Affinity of PET-MRI Tracers for Hypoxic Cells in Breast Cancer: A Systematic Review
Abstract
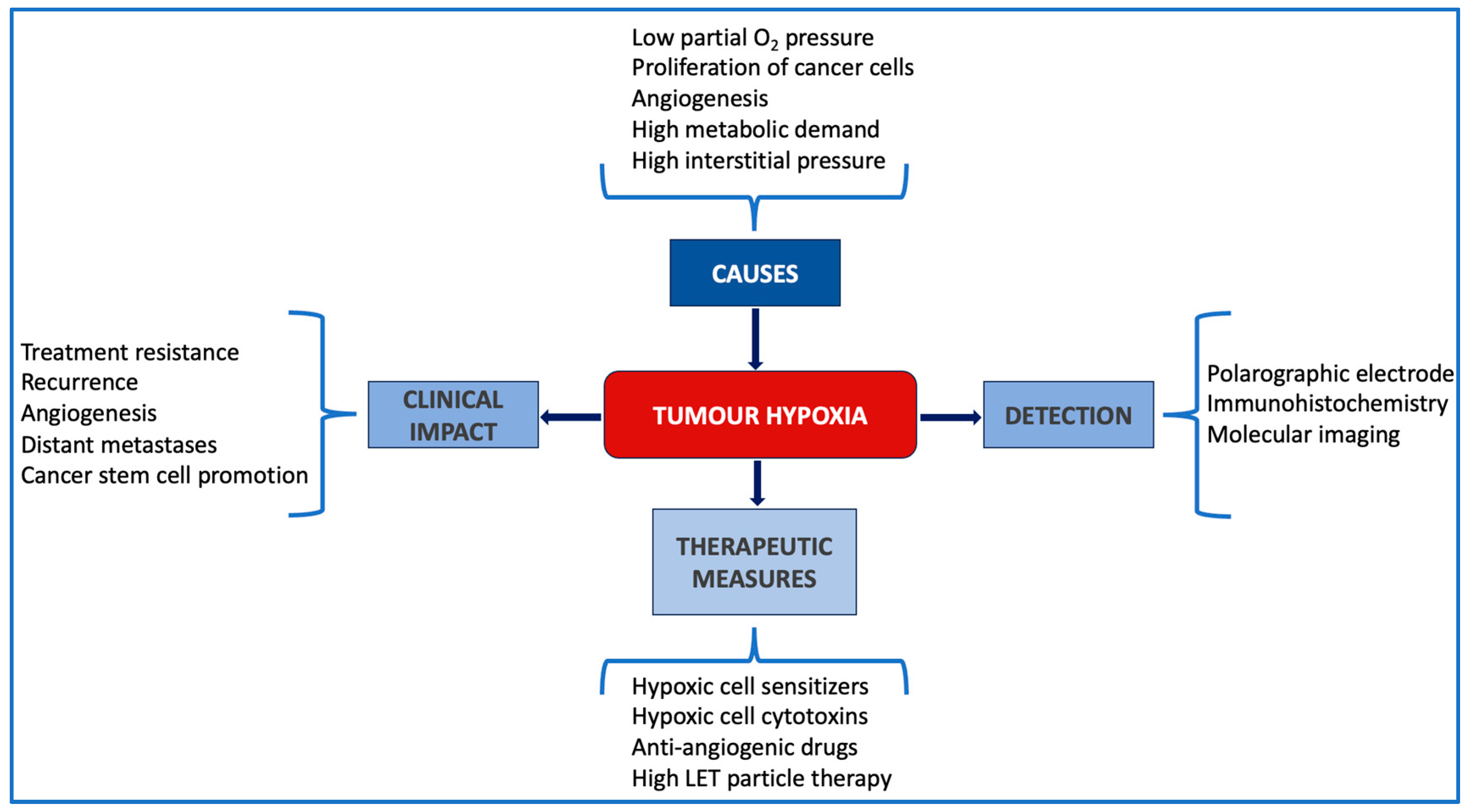
:1. Introduction
1.1. The Challenge of Hypoxia in Today’s Oncology
1.2. Hypoxia in Breast Cancer
2. The Role of Functional Imaging in Detection of Hypoxic Cells in Breast Cancer
2.1. SPECT Imaging
2.2. PET Imaging
2.3. The Potential of MRI to Augment Hypoxic Cell Detection
3. PET-MRI Imaging of Hypoxic Cells in Breast Cancer
3.1. Literature Search
3.2. Pre-Clinical Studies
3.3. Clinical Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomlinson, R.H.; Gray, L.H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer 1955, 9, 539–549. [Google Scholar] [CrossRef]
- Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 2003, 9, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Forster, J.C.; Marcu, L.G.; Bezak, E. Approaches to combat hypoxia in cancer therapy and the potential for in silico models in their evaluation. Phys. Med. 2019, 64, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Facciabene, A.; Peng, X.; Hagemann, I.S.; Balint, K.; Barchetti, A.; Wang, L.P.; Gimotty, P.A.; Gilks, C.B.; Lal, P.; Zhang, L.; et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 2011, 475, 226–230. [Google Scholar] [CrossRef]
- Höckel, M.; Vaupel, P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef]
- Chang, J.; Erler, J. Hypoxia-mediated metastasis. Adv. Exp. Med. Biol. 2014, 772, 55–81. [Google Scholar] [CrossRef]
- Krock, B.L.; Skuli, N.; Simon, M.C. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer 2011, 2, 1117–1133. [Google Scholar] [CrossRef]
- Marcu, L.G.; Marcu, D.; Filip, S.M. In silico study of the impact of cancer stem cell dynamics and radiobiological hypoxia on tumour response to hyperfractionated radiotherapy. Cell Prolif. 2016, 49, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Abd, G.M.; Laird, M.C.; Ku, J.C.; Li, Y. Hypoxia-induced cancer cell reprogramming: A review on how cancer stem cells arise. Front. Oncol. 2023, 13, 1227884. [Google Scholar] [CrossRef]
- Marcu, L.G.; Dell’Oro, M.; Bezak, E. Opportunities in cancer therapies: Deciphering the role of cancer stem cells in tumour repopulation. Int. J. Mol. Sci. 2023, 24, 17258. [Google Scholar] [CrossRef]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L.; Bezak, E.; Allen, B.J. Global comparison of targeted alpha vs targeted beta therapy for cancer: In vitro, in vivo and clinical trials. Crit. Rev. Oncol. Hematol. 2018, 123, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, K.; Holm, C.; Landberg, G. Hypoxia and breast cancer: Prognostic and therapeutic implications. Cell Mol. Life Sci. 2007, 64, 3233–3247. [Google Scholar] [CrossRef] [PubMed]
- Tutzauer, J.; Sjöström, M.; Holmberg, E.; Karlsson, P.; Killander, F.; Leeb-Lundberg, L.M.F.; Malmström, P.; Niméus, E.; Fernö, M.; Jögi, A. Breast cancer hypoxia in relation to prognosis and benefit from radiotherapy after breast-conserving surgery in a large, randomised trial with long-term follow-up. Br. J. Cancer 2022, 126, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.S.; Lee, H.; Park, I.A.; Chung, Y.R.; Im, S.A.; Lee, K.H.; Moon, H.G.; Han, W.; Kim, K.; Kim, T.Y.; et al. Overexpression of HIF1α and CAXI predicts poor outcome in early-stage triple negative breast cancer. Virchows Arch. 2016, 469, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.H.C.; Lee, D.Y.; Lee, B.; Li, H.; Lim, J.C.T.; Lim, J.X.; Yeong, J.P.S.; Lau, H.Y.; Thike, A.A.; Tan, P.H.; et al. Hypoxia-regulated carbonic anhydrase IX (CAIX) protein is an independent prognostic indicator in triple negative breast cancer. Breast Cancer Res. 2022, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Lin, C.E.; Wu, S.C.; Yang, Z.Y.; Chiang, Y.F.; Huang, K.C.; Wang, K.L.; Ali, M.; Shieh, T.M.; Chang, H.Y.; et al. Para-toluenesulfonamide, a novel potent carbonic anhydrase inhibitor, improves hypoxia-induced metastatic breast cancer cell viability and prevents resistance to αPD-1 therapy in triple-negative breast cancer. Biomed. Pharmacother. 2023, 167, 115533. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, J.; Otsuki, T.; Moriya, T.; Sonoo, H. Hypoxia reduces hormone responsiveness of human breast cancer cells. Jpn. J. Cancer Res. 2001, 92, 1093–1101. [Google Scholar] [CrossRef]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef]
- Bashari, M.H.; Fan, F.; Vallet, S.; Sattler, M.; Arn, M.; Luckner-Minden, C.; Schulze-Bergkamen, H.; Zörnig, I.; Marme, F.; Schneeweiss, A.; et al. Mcl-1 confers protection of Her2-positive breast cancer cells to hypoxia: Therapeutic implications. Breast Cancer Res. 2016, 18, 26. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Han, X.; Pan, Y. Analyses of hypoxia-related risk factors and clinical relevance in breast cancer. Front. Oncol. 2024, 14, 1350426. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.J.; Houston, S.; Barrington, S.F.; Fogelman, I. Technetium-99m-labeled HL91 to identify tumor hypoxia: Correlation with fluorine-18-FDG. J. Nucl. Med. 1998, 39, 99–103. [Google Scholar]
- Tatsumi, M.; Yutani, K.; Kusuoka, H.; Nishimura, T. Technetium-99m HL91 uptake as a tumour hypoxia marker: Relationship to tumour blood flow. Eur. J. Nucl. Med. 1999, 26, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Yutani, K.; Kusuoka, H.; Fukuchi, K.; Tatsumi, M.; Nishimura, T. Applicability of 99mTc-HL91, a putative hypoxic tracer, to detection of tumor hypoxia. J. Nucl. Med. 1999, 40, 854–861. [Google Scholar]
- Jeong, Y.J.; Jung, J.W.; Cho, Y.Y.; Park, S.H.; Oh, H.K.; Kang, S. Correlation of hypoxia inducible transcription factor in breast cancer and SUVmax of F-18 FDG PET/CT. Nucl. Med. Rev. Cent. East. Eur. 2017, 20, 32–38. [Google Scholar] [CrossRef]
- Chirla, R.; Marcu, L.G. PET-based quantification of statistical properties of hypoxic tumor subvolumes in head and neck cancer. Phys. Med. 2016, 32, 23–35. [Google Scholar] [CrossRef]
- Yu, W.; Qiao, F.; Su, X.; Zhang, D.; Wang, H.; Jiang, J.; Xu, H. 18F-HX4/18F-FMISO-based micro PET for imaging of tumor hypoxia and radiotherapy-associated changes in mice. Biomed. Pharmacother. 2019, 119, 109454. [Google Scholar] [CrossRef] [PubMed]
- Thureau, S.; Piton, N.; Gouel, P.; Modzelewski, R.; Dujon, A.; Baste, J.M.; Melki, J.; Rinieri, P.; Peillon, C.; Rastelli, O.; et al. First comparison between [18f]-FMISO and [18f]-Faza for preoperative PET imaging of hypoxia in lung cancer. Cancers 2021, 13, 4101. [Google Scholar] [CrossRef]
- Asano, A.; Ueda, S.; Kuji, I.; Yamane, T.; Takeuchi, H.; Hirokawa, E.; Sugitani, I.; Shimada, H.; Hasebe, T.; Osaki, A.; et al. Intracellular hypoxia measured by 18F-fluoromisonidazole positron emission tomography has prognostic impact in patients with estrogen receptor-positive breast cancer. Breast Cancer Res. 2018, 20, 78. [Google Scholar] [CrossRef]
- Shimizu, Y.; Zhao, S.; Yasui, H.; Nishijima, K.I.; Matsumoto, H.; Shiga, T.; Tamaki, N.; Ogawa, M.; Kuge, Y. A novel PET probe “[18F]DiFA” accumulates in hypoxic region via glutathione conjugation following reductive metabolism. Mol. Imaging Biol. 2019, 21, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Nakata, N.; Kiriu, M.; Okumura, Y.; Zhao, S.; Nishijima, K.I.; Shiga, T.; Tamaki, N.; Kuge, Y.; Matsumoto, H. Comparative evaluation of [18F]DiFA and its analogs as novel hypoxia positron emission tomography and [18F]FMISO as the standard. Nucl. Med. Biol. 2019, 70, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.; Yasui, H.; Shiga, T.; Shibata, Y.; Fujimoto, M.; Suzuki, M.; Higashikawa, K.; Miyamoto, N.; Inanami, O.; Kuge, Y. Eribulin improves tumor oxygenation demonstrated by 18F-DiFA hypoxia imaging, leading to radio-sensitization in human cancer xenograft models. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.N.; Wuest, M.; Jans, H.S.; Woodfield, J.; Nario, A.P.; Krys, D.; Dufour, J.; Glubrecht, D.; Bergman, C.; Bernardes, E.S.; et al. Comparison of three 18F-labeled 2-nitroimidazoles for imaging hypoxia in breast cancer xenografts: [18F]FBNA, [18F]FAZA and [18F]FMISO. Nucl. Med. Biol. 2023, 124–125, 108383. [Google Scholar] [CrossRef] [PubMed]
- Braune, A.; Oehme, L.; Freudenberg, R.; Hofheinz, F.; van den Hoff, J.; Kotzerke, J.; Hoberück, S. Comparison of image quality and spatial resolution between 18F, 68Ga, and 64Cu phantom measurements using a digital Biograph Vision PET/CT. EJNMMI Phys. 2022, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Walke, G.R.; Meron, S.; Shenberger, Y.; Gevorkyan-Airapetov, L.; Ruthstein, S. Cellular uptake of the ATSM-Cu(II) complex under hypoxic conditions. Chem. Open 2021, 10, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishna, S.; Agarwal, S.; Parikh, P.M.; Kaur, K.; Panwar, S.; Sharma, S.; Dey, A.; Saxena, K.K.; Chandra, M.; Sud, S. Role of magnetic resonance imaging in breast cancer management. South. Asian J. Cancer 2018, 7, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Bennani-Baiti, B.; Pinker, K.; Zimmermann, M.; Helbich, T.H.; Baltzer, P.A.; Clauser, P.; Kapetas, P.; Bago-Horvath, Z.; Stadlbauer, A. Non-invasive assessment of hypoxia and neovascularization with MRI for identification of aggressive breast cancer. Cancers 2020, 12, 2024. [Google Scholar] [CrossRef] [PubMed]
- Yankeelov, T.E.; Gore, J.C. Dynamic contrast enhanced magnetic resonance imaging in oncology: Theory, data acquisition, analysis, and examples. Cur Med. Imaging Rev. 2009, 3, 91–107. [Google Scholar] [CrossRef]
- Frankhouser, D.E.; Dietze, E.; Mahabal, A.; Seewaldt, V.L. Vascularity and dynamic contrast-enhanced breast magnetic resonance imaging. Front. Radiol. 2021, 1, 735567. [Google Scholar] [CrossRef]
- Zaha, D.C. Significance of immunohistochemistry in breast cancer. World J. Clin. Oncol. 2014, 5, 382–392. [Google Scholar] [CrossRef]
- Andrzejewski, P.; Wengert, G.; Helbich, T.H.; Magometschnigg, H.; Georg, D.; Hacker, M.; Baltzer, P.; Clauser, P.; Kapetas, P.; Georg, P.; et al. Sequential [18F]FDG-[18F]FMISO PET and multiparametric MRI at 3T for insights into breast cancer heterogeneity and correlation with patient outcomes: First clinical experience. Contrast Media Mol. Imaging 2019, 2019, 1307247. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.K.; Woodall, R.; Whisenant, J.G.; Yankeelov, T.E.; Sorace, A.G. Characterizing trastuzumabinduced alterations in intratumoral heterogeneity with quantitative imaging and immunohistochemistry in HER2+ breast cancer. Neoplasia 2019, 21, 17–29. [Google Scholar] [CrossRef]
- Gertsenshteyn, I.; Epel, B.; Barth, E.; Leoni, L.; Markiewicz, A.; Tsai, H.M.; Fan, X.; Giurcanu, M.; Bodero, D.; Zamora, M.; et al. Improving tumor hypoxia location in 18F-Misonidazole PET with dynamic contrast-enhanced MRI using quantitative electron paramagnetic resonance partial oxygen pressure images. Radiol. Imaging Cancer 2021, 3, 200104. [Google Scholar] [CrossRef] [PubMed]
- Zschaeck, S.; Steinbach, J.; Troost, E.G.C. FMISO as a biomarker for clinical radiation oncology. Recent. Results Cancer Res. 2016, 198, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Parkins, K.M.; Krishnamachary, B.; Jacob, D.; Kakkad, S.M.; Solaiyappan, M.; Mishra, A.; Mironchik, Y.; Penet, M.F.; McMahon, M.T.; Knopf, P.; et al. PET/MRI and bioluminescent imaging identify hypoxia as a cause of programmed cell death ligand 1 image heterogeneity. Radiol. Imaging Cancer 2023, 5, 220138. [Google Scholar] [CrossRef]
- Jarrett, A.M.; Bloom, M.J.; Godfrey, W.; Syed, A.K.; Ekrut, D.A.; Ehrlich, L.I.; Yankeelov, T.E.; Sorace, A.G. Mathematical modelling of trastuzumab-induced immune response in an in vivo murine model of HER2+ breast cancer. Math. Med. Biol. 2019, 36, 381–410. [Google Scholar] [CrossRef]
- Sorace, A.G.; Quarles, C.C.; Whisenant, J.G.; Hanker, A.B.; McIntyre, J.O.; Sanchez, V.M.; Yankeelov, T.E. Trastuzumab improves tumor perfusion and vascular delivery of cytotoxic therapy in a murine model of HER2+ breast cancer: Preliminary results. Breast Cancer Res. Treat. 2016, 155, 273–284. [Google Scholar] [CrossRef]
- Sorace, A.G.; Syed, A.K.; Barnes, S.L.; Quarles, C.C.; Sanchez, V.; Kang, H.; Yankeelov, T.E. Quantitative [18F]FMISO PET imaging shows reduction of hypoxia following trastuzumab in a murine model of HER2+ breast cancer. Mol. Imaging Biol. 2017, 19, 130–137. [Google Scholar] [CrossRef]
- Garcia-Foncillas, J.; Martinez, P.; Lahuerta, A.; Cussac, A.L.; Garcia Gonzalez, M.; Sanchez Gomez, R.M.; Alvarez, I.; Anton, A.; Illarramendi, J.J.; De Juan, A.; et al. Dynamic contrast-enhanced MRI versus 18F-misonidazol-PET/CT to predict pathologic response in bevacizumab-based neoadjuvant therapy in breast cancer. J. Clin. Oncol. 2012, 30, 15. [Google Scholar] [CrossRef]
- Ueda, S.; Saeki, T.; Osaki, A.; Yamane, T.; Kuji, I. Bevacizumab induces acute hypoxia and cancer progression in patients with refractory breast cancer: Multimodal functional imaging and multiplex cytokine analysis. Clin. Cancer Res. 2017, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Bozo, J.C.; Manavaki, R.; Woitek, R.; Torheim, T.; Baxter, G.C.; Caracò, C.; Provenzano, E.; Graves, M.J.; Fryer, T.D.; Patterson, A.J.; et al. Hypoxia and perfusion in breast cancer: Simultaneous assessment using PET/MR imaging. Eur. Radiol. 2021, 31, 333–344. [Google Scholar] [CrossRef]
- López-Vega, J.M.; Álvarez, I.; Antón, A.; Illarramendi, J.J.; Llombart, A.; Boni, V.; García-Velloso, M.J.; Martí-Climent, J.M.; Pina, L.; García-Foncillas, J. Early imaging and molecular changes with neoadjuvant bevacizumab in stage II/III breast cancer. Cancers 2021, 13, 3511. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Bozo, J.C.; Manavaki, R.; Miller, J.L.; Brodie, C.; Caracò, C.; Woitek, R.; Baxter, G.C.; Graves, M.J.; Fryer, T.D.; Provenzano, E.; et al. PET/MRI of hypoxia and vascular function in ER-positive breast cancer: Correlations with immunohistochemistry. Eur. Radiol. 2023, 33, 6168–6178. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Wree, A.; Hockel, M.; Leo, C.; Pilch, H.; Vaupel, P. Lack of correlation between expression of HIF-1 protein and oxygenation status in identical tissue areas of squamous cell carcinomas of the uterine cervix. Cancer Res. 2004, 64, 5876–5881. [Google Scholar] [CrossRef] [PubMed]
- Margolis, N.E.; Moy, L.; Sigmund, E.E.; Freed, M.; McKellop, J.; Melsaether, A.N.; Kim, S.G. Assessment of aggressiveness of breast cancer using simultaneous 18F-FDG-PET and DCE-MRI. Clin. Nucl. Med. 2016, 41, e355–e361. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Padhani, A.R.; Taylor, N.J.; Beresford, M.J.; Ah-See, M.L.W.; Stirling, J.J.; d’Arcy, J.A.; Collins, D.J.; Makris, A. Vascular characterisation of triple negative breast carcinomas using dynamic MRI. Eur. Radiol. 2011, 21, 1364–1373. [Google Scholar] [CrossRef]
- Lehmanna, S.; Stiehlb, D.P.; Honerc, M.; Dominiettoa, M.; Keista, R.; Kotevica, I.; Wollenickb, K.; Ametameyc, S.; Wengerb, R.H.; Rudina, M. Longitudinal and multimodal in vivo imaging of tumor hypoxia and its downstream molecular events. Proc. Natl. Acad. Sci. USA 2009, 106, 14004–14009. [Google Scholar] [CrossRef]

| Image Indicators | |
|---|---|
| PET | MRI |
| Ki = tracer influx rate constant | kep = transfer constant from the interstitial space to the blood plasma |
| %HF = % of voxels with Ki values > 2x standard deviation of mean Ki of normoxic tissue | Ktrans = volume transfer constant between blood plasma and the interstitial space (vascular heterogeneity) |
| SUV = standardised uptake value (hypoxia heterogeneity) | νe = extravascular extracellular volume fraction (cellular heterogeneity) |
| DSS = death induced by the disease | νp = plasmatic volume fraction |
| TBR = tumour-to-background ratio calculated as the region of interest SUV normalized to the SUV measured in the patients’ aorta | ADC = apparent diffusion coefficient |
| Immunohistochemistry parameters | |
| HER2 = human epidermal growth factor receptor 2 = prognoses higher incidence of metastases | |
| VEGFR-2 = vascular endothelial growth factor receptor-2 = angiogenesis regulator | |
| CD31 = cluster of differentiation 31 = used to expose the presence of endothelial cells and can evaluate tumour angiogenesis | |
| HIF-1α = hypoxia-inducible factor 1α = regulates genes involved in angiogenesis, pH regulation, migration and invasion which consists in cancer progression | |
| CAIX = hypoxia-induced carbonic anhydrase IX | |
| Ki67 = tumour cell proliferation and growth | |
| HE = haematoxylin and eosin = identify different types of cells, especially ribosomes and cytoplasmic regions rich in RNA | |
| Pimonidazole = nitroimidazole with hypoxic selectivity, is reduced in hypoxic environments and can be used as a hypoxia marker | |
| Pre-Clinical Study (Ref.) | MRI Parameter | PET Tracer | Observations |
|---|---|---|---|
| BT747 tumour-bearing mice (Syed et al., 2019 [43]) | DCE-MRI | 18F-FMISO | Under Trastuzumab: Ktrans: longitudinal increase in vascular heterogeneity on day 4 (K-S distance 0.42) νe: longitudinal increase in cellularity heterogeneity on day 4 (K-S distance 0.32) SUV: longitudinal decrease in hypoxia heterogeneity on day 3 (K-S distance 0.42) and on day 7 (K-S distance 0.46) with narrowing distribution of treated SUV |
| MCa4 mammary carcinoma-bearing mice (Gertsenshteyn et al., 2021 [44]) | DCE-MRI | 18F-FMISO | Ktrans > 0.25 min−1 => higher values => higher perfusion and vascular permeability with high pO2 (14 mm Hg > pO2 > 60 mm Hg) Lower Ktrans => hypoxic pO2 regions on EPR |
| 4T1 triple-negative breast cancer cell line (Parkins et al., 2023 [46]) | DCE-MRI | 64Cu-avelumab | Mouse and human cells exposed to hypoxia expressed an increase in PD-L1 expression. |
| Ref/No. Patients | MRI Parameter | PET Tracer | Results/Validation of Imaging Techniques Against Histopathology |
|---|---|---|---|
| Garcia-Foncillas et al., 2012 [50] 73 patients | - | 18F-FLT and 18F-FMISO | Under bevacizumab: early changes in tumour hypoxia via 18F-FMISO serve as biomarker of pathological response |
| Margolis et al., 2016 [56] 12 patients | Ktrans and kep | 18F-FDG |
|
| Ueda et al., 2017 [51] 28 patients | - | 18F-FDG and 18F-FMISO |
|
| Andrzejewki et al., 2019 [42] 9 patients with 10 breast cancer lesions | - | 18F-FDG and 18F-FMISO | FMISOTBR mean vs. Ki67: r = 0.77 FDGmean vs. Ki67: r = 0.86 FDGmean vs. DSS: r = 0.83 FDGTRBmax/FMISOTRBmax vs. presence/development of metastasis: r = 0.69 FMISOTRB mean vs. DSS: r = 0.64 |
| Carmona-Bozo et al., 2021 [52] 29 patients with 32 breast cancer lesions | Ktrans, kep, νe and νp | 18F-FMISO | Ktrans vs. %HF: r = −0.33, p = 0.04 νe vs. %HF: r = −0.38, p = 0.03 kep vs. %HF: r = 0.02, p = 0.90 kep vs. Ki: r = 0.08, p = 0.65 No correlations between MRI parameters and tumour size %HF vs. pathological size: r = 0.63, p < 0.01 |
| Lopez-Vega et al., 2021 [53] 73 patients, efficacy evaluated in 70 patients | Microvessel density, Ktrans and kep | 18F-FLT and 18F-FMISO | FLT: SUVmax vs. Ki67: ρ = 0.38, p = 0.001 FMISO: SUVmax vs. VEGFR-2: ρ = 0.26, p = 0.02 FMISO: SUVmax vs. microvessel density: no correlation MRI: kep vs. FLT SUVmax: ρ = 0.449, p < 0.01 MRI: Ktrans vs. FLT SUVmax: ρ = 0.414, p < 0.01 |
| Carmona-Bozo et al., 2023 [54] 20 patients with 22 breast cancer lesions | Microvessel density, vessel diameter, Ktrans, kep, νe and νp | 18F-FMISO | Ki vs. microvessel density: slope = −0.016, r = 0.26, p = 0.02 Ki vs. vessel diameter: slope = −0.43, r = 0.23, p = 0.03 Ki vs. CAIX: slope = 1.3 × 10−4, r = 0.40, p < 0.01 No correlation between MRI parameters and HIF-1α or CAIX |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costin, I.-C.; Marcu, L.G. Affinity of PET-MRI Tracers for Hypoxic Cells in Breast Cancer: A Systematic Review. Cells 2024, 13, 1048. https://doi.org/10.3390/cells13121048
Costin I-C, Marcu LG. Affinity of PET-MRI Tracers for Hypoxic Cells in Breast Cancer: A Systematic Review. Cells. 2024; 13(12):1048. https://doi.org/10.3390/cells13121048
Chicago/Turabian StyleCostin, Ioana-Claudia, and Loredana G. Marcu. 2024. "Affinity of PET-MRI Tracers for Hypoxic Cells in Breast Cancer: A Systematic Review" Cells 13, no. 12: 1048. https://doi.org/10.3390/cells13121048
APA StyleCostin, I.-C., & Marcu, L. G. (2024). Affinity of PET-MRI Tracers for Hypoxic Cells in Breast Cancer: A Systematic Review. Cells, 13(12), 1048. https://doi.org/10.3390/cells13121048







