-state functional connectivity. Hum. Brain Mapp. 2023, 44, 6074–6089. [Google Scholar] [CrossRef] [PubMed]
- de Leon Reyes, N.S.; Sierra Diaz, P.; Nogueira, R.; Ruiz-Pino, A.; Nomura, Y.; de Solis, C.A.; Schulkin, J.; Asok, A.; Leroy, F. Corticotropin-releasing hormone signaling from prefrontal cortex to lateral septum suppresses interaction with familiar mice. Cell 2023, 186, 4152–4171.e31. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Fisher, R.A. Introduction: G Protein-coupled Receptors and RGS Proteins. Prog. Mol. Biol. Transl. Sci. 2015, 133, 1–11. [Google Scholar]
- Senese, N.B.; Kandasamy, R.; Kochan, K.E.; Traynor, J.R. Regulator of G-Protein Signaling (RGS) Protein Modulation of Opioid Receptor Signaling as a Potential Target for Pain Management. Front. Mol. Neurosci. 2020, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Ahlers-Dannen, K.E.; Spicer, M.M.; Fisher, R.A. RGS Proteins as Critical Regulators of Motor Function and Their Implications in Parkinson’s Disease. Mol. Pharmacol. 2020, 98, 730–738. [Google Scholar] [CrossRef]
- Martemyanov, K.A.; Arshavsky, V.Y. Biology and functions of the RGS9 isoforms. Prog. Mol. Biol. Transl. Sci. 2009, 86, 205–227. [Google Scholar]
- Rivero, G.; Gabilondo, A.M.; Garcia-Fuster, M.J.; La Harpe, R.; Garcia-Sevilla, J.A.; Meana, J.J. Differential regulation of RGS proteins in the prefrontal cortex of short- and long-term human opiate abusers. Neuropharmacology 2012, 62, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; van Velthoven, C.T.J.; Nguyen, T.N.; Goldy, J.; Sedeno-Cortes, A.E.; Baftizadeh, F.; Bertagnolli, D.; Casper, T.; Chiang, M.; Crichton, K.; et al. A taxonomy of transcriptomic cell types across the isocortex and hippocampal formation. Cell 2021, 184, 3222–3241.e3226. [Google Scholar] [CrossRef]
- Navandar, M.; Martin-Garcia, E.; Maldonado, R.; Lutz, B.; Gerber, S.; Ruiz de Azua, I. Transcriptional signatures in prefrontal cortex confer vulnerability versus resilience to food and cocaine addiction-like behavior. Sci. Rep. 2021, 11, 9076. [Google Scholar] [CrossRef]
- Graham, C.A.; Spedicati, B.; Pelliccione, G.; Gasparini, P.; Concas, M.P. Regulator of G-Protein Signalling 9: A New Candidate Gene for Sweet Food Liking? Foods 2023, 12, 1739. [Google Scholar] [CrossRef]
- Schwarzer, C. 30 years of dynorphins—New insights on their functions in neuropsychiatric diseases. Pharmacol. Ther. 2009, 123, 353–370. [Google Scholar] [CrossRef]
- Bodnar, R.J. Endogenous opiates and behavior: 2021. Peptides 2023, 164, 171004. [Google Scholar] [CrossRef]
- Casello, S.M.; Flores, R.J.; Yarur, H.E.; Wang, H.; Awanyai, M.; Arenivar, M.A.; Jaime-Lara, R.B.; Bravo-Rivera, H.; Tejeda, H.A. Neuropeptide System Regulation of Prefrontal Cortex Circuitry: Implications for Neuropsychiatric Disorders. Front. Neural. Circuits 2022, 16, 796443. [Google Scholar] [CrossRef] [PubMed]
- Tejeda, H.A.; Wang, H.; Flores, R.J.; Yarur, H.E. Dynorphin/Kappa-Opioid Receptor System Modulation of Cortical Circuitry. Handb. Exp. Pharmacol. 2022, 271, 223–253. [Google Scholar] [PubMed]
- Zhou, S.; Yin, Y.; Sheets, P.L. Mouse models of surgical and neuropathic pain produce distinct functional alterations to prodynorphin expressing neurons in the prelimbic cortex. Neurobiol. Pain 2023, 13, 100121. [Google Scholar] [CrossRef] [PubMed]
- Yarur, H.E.; Casello, S.M.; Tsai, V.S.; Enriquez-Traba, J.; Kore, R.; Wang, H.; Arenivar, M.; Tejeda, H.A. Dynorphin/kappa-opioid receptor regulation of excitation-inhibition balance toggles afferent control of prefrontal cortical circuits in a pathway-specific manner. Mol. Psychiatry 2023, 28, 4801–4813. [Google Scholar] [CrossRef]
- Wang, H.; Flores, R.J.; Yarur, H.E.; Limoges, A.; Bravo-Rivera, H.; Casello, S.M.; Loomba, N.; Enriquez-Traba, J.; Arenivar, M.; Wang, Q.; et al. Prefrontal cortical dynorphin peptidergic transmission constrains threat-driven behavioral and network states. bioRxiv 2024, preprint. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.D.; Casello, S.M.; Schattauer, S.S.; Wong, B.A.; Mizuno, G.O.; Mahe, K.; Tian, L.; Land, B.B.; Chavkin, C. Release of endogenous dynorphin opioids in the prefrontal cortex disrupts cognition. Neuropsychopharmacology 2021, 46, 2330–2339. [Google Scholar] [CrossRef] [PubMed]
- Varlinskaya, E.I.; Spear, L.P.; Diaz, M.R. Stress alters social behavior and sensitivity to pharmacological activation of kappa opioid receptors in an age-specific manner in Sprague Dawley rats. Neurobiol. Stress 2018, 9, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Xu, H.; Xue, Y.; An, D.; Li, H.; Chen, W.; Yu, D.; Sun, Y.; Ma, J.; Tang, Y.; et al. 5-HT2CR antagonist/5-HT2CR inverse agonist recovered the increased isolation-induced aggressive behavior of BALB/c mice mediated by ADAR1 (p110) expression and Htr2c RNA editing. Brain Behav. 2018, 8, e00929. [Google Scholar] [CrossRef]
- Popova, N.K.; Tsybko, A.S.; Naumenko, V.S. The Implication of 5-HT Receptor Family Members in Aggression, Depression and Suicide: Similarity and Difference. Int. J. Mol. Sci. 2022, 23, 8814. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Oh, C.M.; Kim, H. Serotonin in the regulation of systemic energy metabolism. J. Diabetes Investig. 2022, 13, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Albert, P.R.; Benkelfat, C.; Descarries, L. The neurobiology of depression--revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2378–2381. [Google Scholar] [CrossRef] [PubMed]
- Nic Dhonnchadha, B.A.; Bourin, M.; Hascoet, M. Anxiolytic-like effects of 5-HT2 ligands on three mouse models of anxiety. Behav. Brain Res. 2003, 140, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Quesseveur, G.; Nguyen, H.T.; Gardier, A.M.; Guiard, B.P. 5-HT2 ligands in the treatment of anxiety and depression. Expert. Opin. Investig. Drugs 2012, 21, 1701–1725. [Google Scholar] [CrossRef]
- Lanfranco, M.F.; Anastasio, N.C.; Seitz, P.K.; Cunningham, K.A. Quantification of RNA editing of the serotonin 2C receptor (5-HT((2)C)R) ex vivo. Methods Enzymol. 2010, 485, 311–328. [Google Scholar]
- Frazer, A.; Hensler, J.G. Serotonin Receptors. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects; Siegel, G.J., Agranoff, B.W., Albers, R.W., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1999. [Google Scholar]
- Serretti, A.; Artioli, P.; De Ronchi, D. The 5-HT2C receptor as a target for mood disorders. Expert. Opin. Ther. Targets 2004, 8, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Merritt, C.R.; Smith, A.E.; Khanipov, K.; Golovko, G.; Dineley, K.T.; Anastasio, N.C.; Cunningham, K.A. Heightened cocaine-seeking in male rats associates with a distinct transcriptomic profile in the medial prefrontal cortex. Front. Pharmacol. 2022, 13, 1022863. [Google Scholar] [CrossRef] [PubMed]
- Barbon, A.; Orlandi, C.; La Via, L.; Caracciolo, L.; Tardito, D.; Musazzi, L.; Mallei, A.; Gennarelli, M.; Racagni, G.; Popoli, M.; et al. Antidepressant treatments change 5-HT2C receptor mRNA expression in rat prefrontal/frontal cortex and hippocampus. Neuropsychobiology 2011, 63, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Castensson, A.; Aberg, K.; McCarthy, S.; Saetre, P.; Andersson, B.; Jazin, E. Serotonin receptor 2C (HTR2C) and schizophrenia: Examination of possible medication and genetic influences on expression levels. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 134B, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M.; Mocaer, E.; Porsolt, R. Antidepressant-like activity of S 20098 (agomelatine) in the forced swimming test in rodents: Involvement of melatonin and serotonin receptors. J. Psychiatry Neurosci. 2004, 29, 126–133. [Google Scholar] [PubMed]
- Fornaro, M.; Prestia, D.; Colicchio, S.; Perugi, G. A systematic, updated review on the antidepressant agomelatine focusing on its melatonergic modulation. Curr. Neuropharmacol. 2010, 8, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Zakaria, R.; Othman, Z.; Lauterbach, E.C.; Acuna-Castroviejo, D. Agomelatine in depressive disorders: Its novel mechanisms of action. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 290–308. [Google Scholar] [CrossRef]
- He, Y.; Brouwers, B.; Liu, H.; Liu, H.; Lawler, K.; Mendes de Oliveira, E.; Lee, D.K.; Yang, Y.; Cox, A.R.; Keogh, J.M.; et al. Human loss-of-function variants in the serotonin 2C receptor associated with obesity and maladaptive behavior. Nat. Med. 2022, 28, 2537–2546. [Google Scholar] [CrossRef]

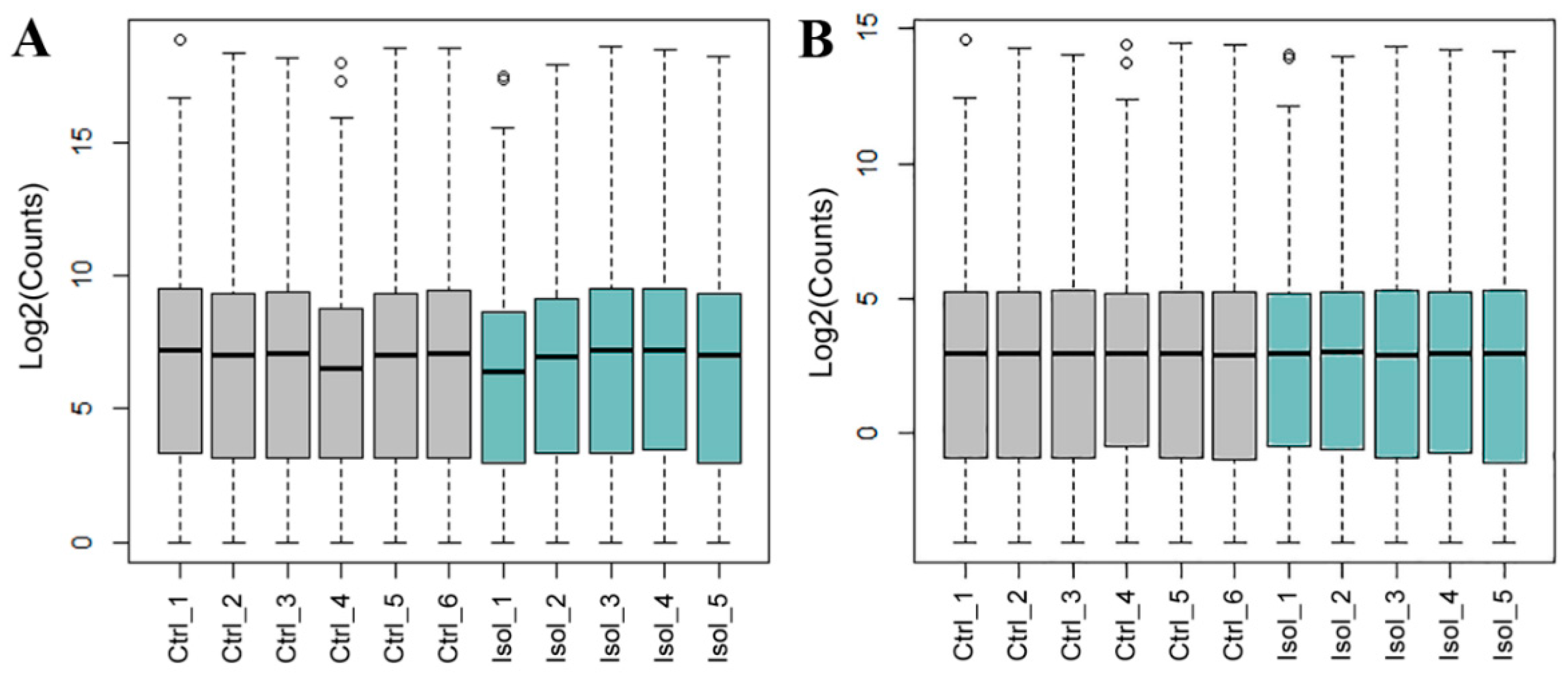


| Sample | RNA Integrity Number (RIN) | Total Clean Reads (M) | Total Mapping Ratio (%) | Uniquely Mapping Ratio (%) | Total Gene Mapping Ratio (%) |
|---|---|---|---|---|---|
| Ctrl_1 | 9.4 | 41.8 | 96.7 | 93.4 | 48.3 |
| Ctrl_2 | 9.5 | 39.3 | 96.2 | 86.9 | 45.3 |
| Ctrl_3 | 9.6 | 34.4 | 97.7 | 93.5 | 51.8 |
| Ctrl_4 | 8.3 | 33.9 | 96.6 | 92.6 | 34.1 |
| Ctrl_5 | 10 | 35.6 | 97.4 | 92.4 | 49.3 |
| Ctrl_6 | 10 | 36.7 | 97.4 | 92.7 | 51.5 |
| Isol_1 | 8.3 | 32.2 | 96.6 | 92.8 | 33.7 |
| Isol_2 | 8.9 | 32.7 | 97.4 | 93.6 | 44.5 |
| Isol_3 | 9.3 | 39.2 | 97.5 | 93.3 | 51.9 |
| Isol_4 | 9.6 | 37.7 | 96.4 | 92.0 | 50.4 |
| Isol_5 | 9.7 | 31.3 | 97.7 | 93.0 | 56.5 |
| Gene_ID | Gene | Protein Name | log2 of Fold Change | p-Value | q-Value | Biological Function | Regulation |
|---|---|---|---|---|---|---|---|
| MSTRG.19509, ENSRNOG00000011111 | Cipc | CLOCK-interacting pacemaker | −6.47 | 5.0 × 10−5 | 0.02 | exhibits circadian regulation in multiple tissues | down |
| MSTRG.700 | N/A | N/A | −4.38 | 5.0 × 10−5 | 0.02 | N/A | down |
| MSTRG.21449, ENSRNOG00000054065 | N/A | N/A | −3.24 | 5.0 × 10−5 | 0.02 | N/A | down |
| MSTRG.13085, ENSRNOG00000009577 | Ndst4 | N-deacetylase and N-sulfotransferase 4 | −2.16 | 5.0 × 10−5 | 0.02 | catalyzes the transfer of a sulfate group from 3′-phosphoadenosine 5′-phosphosulfate to the hydroxyl group of an acceptor | down |
| MSTRG.13636, ENSRNOG00000001302 | Adora2a | adenosine A2a receptor | −1.81 | 5.0 × 10−5 | 0.02 | receptor for adenosine | down |
| MSTRG.106, ENSRNOG00000012311 | Slc35d3 | solute carrier family 35, member D3 | −1.78 | 5.0 × 10−5 | 0.02 | carbohydrate transport and pyrimidine nucleotide-sugar transmembrane transport | down |
| MSTRG.4488, ENSRNOG00000003800 | Rgs9 | regulator of G-protein signalling 9 | −1.65 | 5.0 × 10−5 | 0.02 | inhibits signal transduction b | down |
| MSTRG.19469, ENSRNOG00000027645 | Syndig1l | synapse differentiation-inducing 1-like | −1.61 | 5.0 × 10−5 | 0.02 | predicted to be an integral component of the membrane | down |
| MSTRG.13873, ENSRNOG00000049580 | Gpr6 | G protein-coupled receptor 6 | −1.43 | 5.0 × 10−5 | 0.02 | activate cyclic AMP, promotes neurite outgrowth and blocks myelin inhibition in neurons | down |
| MSTRG.21857, ENSRNOG00000008428 | Drd2 | dopamine receptor D2 | −1.4 | 5.0 × 10−5 | 0.02 | mediated by G proteins which inhibit adenylyl cyclase | down |
| MSTRG.17137, ENSRNOG00000007647 | Oprk1 | opioid receptor, kappa 1 | −1.38 | 5.0 × 10−5 | 0.02 | receptor for endogenous alpha-neoendorphins and dynorphins | down |
| MSTRG.2540, ENSRNOG00000030180 | Lrrc10b | leucine-rich repeat containing 10B | −1.33 | 5.0 × 10−5 | 0.02 | required for endogenous cardiac regeneration | down |
| MSTRG.17163, ENSRNOG00000008943 | Penk | proenkephalin | −1.27 | 5.0 × 10−5 | 0.02 | increases glutamate release and decreases GABA concentration | down |
| MSTRG.21754, ENSRNOG00000035548 | Mir3596a | microRNA 3596a | −1.27 | 5.0 × 10−5 | 0.02 | N/A | down |
| MSTRG.19142, ENSRNOG00000005286 | Coch | cochlin | −1.11 | 5.0 × 10−5 | 0.02 | plays a role in the control of cell shape and motility in the trabecular meshwork | down |
| MSTRG.21176, ENSRNOG00000041492 | Mir1249 | microRNA 1249 | −1.11 | 5.0 × 10−5 | 0.02 | regulatory role in inflammation, tumor progression, and cell differentiation | down |
| MSTRG.16758, ENSRNOG00000012876 | Slc6a13 | sodium- and chloride-dependent GABA transporter 2 | −1.08 | 5.0 × 10−5 | 0.02 | mediates sodium- and chloride-dependent transport of GABA | down |
| MSTRG.12399, ENSRNOG00000014793 | Gpr149 | G protein-coupled receptor 149 | −1.07 | 1.0 × 10−4 | 0.04 | chemical synaptic transmission, G-protein coupled receptor signalling pathway, and neuropeptide signalling pathway | down |
| MSTRG.9545, ENSRNOG00000023688 | Drd1 | dopamine receptor D1 | −1.05 | 5.0 × 10−5 | 0.02 | encodes the D1 subtype of the dopamine receptor | down |
| MSTRG.14132 | N/A | N/A | −1.03 | 5.0 × 10−5 | 0.02 | N/A | down |
| MSTRG.23800 | N/A | N/A | −0.92 | 1.0 × 10−4 | 0.04 | N/A | down |
| MSTRG.2495, ENSRNOG00000054315 | Snord27 | small nucleolar RNA, C/D box 27 | −0.91 | 5.0 × 10−5 | 0.02 | conversion of one or more primary RNA transcripts into one or more mature RNA molecules | down |
| MSTRG.7907, ENSRNOG00000024061 | Rarb | retinoic acid receptor, beta | −0.89 | 5.0 × 10−5 | 0.02 | binds retinoic acid, the biologically active form of vitamin A | down |
| MSTRG.8731, ENSRNOG00000013330 | Cdhr1 | cadherin-related family member 1 | −0.89 | 5.0 × 10−5 | 0.02 | it is a photoreceptor-specific cadherin that plays a role in outer segment disc morphogenesis | down |
| MSTRG.16061, ENSRNOG00000011184 | Slc13a4 | sulphate transporter 1 | −0.87 | 5.0 × 10−5 | 0.02 | predicted to enable sodium:sulfate symporter activity and involved in anion transmembrane transport | down |
| MSTRG.13986, ENSRNOG00000015550 | Ptgds | prostaglandin D2 synthase | −0.82 | 5.0 × 10−5 | 0.02 | catalyzes the conversion of PGH2 to PGD2 | down |
| MSTRG.15056, ENSRNOG00000026036 | Pdyn | prodynorphin | −0.81 | 5.0 × 10−5 | 0.02 | Leu-enkephalins compete with and mimics the effects of opiate drugs | down |
| MSTRG.23424, ENSRNOG00000016957 | Igfbp2 | insulin-like growth factor binding protein 2 | −0.77 | 5.0 × 10−5 | 0.02 | inhibits IGF-mediated growth and developmental rates | down |
| MSTRG.15889, ENSRNOG00000007374 | Tac1 | tachykinin, precursor 1 | −0.76 | 5.0 × 10−5 | 0.02 | a neuropeptide, which excites neurons to evoke behavioural responses | down |
| MSTRG.24554, ENSRNOG00000030877 | Htr2c | 5-hydroxytryptamine receptor 2C | −0.75 | 5.0 × 10−5 | 0.02 | G-protein coupled receptor for 5-hydroxytryptamine | down |
| MSTRG.4265, ENSRNOG00000028404 | Ppp1r1b | protein phosphatase 1, regulatory subunit 1B | −0.74 | 5.0 × 10−5 | 0.02 | inhibitor of protein-phosphatase 1 | down |
| MSTRG.11897 | N/A | N/A | −0.71 | 1.0 × 10−4 | 0.04 | N/A | down |
| MSTRG.16148, ENSRNOG00000006228 | Pdia4 | protein disulfide isomerase family A, member 4 | 7.68 | 5.0 × 10−5 | 0.02 | a member of the disulfide isomerase family of endoplasmic reticulum proteins that catalyze protein folding and thiol-disulfide interchange reactions | up |
| MSTRG.14025, ENSRNOG00000028021 | Rps13 | ribosomal protein S13 | 4.11 | 5.0 × 10−5 | 0.02 | component of the small ribosomal subunit | up |
| MSTRG.19735, ENSRNOG00000035600 | Mir154 | microRNA 154 | 3.24 | 5.0 × 10−5 | 0.02 | inhibits cell proliferation, migration, and invasion | up |
| MSTRG.22465, ENSRNOG00000030714 | Bsn | bassoon | 3.04 | 5.0 × 10−5 | 0.02 | involved in organizing the presynaptic cytoskeleton | up |
| MSTRG.3480, ENSRNOG00000032825 | Rpl30 | ribosomal protein L30 | 1.91 | 5.0 × 10−5 | 0.02 | component of the large ribosomal subunit | up |
| MSTRG.10334, ENSRNOG00000056958 | Snora21 | small nucleolar RNA, H/ACA box 21 | 1.89 | 5.0 × 10−5 | 0.02 | plays an important role in cancer progression | up |
| MSTRG.23546 | N/A | N/A | 1.47 | 5.0 × 10−5 | 0.02 | N/A | up |
| MSTRG.9257 | N/A | N/A | 1.21 | 5.0 × 10−5 | 0.02 | N/A | up |
| MSTRG.16897, ENSRNOG00000007830 | Apold1 | apolipoprotein L domain containing 1 | 1.01 | 5.0 × 10−5 | 0.02 | plays a role in the regulation of endothelial cell signalling and vascular function | up |
| MSTRG.20900, ENSRNOG00000043465 | Arc | activity-regulated cytoskeleton-associated protein | 0.87 | 5.0 × 10−5 | 0.02 | master regulator of synaptic plasticity | up |
| MSTRG.21400, ENSRNOG00000007607 | Nr4a1 | nuclear receptor subfamily 4, group A, member 1 | 0.85 | 5.0 × 10−5 | 0.02 | orphan nuclear receptor | up |
| MSTRG.3306, ENSRNOG00000003977 | Dusp1 | Dual specificity protein | 0.79 | 5.0 × 10−5 | 0.02 | dual specificity phosphatase | up |
| MSTRG.20616, ENSRNOG00000049283 | N/A | N/A | 0.64 | 5.0 × 10−5 | 0.02 | N/A | up |
| MSTRG.14769, ENSRNOG00000009549 | Fbxo3 | F-box protein 3 | 0.57 | 5.0 × 10−5 | 0.02 | promotes the proteasomal degradation of Smurf1 | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csikós, V.; Dóra, F.; Láng, T.; Darai, L.; Szendi, V.; Tóth, A.; Cservenák, M.; Dobolyi, A. Social Isolation Induces Changes in the Monoaminergic Signalling in the Rat Medial Prefrontal Cortex. Cells 2024, 13, 1043. https://doi.org/10.3390/cells13121043
Csikós V, Dóra F, Láng T, Darai L, Szendi V, Tóth A, Cservenák M, Dobolyi A. Social Isolation Induces Changes in the Monoaminergic Signalling in the Rat Medial Prefrontal Cortex. Cells. 2024; 13(12):1043. https://doi.org/10.3390/cells13121043
Chicago/Turabian StyleCsikós, Vivien, Fanni Dóra, Tamás Láng, Luca Darai, Vivien Szendi, Attila Tóth, Melinda Cservenák, and Arpád Dobolyi. 2024. "Social Isolation Induces Changes in the Monoaminergic Signalling in the Rat Medial Prefrontal Cortex" Cells 13, no. 12: 1043. https://doi.org/10.3390/cells13121043
APA StyleCsikós, V., Dóra, F., Láng, T., Darai, L., Szendi, V., Tóth, A., Cservenák, M., & Dobolyi, A. (2024). Social Isolation Induces Changes in the Monoaminergic Signalling in the Rat Medial Prefrontal Cortex. Cells, 13(12), 1043. https://doi.org/10.3390/cells13121043








