Voltage-Gated Ion Channels Are Transcriptional Targets of Sox10 during Oligodendrocyte Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids
2.2. Cell Culture, Luciferase Assays
2.3. Quantitative RT-PCR
2.4. Chromatin Immunoprecipitation (ChIP)
2.5. Husbandry and Breeding of Mice
2.6. In Situ Hybridization and Immunohistochemistry
2.7. Statistical Analysis
3. Results
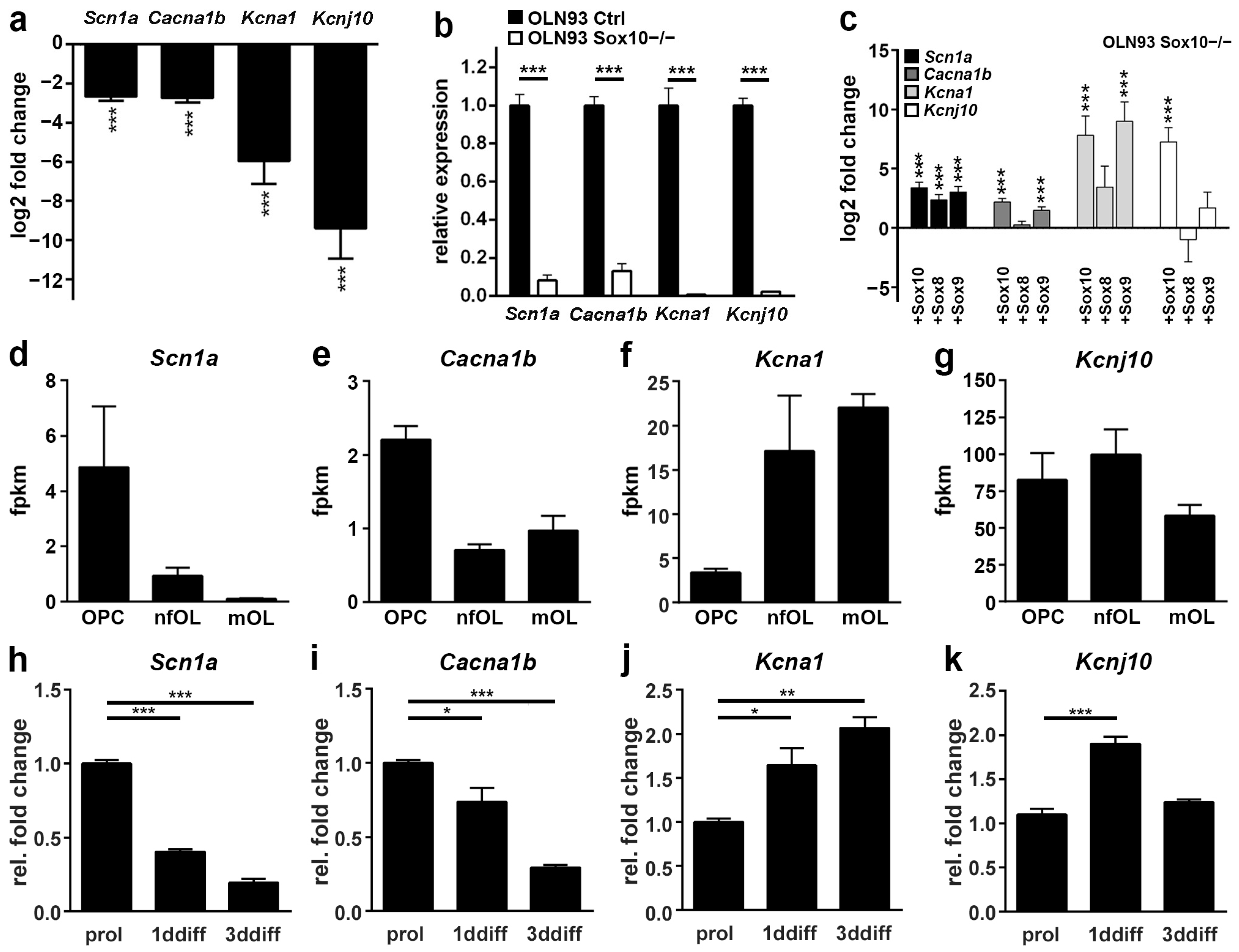
3.1. Voltage-Gated Ion Channels Are Downregulated upon Sox10 Inactivation in an Oligodendroglial Cell Line
3.2. The Scn1a Promoter and a Putative Regulatory Region at +145 kb Are Transcriptionally Activated by Sox10
3.3. The Cacna1b Promoter and a Putative Regulatory Region at +78 kb Are Transcriptionally Activated by Sox10
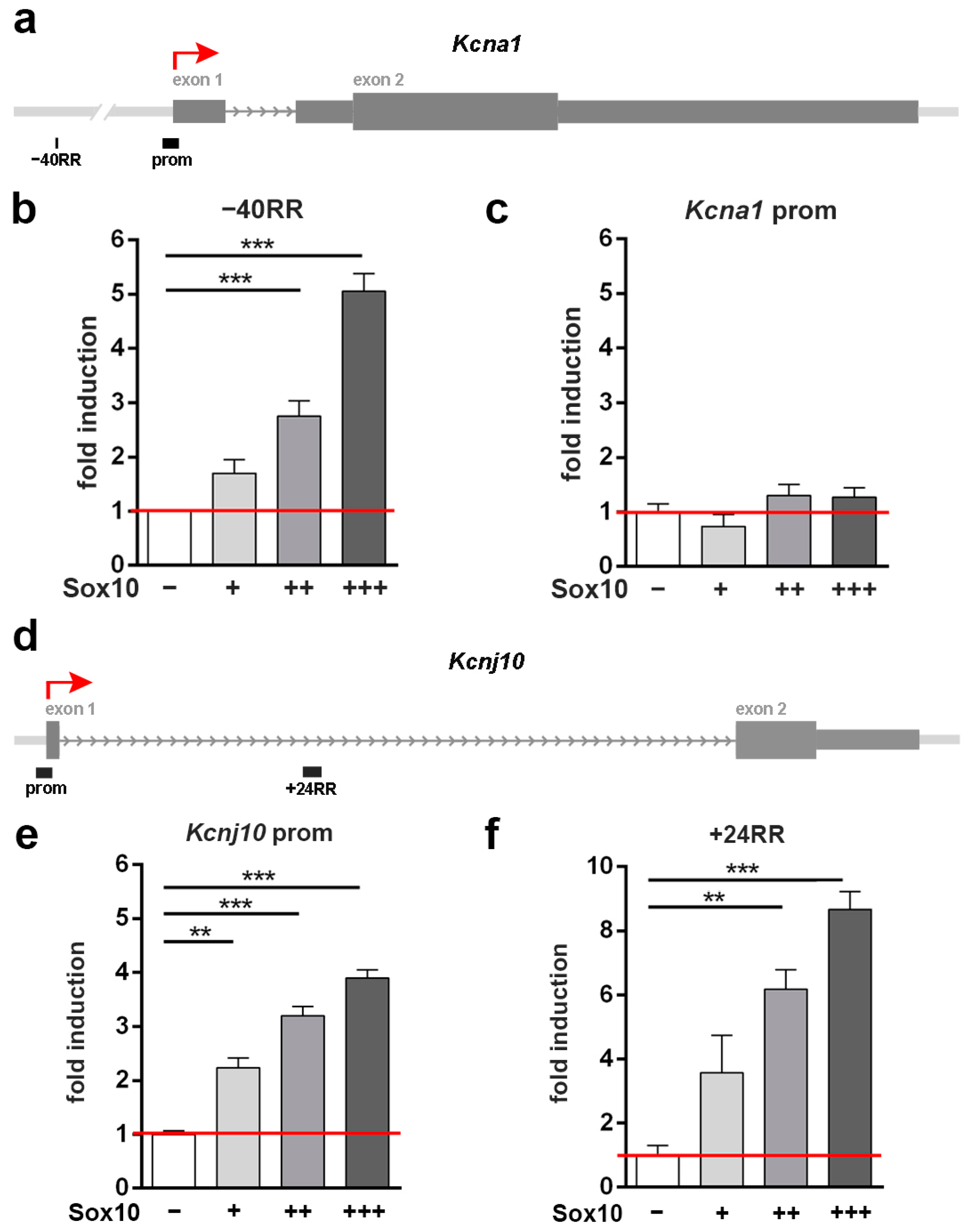
3.4. A Putative Regulatory Region 40 kb in Front of the Kcna1 Gene Is Transcriptionally Activated by Sox10
3.5. The Kcnj10 Promoter and a Putative Regulatory Region at +24 kb Are Transcriptionally Activated by Sox10
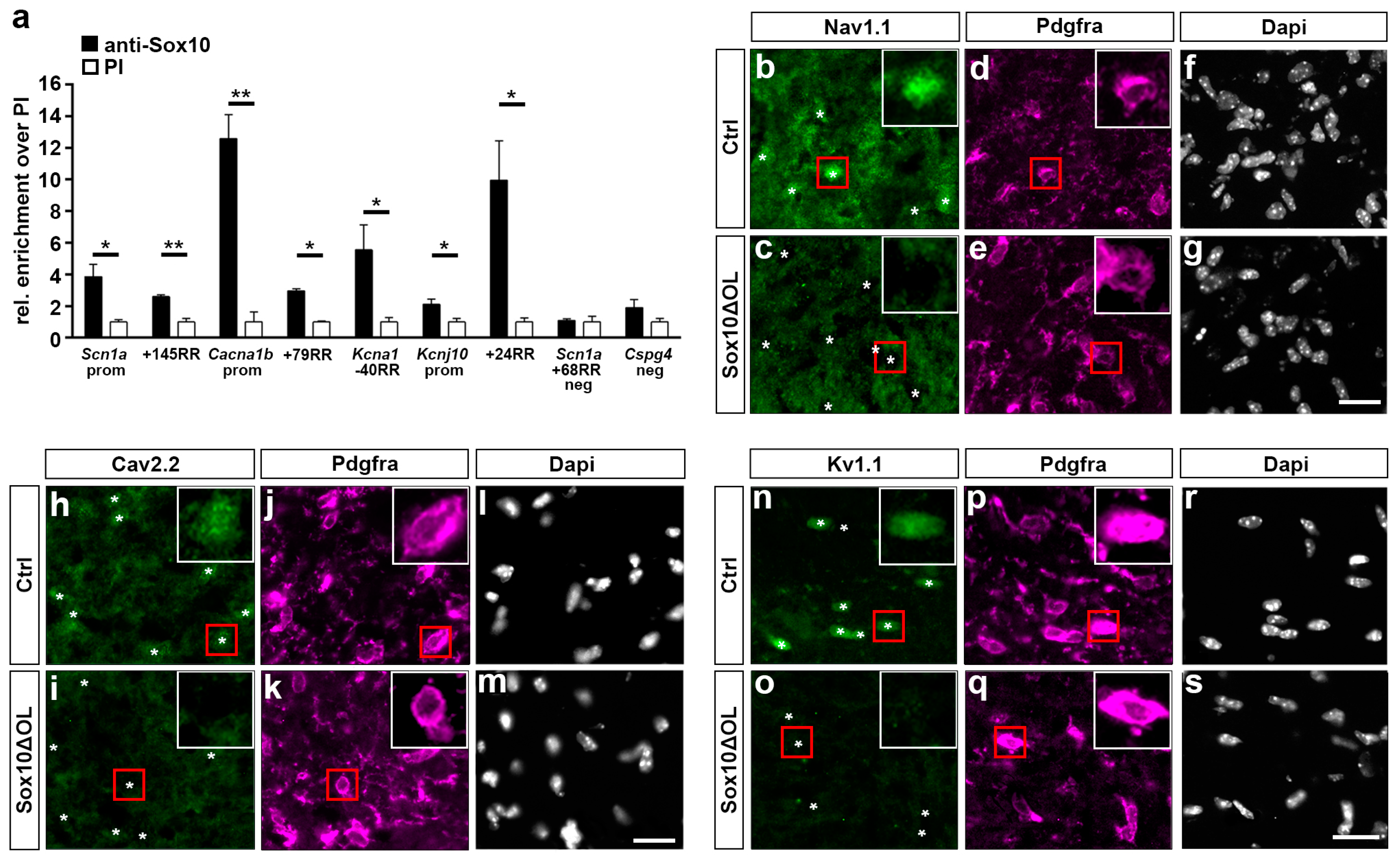
3.6. Regulatory Regions of Voltage-Gated Ion Channel Genes Are Bound by Sox10 In Vivo
3.7. Oligodendroglial Expression of Voltage-Gated Ion Channel Genes Is Severely Affected In Vivo following Sox10 Inactivation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakiri, Y.; Attwell, D.; Karadottir, R. Electrical signalling properties of oligodendrocyte precursor cells. Neuron Glia Biol. 2009, 5, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Cherchi, F.; Bulli, I.; Venturini, M.; Pugliese, A.M.; Coppi, E. Ion Channels as New Attractive Targets to Improve Re-Myelination Processes in the Brain. Int. J. Mol. Sci. 2021, 22, 7277. [Google Scholar] [CrossRef] [PubMed]
- Paez, P.M.; Fulton, D.; Colwell, C.S.; Campagnoni, A.T. Voltage-operated Ca(2+) and Na(+) channels in the oligodendrocyte lineage. J. Neurosci. Res. 2009, 87, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Krasnow, A.M.; Attwell, D. NMDA Receptors: Power Switches for Oligodendrocytes. Neuron 2016, 91, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Karadottir, R.; Hamilton, N.B.; Bakiri, Y.; Attwell, D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat. Neurosci. 2008, 11, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.P.; Li, X.Y.; Zhou, B.; Shen, W.; Zhang, Z.J.; Xu, T.L.; Duan, S. Ca(2+) signaling evoked by activation of Na(+) channels and Na(+)/Ca(2+) exchangers is required for GABA-induced NG2 cell migration. J. Cell Biol. 2009, 186, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Lynch, D.T.; Schools, G.P.; Feustel, P.J.; Kimelberg, H.K.; Zhou, M. Sodium channel currents in rat hippocampal NG2 glia: Characterization and contribution to resting membrane potential. Neuroscience 2007, 150, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Cheli, V.T.; Santiago Gonzalez, D.A.; Spreuer, V.; Paez, P.M. Voltage-gated Ca2+ entry promotes oligodendrocyte progenitor cell maturation and myelination in vitro. Exp. Neurol. 2015, 265, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Coppi, E.; Maraula, G.; Fumagalli, M.; Failli, P.; Cellai, L.; Bonfanti, E.; Mazzoni, L.; Coppini, R.; Abbracchio, M.P.; Pedata, F.; et al. UDP-glucose enhances outward K(+) currents necessary for cell differentiation and stimulates cell migration by activating the GPR17 receptor in oligodendrocyte precursors. Glia 2013, 61, 1155–1171. [Google Scholar] [CrossRef]
- Krasnow, A.M.; Ford, M.C.; Valdivia, L.E.; Wilson, S.W.; Attwell, D. Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo. Nat. Neurosci. 2018, 21, 24–28. [Google Scholar] [CrossRef]
- Agrawal, S.K.; Nashmi, R.; Fehlings, M.G. Role of L- and N-type calcium channels in the pathophysiology of traumatic spinal cord white matter injury. Neuroscience 2000, 99, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Kornek, B.; Storch, M.K.; Weissert, R.; Wallstroem, E.; Stefferl, A.; Olsson, T.; Linington, C.; Schmidbauer, M.; Lassmann, H. Multiple sclerosis and chronic autoimmune encephalomyelitis: A comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am. J. Pathol. 2000, 157, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Larson, V.A.; Mironova, Y.; Vanderpool, K.G.; Waisman, A.; Rash, J.E.; Agarwal, A.; Bergles, D.E. Oligodendrocytes control potassium accumulation in white matter and seizure susceptibility. eLife 2018, 7, e34829. [Google Scholar] [CrossRef]
- Schirmer, L.; Mobius, W.; Zhao, C.; Cruz-Herranz, A.; Ben Haim, L.; Cordano, C.; Shiow, L.R.; Kelley, K.W.; Sadowski, B.; Timmons, G.; et al. Oligodendrocyte-encoded Kir4.1 function is required for axonal integrity. eLife 2018, 7, e36428. [Google Scholar] [CrossRef] [PubMed]
- Ngo, C.; Kothary, R. MicroRNAs in oligodendrocyte development and remyelination. J. Neurochem. 2022, 162, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Parras, C.; Marie, C.; Zhao, C.; Lu, Q.R. Chromatin remodelers in oligodendroglia. Glia 2020, 68, 1604–1618. [Google Scholar] [CrossRef] [PubMed]
- Sock, E.; Wegner, M. Using the lineage determinants Olig2 and Sox10 to explore transcriptional regulation of oligodendrocyte development. Dev. Neurobiol. 2021, 81, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Selcen, I.; Prentice, E.; Casaccia, P. The epigenetic landscape of oligodendrocyte lineage cells. Ann. N. Y. Acad. Sci. 2023, 1522, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Rowitch, D.H.; Lu, Q.R.; Kessaris, N.; Richardson, W.D. An ‘oligarchy’ rules neural development. Trends Neurosci. 2002, 25, 417–422. [Google Scholar] [CrossRef]
- Li, H.; Richardson, W.D. Evolution of the CNS myelin gene regulatory program. Brain Res. 2016, 1641, 111–121. [Google Scholar] [CrossRef]
- Aprato, J.; Sock, E.; Weider, M.; Elsesser, O.; Frob, F.; Wegner, M. Myrf guides target gene selection of transcription factor Sox10 during oligodendroglial development. Nucleic Acids Res. 2020, 48, 1254–1270. [Google Scholar] [CrossRef] [PubMed]
- Reiprich, S.; Cantone, M.; Weider, M.; Baroti, T.; Wittstatt, J.; Schmitt, C.; Kuspert, M.; Vera, J.; Wegner, M. Transcription factor Sox10 regulates oligodendroglial Sox9 levels via microRNAs. Glia 2017, 65, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Stolt, C.C.; Rehberg, S.; Ader, M.; Lommes, P.; Riethmacher, D.; Schachner, M.; Bartsch, U.; Wegner, M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002, 16, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Richter-Landsberg, C.; Heinrich, M. OLN-93: A new permanent oligodendroglia cell line derived from primary rat brain glial cultures. J. Neurosci. Res. 1996, 45, 161–173. [Google Scholar] [CrossRef]
- Aberle, T.; Piefke, S.; Hillgartner, S.; Tamm, E.R.; Wegner, M.; Kuspert, M. Transcription factor Zfp276 drives oligodendroglial differentiation and myelination by switching off the progenitor cell program. Nucleic Acids Res. 2022, 50, 1951–1968. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.D.; DeVellis, J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 1980, 85, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cheng, X.; Shen, J.; Xie, B.; Zhao, X.; Zhang, Z.; Cao, Q.; Shen, Y.; Qiu, M. A Novel Approach for Amplification and Purification of Mouse Oligodendrocyte Progenitor Cells. Front. Cell. Neurosci. 2016, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Stolt, C.C.; Lommes, P.; Sock, E.; Chaboissier, M.-C.; Schedl, A.; Wegner, M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003, 17, 1677–1689. [Google Scholar] [CrossRef]
- Finzsch, M.; Schreiner, S.; Kichko, T.; Reeh, P.; Tamm, E.R.; Bösl, M.R.; Meijer, D.; Wegner, M. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J. Cell Biol. 2010, 189, 701–712. [Google Scholar] [CrossRef]
- Dessaud, E.; Yang, L.L.; Hill, K.; Cox, B.; Ulloa, F.; Ribeiro, A.; Mynett, A.; Novitch, B.G.; Briscoe, J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature 2007, 450, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Ahlberg, P.E.; Kessaris, N.; Iannarelli, P.; Dennehy, U.; Richardson, W.D.; McMahon, A.P.; Koentges, G. Neural crest origins of the neck and shoulder. Nature 2005, 436, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Anido, C.; Sun, G.; Koenning, M.; Srinivasan, R.; Hung, H.A.; Emery, B.; Keles, S.; Svaren, J. Differential Sox10 genomic occupancy in myelinating glia. Glia 2015, 63, 1897–1914. [Google Scholar] [CrossRef] [PubMed]
- Finzsch, M.; Stolt, C.C.; Lommes, P.; Wegner, M. Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor {alpha} expression. Development 2008, 135, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, H.; Wood, W.M.; Patel, K.D.; Factor, D.C.; Boshans, L.L.; Nomura, T.; Tesar, P.J.; Ono, K.; Nishiyama, A. NG2 expression in NG2 glia is regulated by binding of SoxE and bHLH transcription factors to a Cspg4 intronic enhancer. Glia 2018, 66, 2684–2699. [Google Scholar] [CrossRef]
- Bernhardt, C.; Sock, E.; Frob, F.; Hillgartner, S.; Nemer, M.; Wegner, M. KLF9 and KLF13 transcription factors boost myelin gene expression in oligodendrocytes as partners of SOX10 and MYRF. Nucleic Acids Res. 2022, 50, 11509–11528. [Google Scholar] [CrossRef] [PubMed]
- Onwuli, D.O.; Beltran-Alvarez, P. An update on transcriptional and post-translational regulation of brain voltage-gated sodium channels. Amino Acids 2016, 48, 641–651. [Google Scholar] [CrossRef]
- van Loo, K.M.J.; Becker, A.J. Transcriptional Regulation of Channelopathies in Genetic and Acquired Epilepsies. Front. Cell. Neurosci. 2019, 13, 587. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, C.; Aberle, T.; Sock, E.; Brunner, J.; Küspert, M.; Hillgärtner, S.; Wüst, H.M.; Wegner, M. Voltage-Gated Ion Channels Are Transcriptional Targets of Sox10 during Oligodendrocyte Development. Cells 2024, 13, 1159. https://doi.org/10.3390/cells13131159
Peters C, Aberle T, Sock E, Brunner J, Küspert M, Hillgärtner S, Wüst HM, Wegner M. Voltage-Gated Ion Channels Are Transcriptional Targets of Sox10 during Oligodendrocyte Development. Cells. 2024; 13(13):1159. https://doi.org/10.3390/cells13131159
Chicago/Turabian StylePeters, Christian, Tim Aberle, Elisabeth Sock, Jessica Brunner, Melanie Küspert, Simone Hillgärtner, Hannah M. Wüst, and Michael Wegner. 2024. "Voltage-Gated Ion Channels Are Transcriptional Targets of Sox10 during Oligodendrocyte Development" Cells 13, no. 13: 1159. https://doi.org/10.3390/cells13131159
APA StylePeters, C., Aberle, T., Sock, E., Brunner, J., Küspert, M., Hillgärtner, S., Wüst, H. M., & Wegner, M. (2024). Voltage-Gated Ion Channels Are Transcriptional Targets of Sox10 during Oligodendrocyte Development. Cells, 13(13), 1159. https://doi.org/10.3390/cells13131159






