Non-Coding RNAs in Neurological and Neuropsychiatric Disorders: Unraveling the Hidden Players in Disease Pathogenesis
Abstract
:1. Introduction
2. Non-Coding RNAs: Mechanisms of Action and Roles in Brain Molecular and Cellular Events
3. Role of ncRNAs in Specific Brain Disorders
3.1. Alzheimer’s Disease (AD)
3.2. Parkinson’s Disease (PD)
3.3. Amyotrophic Lateral Sclerosis (ALS)
3.4. Huntington’s Disease (HD)
3.5. Multiple Sclerosis (MS)
3.6. Stroke
3.7. Epilepsy
3.8. Brain Tumors
3.9. Neurodevelopmental Psychiatric Disorders
4. Diagnostic and Therapeutic Implications of ncRNAs
5. Challenges and Perspectives
Funding
Conflicts of Interest
References
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. Available online: https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2018.00402 (accessed on 1 April 2024). [CrossRef]
- Xu, K.; Lin, J.; Zandi, R.; Roth, J.A.; Ji, L. MicroRNA-mediated target mRNA cleavage and 3′-uridylation in human cells. Sci. Rep. 2016, 6, 30242. [Google Scholar] [CrossRef]
- Jie, M.; Feng, T.; Huang, W.; Zhang, M.; Feng, Y.; Jiang, H.; Wen, Z. Subcellular Localization of miRNAs and Implications in Cellular Homeostasis. Genes 2021, 12, 856. [Google Scholar] [CrossRef]
- Leung, A.K.L. The Whereabouts of microRNA Actions: Cytoplasm and Beyond. Trends Cell Biol. 2015, 25, 601–610. [Google Scholar] [CrossRef]
- Wong, H.-K.A.; Veremeyko, T.; Patel, N.; Lemere, C.A.; Walsh, D.M.; Esau, C.; Vanderburg, C.; Krichevsky, A.M. De-repression of FOXO3a death axis by microRNA-132 and -212 causes neuronal apoptosis in Alzheimer’s disease. Hum. Mol. Genet. 2013, 22, 3077–3092. [Google Scholar] [CrossRef]
- Mu, C.; Gao, M.; Xu, W.; Sun, X.; Chen, T.; Xu, H.; Qiu, H. Mechanisms of microRNA-132 in central neurodegenerative diseases: A comprehensive review. Biomed. Pharmacother. 2024, 170, 116029. [Google Scholar] [CrossRef]
- Chen, D.; Hu, S.; Wu, Z.; Liu, J.; Li, S. The Role of MiR-132 in Regulating Neural Stem Cell Proliferation, Differentiation and Neuronal Maturation. Cell. Physiol. Biochem. 2018, 47, 2319–2330. [Google Scholar] [CrossRef]
- El Fatimy, R.; Li, S.; Chen, Z.; Mushannen, T.; Gongala, S.; Wei, Z.; Balu, D.T.; Rabinovsky, R.; Cantlon, A.; Elkhal, A.; et al. MicroRNA-132 provides neuroprotection for tauopathies via multiple signaling pathways. Acta Neuropathol. 2018, 136, 537–555. [Google Scholar] [CrossRef]
- Sala, C.; Segal, M. Dendritic Spines: The Locus of Structural and Functional Plasticity. Physiol. Rev. 2014, 94, 141–188. [Google Scholar] [CrossRef]
- Qian, Y.; Song, J.; Ouyang, Y.; Han, Q.; Chen, W.; Zhao, X.; Xie, Y.; Chen, Y.; Yuan, W.; Fan, C. Advances in Roles of miR-132 in the Nervous System. Front. Pharmacol. 2017, 8, 770. Available online: https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2017.00770 (accessed on 1 April 2024). [CrossRef]
- Scott, H.L.; Tamagnini, F.; Narduzzo, K.E.; Howarth, J.L.; Lee, Y.B.; Wong, L.F.; Brown, M.W.; Warburton, E.C.; Bashir, Z.I.; Uney, J.B. MicroRNA-132 regulates recognition memory and synaptic plasticity in the perirhinal cortex. Eur. J. Neurosci. 2012, 36, 2941–2948. [Google Scholar] [CrossRef]
- Yang, R.; Yang, B.; Liu, W.; Tan, C.; Chen, H.; Wang, X. Emerging role of non-coding RNAs in neuroinflammation mediated by microglia and astrocytes. J. Neuroinflammation 2023, 20, 173. [Google Scholar] [CrossRef]
- Gong, X.; Huang, M.; Chen, L. Mechanism of miR-132-3p Promoting Neuroinflammation and Dopaminergic Neurodegeneration in Parkinson’s Disease. eNeuro 2022, 9, ENEURO.0393-21.2021. [Google Scholar] [CrossRef]
- Walgrave, H.; Penning, A.; Tosoni, G.; Snoeck, S.; Davie, K.; Davis, E.; Wolfs, L.; Sierksma, A.; Mars, M.; Bu, T.; et al. microRNA-132 regulates gene expression programs involved in microglial homeostasis. iScience 2023, 26, 106829. [Google Scholar] [CrossRef]
- Shaked, I.; Meerson, A.; Wolf, Y.; Avni, R.; Greenberg, D.; Gilboa-Geffen, A.; Soreq, H. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity 2009, 31, 965–973. [Google Scholar] [CrossRef]
- Mishra, N.; Friedson, L.; Hanin, G.; Bekenstein, U.; Volovich, M.; Bennett, E.R.; Greenberg, D.S.; Soreq, H. Antisense miR-132 blockade via the AChE-R splice variant mitigates cortical inflammation. Sci. Rep. 2017, 7, 42755. [Google Scholar] [CrossRef]
- Mokabber, H.; Najafzadeh, N.; Mohammadzadeh Vardin, M. miR-124 promotes neural differentiation in mouse bulge stem cells by repressing Ptbp1 and Sox9. J. Cell. Physiol. 2019, 234, 8941–8950. [Google Scholar] [CrossRef]
- Lang, M.-F.; Shi, Y. Dynamic Roles of microRNAs in Neurogenesis. Front. Neurosci. 2012, 6, 71. [Google Scholar] [CrossRef]
- Schieweck, R.; Ninkovic, J.; Kiebler, M.A. RNA-binding proteins balance brain function in health and disease. Physiol. Rev. 2021, 101, 1309–1370. [Google Scholar] [CrossRef]
- Yu, J.Y.; Chung, K.H.; Deo, M.; Thompson, R.C.; Turner, D.L. MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Exp. Cell Res. 2008, 314, 2618–2633. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Y.; Fan, K.; Luo, J.; Yang, Y.; Ma, Y. MiR-124 Reduced Neuroinflammation after Traumatic Brain Injury by Inhibiting TRAF6. Neuroimmunomodulation 2023, 30, 55–68. [Google Scholar] [CrossRef]
- Zhao, J.; He, Z.; Wang, J. MicroRNA-124: A Key Player in Microglia-Mediated Inflammation in Neurological Diseases. Front. Cell. Neurosci. 2021, 15, 771898. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.D.; Sun, M.C.; Gu, W.D.; Geng, H.Z. Over-expression of mir-124 inhibits MMP-9 expression and decreases invasion of renal cell carcinoma cells. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6308–6314. [Google Scholar] [CrossRef]
- Silber, J.; Lim, D.A.; Petritsch, C.; Persson, A.I.; Maunakea, A.K.; Yu, M.; Vandenberg, S.R.; Ginzinger, D.G.; James, C.D.; Costello, J.F.; et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008, 6, 14. [Google Scholar] [CrossRef]
- Meza-Sosa, K.F.; Pedraza-Alva, G.; Pérez-Martínez, L. microRNAs: Key triggers of neuronal cell fate. Front. Cell Neurosci. 2014, 8, 175. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Zhang, C.; Ge, H.; Yin, Y.; Feng, H.; Hu, R. MicroRNAs as Big Regulators of Neural Stem/Progenitor Cell Proliferation, Differentiation and Migration: A Potential Treatment for Stroke. Curr. Pharm. Des. 2017, 23, 2252–2257. [Google Scholar] [CrossRef]
- Ma, J.; Shang, S.; Wang, J.; Zhang, T.; Nie, F.; Song, X.; Zhao, H.; Zhu, C.; Zhang, R.; Hao, D. Identification of miR-22-3p, miR-92a-3p, and miR-137 in peripheral blood as biomarker for schizophrenia. Psychiatry Res. 2018, 265, 70–76. [Google Scholar] [CrossRef]
- Thomas, K.T.; Gross, C.; Bassell, G.J. microRNAs Sculpt Neuronal Communication in a Tight Balance That Is Lost in Neurological Disease. Front. Mol. Neurosci. 2018, 11, 455. Available online: https://www.frontiersin.org/articles/10.3389/fnmol.2018.00455 (accessed on 20 April 2024). [CrossRef]
- Aloi, M.S.; Prater, K.E.; Sánchez, R.E.A.; Beck, A.; Pathan, J.L.; Davidson, S.; Wilson, A.; Keene, C.D.; de la Iglesia, H.; Jayadev, S.; et al. Microglia specific deletion of miR-155 in Alzheimer’s disease mouse models reduces amyloid-β pathology but causes hyperexcitability and seizures. J. Neuroinflammation 2023, 20, 60. [Google Scholar] [CrossRef]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An Important Regulator of Neuroinflammation. Int. J. Mol. Sci. 2021, 23, 90. [Google Scholar] [CrossRef]
- Cichon, C.; Sabharwal, H.; Ruter, C.; Schmidt, M.A. MicroRNAs regulate tightjunction proteins and modulate epithelial/endothelial barrier functions. Tissue Barriers 2014, 2, e944446. [Google Scholar] [CrossRef]
- Pena-Philippides, J.C.; Gardiner, A.; Caballero-Garrido, E.; Pan, R.; Zhu, Y.; Roitbak, T. Inhibition of MicroRNA-155 Supports Endothelial Tight Junction Integrity Following Oxygen-Glucose Deprivation. J. Am. Heart Assoc. 2018, 7, e009244. [Google Scholar] [CrossRef]
- Coolen, M.; Katz, S.; Bally-Cuif, L. miR-9: A versatile regulator of neurogenesis. Front. Cell Neurosci. 2013, 7, 220. [Google Scholar] [CrossRef]
- Packer, A.N.; Xing, Y.; Harper, S.Q.; Jones, L.; Davidson, B.L. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington’s disease. J. Neurosci. 2008, 28, 14341–14346. [Google Scholar] [CrossRef]
- Yao, H.; Ma, R.; Yang, L.; Hu, G.; Chen, X.; Duan, M.; Kook, Y.; Niu, F.; Liao, K.; Fu, M.; et al. MiR-9 promotes microglial activation by targeting MCPIP1. Nat. Commun. 2014, 5, 4386. [Google Scholar] [CrossRef]
- Jobe, E.M.; McQuate, A.L.; Zhao, X. Crosstalk among Epigenetic Pathways Regulates Neurogenesis. Front. Neurosci. 2012, 6, 59. [Google Scholar] [CrossRef]
- Baby, N.; Alagappan, N.; Dheen, S.T.; Sajikumar, S. MicroRNA-134-5p inhibition rescues long-term plasticity and synaptic tagging/capture in an Aβ(1–42)-induced model of Alzheimer’s disease. Aging Cell 2020, 19, e13046. [Google Scholar] [CrossRef]
- Huang, W.; Liu, X.; Cao, J.; Meng, F.; Li, M.; Chen, B.; Zhang, J. miR-134 regulates ischemia/reperfusion injury-induced neuronal cell death by regulating CREB signaling. J. Mol. Neurosci. 2015, 55, 821–829. [Google Scholar] [CrossRef]
- Schratt, G.M.; Tuebing, F.; Nigh, E.A.; Kane, C.G.; Sabatini, M.E.; Kiebler, M.; Greenberg, M.E. A brain-specific microRNA regulates dendritic spine development. Nature 2006, 439, 283–289. [Google Scholar] [CrossRef]
- Fiore, R.; Rajman, M.; Schwale, C.; Bicker, S.; Antoniou, A.; Bruehl, C.; Draguhn, A.; Schratt, G. MiR-134-dependent regulation of Pumilio-2 is necessary for homeostatic synaptic depression. EMBO J. 2014, 33, 2231–2246. [Google Scholar] [CrossRef]
- Zampa, F.; Bicker, S.; Schratt, G. Activity-Dependent Pre-miR-134 Dendritic Localization Is Required for Hippocampal Neuron Dendritogenesis. Front. Mol. Neurosci. 2018, 11, 171. [Google Scholar] [CrossRef]
- Leontariti, M.; Avgeris, M.; Katsarou, M.S.; Drakoulis, N.; Siatouni, A.; Verentzioti, A.; Alexoudi, A.; Fytraki, A.; Patrikelis, P.; Vassilacopoulou, D.; et al. Circulating miR-146a and miR-134 in predicting drug-resistant epilepsy in patients with focal impaired awareness seizures. Epilepsia 2020, 61, 959–970. [Google Scholar] [CrossRef]
- Morris, G.; Reschke, C.; Henshall, D. Targeting microRNA-134 for seizure control and disease modification in epilepsy. EBioMedicine 2019, 45, 646–654. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, L.; Li, R.; Liu, N.; Huang, X.; Wong, G. PIWI-interacting RNAs in human diseases: Databases and computational models. Brief. Bioinform. 2022, 23, bbac217. [Google Scholar] [CrossRef]
- Yamanaka, S.; Siomi, M.C.; Siomi, H. piRNA clusters and open chromatin structure. Mobile DNA 2014, 5, 22. [Google Scholar] [CrossRef]
- Fu, A.; Jacobs, D.I.; Zhu, Y. Epigenome-wide analysis of piRNAs in gene-specific DNA methylation. RNA Biol. 2014, 11, 1301–1312. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, Y.; Cao, X.; Tan, W.; Yu, J.; Lu, Y.; Kang, R.; Wang, X.; Li, E. The epigenetic regulatory mechanism of PIWI/piRNAs in human cancers. Mol. Cancer 2023, 22, 45. [Google Scholar] [CrossRef]
- Bozzetti, M.P.; Specchia, V.; Cattenoz, P.B.; Laneve, P.; Geusa, A.; Sahin, H.B.; Di Tommaso, S.; Friscini, A.; Massari, S.; Diebold, C.; et al. The Drosophila fragile X mental retardation protein participates in the piRNA pathway. J. Cell Sci. 2015, 128, 2070–2084. [Google Scholar] [CrossRef]
- Chavda, V.; Madhwani, K.; Chaurasia, B. PiWi RNA in Neurodevelopment and Neurodegenerative Disorders. Curr. Mol. Pharmacol. 2022, 15, 517–531. [Google Scholar] [CrossRef]
- Kim, K.W. PIWI Proteins and piRNAs in the Nervous System. Mol. Cells 2019, 42, 828–835. [Google Scholar] [CrossRef]
- Sato, K.; Takayama, K.-I.; Inoue, S. Role of piRNA biogenesis and its neuronal function in the development of neurodegenerative diseases. Front. Aging Neurosci. 2023, 15, 1157818. [Google Scholar] [CrossRef]
- Rajasethupathy, P.; Antonov, I.; Sheridan, R.; Frey, S.; Sander, C.; Tuschl, T.; Kandel, E.R. A role for neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity. Cell 2012, 149, 693–707. [Google Scholar] [CrossRef]
- Spadaro, P.; Bredy, T.W. Emerging role of non-coding RNA in neural plasticity, cognitive function, and neuropsychiatric disorders. Front. Genet. 2012, 3, 132. [Google Scholar] [CrossRef]
- Ahmad, P.; Bensaoud, C.; Mekki, I.; Rehman, M.U.; Kotsyfakis, M. Long Non-Coding RNAs and Their Potential Roles in the Vector–Host–Pathogen Triad. Life 2021, 11, 56. [Google Scholar] [CrossRef]
- Latgé, G.; Poulet, C.; Bours, V.; Josse, C.; Jerusalem, G. Natural Antisense Transcripts: Molecular Mechanisms and Implications in Breast Cancers. Int. J. Mol. Sci. 2018, 19, 123. [Google Scholar] [CrossRef]
- Ransohoff, J.D.; Wei, Y.; Khavari, P.A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018, 19, 143–157. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Marchese, F.P.; Raimondi, I.; Huarte, M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017, 18, 206. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Shibayama, Y.; Fanucchi, S.; Magagula, L.; Mhlanga, M.M. lncRNA and gene looping: What’s the connection? Transcription 2014, 5, e28658. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Yang, H.; Xu, Y.; Zhou, X.; Zhang, X.; Xie, Z.; Bi, J. The effect of BACE1-AS on β-amyloid generation by regulating BACE1 mRNA expression. BMC Mol. Biol. 2019, 20, 23. [Google Scholar] [CrossRef]
- Taiana, E.; Ronchetti, D.; Todoerti, K.; Nobili, L.; Tassone, P.; Amodio, N.; Neri, A. LncRNA NEAT1 in Paraspeckles: A Structural Scaffold for Cellular DNA Damage Response Systems? Noncoding RNA 2020, 6, 26. [Google Scholar] [CrossRef]
- Hirose, T.; Virnicchi, G.; Tanigawa, A.; Naganuma, T.; Li, R.; Kimura, H.; Yokoi, T.; Nakagawa, S.; Bénard, M.; Fox, A.H.; et al. NEAT1 long noncoding RNA regulates transcription via protein sequestration within subnuclear bodies. Mol. Biol. Cell 2014, 25, 169–183. [Google Scholar] [CrossRef]
- Li, K.; Wang, Z. lncRNA NEAT1: Key player in neurodegenerative diseases. Ageing Res. Rev. 2023, 86, 101878. [Google Scholar] [CrossRef]
- Sayad, A.; Omrani, M.D.; Fallah, H.; Taheri, M.; Ghafouri-Fard, S. Aberrant Expression of Long Non-coding RNAs in Peripheral Blood of Autistic Patients. J. Mol. Neurosci. 2019, 67, 276–281. [Google Scholar] [CrossRef]
- He, C.; Jiang, B.; Ma, J.; Li, Q. Aberrant NEAT1 expression is associated with clinical outcome in high grade glioma patients. APMIS 2016, 124, 169–174. [Google Scholar] [CrossRef]
- Tsai, M.C.; Manor, O.; Wan, Y.; Mosammaparast, N.; Wang, J.K.; Lan, F.; Shi, Y.; Segal, E.; Chang, H.Y. Long Noncoding RNA as Modular Scaffold of Histone Modification Complexes. Science 2010, 329, 689–693. [Google Scholar] [CrossRef]
- Pahlevan Kakhki, M.; Nikravesh, A.; Shirvani Farsani, Z.; Sahraian, M.A.; Behmanesh, M. HOTAIR but not ANRIL long non-coding RNA contributes to the pathogenesis of multiple sclerosis. Immunology 2018, 153, 479–487. [Google Scholar] [CrossRef]
- Duan, C.; Liu, Y.; Li, Y.; Chen, H.; Liu, X.; Chen, X.; Yue, J.; Zhou, X.; Yang, J. Sulfasalazine alters microglia phenotype by competing endogenous RNA effect of miR-136-5p and long non-coding RNA HOTAIR in cuprizone-induced demyelination. Biochem. Pharmacol. 2018, 155, 110–123. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, N.; Yang, L.; Tang, J.; Wang, Y.; Mei, M. A Brief Review of circRNA Biogenesis, Detection, and Function. Curr. Genom. 2021, 22, 485–495. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, functions and interactions with proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef]
- Yu, C.Y.; Kuo, H.C. The emerging roles and functions of circular RNAs and their generation. J. Biomed. Sci. 2019, 26, 29. [Google Scholar] [CrossRef]
- D’Ambra, E.; Capauto, D.; Morlando, M. Exploring the Regulatory Role of Circular RNAs in Neurodegenerative Disorders. Int. J. Mol. Sci. 2019, 20, 5477. [Google Scholar] [CrossRef]
- Mehta, S.L.; Dempsey, R.J.; Vemuganti, R. Role of circular RNAs in brain development and CNS diseases. Prog. Neurobiol. 2020, 186, 101746. [Google Scholar] [CrossRef]
- Mehta, S.L.; Chokkalla, A.K.; Bathula, S.; Arruri, V.; Chelluboina, B.; Vemuganti, R. CDR1as regulates α-synuclein-mediated ischemic brain damage by controlling miR-7 availability. Mol. Ther. Nucleic Acids 2022, 31, 57–67. [Google Scholar] [CrossRef]
- Scoyni, F.; Sitnikova, V.; Giudice, L.; Korhonen, P.; Trevisan, D.M.; Hernandez de Sande, A.; Gomez-Budia, M.; Giniatullina, R.; Ugidos, I.F.; Dhungana, H.; et al. ciRS-7 and miR-7 regulate ischemia-induced neuronal death via glutamatergic signaling. Cell Rep. 2024, 43, 113862. [Google Scholar] [CrossRef]
- Chen, G.; Shan, X.; Li, L.; Dong, L.; Huang, G.; Tao, H. circHIPK3 regulates apoptosis and mitochondrial dysfunction induced by ischemic stroke in mice by sponging miR-148b-3p via CDK5R1/SIRT1. Exp. Neurol. 2022, 355, 114115. [Google Scholar] [CrossRef]
- Yin, X.; Zheng, W.; He, L.; Mu, S.; Shen, Y.; Wang, J. CircHIPK3 alleviates inflammatory response and neuronal apoptosis via regulating miR-382-5p/DUSP1 axis in spinal cord injury. Transpl. Immunol. 2022, 73, 101612. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, Y.; Han, B.; Bai, Y.; Zhou, R.; Gan, G.; Chao, J.; Hu, G.; Yao, H. Circular RNA HIPK2 regulates astrocyte activation via cooperation of autophagy and ER stress by targeting MIR124-2HG. Autophagy 2017, 13, 1722–1741. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Y.; Han, B.; Yang, L.; Chen, X.; Huang, R.; Wu, F.; Chao, J.; Liu, P.; Hu, G.; et al. Circular RNA DLGAP4 Ameliorates Ischemic Stroke Outcomes by Targeting miR-143 to Regulate Endothelial-Mesenchymal Transition Associated with Blood-Brain Barrier Integrity. J. Neurosci. 2018, 38, 32–50. [Google Scholar] [CrossRef]
- Huang, G.; Zhu, H.; Wu, S.; Cui, M.; Xu, T. Long Noncoding RNA Can Be a Probable Mechanism and a Novel Target for Diagnosis and Therapy in Fragile X Syndrome. Front. Genet. 2019, 10, 446. [Google Scholar] [CrossRef]
- Garikipati, V.N.S.; Uchida, S. Elucidating the Functions of Non-Coding RNAs from the Perspective of RNA Modifications. Non-Coding RNA 2021, 7, 31. [Google Scholar] [CrossRef]
- Sarkar, S.; Jun, S.; Rellick, S.; Quintana, D.D.; Cavendish, J.Z.; Simpkins, J.W. Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain Res. 2016, 1646, 139–151. [Google Scholar] [CrossRef]
- Fotuhi, S.N.; Khalaj-Kondori, M.; Hoseinpour Feizi, M.A.; Talebi, M. Long Non-coding RNA BACE1-AS May Serve as an Alzheimer’s Disease Blood-Based Biomarker. J. Mol. Neurosci. 2019, 69, 351–359. [Google Scholar] [CrossRef]
- Gentile, G.; Morello, G.; La Cognata, V.; Guarnaccia, M.; Conforti, F.L.; Cavallaro, S. Dysregulated miRNAs as Biomarkers and Therapeutical Targets in Neurodegenerative Diseases. J. Pers. Med. 2022, 12, 770. [Google Scholar] [CrossRef]
- Chanda, K.; Jana, N.R.; Mukhopadhyay, D. Long non-coding RNA MALAT1 protects against Aβ1–42 induced toxicity by regulating the expression of receptor tyrosine kinase EPHA2 via quenching miR-200a/26a/26b in Alzheimer’s disease. Life Sci. 2022, 302, 120652. [Google Scholar] [CrossRef]
- Qiu, W.; Guo, X.; Lin, X.; Yang, Q.; Zhang, W.; Zhang, Y.; Zuo, L.; Zhu, Y.; Li, C.R.; Ma, C.; et al. Transcriptome-wide piRNA profiling in human brains of Alzheimer’s disease. Neurobiol. Aging 2017, 57, 170–177. [Google Scholar] [CrossRef]
- Mao, Q.; Fan, L.; Wang, X.; Lin, X.; Cao, Y.; Zheng, C.; Zhang, Y.; Zhang, H.; Garcia-Milian, R.; Kang, L.; et al. Transcriptome-wide piRNA profiling in human brains for aging genetic factors. Jacobs J. Genet. 2019, 4, 14. [Google Scholar]
- Chen, Q.; Deng, N.; Lu, K.; Liao, Q.; Long, X.; Gou, D.; Bi, F.; Zhou, J. Elevated plasma miR-133b and miR-221-3p as biomarkers for early Parkinson’s disease. Sci. Rep. 2021, 11, 15268. [Google Scholar] [CrossRef]
- Zhang, T.; Wong, G. Dysregulation of human somatic piRNA expression in Parkinson’s disease subtypes and stages. Int. J. Mol. Sci. 2022, 23, 2469. [Google Scholar] [CrossRef]
- Cao, B.; Wang, T.; Qu, Q.; Kang, T.; Yang, Q. Long Noncoding RNA SNHG1 Promotes Neuroinflammation in Parkinson’s Disease via Regulating miR-7/NLRP3 Pathway. Neuroscience 2018, 388, 118–127. [Google Scholar] [CrossRef]
- Xiao, X.; Tan, Z.; Jia, M.; Zhou, X.; Wu, K.; Ding, Y.; Li, W. Long Noncoding RNA SNHG1 Knockdown Ameliorates Apoptosis, Oxidative Stress and Inflammation in Models of Parkinson’s Disease by Inhibiting the miR-125b-5p/MAPK1 Axis. Neuropsychiatr. Dis. Treat. 2021, 17, 1153–1163. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Zhang, Y.; Zhao, J. LncRNA SNHG1 promotes neuronal injury in Parkinson’s disease cell model by miR-181a-5p/CXCL12 axis. J. Mol. Histol. 2021, 52, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ye, Y.; Mao, H.; Yao, L.; Sun, X.; Wang, B.; Zhang, H.; Xie, L.; Zhang, H.; Zhang, Y.; et al. Downregulated lncRNA-SNHG1 enhances autophagy and prevents cell death through the miR-221/222/p27/mTOR pathway in Parkinson’s disease. Exp. Cell Res. 2019, 384, 111614. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Guo, Y.; Rong, H.; Liu, T. The long noncoding RNA HOTAIR promotes Parkinson’s disease by upregulating LRRK2 expression. Oncotarget 2017, 8, 24449–24456. [Google Scholar] [CrossRef]
- Liu, S.; Cui, B.; Dai, Z.X.; Shi, P.K.; Wang, Z.H.; Guo, Y.Y. Long Non-coding RNA HOTAIR Promotes Parkinson’s Disease Induced by MPTP Through up-regulating the expression of LRRK2. Curr. Neurovasc. Res. 2016, 13, 115–120. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Wang, Z.H.; Zhang, J.L.; Duan, Y.L.; Li, G.F.; Zheng, D.L. Beta-asarone protects against MPTP-induced Parkinson’s disease via regulating long non-coding RNA MALAT1 and inhibiting α-synuclein protein expression. Biomed. Pharmacother. 2016, 83, 153–159. [Google Scholar] [CrossRef]
- Ma, G.; Wang, Y.; Li, Y.; Cui, L.; Zhao, Y.; Zhao, B.; Li, K. MiR-206, a key modulator of skeletal muscle development and disease. Int. J. Biol. Sci. 2015, 11, 345–352. [Google Scholar] [CrossRef]
- Toivonen, J.M.; Manzano, R.; Oliván, S.; Zaragoza, P.; García-Redondo, A.; Osta, R. MicroRNA-206: A potential circulating biomarker candidate for amyotrophic lateral sclerosis. PLoS ONE 2014, 9, e89065. [Google Scholar] [CrossRef]
- Abdelhamid, R.F.; Ogawa, K.; Beck, G.; Ikenaka, K.; Takeuchi, E.; Yasumizu, Y.; Jinno, J.; Kimura, Y.; Baba, K.; Nagai, Y.; et al. piRNA/PIWI Protein Complex as a Potential Biomarker in Sporadic Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2022, 59, 1693–1705. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Nakagawa, S.; Hirose, T.; Okano, H.J.; Takao, M.; Shibata, S.; Suyama, S.; Kuwako, K.; Imai, T.; Murayama, S.; et al. The long non-coding RNA nuclear-enriched abundant transcript 1_2 induces paraspeckle formation in the motor neuron during the early phase of amyotrophic lateral sclerosis. Mol. Brain 2013, 6, 31. [Google Scholar] [CrossRef]
- Panero, R.; Rinaldi, A.; Memoli, D.; Nassa, G.; Ravo, M.; Rizzo, F.; Tarallo, R.; Milanesi, L.; Weisz, A.; Giurato, G. iSmaRT: A toolkit for a comprehensive analysis of small RNA-Seq data. Bioinformatics 2017, 33, 938–940. [Google Scholar] [CrossRef]
- Tao, Y.; Mercaldo, N.; Duffy, A.; Dayananthan, A.; Wheelock, V.L.; Rosas, H.D. Circulating miRNA Signatures in Early-Stage Huntington’s Disease. 12 January 2023. PREPRINT (Version 1). Available online: https://www.researchsquare.com/article/rs-2440808/v1 (accessed on 20 April 2024). [CrossRef]
- Helwak, A.; Kudla, G.; Dudnakova, T.; Tollervey, D. Mapping the Human miRNA Interactome by CLASH Reveals Frequent Noncanonical Binding. Cell 2013, 153, 654. [Google Scholar] [CrossRef]
- Wang, Z.M.; Dong, X.Y.; Cong, S.Y. Bioinformatic analysis of a microRNA regulatory network in Huntington’s disease. J. Integr. Neurosci. 2020, 19, 641–650. [Google Scholar] [CrossRef]
- Chanda, K.; Das, S.; Chakraborty, J.; Bucha, S.; Maitra, A.; Chatterjee, R.; Mukhopadhyay, D.; Bhattacharyya, N.P. Altered levels of long NcRNAs Meg3 and Neat1 in cell and animal models of Huntington’s disease. RNA Biol. 2018, 15, 1348–1363. [Google Scholar] [CrossRef]
- Francelle, L.; Galvan, L.; Gaillard, M.C.; Petit, F.; Bernay, B.; Guillermier, M.; Bonvento, G.; Dufour, N.; Elalouf, J.M.; Hantraye, P.; et al. Striatal long noncoding RNA Abhd11os is neuroprotective against an N-terminal fragment of mutant huntingtin in vivo. Neurobiol. Aging 2015, 36, 1601.e7–16. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, J.S.; Lee, S.T.; Im, W.; Lee, M.; Byun, J.I.; Jung, K.H.; Park, K.I.; Jung, K.Y.; Lee, S.K.; Chu, K.; et al. Altered Expression of the Long Noncoding RNA NEAT1 in Huntington’s Disease. Mol. Neurobiol. 2017, 54, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Spengler, R.M.; Keiser, M.S.; Monteys, A.M.; Rieders, J.M.; Ramachandran, S.; Davidson, B.L. The long non-coding RNA NEAT1 is elevated in polyglutamine repeat expansion diseases and protects from disease gene-dependent toxicities. Hum. Mol. Genet. 2018, 27, 4303–4314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mameli, G.; Arru, G.; Caggiu, E.; Niegowska, M.; Leoni, S.; Madeddu, G.; Babudieri, S.; Sechi, G.P.; Sechi, L.A. Natalizumab therapy modulates miR-155, miR-26a and proinflammatory cytokine expression in MS patients. PLoS ONE 2016, 11, e0157153. [Google Scholar] [CrossRef]
- Louafi, F.; Martinez-Nunez, R.T.; Sanchez-Elsner, T. MicroRNA-155 targets SMAD2 and modulates the response of macrophages to transforming growth factor-{beta}. J. Biol. Chem. 2010, 285, 41328–41336. [Google Scholar] [CrossRef]
- McCoy, C.E. miR-155 Dysregulation and Therapeutic Intervention in Multiple Sclerosis. In Regulation of Inflammatory Signaling in Health and Disease. Advances in Experimental Medicine and Biology; Xu, D., Ed.; Springer: Singapore, 2017; Volume 1024. [Google Scholar] [CrossRef]
- Fattahi, M.; Eskandari, N.; Sotoodehnejadnematalahi, F.; Shaygannejad, V.; Kazemi, M. Comparison of The Expression of miR-326 between Interferon beta Responders and Non-Responders in Relapsing-Remitting Multiple Sclerosis. Cell J. 2020, 22, 92–95. [Google Scholar] [CrossRef]
- Karimi, E.; Azari, H.; Tahmasebi, A.; Nikpoor, A.R.; Negahi, A.A.; Sanadgol, N.; Shekari, M.; Mousavi, P. LncRNA-miRNA network analysis across the Th17 cell line reveals biomarker potency of lncRNA NEAT1 and KCNQ1OT1 in multiple sclerosis. J. Cell. Mol. Med. 2022, 26, 2351–2362. [Google Scholar] [CrossRef]
- Senousy, M.A.; Shaker, O.G.; Sayed, N.H.; Fathy, N.; Kortam, M.A. LncRNA GAS5 and miR-137 Polymorphisms and Expression are Associated with Multiple Sclerosis Risk: Mechanistic Insights and Potential Clinical Impact. ACS Chem. Neurosci. 2020, 11, 1651–1660. [Google Scholar] [CrossRef]
- Adly Sadik, N.; Ahmed Rashed, L.; Ahmed Abd-El Mawla, M. Circulating miR-155 and JAK2/STAT3 Axis in Acute Ischemic Stroke Patients and Its Relation to Post-Ischemic Inflammation and Associated Ischemic Stroke Risk Factors. Int. J. Gen. Med. 2021, 14, 1469–1484. [Google Scholar] [CrossRef]
- Fathy, N.; Kortam, M.A.; Shaker, O.G.; Sayed, N.H. Long noncoding RNAs MALAT1 and ANRIL gene variants and the risk of cerebral ischemic stroke: An association study. ACS Chem. Neurosci. 2021, 12, 1351–1362. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Moalemnia, A.; Anbiyaee, O.; Farzaneh, M.; Ghaderi, S. LncRNA MALAT1 and Ischemic Stroke: Pathogenesis and Opportunities. Mol. Neurobiol. 2023, 1–12. [Google Scholar] [CrossRef]
- Ali, M.A.; Shaker, O.G.; Khalifa, A.A.; Ezzat, E.M.; Elghobary, H.A.; Abdel Mawla, T.S.; Elkhateeb, A.F.; Elebiary, A.M.A.; Elamir, A.M. LncRNAs NEAT1, HOTAIR, and GAS5 expression in hypertensive and non-hypertensive associated cerebrovascular stroke patients, and its link to clinical characteristics and severity score of the disease. Noncoding RNA Res. 2022, 8, 96–108. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, T.; Liu, Y.; Li, Y.; Zhou, S.; Song, D.; Zhao, Y.; Feng, R.; Zhang, X.; Li, L.; et al. PAX6 downregulates miR-124 expression to promote cell migration during embryonic stem cell differentiation. Stem Cells Dev. 2014, 23, 2297–2310. [Google Scholar] [CrossRef]
- Wang, G.; Han, B.; Shen, L.; Wu, S.; Yang, L.; Liao, J.; Wu, F.; Li, M.; Leng, S.; Zang, F.; et al. Silencing of circular RNA HIPK2 in neural stem cells enhances functional recovery following ischaemic stroke. EBioMedicine 2020, 52, 102660. [Google Scholar] [CrossRef]
- Jimenez-Mateos, E.M.; Engel, T.; Merino-Serrais, P.; McKiernan, R.C.; Tanaka, K.; Mouri, G.; Sano, T.; O’Tuathaigh, C.; Waddington, J.L.; Prenter, S.; et al. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat. Med. 2012, 18, 1087–1094. [Google Scholar] [CrossRef]
- Zhong, J.; Chuang, S.C.; Bianchi, R.; Zhao, W.; Lee, H.; Fenton, A.A.; Wong, R.K.; Tiedge, H. BC1 regulation of metabotropic glutamate receptor-mediated neuronal excitability. J. Neurosci. 2009, 29, 9977–9986. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schneider, M.F.; Vogt, M.; Scheuermann, J.; Müller, V.; Fischer-Hentrich, A.H.L.; Kremer, T.; Lugert, S.; Metzger, F.; Kudernatsch, M.; Kluger, G.; et al. Brain expression profiles of two SCN1A antisense RNAs in children and adolescents with epilepsy. Transl. Neurosci. 2024, 15, 20220330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piwecka, M.; Glažar, P.; Hernandez-Miranda, L.R.; Memczak, S.; Wolf, S.A.; Rybak-Wolf, A.; Filipchyk, A.; Klironomos, F.; Cerda Jara, C.A.; Fenske, P.; et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science 2017, 357, eaam8526. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.J.; Tang, H.M.; To, S.S. Targeting strategies on miRNA-21 and PDCD4 for glioblastoma. Arch. Biochem. Biophys. 2015, 580, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Cheung, W.K.C.; Ng, S.S.; Jiang, X.; Jiang, S.; Sze, J.; Leung, G.K.K.; Lu, G.; Chan, D.T.M.; Bian, X.W.; et al. Loss of brain-enriched miR-124 microRNA enhances stem-like traits and invasiveness of glioma cells. J. Biol. Chem. 2012, 287, 9962–9971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanuki, R.; Yamamura, T. Tumor Suppressive Effects of miR-124 and Its Function in Neuronal Development. Int. J. Mol. Sci. 2021, 22, 5919. [Google Scholar] [CrossRef]
- Zhou, X.; Ren, Y.; Zhang, J.; Zhang, C.; Zhang, K.; Han, L.; Kong, L.; Wei, J.; Chen, L.; Yang, J.; et al. HOTAIR is a therapeutic target in glioblastoma. Oncotarget 2015, 6, 8353–8365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, H.; Wang, X.; Feng, X.; Li, X.; Pan, L.; Liu, J.; Wang, F.; Yuan, Z.; Yang, L.; Yu, J.; et al. Long non-coding RNA MEG3 regulates proliferation, apoptosis, and autophagy and is associated with prognosis in glioma. J. Neurooncol. 2018, 140, 281–288. [Google Scholar] [CrossRef]
- Li, H.; Xue, Y.; Ma, J.; Shao, L.; Wang, D.; Zheng, J.; Liu, X.; Yang, C.; He, Q.; Ruan, X.; et al. SNHG1 promotes malignant biological behaviors of glioma cells via microRNA-154-5p/miR-376b-3p- FOXP2- KDM5B participating positive feedback loop. J. Exp. Clin. Cancer Res. 2019, 38, 59. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Liu, Y.; Xu, G.; Hei, Y.; Lu, X.; Liu, W. Long non-coding RNA NEAT1 regulates glioma cell proliferation and apoptosis by competitively binding to microRNA-324-5p and upregulating KCTD20 expression. Oncol. Rep. 2021, 46, 125. [Google Scholar] [CrossRef]
- Lv, T.; Miao, Y.F.; Jin, K.; Han, S.; Xu, T.Q.; Qiu, Z.L.; Zhang, X.H. Dysregulated circular RNAs in medulloblastoma regulate proliferation and growth of tumor cells via host genes. Cancer Med. 2018, 7, 6147–6157. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Z.M.; Tan, W.; Wang, X.; Li, Y.; Bai, B.; Li, Y.; Zhang, S.F.; Yan, H.L.; Chen, Z.L.; et al. Partial loss of psychiatric risk gene Mir137 in mice causes repetitive behavior and impairs sociability and learning via increased Pde10a. Nat. Neurosci. 2018, 21, 1689–1703. [Google Scholar] [CrossRef]
- de Sena Cortabitarte, A.; Berkel, S.; Cristian, F.B.; Fischer, C.; Rappold, G.A. A direct regulatory link between microRNA-137 and SHANK2: Implications for neuropsychiatric disorders. J. Neurodev. Disord. 2018, 10, 15. [Google Scholar] [CrossRef]
- Cogill, S.B.; Srivastava, A.K.; Yang, M.Q.; Wang, L. Co-expression of long non-coding RNAs and autism risk genes in the developing human brain. BMC Syst Biol 2018, 12 (Suppl. 7). [Google Scholar] [CrossRef]
- Luo, T.; Liu, P.; Wang, X.Y.; Li, L.Z.; Zhao, L.P.; Huang, J.; Li, Y.M.; Ou, J.L.; Peng, X.Q. Effect of the autism-associated lncRNA Shank2-AS on architecture and growth of neurons. J. Cell Biochem. 2019, 120, 1754–1762. [Google Scholar] [CrossRef]
- Briggs, J.A.; Wolvetang, E.J.; Mattick, J.S.; Rinn, J.L.; Barry, G. Mechanisms of long non-coding RNAs in mammalian nervous system development, plasticity, disease, and evolution. Neuron 2015, 88, 861–877. [Google Scholar] [CrossRef]
- Miller, B.H.; Zeier, Z.; Xi, L.; Lanz, T.A.; Deng, S.; Strathmann, J.; Willoughby, D.; Kenny, P.J.; Elsworth, J.D.; Lawrence, M.S.; et al. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc. Natl. Acad. Sci. USA 2012, 109, 3125–3130. [Google Scholar] [CrossRef]
- Yu, H.C.; Wu, J.; Zhang, H.X.; Zhang, G.L.; Sui, J.; Tong, W.W.; Zhang, X.Y.; Nie, L.L.; Duan, J.H.; Zhang, L.R.; et al. Alterations of miR-132 are novel diagnostic biomarkers in peripheral blood of schizophrenia patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 63, 23–29. [Google Scholar] [CrossRef]
- Hauberg, M.E.; Roussos, P.; Grove, J.; Børglum, A.D.; Mattheisen, M. Analyzing the role of microRNAs in schizophrenia in the context of common genetic risk variants. JAMA Psychiatry 2016, 73, 369–377. [Google Scholar] [CrossRef]
- Safari, M.R.; Komaki, A.; Arsang-Jang, S.; Taheri, M.; Ghafouri-Fard, S. Expression Pattern of Long Non-coding RNAs in Schizophrenic Patients. Cell. Mol. Neurobiol. 2019, 39, 211–221. [Google Scholar] [CrossRef]
- Wu, G.; Du, X.; Li, Z.; Du, Y.; Lv, J.; Li, X.; Xu, Y.; Liu, S. The emerging role of long non-coding RNAs in schizophrenia. Front. Psychiatry. 2022, 13, 995956. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, F.; Wang, X.; Shugart, Y.Y.; Zhao, Y.; Li, X.; Liu, Z.; Sun, N.; Yang, C.; Zhang, K.; et al. Diagnostic value of blood-derived microRNAs for schizophrenia: Results of a meta-analysis and validation. Sci. Rep. 2017, 7, 15328. [Google Scholar] [CrossRef]
- Song, M.F.; Dong, J.Z.; Wang, Y.W.; He, J.; Ju, X.; Zhang, L.; Zhang, Y.H.; Shi, J.F.; Lv, Y.Y. CSF miR-16 is decreased in major depression patients and its neutralization in rats induces depression-like behaviors via a serotonin transmitter system. J. Affect. Disord. 2015, 178, 25–31. [Google Scholar] [CrossRef]
- Shkundin, A.; Halaris, A. Associations of BDNF/BDNF-AS SNPs with Depression, Schizophrenia, and Bipolar Disorder. J. Pers. Med. 2023, 13, 1395. [Google Scholar] [CrossRef]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of siRNA. Int. J. Biomed. Sci. 2017, 13, 48–57. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef]
- Min, H.S.; Kim, H.J.; Naito, M.; Ogura, S.; Toh, K.; Hayashi, K.; Kim, B.S.; Fukushima, S.; Anraku, Y.; Miyata, K.; et al. Systemic Brain Delivery of Antisense Oligonucleotides across the Blood-Brain Barrier with a Glucose-Coated Polymeric Nanocarrier. Angew. Chem. Int. Ed. Engl. 2020, 59, 8173–8180. [Google Scholar] [CrossRef]
- Lam, J.K.; Chow, M.Y.; Zhang, Y.; Leung, S.W. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ly, C.V.; Miller, T.M. Emerging antisense oligonucleotide and viral therapies for amyotrophic lateral sclerosis. Curr. Opin. Neurol. 2018, 31, 648–654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
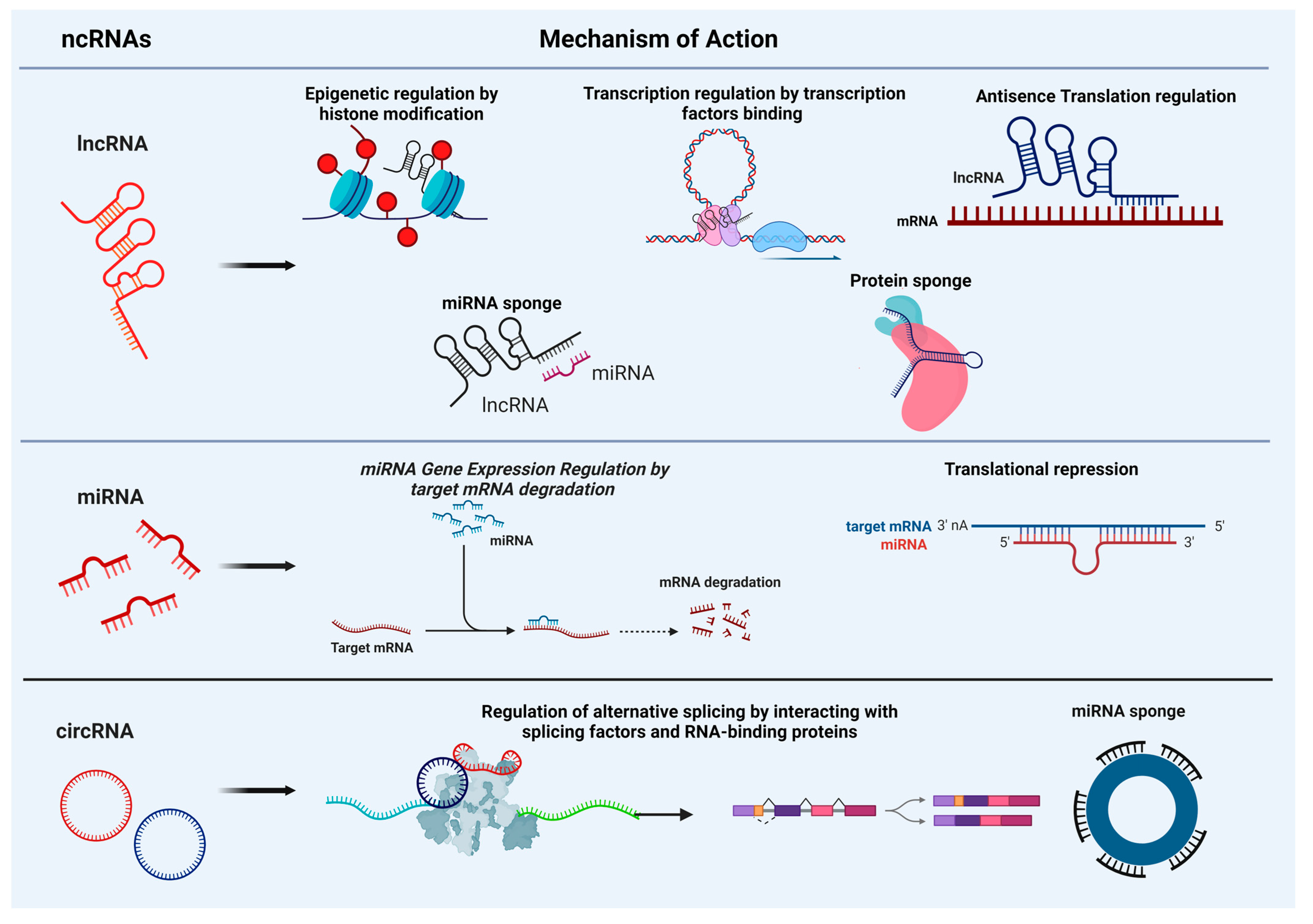
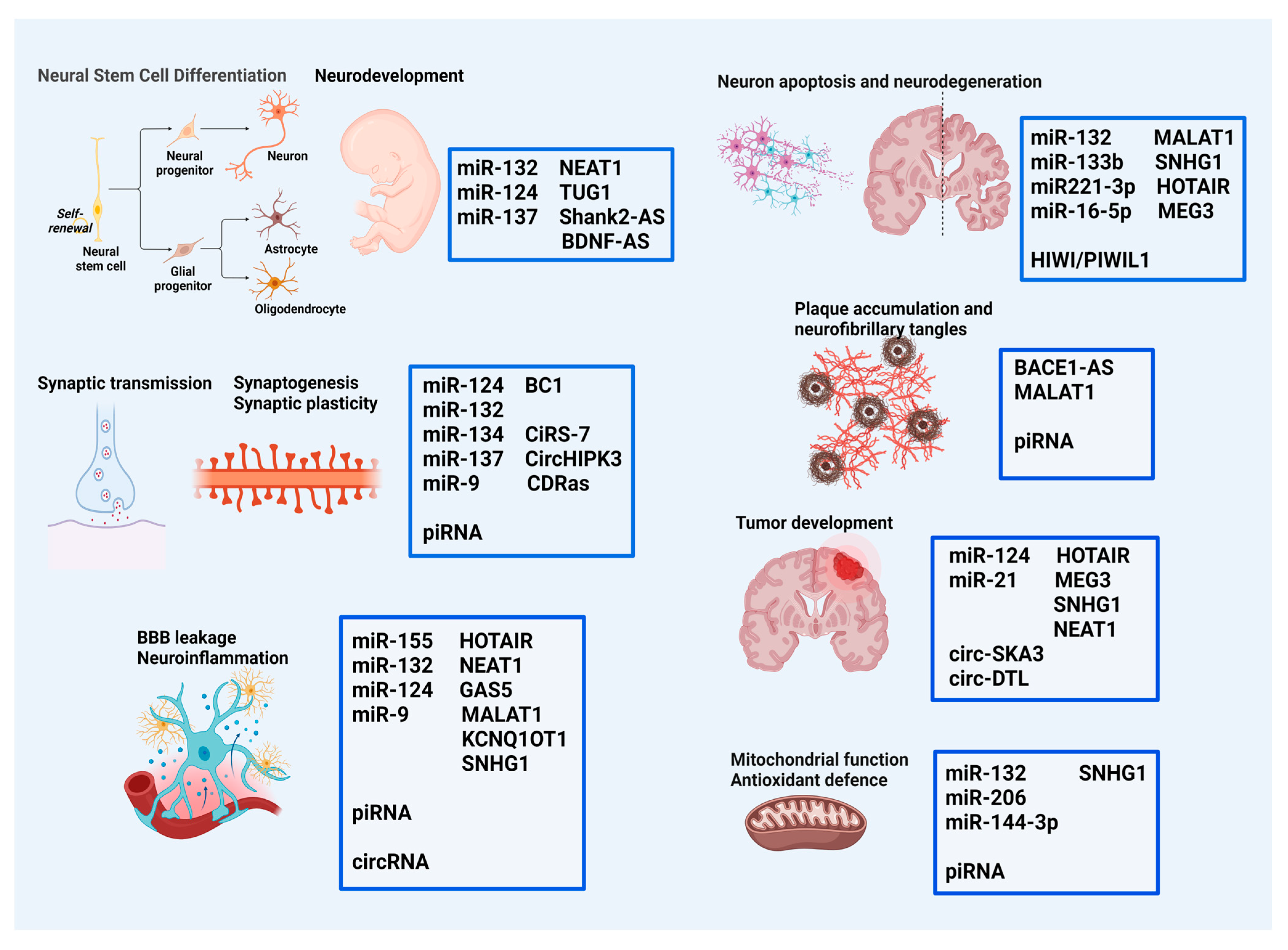
| miRNA | Target | Cellular/Molecular Events | Disorders/Pathological Conditions |
|---|---|---|---|
| miR-132 | MeCP2 p250GAP CREB FOXO3 RhoA GABAR EPAC1 Il-6 p300 FOXO3 AchE | Neurite outgrowth Dendritic arborization Synapse formation Synaptic plasticity Neuronal excitability Glia activation Apoptosis Oxidative stress Inflammation | Cognitive deficit Neurodegeneration Neurodevelopmental disorders Stroke Traumatic brain injury |
| miR-124 | Sox9 PTBP1 LIMK1 NRXN1 KCC2 C/EBP-α GFAP CDK6 BCL2L12 MMP-9 SNAI2 | Neural stem cell maintaining Neural differentiation Synaptic plasticity Neuronal connectivity Neuroinflammation Tumor suppression | Cognitive decline Neurodegeneration Stroke Brain tumors |
| miR-137 | Ezh2 Sox2 Tbr2 NRG1 GRIN2A PSD95 | Neurogenesis Synaptic function Plasticity | Cognitive dysfunction |
| miR-155 | SOCS1 SHIP1 Claudin 1 ZO-1 | Neuroinflammation BBB integrity | Neurodegenerative disorders Cerebral ischemia |
| miR-9 | TLX FOXG1 REST NFkβ GFAP HDAC5 MECP2 | Neurogenesis Neural differentiation Neuroinflammation Chromatin remodeling Transcription activity | Neurodegeneration Stroke Traumatic brain injury |
| miR-134 | LIMK1 CREB Pumilio DCX | Synaptic plasticity Dendritic spine morphology GABA-ergic signaling | Epilepsy Schizophrenia |
| miR-34a | NFkb STAT1 c-Fos CREB P53 | Synaptic plasticity Glycolysis Oxidative phosphorylation | Neurodegeneration |
| miR-21 | PTEN PDCD4 | Oncogenic effect | Glioblastoma |
| miR-206 | HDAC4, BDNF, MEF2 | Translation, protein aggregation, oxidative stress Regeneration of neuromuscular synapses | ALS |
| miR-16-5p | BDNF | Apoptosis | Huntington’s disease |
| lncRNA | Target | Cellular/Molecular Events | Disorders/Pathological Conditions |
|---|---|---|---|
| BACE1-AS | BACE1 | Aβ production | Alzheimer’s disease |
| NEAT1 | Paraspeckels SFPQ | Transcriptional activity RNA processing mRNA splicing Cell cycle progression EMT DNA damage response pathways | Brain tumors Stroke |
| HOTAIR | Chromatin-modifying complexes—PRC2, LSD1 | Histone methylation and acetylation Neuroinflammatory responses | Neurodevelopmental disorders Neurodegeneration Multiple sclerosis Stroke |
| SNHG1 | Molecular sponge of miR-125b-5p, miR-216a-3p, miR-7, miR-194 | Neuroinflammation Apoptosis Autophagy | Parkinson’s disease Glioma |
| MALAT1 | Recruitment of SR family pre-mRNA-splicing factor | Regulation of gene expression, alternative splicing, epigenetic modifications Synapse formation Oxidative stress, apoptosis, neuroinflammation | Neurodegeneration AD, PD Brain tumors Stroke |
| GAS5 | Inhibition of TRF4 by binding to PRC2 | Inhibition of M2 polarization | Multiple sclerosis |
| BC1 | Translational repressor in ImGluR-stimulated pathways | Synaptic transmission | Epilepsy |
| MEG3 | miRNA sponge | Tumor suppressor Cell proliferation, apoptosis, and autophagy. | Glioma |
| TUG1 | miR-9 inhibition | Proliferation, migration, differentiation of NPC | ASD Schizophrenia |
| BDNF-AS | Inhibits BDNF expression | Neuronal growth and differentiation, synaptic plasticity | Neurodevelopmental diseases |
| SHANK-AS | Inhibit SHANK2 expression | Neurite number and length, apoptosis | Neurodevelopmental diseases |
| KCNQ1OT1 | Binds PRC2 and G9a | Epigenetic regulation of immune cells functions and inflammatory responses | Multiple sclerosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilieva, M.S. Non-Coding RNAs in Neurological and Neuropsychiatric Disorders: Unraveling the Hidden Players in Disease Pathogenesis. Cells 2024, 13, 1063. https://doi.org/10.3390/cells13121063
Ilieva MS. Non-Coding RNAs in Neurological and Neuropsychiatric Disorders: Unraveling the Hidden Players in Disease Pathogenesis. Cells. 2024; 13(12):1063. https://doi.org/10.3390/cells13121063
Chicago/Turabian StyleIlieva, Mirolyuba Simeonova. 2024. "Non-Coding RNAs in Neurological and Neuropsychiatric Disorders: Unraveling the Hidden Players in Disease Pathogenesis" Cells 13, no. 12: 1063. https://doi.org/10.3390/cells13121063
APA StyleIlieva, M. S. (2024). Non-Coding RNAs in Neurological and Neuropsychiatric Disorders: Unraveling the Hidden Players in Disease Pathogenesis. Cells, 13(12), 1063. https://doi.org/10.3390/cells13121063






