Resveratrol Induces Myotube Development by Altering Circadian Metabolism via the SIRT1-AMPK-PP2A Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Cell Viability Assay
2.3. Western Blot Analyses
2.4. RNA Extraction and Quantitative Real-Time PCR
2.5. Statistical Analyses
3. Results
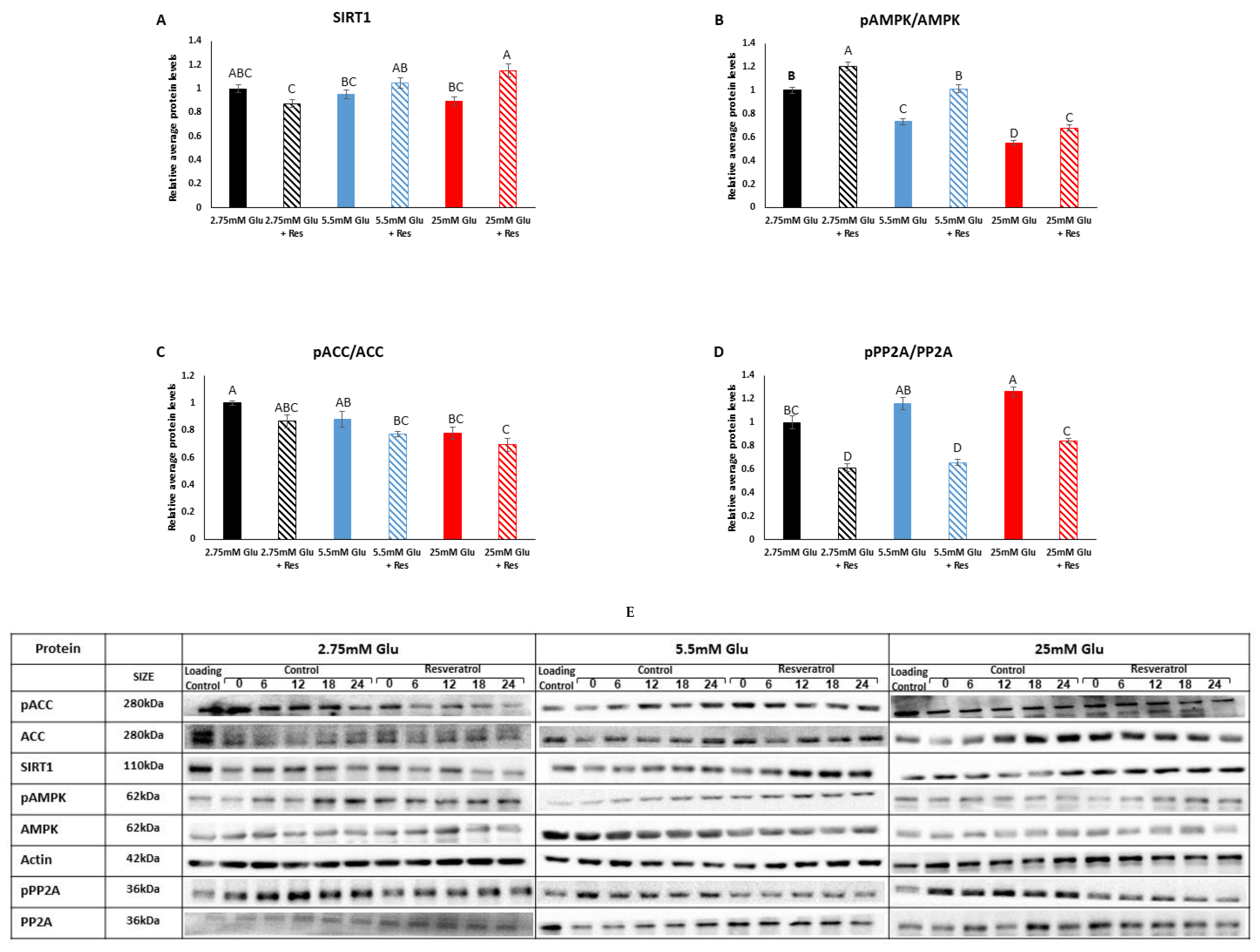
3.1. Effect of Resveratrol on the SIRT1-AMPK-PP2A Axis in Myotubes
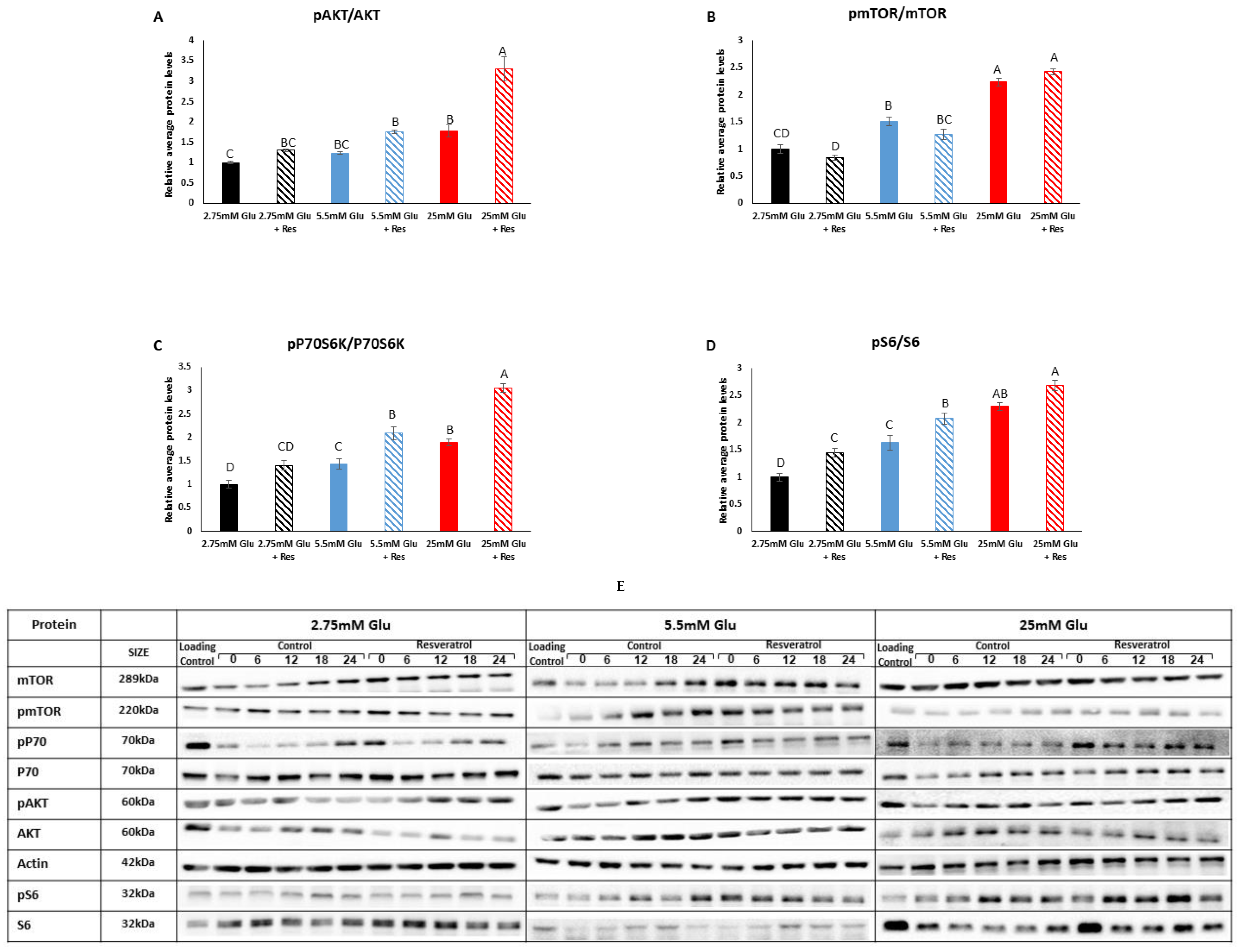
3.2. Effect of Resveratrol on the mTOR Signaling Pathway in Myotubes
3.3. Effect of Resveratrol on Circadian Rhythms in Myotubes
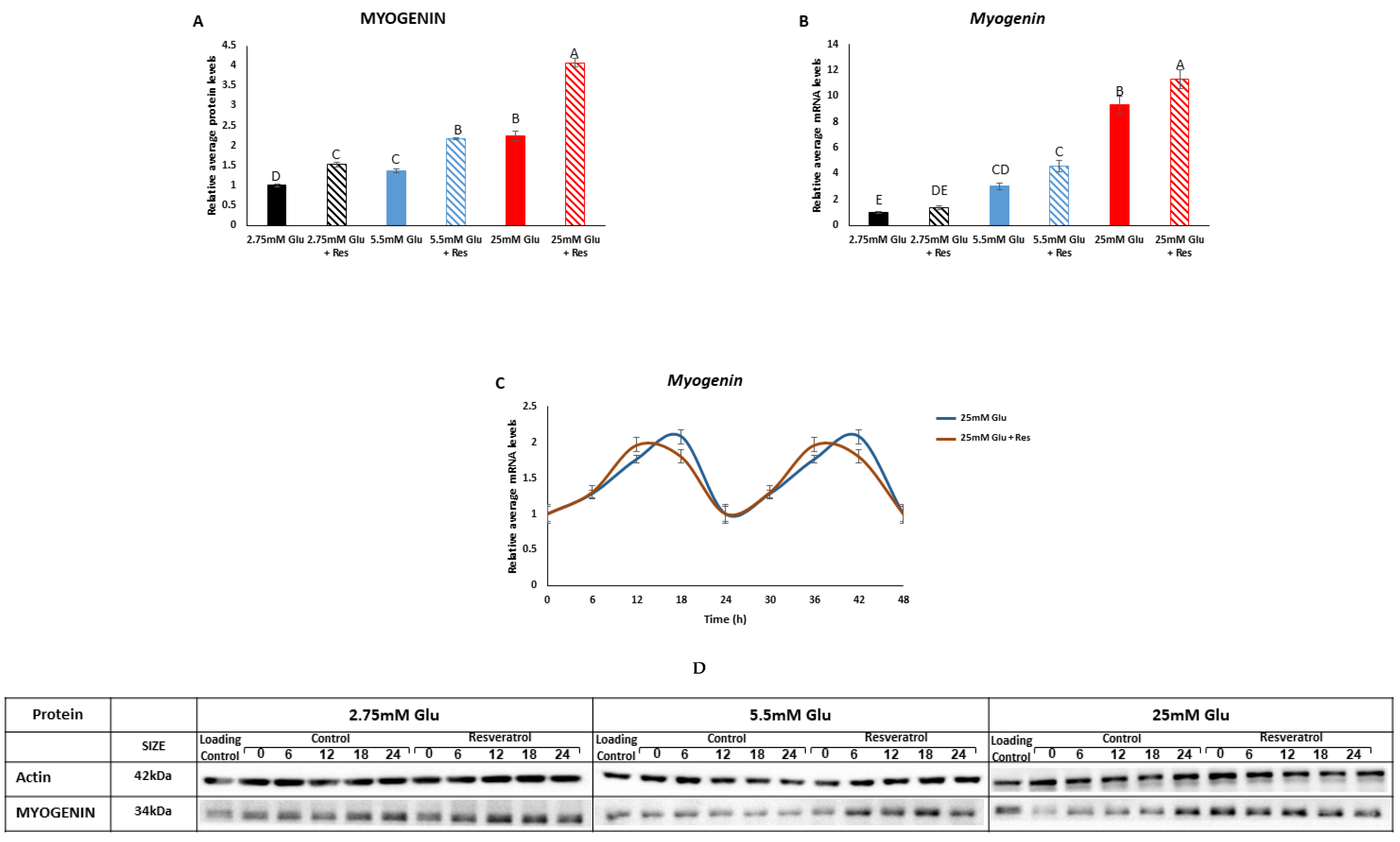
3.4. Effect of Resveratrol on Myotube Development
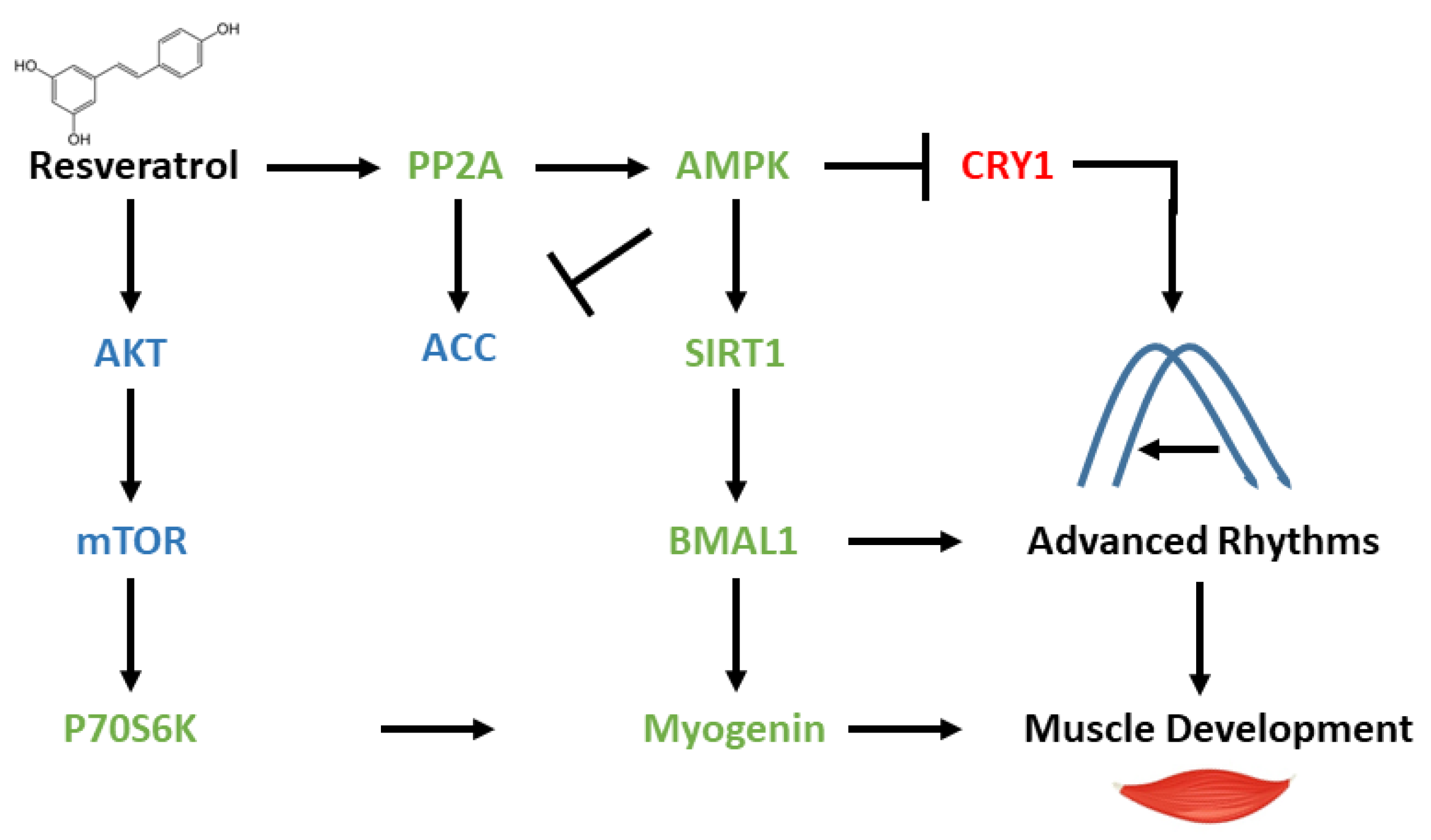
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Devlin, P.F. Signs of the time: Environmental input to the circadian clock. J. Exp. Bot. 2002, 53, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U. Timing to perfection: The biology of central and peripheral circadian clocks. Neuron 2012, 74, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Mahan, K.; Sassone-Corsi, P. Metabolism and the circadian clock converge. Physiol. Rev. 2013, 93, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Froy, O. Metabolism and circadian rhythms—Implications for obesity. Endocr. Rev. 2010, 31, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Froy, O.; Garaulet, M. The Circadian Clock in White and Brown Adipose Tissue: Mechanistic, Endocrine, and Clinical Aspects. Endocr. Rev. 2018, 39, 261–273. [Google Scholar] [CrossRef]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Marhuenda-Muñoz, M.; Storniolo, C.E.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutrients 2018, 10, 1892. [Google Scholar] [CrossRef] [PubMed]
- Mikstacka, R.; Rimando, A.M.; Ignatowicz, E. Antioxidant effect of trans-resveratrol, pterostilbene, quercetin and their combinations in human erythrocytes in vitro. Plant Foods Hum. Nutr. 2010, 65, 57–63. [Google Scholar] [CrossRef]
- Chatam, O.; Chapnik, N.; Froy, O. Resveratrol Induces the Fasting State and Alters Circadian Metabolism in Hepatocytes. Plant Foods Hum. Nutr. 2022, 77, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Spaleniak, W.; Cuendet, M. Resveratrol as a circadian clock modulator: Mechanisms of action and therapeutic applications. Mol. Biol. Rep. 2023, 50, 6159–6170. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Ho, C.T.; Chen, Y.K. Biological actions and molecular effects of resveratrol, pterostilbene, and 3′-hydroxypterostilbene. J. Food Drug Anal. 2017, 25, 134–147. [Google Scholar] [CrossRef]
- Okada, Y.; Okada, M. Quercetin, caffeic acid and resveratrol regulate circadian clock genes and aging-related genes in young and old human lung fibroblast cells. Mol. Biol. Rep. 2020, 47, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Guarente, L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 2013, 153, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Anand, S.K.; Singh, N.; Dwivedi, U.N.; Kakkar, P. AMP-activated protein kinase: An energy sensor and survival mechanism in the reinstatement of metabolic homeostasis. Exp. Cell Res. 2023, 428, 113614. [Google Scholar] [CrossRef] [PubMed]
- Martinet, W.; De Loof, H.; De Meyer, G.R.Y. mTOR inhibition: A promising strategy for stabilization of atherosclerotic plaques. Atherosclerosis 2014, 233, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Weikel, K.A.; Cacicedo, J.M.; Ido, Y. Resveratrol-Induced AMP-Activated Protein Kinase Activation Is Cell-Type Dependent: Lessons from Basic Research for Clinical Application. Nutrients 2017, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.T.; Coustry, F.; Veerisetty, A.C.; Hossain, M.G.; Posey, K.L. Resveratrol Reduces COMPopathy in Mice Through Activation of Autophagy. JBMR Plus 2021, 5, e10456. [Google Scholar] [CrossRef]
- Guo, S.; Chen, C.; Ji, F.; Mao, L.; Xie, Y. PP2A catalytic subunit silence by microRNA-429 activates AMPK and protects osteoblastic cells from dexamethasone. Biochem. Biophys. Res. Commun. 2017, 487, 660–665. [Google Scholar] [CrossRef]
- Perera, N.D.; Sheean, R.K.; Scott, J.W.; Kemp, B.E.; Horne, M.K.; Turner, B.J. Mutant TDP-43 deregulates AMPK activation by PP2A in ALS models. PLoS ONE 2014, 9, e95549. [Google Scholar] [CrossRef] [PubMed]
- Lipton, J.O.; Yuan, E.D.; Boyle, L.M.; Ebrahimi-Fakhari, D.; Kwiatkowski, E.; Nathan, A.; Güttler, T.; Davis, F.; Asara, J.M.; Sahin, M. The Circadian Protein BMAL1 Regulates Translation in Response to S6K1-Mediated Phosphorylation. Cell 2015, 161, 1138–1151. [Google Scholar] [CrossRef]
- Keerthana, C.K.; Rayginia, T.P.; Shifana, S.C.; Anto, N.P.; Kalimuthu, K.; Isakov, N.; Anto, R.J. The role of AMPK in cancer metabolism and its impact on the immunomodulation of the tumor microenvironment. Front. Immunol. 2023, 14, 1114582. [Google Scholar] [CrossRef]
- Luo, D.X.; Tong, D.J.; Rajput, S.; Wang, C.; Liao, D.F.; Cao, D.; Maser, E. Targeting acetyl-CoA carboxylases: Small molecular inhibitors and their therapeutic potential. Recent. Pat. Anticancer Drug. Discov. 2012, 7, 168–184. [Google Scholar] [CrossRef]
- Schweiger, S.; Matthes, F.; Posey, K.; Kickstein, E.; Weber, S.; Hettich, M.M.; Pfurtscheller, S.; Ehninger, D.; Schneider, R.; Krauß, S. Resveratrol induces dephosphorylation of Tau by interfering with the MID1-PP2A complex. Sci. Rep. 2017, 7, 13753. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.; Sun, C.; Zhang, H.; Xu, C.; Liu, W.; Gao, W.; Huang, S.; Chen, L. Resveratrol prevents cadmium activation of Erk1/2 and JNK pathways from neuronal cell death via protein phosphatases 2A and 5. J. Neurochem. 2015, 135, 466–478. [Google Scholar] [CrossRef]
- Lu, C.; Xing, H.; Yang, L.; Chen, K.; Shu, L.; Zhao, X.; Song, G. Resveratrol Ameliorates High-Fat-Diet-Induced Abnormalities in Hepatic Glucose Metabolism in Mice via the AMP-Activated Protein Kinase Pathway. Evid. Based Complement. Alternat. Med. 2021, 2021, 6616906. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.K.; Liu, H.Y.; Francisco, J.; Pandya, D.; Donigan, M.; Gallo-Ebert, C.; Giordano, C.; Bata, A.; Nickels, J.T. Inhibition of AMP Kinase by the Protein Phosphatase 2A Heterotrimer, PP2APpp2r2d. J. Biol. Chem. 2015, 290, 10588–10598. [Google Scholar] [CrossRef] [PubMed]
- Shati, A.A.; Alfaifi, M.Y. Trans-resveratrol Inhibits Tau Phosphorylation in the Brains of Control and Cadmium Chloride-Treated Rats by Activating PP2A and PI3K/Akt Induced-Inhibition of GSK3beta. Neurochem. Res. 2019, 44, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Baek, A.; Hwang, J.E.; Choi, Y.A.; Jeong, J.; Lee, M.S.; Cho, D.H.; Lim, J.S.; Kim, K.I.; Yang, Y. Adiponectin-activated AMPK stimulates dephosphorylation of AKT through protein phosphatase 2A activation. Cancer Res. 2009, 69, 4018–4026. [Google Scholar] [CrossRef]
- Chen, B.; Li, J.; Zhu, H. AMP-activated protein kinase attenuates oxLDL uptake in macrophages through PP2A/NF-kappaB/LOX-1 pathway. Vascul. Pharmacol. 2016, 85, 1–10. [Google Scholar] [CrossRef]
- Hong, K.; Lou, L.; Gupta, S.; Ribeiro-Neto, F.; Altschuler, D.L. A novel Epac-Rap-PP2A signaling module controls cAMP-dependent Akt regulation. J. Biol. Chem. 2008, 283, 23129–23138. [Google Scholar] [CrossRef]
- Tohmé, R.; Izadmehr, S.; Gandhe, S.; Tabaro, G.; Vallabhaneni, S.; Thomas, A.; Vasireddi, N.; Dhawan, N.S.; Ma’ayan, A.; Sharma, N.; et al. Direct activation of PP2A for the treatment of tyrosine kinase inhibitor-resistant lung adenocarcinoma. JCI Insight 2019, 4, e125693. [Google Scholar] [CrossRef]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids 2016, 48, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; Pereira, S.L.; Edens, N.K.; Hao, Y.; Bennett, B.T. beta-Hydroxy-beta-methylbutyrate (HMB) enhances the proliferation of satellite cells in fast muscles of aged rats during recovery from disuse atrophy. Exp. Gerontol. 2013, 48, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, G.D.; Rosa, J.C.; Lira, F.S.; Zanchi, N.E.; Ropelle, E.R.; Oyama, L.M.; Oller do Nascimento, C.M.; de Mello, M.T.; Tufik, S.; Santos, R.V. beta-Hydroxy-beta-methylbutyrate (HMbeta) supplementation stimulates skeletal muscle hypertrophy in rats via the mTOR pathway. Nutr. Metab. 2011, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Eley, H.L.; Russell, S.T.; Baxter, J.H.; Mukerji, P.; Tisdale, M.J. Signaling pathways initiated by beta-hydroxy-beta-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E923–E931. [Google Scholar] [CrossRef] [PubMed]
- Sakushima, K.; Yoshikawa, M.; Osaki, T.; Miyamoto, N.; Hashimoto, T. Moderate hypoxia promotes skeletal muscle cell growth and hypertrophy in C2C12 cells. Biochem. Biophys. Res. Commun. 2020, 525, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Asfour, H.A.; Allouh, M.Z.; Said, R.S. Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery. Exp. Biol. Med. 2018, 243, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Hasty, P.; Bradley, A.; Morris, J.H.; Edmondson, D.G.; Venuti, J.M.; Olson, E.N.; Klein, W.H. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 1993, 364, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Myer, A.; Olson, E.N.; Klein, W.H. MyoD cannot compensate for the absence of myogenin during skeletal muscle differentiation in murine embryonic stem cells. Dev. Biol. 2001, 229, 340–350. [Google Scholar] [CrossRef]
- Dadon-Freiberg, M.; Chapnik, N.; Froy, O. REV-ERBalpha activates the mTOR signalling pathway and promotes myotubes differentiation. Biol. Cell 2020, 112, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, E.K. AMP-activated protein kinase as a key molecular link between metabolism and clockwork. Exp. Mol. Med. 2013, 45, e33. [Google Scholar] [CrossRef]
- Lamia, K.A.; Sachdeva, U.M.; DiTacchio, L.; Williams, E.C.; Alvarez, J.G.; Egan, D.F.; Vasquez, D.S.; Juguilon, H.; Panda, S.; Shaw, R.J.; et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 2009, 326, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.L.; Zhang, X.; McCarthy, J.J.; McDearmon, E.L.; Hornberger, T.A.; Russell, B.; Campbell, K.S.; Arbogast, S.; Reid, M.B.; Walker, J.R.; et al. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc. Natl. Acad. Sci. USA 2010, 107, 19090–19095. [Google Scholar] [CrossRef] [PubMed]
- Bozek, K.; Relógio, A.; Kielbasa, S.M.; Heine, M.; Dame, C.; Kramer, A.; Herzel, H. Regulation of clock-controlled genes in mammals. PLoS ONE 2009, 4, e4882. [Google Scholar] [CrossRef] [PubMed]
- Harfmann, B.D.; Schroder, E.A.; Esser, K.A. Circadian rhythms, the molecular clock, and skeletal muscle. J. Biol. Rhythms 2015, 30, 84–94. [Google Scholar] [CrossRef]
- Shavlakadze, T.; Anwari, T.; Soffe, Z.; Cozens, G.; Mark, P.J.; Gondro, C.; Grounds, M.D. Impact of fasting on the rhythmic expression of myogenic and metabolic factors in skeletal muscle of adult mice. Am. J. Physiol. Cell Physiol. 2013, 305, C26–C35. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avital-Cohen, N.; Chapnik, N.; Froy, O. Resveratrol Induces Myotube Development by Altering Circadian Metabolism via the SIRT1-AMPK-PP2A Axis. Cells 2024, 13, 1069. https://doi.org/10.3390/cells13121069
Avital-Cohen N, Chapnik N, Froy O. Resveratrol Induces Myotube Development by Altering Circadian Metabolism via the SIRT1-AMPK-PP2A Axis. Cells. 2024; 13(12):1069. https://doi.org/10.3390/cells13121069
Chicago/Turabian StyleAvital-Cohen, Natalie, Nava Chapnik, and Oren Froy. 2024. "Resveratrol Induces Myotube Development by Altering Circadian Metabolism via the SIRT1-AMPK-PP2A Axis" Cells 13, no. 12: 1069. https://doi.org/10.3390/cells13121069




