The Influence of Metabolic Risk Factors on the Inflammatory Response Triggered by Myocardial Infarction: Bridging Pathophysiology to Treatment
Abstract
1. Introduction
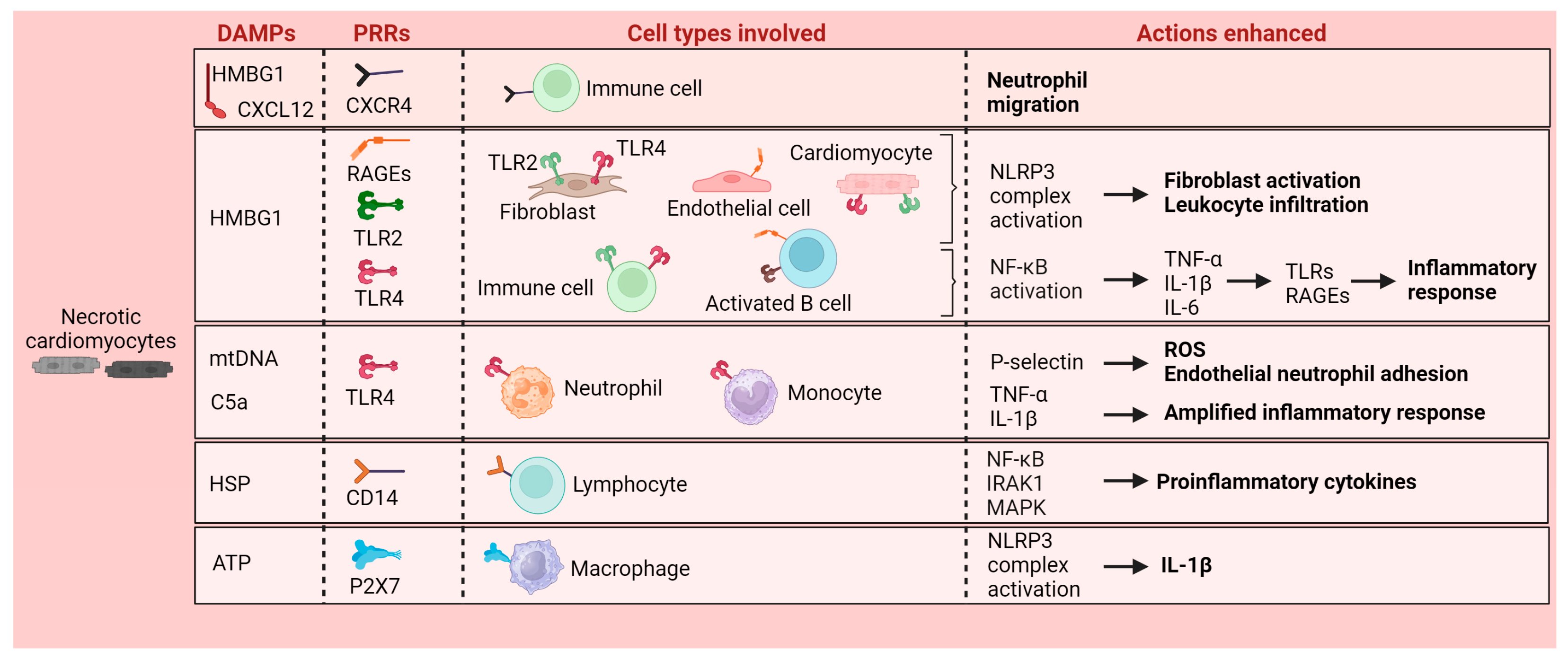
2. Local Inflammatory Response Triggered by Myocardial Infarction
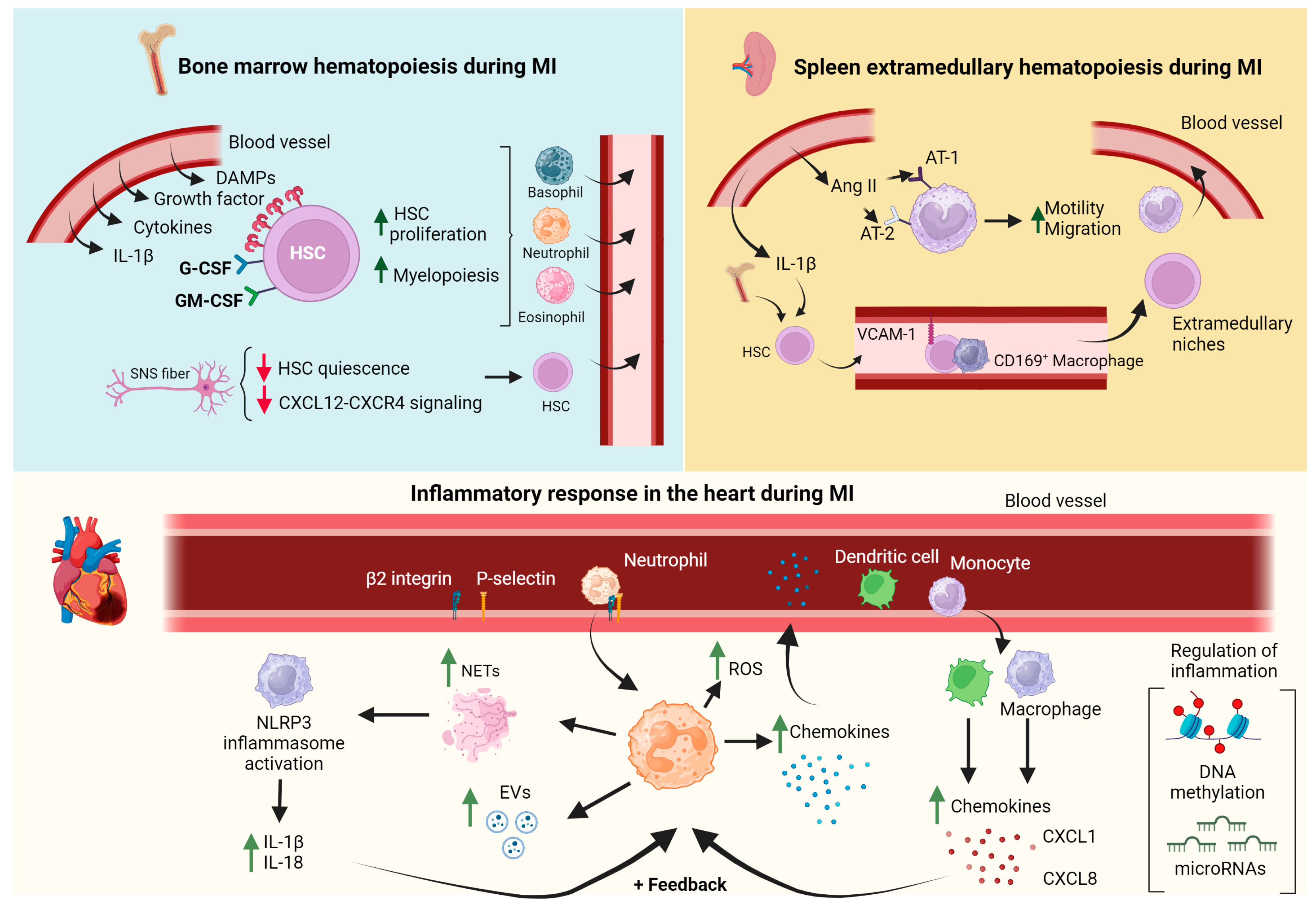
3. The Involvement of the Bone Marrow and Spleen in the Systemic Inflammatory Response following Myocardial Infarction
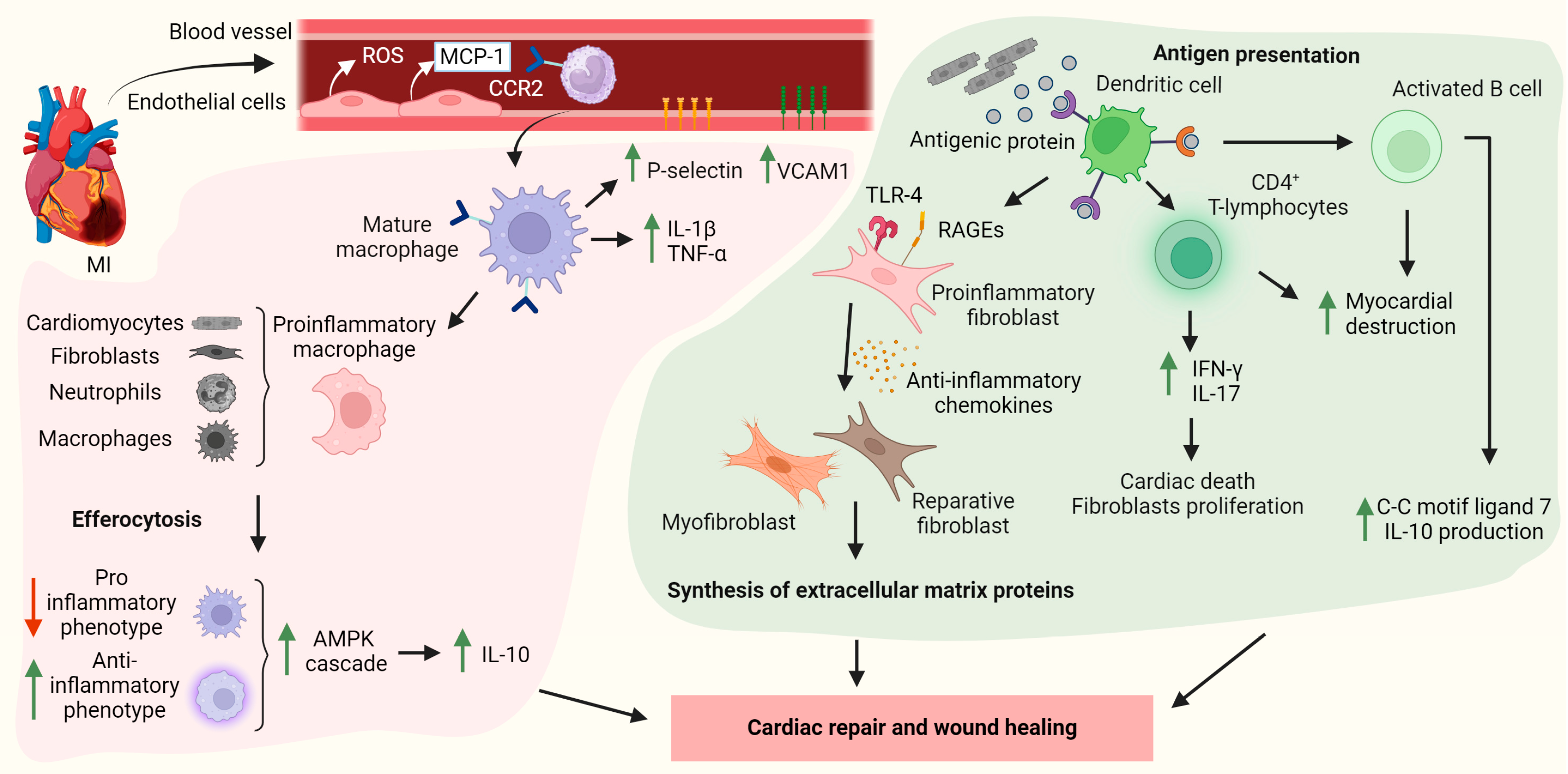
4. The Migration and Recruitment of Immune Cells to the Infarcted Heart
4.1. Neutrophils
4.2. Monocyte/Macrophages
4.3. Dendritic Cells
4.4. Lymphocytes
5. The Impact of Metabolic Cardiovascular Risk Factors on the Myocardial Infarction-Induced Inflammatory Response
6. Impact of Lifestyle Changes and Therapeutic Approaches
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The Global Prevalence of Myocardial Infarction: A Systematic Review and Meta-Analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef]
- Vilahur, G.; Badimon, J.J.; Bugiardini, R.; Badimon, L. Perspectives: The Burden of Cardiovascular Risk Factors and Coronary Heart Disease in Europe and Worldwide. Eur. Heart J. Suppl. 2014, 16, A7–A11. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The Inflammatory Response in Myocardial Injury, Repair, and Remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Vilahur, G.; Juan-Babot, O.; Peña, E.; Oñate, B.; Casaní, L.; Badimon, L. Molecular and Cellular Mechanisms Involved in Cardiac Remodeling after Acute Myocardial Infarction. JMCC 2011, 50, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Cabrera-Fuentes, H.A.; Devaux, Y.; Frangogiannis, N.G.; Frantz, S.; Guzik, T.; Liehn, E.A.; Gomes, C.P.C.; Schulz, R.; Hausenloy, D.J. Immune Cells as Targets for Cardioprotection: New Players and Novel Therapeutic Opportunities. Cardiovasc. Res. 2019, 115, 1117–1130. [Google Scholar] [CrossRef]
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Josef, K.; Adámková, V.; Wohlfahrt, P. Heart Failure after Myocardial Infarction: Incidence and Predictors. ESC Heart Fail. 2021, 8, 222–237. [Google Scholar] [CrossRef]
- Gerdts, E.; Regitz-Zagrosek, V. Sex Differences in Cardiometabolic Disorders. Nat. Med. 2019, 25, 1657–1666. [Google Scholar] [CrossRef]
- Teo, K.K.; Rafiq, T. Cardiovascular Risk Factors and Prevention: A Perspective from Developing Countries. Can. J. Cardiol. 2021, 37, 733–743. [Google Scholar] [CrossRef]
- Vilahur, G.; Casani, L.; Juan-Babot, O.; Guerra, J.M.; Badimon, L. Infiltrated Cardiac Lipids Impair Myofibroblast-Induced Healing of the Myocardial Scar Post-Myocardial Infarction. Atherosclerosis 2012, 224, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Schulz, R.; Badimon, L.; Adameová, A.; Kleinbongard, P.; Lecour, S.; Nikolaou, P.; Falcão-Pires, I.; Vilahur, G.; Woudberg, N.; et al. Hyperlipidaemia and Cardioprotection: Animal Models for Translational Studies. Br. J. Pharmacol. 2020, 177, 5287–5311. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N. The Immune System and Cardiac Repair. Pharmacol. Res. 2008, 58, 88–111. [Google Scholar] [CrossRef] [PubMed]
- Anzai, A.; Ko, S.; Fukuda, K. Immune and Inflammatory Networks in Myocardial Infarction: Current Research and Its Potential Implications for the Clinic. IJMS 2022, 23, 5214. [Google Scholar] [CrossRef] [PubMed]
- Foglio, E.; Pellegrini, L.; Russo, M.A.; Limana, F. HMGB1-Mediated Activation of the Inflammatory-Reparative Response Following Myocardial Infarction. Cells 2022, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Jaén, R.I.; Val-Blasco, A.; Prieto, P.; Gil-Fernández, M.; Smani, T.; López-Sendón, J.L.; Delgado, C.; Boscá, L.; Fernández-Velasco, M. Innate Immune Receptors, Key Actors in Cardiovascular Diseases. JACC Basic Transl. Sci. 2020, 5, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G.; Badimon, L. Ischemia/Reperfusion Activates Myocardial Innate Immune Response: The Key Role of the Toll-like Receptor. Front. Physiol. 2014, 5, 496. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-C.; Ghanem, A.; Stapel, H.; Tiemann, K.; Knuefermann, P.; Hoeft, A.; Meyer, R.; Grohé, C.; Knowlton, A.A.; Baumgarten, G. Toll-like Receptor 4 Deficiency: Smaller Infarcts, but Nogain in Function. BMC Physiol. 2007, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Jäger, S.; Sobirey, M.; Escher, F.; Yaulema-Riss, A.; Westermann, D.; Karatas, A.; Heimesaat, M.M.; Bereswill, S.; Dragun, D.; et al. Toll-Like Receptor-4 Modulates Survival by Induction of Left Ventricular Remodeling after Myocardial Infarction in Mice. J. Immun. 2008, 180, 6954–6961. [Google Scholar] [CrossRef]
- Bucciarelli, L.G.; Ananthakrishnan, R.; Hwang, Y.C.; Kaneko, M.; Song, F.; Sell, D.R.; Strauch, C.; Monnier, V.M.; Yan, S.F.; Schmidt, A.M.; et al. RAGE and Modulation of Ischemic Injury in the Diabetic Myocardium. Diabetes 2008, 57, 1941–1951. [Google Scholar] [CrossRef]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. The Receptor for Advanced Glycation Endproducts (RAGE) and Cardiovascular Disease. Expert Rev. Mol. Med. 2009, 11, e9. [Google Scholar] [CrossRef]
- Hohensinner, P.J.; Niessner, A.; Huber, K.; Weyand, C.M.; Wojta, J. Inflammation and Cardiac Outcome. Curr. Opin. Infect. Dis. 2011, 24, 259–264. [Google Scholar] [CrossRef]
- Chen, B.; Frangogiannis, N.G. Chemokines in Myocardial Infarction. J. Cardiovasc. Trans. Res. 2021, 14, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Torp, M.-K.; Vaage, J.; Stensløkken, K.-O. Mitochondria-Derived Damage-Associated Molecular Patterns and Inflammation in the Ischemic-Reperfused Heart. Acta Physiol. 2023, 237, e13920. [Google Scholar] [CrossRef] [PubMed]
- Longnus, S.L.; Rutishauser, N.; Gillespie, M.N.; Reichlin, T.; Carrel, T.P.; Sanz, M.N. Mitochondrial Damage-Associated Molecular Patterns as Potential Biomarkers in DCD Heart Transplantation: Lessons from Myocardial Infarction and Cardiac Arrest. Transplant. Direct 2021, 8, e1265. [Google Scholar] [CrossRef] [PubMed]
- Barbalata, T.; Scarlatescu, A.I.; Sanda, G.M.; Toma, L.; Stancu, C.S.; Dorobantu, M.; Micheu, M.M.; Sima, A.V.; Niculescu, L.S. Mitochondrial DNA Together with miR-142-3p in Plasma Can Predict Unfavorable Outcomes in Patients after Acute Myocardial Infarction. IJMS 2022, 23, 9947. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; Guo, L.; Wei, Z.; Wang, X.; Zhao, L.; Kang, L.; Wang, K.; Zhou, W.; Cheng, S.; Yin, S.; et al. Platelet Mitochondrial DNA Methylation: A Novel Biomarker for Myocardial Infarction—A Preliminary Study. Int. J. Cardiol. 2024, 398, 131606. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The Immune System and the Remodeling Infarcted Heart: Cell Biological Insights and Therapeutic Opportunities. J. Cardiovasc. Pharmacol. 2014, 63, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, T.; Mandal, A.; Mandal, M.; Das, S.; Chakraborti, S. Complement Activation in Heart Diseases: Role of Oxidants. Cell. Signal. 2000, 12, 607–617. [Google Scholar] [CrossRef]
- Toldo, S.; Abbate, A. The Role of the NLRP3 Inflammasome and Pyroptosis in Cardiovascular Diseases. Nat. Rev. Cardiol. 2023, 21, 219–237. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Liu, Y.; Liu, Z.; Wang, D.; Cheng, Y. Therapeutic Perspectives of Heat Shock Proteins and Their Protein-Protein Interactions in Myocardial Infarction. Pharmacol. Res. 2020, 160, 105162. [Google Scholar] [CrossRef]
- Chen, Z.; He, L.; Li, L.; Chen, L. The P2X7 Purinergic Receptor: An Emerging Therapeutic Target in Cardiovascular Diseases. Clin. Chim. Acta 2018, 479, 196–207. [Google Scholar] [CrossRef]
- Yin, J.; Wang, Y.; Hu, H.; Li, X.; Xue, M.; Cheng, W.; Wang, Y.; Li, X.; Yang, N.; Shi, Y.; et al. P2X7 Receptor Inhibition Attenuated Sympathetic Nerve Sprouting after Myocardial Infarction via the NLRP3/IL-1β Pathway. J. Cell. Mol. Med. 2017, 21, 2695–2710. [Google Scholar] [CrossRef]
- Zapata-Martínez, L.; Águila, S.; De Los Reyes-García, A.M.; Carrillo-Tornel, S.; Lozano, M.L.; González-Conejero, R.; Martínez, C. Inflammatory microRNAs in Cardiovascular Pathology: Another Brick in the Wall. Front. Immunol. 2023, 14, 1196104. [Google Scholar] [CrossRef]
- Varzideh, F.; Kansakar, U.; Donkor, K.; Wilson, S.; Jankauskas, S.S.; Mone, P.; Wang, X.; Lombardi, A.; Santulli, G. Cardiac Remodeling after Myocardial Infarction: Functional Contribution of microRNAs to Inflammation and Fibrosis. Front. Cardiovasc. Med. 2022, 9, 863238. [Google Scholar] [CrossRef]
- Sessa, F.; Salerno, M.; Esposito, M.; Cocimano, G.; Pisanelli, D.; Malik, A.; Khan, A.A.; Pomara, C. New Insight into Mechanisms of Cardiovascular Diseases: An Integrative Analysis Approach to Identify TheranoMiRNAs. IJMS 2023, 24, 6781. [Google Scholar] [CrossRef]
- Sessa, F.; Salerno, M.; Esposito, M.; Cocimano, G.; Pomara, C. miRNA Dysregulation in Cardiovascular Diseases: Current Opinion and Future Perspectives. IJMS 2023, 24, 5192. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Wang, W.; Wang, Q.; Maduray, K.; Hao, L.; Zhong, J. A Review on Regulation of DNA Methylation during Post-Myocardial Infarction. Front. Pharmacol. 2024, 15, 1267585. [Google Scholar] [CrossRef]
- Fang, L.; Moore, X.-L.; Dart, A.M.; Wang, L.-M. Systemic Inflammatory Response Following Acute Myocardial Infarction. J. Geriatr. Cardiol. 2015, 12, 305–312. [Google Scholar] [CrossRef]
- Vilahur, G.; Hernández-Vera, R.; Molins, B.; Casaní, L.; Duran, X.; Padró, T.; Badimon, L. Short-term Myocardial Ischemia Induces Cardiac Modified C-reactive Protein Expression and Proinflammatory Gene (Cyclo-oxygenase-2, Monocyte Chemoattractant Protein-1, and Tissue Factor) Upregulation in Peripheral Blood Mononuclear Cells. J. Thromb. Haemost. 2009, 7, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Scadden, D.T. The Bone Marrow Niche for Haematopoietic Stem Cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef]
- Poller, W.C.; Nahrendorf, M.; Swirski, F.K. Hematopoiesis and Cardiovascular Disease. Circ. Res. 2020, 126, 1061–1085. [Google Scholar] [CrossRef]
- Sager, H.B.; Heidt, T.; Hulsmans, M.; Dutta, P.; Courties, G.; Sebas, M.; Wojtkiewicz, G.R.; Tricot, B.; Iwamoto, Y.; Sun, Y.; et al. Targeting Interleukin-1β Reduces Leukocyte Production after Acute Myocardial Infarction. Circulation 2015, 132, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- Crane, G.M.; Jeffery, E.; Morrison, S.J. Adult Haematopoietic Stem Cell Niches. Nat. Rev. Immunol. 2017, 17, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Anzai, A.; Choi, J.L.; He, S.; Fenn, A.M.; Nairz, M.; Rattik, S.; McAlpine, C.S.; Mindur, J.E.; Chan, C.T.; Iwamoto, Y.; et al. The Infarcted Myocardium Solicits GM-CSF for the Detrimental Oversupply of Inflammatory Leukocytes. J. Exp. Med. 2017, 214, 3293–3310. [Google Scholar] [CrossRef] [PubMed]
- Seung, H.; Wrobel, J.; Wadle, C.; Bühler, T.; Chiang, D.; Rettkowski, J.; Cabezas-Wallscheid, N.; Hechler, B.; Stachon, P.; Maier, A.; et al. P2Y12-dependent Activation of Hematopoietic Stem and Progenitor Cells Promotes Emergency Hematopoiesis after Myocardial Infarction. Basic. Res. Cardiol. 2022, 117, 16. [Google Scholar] [CrossRef] [PubMed]
- Rohde, D.; Vandoorne, K.; Lee, I.-H.; Grune, J.; Zhang, S.; McAlpine, C.S.; Schloss, M.J.; Nayar, R.; Courties, G.; Frodermann, V.; et al. Bone Marrow Endothelial Dysfunction Promotes Myeloid Cell Expansion in Cardiovascular Disease. Nat. Cardiovasc. Res. 2021, 1, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Maryanovich, M.; Takeishi, S.; Frenette, P.S. Neural Regulation of Bone and Bone Marrow. Cold Spring Harb. Perspect. Med. 2018, 8, a031344. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M. Myeloid Cell Contributions to Cardiovascular Health and Disease. Nat. Med. 2018, 24, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, T.; Sugiyama, T.; Omatsu, Y.; Watanabe, H.; Kondoh, G.; Nagasawa, T. Ebf3+ Niche-Derived CXCL12 Is Required for the Localization and Maintenance of Hematopoietic Stem Cells. Nat. Commun. 2023, 14, 6402. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Courties, G.; Wei, Y.; Leuschner, F.; Gorbatov, R.; Robbins, C.S.; Iwamoto, Y.; Thompson, B.; Carlson, A.L.; Heidt, T.; et al. Myocardial Infarction Accelerates Atherosclerosis. Nature 2012, 487, 325–329. [Google Scholar] [CrossRef]
- Comazzetto, S.; Shen, B.; Morrison, S.J. Niches That Regulate Stem Cells and Hematopoiesis in Adult Bone Marrow. Dev. Cell 2021, 56, 1848–1860. [Google Scholar] [CrossRef]
- Fernández-García, V.; González-Ramos, S.; Martín-Sanz, P.; Castrillo, A.; Boscá, L. Contribution of Extramedullary Hematopoiesis to Atherosclerosis. The Spleen as a Neglected Hub of Inflammatory Cells. Front. Immunol. 2020, 11, 586527. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.-L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of Splenic Reservoir Monocytes and Their Deployment to Inflammatory Sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef]
- Leuschner, F.; Panizzi, P.; Chico-Calero, I.; Lee, W.W.; Ueno, T.; Cortez-Retamozo, V.; Waterman, P.; Gorbatov, R.; Marinelli, B.; Iwamoto, Y.; et al. Angiotensin-Converting Enzyme Inhibition Prevents the Release of Monocytes from Their Splenic Reservoir in Mice with Myocardial Infarction. Circ. Res. 2010, 107, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, F.; Rauch, P.J.; Ueno, T.; Gorbatov, R.; Marinelli, B.; Lee, W.W.; Dutta, P.; Wei, Y.; Robbins, C.; Iwamoto, Y.; et al. Rapid Monocyte Kinetics in Acute Myocardial Infarction Are Sustained by Extramedullary Monocytopoiesis. J. Exp. Med. 2012, 209, 123–137. [Google Scholar] [CrossRef]
- Dutta, P.; Hoyer, F.F.; Grigoryeva, L.S.; Sager, H.B.; Leuschner, F.; Courties, G.; Borodovsky, A.; Novobrantseva, T.; Ruda, V.M.; Fitzgerald, K.; et al. Macrophages Retain Hematopoietic Stem Cells in the Spleen via VCAM-1. J. Exp. Med. 2015, 212, 497–512. [Google Scholar] [CrossRef]
- Tawakol, A.; Ishai, A.; Takx, R.A.; Figueroa, A.L.; Ali, A.; Kaiser, Y.; Truong, Q.A.; Solomon, C.J.; Calcagno, C.; Mani, V.; et al. Relation between Resting Amygdalar Activity and Cardiovascular Events: A Longitudinal and Cohort Study. Lancet 2017, 389, 834–845. [Google Scholar] [CrossRef]
- Ma, Y.; Yabluchanskiy, A.; Lindsey, M.L. Neutrophil Roles in Left Ventricular Remodeling Following Myocardial Infarction. Fibrogenesis Tissue Repair 2013, 6, 11. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role during Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.A.; Sun, J.; Wang, J. Leukocyte-Mediated Cardiac Repair after Myocardial Infarction in Non-Regenerative vs. Regenerative Systems. JCDD 2022, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y. Role of Neutrophils in Cardiac Injury and Repair Following Myocardial Infarction. Cells 2021, 10, 1676. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, X.; Chatterjee, V.; Meegan, J.E.; Beard, R.S., Jr.; Yuan, S.Y. Role of Neutrophil Extracellular Traps and Vesicles in Regulating Vascular Endothelial Permeability. Front. Immunol. 2019, 10, 1037. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Kim, P.; Leuschner, F.; Gorbatov, R.; Kim, J.K.; Ueno, T.; Nahrendorf, M.; Yun, S.H. Endoscopic Time-Lapse Imaging of Immune Cells in Infarcted Mouse Hearts. Circ. Res. 2013, 112, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Stierschneider, A.; Wiesner, C. Shedding Light on the Molecular and Regulatory Mechanisms of TLR4 Signaling in Endothelial Cells under Physiological and Inflamed Conditions. Front. Immunol. 2023, 14, 1264889. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Bosch, M.; Simón-Codina, B.; Jiménez, W.; Edelman, E.R.; Melgar-Lesmes, P. Monocyte-Endothelial Cell Interactions in Vascular and Tissue Remodeling. Front. Immunol. 2023, 14, 1196033. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Swirski, F.K.; Aikawa, E.; Stangenberg, L.; Wurdinger, T.; Figueiredo, J.-L.; Libby, P.; Weissleder, R.; Pittet, M.J. The Healing Myocardium Sequentially Mobilizes Two Monocyte Subsets with Divergent and Complementary Functions. J. Exp. Med. 2007, 204, 3037–3047. [Google Scholar] [CrossRef] [PubMed]
- Lafuse, W.P.; Wozniak, D.J.; Rajaram, M.V.S. Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair. Cells 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, G.; Schneider, C.; Wong, N.; Bredemeyer, A.; Hulsmans, M.; Nahrendorf, M.; Epelman, S.; Kreisel, D.; Liu, Y.; Itoh, A.; et al. The Human Heart Contains Distinct Macrophage Subsets with Divergent Origins and Functions. Nat. Med. 2018, 24, 1234–1245. [Google Scholar] [CrossRef]
- Bajpai, G.; Bredemeyer, A.; Li, W.; Zaitsev, K.; Koenig, A.; Lokshina, I.; Mohan, J.; Ivey, B.; Hsiao, H.-M.; Weinheimer, C.; et al. Tissue Resident CCR2− and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ. Res. 2019, 2, 263–278. [Google Scholar] [CrossRef]
- Chen, B.; Brickshawana, A.; Frangogiannis, N.G. The Functional Heterogeneity of Resident Cardiac Macrophages in Myocardial Injury: CCR2+ Cells Promote Inflammation, Whereas CCR2− Cells Protect. Circ. Res. 2019, 124, 183–185. [Google Scholar] [CrossRef]
- Hitscherich, P.; Lee, E.J. Crosstalk between Cardiac Cells and Macrophages Postmyocardial Infarction: Insights from In Vitro Studies. Tissue Eng. Part B Rev. 2021, 27, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Kologrivova, I.; Shtatolkina, M.; Suslova, T.; Ryabov, V. Cells of the Immune System in Cardiac Remodeling: Main Players in Resolution of Inflammation and Repair after Myocardial Infarction. Front. Immunol. 2021, 12, 664457. [Google Scholar] [CrossRef] [PubMed]
- Wan, E.; Yeap, X.Y.; Dehn, S.; Terry, R.; Novak, M.; Zhang, S.; Iwata, S.; Han, X.; Homma, S.; Drosatos, K.; et al. Enhanced Efferocytosis of Apoptotic Cardiomyocytes through Myeloid-Epithelial-Reproductive Tyrosine Kinase Links Acute Inflammation Resolution to Cardiac Repair after Infarction. Circ. Res. 2013, 113, 1004–1012. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Inflammation in Cardiac Injury, Repair and Regeneration. Curr. Opin. Cardiol. 2015, 30, 240–245. [Google Scholar] [CrossRef]
- Shiraishi, M.; Shintani, Y.; Shintani, Y.; Ishida, H.; Saba, R.; Yamaguchi, A.; Adachi, H.; Yashiro, K.; Suzuki, K. Alternatively Activated Macrophages Determine Repair of the Infarcted Adult Murine Heart. J. Clin. Investig. 2016, 126, 2151–2166. [Google Scholar] [CrossRef]
- Dick, S.A.; Macklin, J.A.; Nejat, S.; Momen, A.; Clemente-Casares, X.; Althagafi, M.G.; Chen, J.; Kantores, C.; Hosseinzadeh, S.; Aronoff, L.; et al. Self-Renewing Resident Cardiac Macrophages Limit Adverse Remodeling Following Myocardial Infarction. Nat. Immunol. 2019, 20, 29–39. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Yu, M.; Yuan, W. Identification of Hub Genes in the Remodeling of Non-Infarcted Myocardium Following Acute Myocardial Infarction. JCDD 2022, 9, 409. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, W.; Hu, T.; Peng, H.; Hu, F.; Yuan, Y.; Liu, X.; Lai, S.; Zhou, J.; Dong, X. Unveiling Macrophage Diversity in Myocardial Ischemia-Reperfusion Injury: Identification of a Distinct Lipid-Associated Macrophage Subset. Front. Immunol. 2024, 15, 1335333. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Jeong, S.-J.; Kim, S.; Chalifour, L.; Yun, T.J.; Miah, M.A.; Li, B.; Majdoubi, A.; Sabourin, A.; Keler, T.; et al. Conventional Dendritic Cells Impair Recovery after Myocardial Infarction. J. Immunol. 2018, 201, 1784–1798. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, H.; Hanna, A.; Humeres, C.; Frangogiannis, N.G. Properties and Functions of Fibroblasts and Myofibroblasts in Myocardial Infarction. Cells 2022, 11, 1386. [Google Scholar] [CrossRef]
- Shinde, A.V.; Frangogiannis, N.G. Fibroblasts in Myocardial Infarction: A Role in Inflammation and Repair. J. Mol. Cell. Cardiol. 2014, 70, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Khalil, H.; Kanisicak, O.; Boyer, J.G.; Vagnozzi, R.J.; Maliken, B.D.; Sargent, M.A.; Prasad, V.; Valiente-Alandi, I.; Blaxall, B.C.; et al. Specialized Fibroblast Differentiated States Underlie Scar Formation in the Infarcted Mouse Heart. J. Clin. Investig. 2018, 128, 2127–2143. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.; Frantz, S. Role of T-Cells in Myocardial Infarction. Eur. Heart J. 2016, 37, 873–879. [Google Scholar] [CrossRef]
- Yan, X.; Shichita, T.; Katsumata, Y.; Matsuhashi, T.; Ito, H.; Ito, K.; Anzai, A.; Endo, J.; Tamura, Y.; Kimura, K.; et al. Deleterious Effect of the IL-23/IL-17A Axis and γδT Cells on Left Ventricular Remodeling after Myocardial Infarction. JAHA 2012, 1, e004408. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-H.; Cheng, X. Autoimmunity in Myocardial Infarction. Int. J. Cardiol. 2006, 112, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Ilatovskaya, D.V.; Pitts, C.; Clayton, J.; Domondon, M.; Troncoso, M.; Pippin, S.; DeLeon-Pennell, K.Y. CD8 + T-Cells Negatively Regulate Inflammation Post-Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H581–H596. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.; Frantz, S. Role of Lymphocytes in Myocardial Injury, Healing, and Remodeling after Myocardial Infarction. Circ. Res. 2015, 116, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-R.A.; O’Koren, E.G.; Hotten, D.F.; Kan, M.J.; Kopin, D.; Nelson, E.R.; Que, L.; Gunn, M.D. A Protocol for the Comprehensive Flow Cytometric Analysis of Immune Cells in Normal and Inflamed Murine Non-Lymphoid Tissues. PLoS ONE 2016, 11, e0150606. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.S.; Alicot, E.M.; Schuerpf, F.; Chiu, I.; Li, J.; Moore, F.D.; Carroll, M.C. Blockade of Self-Reactive IgM Significantly Reduces Injury in a Murine Model of Acute Myocardial Infarction. Cardiovasc. Res. 2010, 87, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Zouggari, Y.; Ait-Oufella, H.; Bonnin, P.; Simon, T.; Sage, A.P.; Guérin, C.; Vilar, J.; Caligiuri, G.; Tsiantoulas, D.; Laurans, L.; et al. B Lymphocytes Trigger Monocyte Mobilization and Impair Heart Function after Acute Myocardial Infarction. Nat. Med. 2013, 19, 1273–1280. [Google Scholar] [CrossRef]
- Wu, L.; Dalal, R.; Cao, C.D.; Postoak, J.L.; Yang, G.; Zhang, Q.; Wang, Z.; Lal, H.; Van Kaer, L. IL-10–Producing B Cells Are Enriched in Murine Pericardial Adipose Tissues and Ameliorate the Outcome of Acute Myocardial Infarction. Proc. Natl. Acad. Sci. USA 2019, 116, 21673–21684. [Google Scholar] [CrossRef] [PubMed]
- Pluijmert, N.J.; Den Haan, M.C.; Van Zuylen, V.L.; Steendijk, P.; De Boer, H.C.; Van Zonneveld, A.J.; Fibbe, W.E.; Schalij, M.J.; Quax, P.H.A.; Atsma, D.E. Hypercholesterolemia Affects Cardiac Function, Infarct Size and Inflammation in APOE*3-Leiden Mice Following Myocardial Ischemia-Reperfusion Injury. PLoS ONE 2019, 14, e0217582. [Google Scholar] [CrossRef]
- Mączewski, M.; Mączewska, J. Hypercholesterolemia Exacerbates Ventricular Remodeling in the Rat Model of Myocardial Infarction. J. Card. Fail. 2006, 12, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Mitakou, S.; Paraschos, S.; Efentakis, P.; Magiatis, P.; Kaklamanis, L.; Halabalaki, M.; Skaltsounis, L.; Iliodromitis, E.K. “Pistacia Lentiscus L.” Reduces the Infarct Size in Normal Fed Anesthetized Rabbits and Possess Antiatheromatic and Hypolipidemic Activity in Cholesterol Fed Rabbits. Phytomedicine 2016, 23, 1220–1226. [Google Scholar] [CrossRef]
- Osipov, R.M.; Bianchi, C.; Feng, J.; Clements, R.T.; Liu, Y.; Robich, M.P.; Glazer, H.P.; Sodha, N.R.; Sellke, F.W. Effect of Hypercholesterolemia on Myocardial Necrosis and Apoptosis in the Setting of Ischemia-Reperfusion. Circulation 2009, 120, S22–S30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-L.; Yang, Y.-J.; You, S.-J.; Cui, C.-J.; Gao, R.-L. Different Effects of Postconditioning on Myocardial No-Reflow in the Normal and Hypercholesterolemic Mini-Swines. Microvasc. Res. 2007, 73, 137–142. [Google Scholar] [CrossRef]
- Ferdinandy, P.; Andreadou, I.; Baxter, G.F.; Bøtker, H.E.; Davidson, S.M.; Dobrev, D.; Gersh, B.J.; Heusch, G.; Lecour, S.; Ruiz-Meana, M.; et al. Interaction of Cardiovascular Nonmodifiable Risk Factors, Comorbidities and Comedications with Ischemia/Reperfusion Injury and Cardioprotection by Pharmacological Treatments and Ischemic Conditioning. Pharmacol. Rev. 2023, 75, 159–216. [Google Scholar] [CrossRef]
- Ben-Aicha, S.; Badimon, L.; Vilahur, G. Advances in HDL: Much More than Lipid Transporters. IJMS 2020, 21, 732. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Vilahur, G.; Yadegar, D.; Viles-Gonzalez, J.; Badimon, J. The Role of High-Density Lipoprotein Cholesterol in the Prevention and Possible Treatment of Cardiovascular Diseases. CMM 2006, 6, 571–587. [Google Scholar] [CrossRef]
- Ben-Aicha, S.; Escate, R.; Casaní, L.; Padró, T.; Peña, E.; Arderiu, G.; Mendieta, G.; Badimón, L.; Vilahur, G. High-Density Lipoprotein Remodelled in Hypercholesterolaemic Blood Induce Epigenetically Driven down-Regulation of Endothelial HIF-1α Expression in a Preclinical Animal Model. Cardiovasc. Res. 2020, 116, 1288–1299. [Google Scholar] [CrossRef]
- Ben-Aicha, S.; Casaní, L.; Muñoz-García, N.; Joan-Babot, O.; Peña, E.; Aržanauskaitė, M.; Gutierrez, M.; Mendieta, G.; Padró, T.; Badimon, L.; et al. HDL (High-Density Lipoprotein) Remodeling and Magnetic Resonance Imaging–Assessed Atherosclerotic Plaque Burden: Study in a Preclinical Experimental Model. ATVB 2020, 40, 2481–2493. [Google Scholar] [CrossRef] [PubMed]
- Padró, T.; Cubedo, J.; Camino, S.; Béjar, M.T.; Ben-Aicha, S.; Mendieta, G.; Escolà-Gil, J.C.; Escate, R.; Gutiérrez, M.; Casani, L.; et al. Detrimental Effect of Hypercholesterolemia on High-Density Lipoprotein Particle Remodeling in Pigs. J. Am. Coll. Cardiol. 2017, 70, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Schoch, L.; Sutelman, P.; Suades, R.; Casani, L.; Padro, T.; Badimon, L.; Vilahur, G. Hypercholesterolemia-Induced HDL Dysfunction Can Be Reversed: The Impact of Diet and Statin Treatment in a Preclinical Animal Model. IJMS 2022, 23, 8596. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjærg-Hansen, A. Nonfasting Triglycerides and Risk of Myocardial Infarction, Ischemic Heart Disease, and Death in Men and Women. JAMA 2007, 298, 299. [Google Scholar] [CrossRef] [PubMed]
- Ting, H.J.; Stice, J.P.; Schaff, U.Y.; Hui, D.Y.; Rutledge, J.C.; Knowlton, A.A.; Passerini, A.G.; Simon, S.I. Triglyceride-Rich Lipoproteins Prime Aortic Endothelium for an Enhanced Inflammatory Response to Tumor Necrosis Factor-α. Circ. Res. 2007, 100, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Farnier, M.; Zeller, M.; Masson, D.; Cottin, Y. Triglycerides and Risk of Atherosclerotic Cardiovascular Disease: An Update. Arch. Cardiovasc. Dis. 2021, 114, 132–139. [Google Scholar] [CrossRef]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Langsted, A.; Nordestgaard, B.G. Dual Elevated Remnant Cholesterol and C-Reactive Protein in Myocardial Infarction, Atherosclerotic Cardiovascular Disease, and Mortality. Atherosclerosis 2023, 379, 117141. [Google Scholar] [CrossRef]
- Mastrocola, R.; Collino, M.; Penna, C.; Nigro, D.; Chiazza, F.; Fracasso, V.; Tullio, F.; Alloatti, G.; Pagliaro, P.; Aragno, M. Maladaptive Modulations of NLRP3 Inflammasome and Cardioprotective Pathways Are Involved in Diet-Induced Exacerbation of Myocardial Ischemia/Reperfusion Injury in Mice. Oxidative Med. Cell. Longev. 2016, 2016, 3480637. [Google Scholar] [CrossRef]
- Padró, T.; Vilahur, G.; Badimon, L. Dyslipidemias and Microcirculation. Curr. Pharm. Des. 2018, 24, 2921–2926. [Google Scholar] [CrossRef]
- Babbar, L.; Mahadevan, N.; Balakumar, P. Fenofibrate Attenuates Impaired Ischemic Preconditioning-Mediated Cardioprotection in the Fructose-Fed Hypertriglyceridemic Rat Heart. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013, 386, 319–329. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Bansal, S.; Burman, A.; Tripathi, A.K. Advanced Glycation End Products: Key Mediator and Therapeutic Target of Cardiovascular Complications in Diabetes. World J. Diabetes 2023, 14, 1146–1162. [Google Scholar] [CrossRef] [PubMed]
- Uchasova, E.; Gruzdeva; Belik, E.; Shurygina; Barbarash, O.; Dyleva, Y. Plasminogen Activator Inhibitor-1, Free Fatty Acids, and Insulin Resistance in Patients with Myocardial Infarction. DMSO 2013, 6, 293–301. [Google Scholar] [CrossRef][Green Version]
- Madrazo, J.A.; Kelly, D.P. The PPAR Trio: Regulators of Myocardial Energy Metabolism in Health and Disease. J. Cell. Mol. Med. 2008, 44, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Battiprolu, P.K.; Hojayev, B.; Jiang, N.; Wang, Z.V.; Luo, X.; Iglewski, M.; Shelton, J.M.; Gerard, R.D.; Rothermel, B.A.; Gillette, T.G.; et al. Metabolic Stress–Induced Activation of FoxO1 Triggers Diabetic Cardiomyopathy in Mice. J. Clin. Investig. 2012, 122, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-M.; Kim, J.-J.; Kim, H.J.; Shong, M.; Ku, B.J.; Jo, E.-K. Upregulated NLRP3 Inflammasome Activation in Patients with Type 2 Diabetes. Diabetes 2013, 62, 194–204. [Google Scholar] [CrossRef]
- Luo, B.; Li, B.; Wang, W.; Liu, X.; Xia, Y.; Zhang, C.; Zhang, M.; Zhang, Y.; An, F. NLRP3 Gene Silencing Ameliorates Diabetic Cardiomyopathy in a Type 2 Diabetes Rat Model. PLoS ONE 2014, 9, e104771. [Google Scholar] [CrossRef]
- Tocci, G.; Figliuzzi, I.; Presta, V.; Miceli, F.; Citoni, B.; Coluccia, R.; Musumeci, M.B.; Ferrucci, A.; Volpe, M. Therapeutic Approach to Hypertension Urgencies and Emergencies during Acute Coronary Syndrome. High. Blood Press. Cardiovasc. Prev. 2018, 25, 253–259. [Google Scholar] [CrossRef]
- Mehdipoor, G.; Redfors, B.; Chen, S.; Gkargkoulas, F.; Zhang, Z.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; et al. Hypertension, Microvascular Obstruction and Infarct Size in Patients with STEMI Undergoing PCI: Pooled Analysis from 7 Cardiac Magnetic Resonance Imaging Studies. Am. Heart J. 2024, 271, 148–155. [Google Scholar] [CrossRef]
- Agita, A.; Thaha, M. Inflammation, Immunity, and Hypertension. Acta Med. Indones. 2017, 49, 158–165. [Google Scholar] [PubMed]
- Vahldieck, C.; Cianflone, E.; Fels, B.; Löning, S.; Depelmann, P.; Sabatino, J.; Salerno, N.; Karsten, C.M.; Torella, D.; Weil, J.; et al. Endothelial Glycocalyx and Cardiomyocyte Damage Is Prevented by Recombinant Syndecan-1 in Acute Myocardial Infarction. AJP 2023, 193, 474–492. [Google Scholar] [CrossRef] [PubMed]
- Civieri, G.; Iop, L.; Tona, F. Antibodies against Angiotensin II Type 1 and Endothelin 1 Type A Receptors in Cardiovascular Pathologies. IJMS 2022, 23, 927. [Google Scholar] [CrossRef] [PubMed]
- Bandoni, R.L.; Bricher Choque, P.N.; Dellê, H.; De Moraes, T.L.; Porter, M.H.M.; Da Silva, B.D.; Neves, G.A.; Irigoyen, M.-C.; De Angelis, K.; Pavlov, V.A.; et al. Cholinergic Stimulation with Pyridostigmine Modulates a Heart-Spleen Axis after Acute Myocardial Infarction in Spontaneous Hypertensive Rats. Sci. Rep. 2021, 11, 9563. [Google Scholar] [CrossRef] [PubMed]
- Neckář, J.; Alánová, P.; Olejníčková, V.; Papoušek, F.; Hejnová, L.; Šilhavý, J.; Behuliak, M.; Bencze, M.; Hrdlička, J.; Vecka, M.; et al. Excess Ischemic Tachyarrhythmias Trigger Protection against Myocardial Infarction in Hypertensive Rats. Clin. Sci. 2021, 135, 2143–2163. [Google Scholar] [CrossRef] [PubMed]
- Mambrini, S.P.; Menichetti, F.; Ravella, S.; Pellizzari, M.; De Amicis, R.; Foppiani, A.; Battezzati, A.; Bertoli, S.; Leone, A. Ultra-Processed Food Consumption and Incidence of Obesity and Cardiometabolic Risk Factors in Adults: A Systematic Review of Prospective Studies. Nutrients 2023, 15, 2583. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health: A Critical Review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G.; Padro, T. Nutraceuticals and Atherosclerosis: Human Trials. Cardiovasc. Ther. 2010, 28, 202–215. [Google Scholar] [CrossRef]
- Babio, N.; Toledo, E.; Estruch, R.; Ros, E.; Martínez-González, M.A.; Castañer, O.; Bulló, M.; DPharm, D.C.; Arós, F.; Gómez-Gracia, E.; et al. Mediterranean Diets and Metabolic Syndrome Status in the PREDIMED Randomized Trial. CMAJ 2014, 186, E649–E657. [Google Scholar] [CrossRef]
- Esposito, K.; Marfella, R.; Ciotola, M.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a Mediterranean-Style Diet on Endothelial Dysfunction and Markers of Vascular Inflammation in the Metabolic Syndrome. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef]
- Magnoni, M.; Scarano, P.; Vergani, V.; Berteotti, M.; Gallone, G.; Cristell, N.; Maseri, A.; Cianflone, D. Impact of Adherence to a Mediterranean Diet Pattern on Patients with First Acute Myocardial Infarction. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 574–580. [Google Scholar] [CrossRef]
- Zununi Vahed, S.; Barzegari, A.; Zuluaga, M.; Letourneur, D.; Pavon-Djavid, G. Myocardial Infarction and Gut Microbiota: An Incidental Connection. Pharmacol. Res. 2018, 129, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Tanaka, S.; Heianza, Y.; Fujihara, K.; Horikawa, C.; Shimano, H.; Saito, K.; Yamada, N.; Ohashi, Y.; Sone, H. Association between Physical Activity and Risk of All-Cause Mortality and Cardiovascular Disease in Patients with Diabetes. Diabetes Care 2013, 36, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Naci, H.; Salcher-Konrad, M.; Dias, S.; Blum, M.R.; Sahoo, S.A.; Nunan, D.; Ioannidis, J.P.A. How Does Exercise Treatment Compare with Antihypertensive Medications? A Network Meta-Analysis of 391 Randomised Controlled Trials Assessing Exercise and Medication Effects on Systolic Blood Pressure. Br. J. Sports Med. 2019, 53, 859–869. [Google Scholar] [CrossRef]
- Ozemek, C.; Laddu, D.R.; Arena, R.; Lavie, C.J. The Role of Diet for Prevention and Management of Hypertension. Curr. Opin. Cardiol. 2018, 33, 388–393. [Google Scholar] [CrossRef]
- Chen, C.-W.; Chang, C.-W.; Lin, Y.-C.; Chen, W.-T.; Chien, L.-N.; Huang, C.-Y. Comparison of Clinical Outcomes of Angiotensin Receptor Blockers with Angiotensin-Converting Enzyme Inhibitors in Patients with Acute Myocardial Infarction. PLoS ONE 2023, 18, e0290251. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.J.; Fegers-Wustrow, I.; Halle, M.; Haykowsky, M.J.; Chung, E.H.; Kovacic, J.C. Exercise for Primary and Secondary Prevention of Cardiovascular Disease. J. Am. Coll. Cardiol. 2022, 80, 1091–1106. [Google Scholar] [CrossRef]
- Puhl, S.-L.; Müller, A.; Wagner, M.; Devaux, Y.; Böhm, M.; Wagner, D.R.; Maack, C. Exercise Attenuates Inflammation and Limits Scar Thinning after Myocardial Infarction in Mice. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H345–H359. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, P.; Bergamaschi, L.; Santulli, G.; Gallinoro, E.; Cesaro, A.; Gragnano, F.; Sardu, C.; Mileva, N.; Foà, A.; Armillotta, M.; et al. Infarct Size, Inflammatory Burden, and Admission Hyperglycemia in Diabetic Patients with Acute Myocardial Infarction Treated with SGLT2-Inhibitors: A Multicenter International Registry. Cardiovasc. Diabetol. 2022, 21, 77. [Google Scholar] [CrossRef]
- Boden, W.E.; Bhatt, D.L.; Toth, P.P.; Ray, K.K.; Chapman, M.J.; Lüscher, T.F. Profound Reductions in First and Total Cardiovascular Events with Icosapent Ethyl in the REDUCE-IT Trial: Why These Results Usher in a New Era in Dyslipidaemia Therapeutics. Eur. Heart J. 2020, 41, 2304–2312. [Google Scholar] [CrossRef]
- Budoff, M.J.; Bhatt, D.L.; Kinninger, A.; Lakshmanan, S.; Muhlestein, J.B.; Le, V.T.; May, H.T.; Shaikh, K.; Shekar, C.; Roy, S.K.; et al. Effect of Icosapent Ethyl on Progression of Coronary Atherosclerosis in Patients with Elevated Triglycerides on Statin Therapy: Final Results of the EVAPORATE Trial. Eur. Heart J. 2020, 41, 3925–3932. [Google Scholar] [CrossRef]
- Toso, A.; Leoncini, M.; De Servi, S. Statins and Myocardial Infarction: From Secondary ‘Prevention’ to Early ‘Treatment’. J. Cardiovasc. Med. 2019, 20, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Mendieta, G.; Ben-Aicha, S.; Casani, L.; Badimon, L.; Sabate, M.; Vilahur, G. Molecular Pathways Involved in the Cardioprotective Effects of Intravenous Statin Administration during Ischemia. Basic Res. Cardiol. 2020, 115, 2. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yang, C.; Yang, G.; Du, H.; Zhao, H.; Liu, H. Effects of Atorvastatin Doses on Serum Level of Procalcitonin and Predictors for Major Adverse Cardiovascular Events in Patients with Acute Myocardial Infarction: A Pilot Study and Post Hoc Analysis. Coron. Artery Dis. 2022, 33, e87–e93. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Z.; Guo, J.; Peng, X.; Zheng, Z.; Chen, H.; Liu, H.; Ma, Y.; Zhu, J. Atorvastatin-Induced Tolerogenic Dendritic Cells Improve Cardiac Remodeling by Suppressing TLR-4/NF-κB Activation after Myocardial Infarction. Inflamm. Res. 2023, 72, 13–25. [Google Scholar] [CrossRef]
- Mao, Y.; Koga, J.; Tokutome, M.; Matoba, T.; Ikeda, G.; Nakano, K.; Egashira, K. Nanoparticle-Mediated Delivery of Pitavastatin to Monocytes/Macrophages Inhibits Left Ventricular Remodeling after Acute Myocardial Infarction by Inhibiting Monocyte-Mediated Inflammation. Int. Heart J. 2017, 58, 615–623. [Google Scholar] [CrossRef]
- Ning, Y.; Huang, P.; Chen, G.; Xiong, Y.; Gong, Z.; Wu, C.; Xu, J.; Jiang, W.; Li, X.; Tang, R.; et al. Atorvastatin-Pretreated Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Cardiac Repair after Myocardial Infarction via Shifting Macrophage Polarization by Targeting microRNA-139-3p/Stat1 Pathway. BMC Med. 2023, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zeng, Y.; Hu, Z.; Liang, H. PCSK9 Promotes the Secretion of Pro-Inflammatory Cytokines by Macrophages to Aggravate H/Rinduced Cardiomyocyte Injury via Activating NF-kB Signalling. Gen. Physiol. Biophys. 2020, 39, 123–134. [Google Scholar] [CrossRef]
- Badimon, L.; Luquero, A.; Crespo, J.; Peña, E.; Borrell-Pages, M. PCSK9 and LRP5 in Macrophage Lipid Internalization and Inflammation. Cardiovasc. Res. 2021, 117, 2054–2068. [Google Scholar] [CrossRef]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Paolisso, P.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; Ferraraccio, F.; Panarese, I.; et al. Evidence of an Anti-Inflammatory Effect of PCSK9 Inhibitors within the Human Atherosclerotic Plaque. Atherosclerosis 2023, 378, 117180. [Google Scholar] [CrossRef]
- Tuñón, J.; Bäck, M.; Badimón, L.; Bochaton-Piallat, M.-L.; Cariou, B.; Daemen, M.J.; Egido, J.; Evans, P.C.; Francis, S.E.; Ketelhuth, D.F.; et al. Interplay between Hypercholesterolaemia and Inflammation in Atherosclerosis: Translating Experimental Targets into Clinical Practice. Eur. J. Prev. Cardiol. 2018, 25, 948–955. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Regalado, L.; Alcover, S.; Badimon, L.; Vilahur, G. The Influence of Metabolic Risk Factors on the Inflammatory Response Triggered by Myocardial Infarction: Bridging Pathophysiology to Treatment. Cells 2024, 13, 1125. https://doi.org/10.3390/cells13131125
Ramos-Regalado L, Alcover S, Badimon L, Vilahur G. The Influence of Metabolic Risk Factors on the Inflammatory Response Triggered by Myocardial Infarction: Bridging Pathophysiology to Treatment. Cells. 2024; 13(13):1125. https://doi.org/10.3390/cells13131125
Chicago/Turabian StyleRamos-Regalado, Lisaidy, Sebastià Alcover, Lina Badimon, and Gemma Vilahur. 2024. "The Influence of Metabolic Risk Factors on the Inflammatory Response Triggered by Myocardial Infarction: Bridging Pathophysiology to Treatment" Cells 13, no. 13: 1125. https://doi.org/10.3390/cells13131125
APA StyleRamos-Regalado, L., Alcover, S., Badimon, L., & Vilahur, G. (2024). The Influence of Metabolic Risk Factors on the Inflammatory Response Triggered by Myocardial Infarction: Bridging Pathophysiology to Treatment. Cells, 13(13), 1125. https://doi.org/10.3390/cells13131125







