Upregulation of Transferrin Receptor 1 (TfR1) but Not Glucose Transporter 1 (GLUT1) or CD98hc at the Blood–Brain Barrier in Response to Valproic Acid
Abstract
1. Introduction
2. Material and Method
2.1. Isolation of BCECs
2.2. Isolation of Mixed Glia Cells
2.3. Construction of an In Vitro Co-Culture BBB Model
2.4. In Vivo Studies
2.5. Labeling of Anti-TfR1 Antibodies with 1.4 nm Gold Nanoparticles
2.6. VPA Administration In Vivo
2.7. Uptake at the BBB of Nanogold-Conjugated Anti-TfR1 Antibodies
2.8. Immunocytochemistry
2.9. Capillary Depletion
2.10. Quantification of Tissue Gold Content
2.11. Probe-Based RT-qPCR
2.12. ELISA
2.13. Statistical Analysis
3. Result
3.1. VPA Administration Upregulates Tfrc in BCECs In Vitro
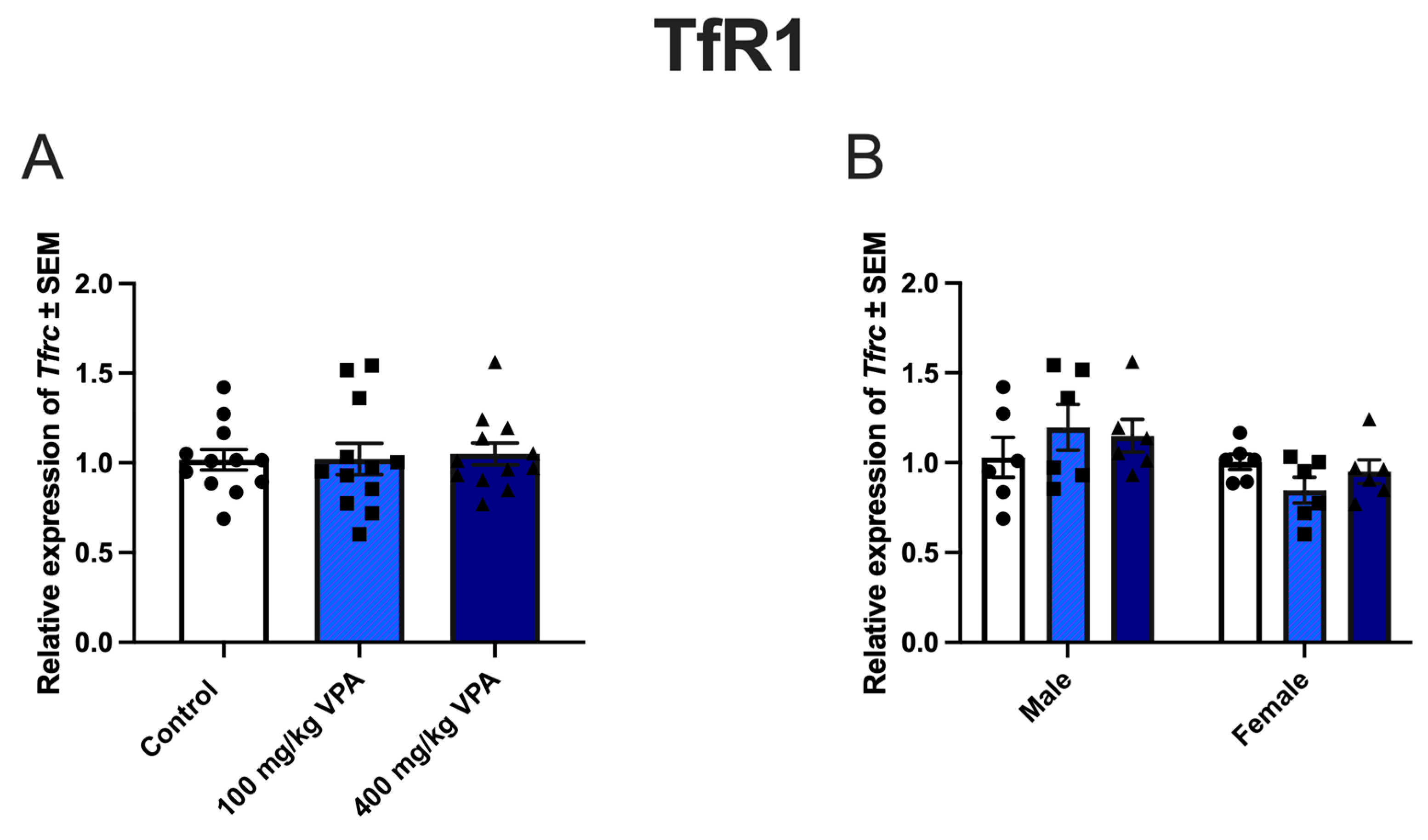
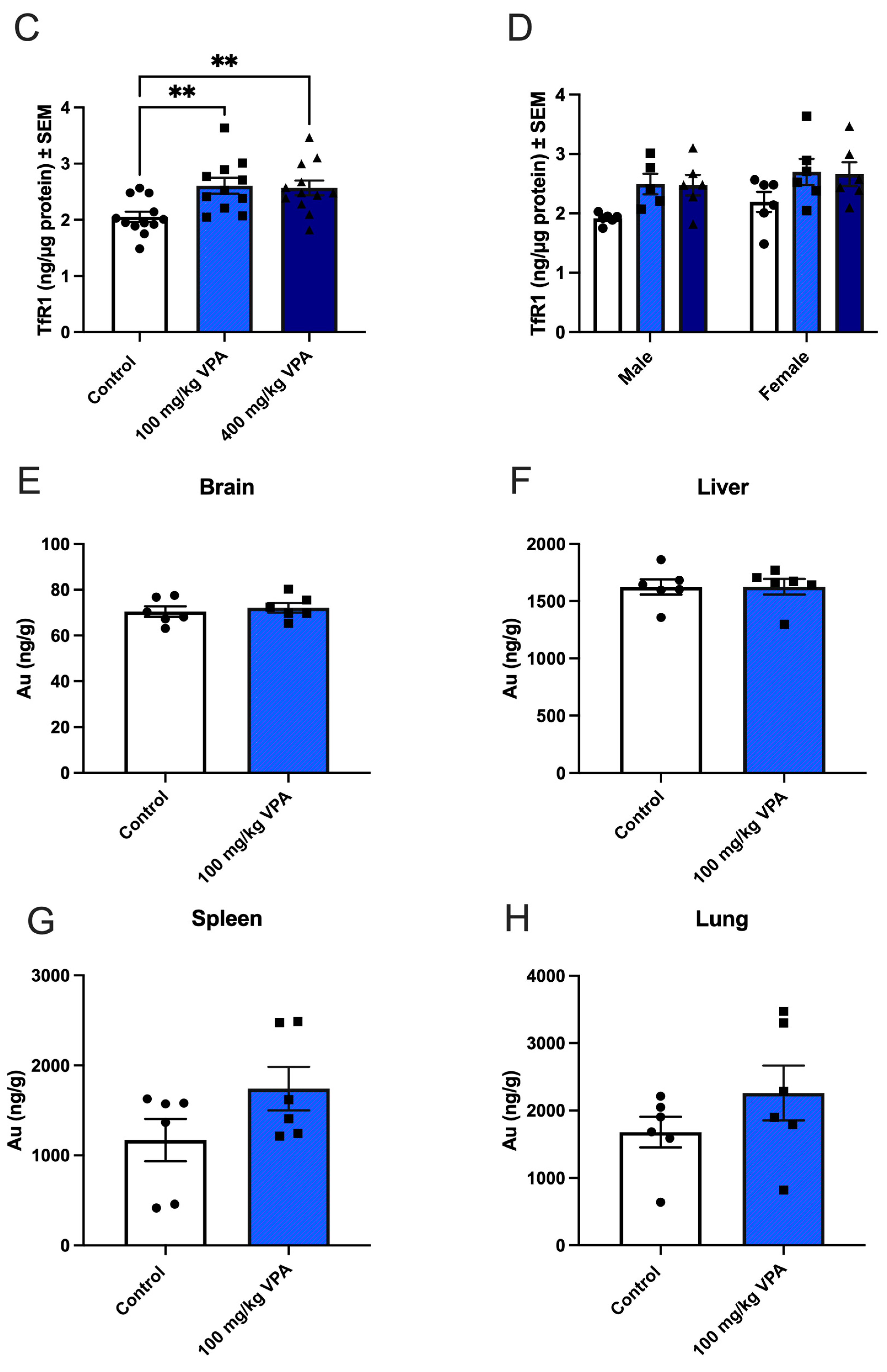
3.2. VPA Induces TfR1 Expression In Vivo
3.3. Uptake of Gold-Labeled Anti-TfR1 Antibodies
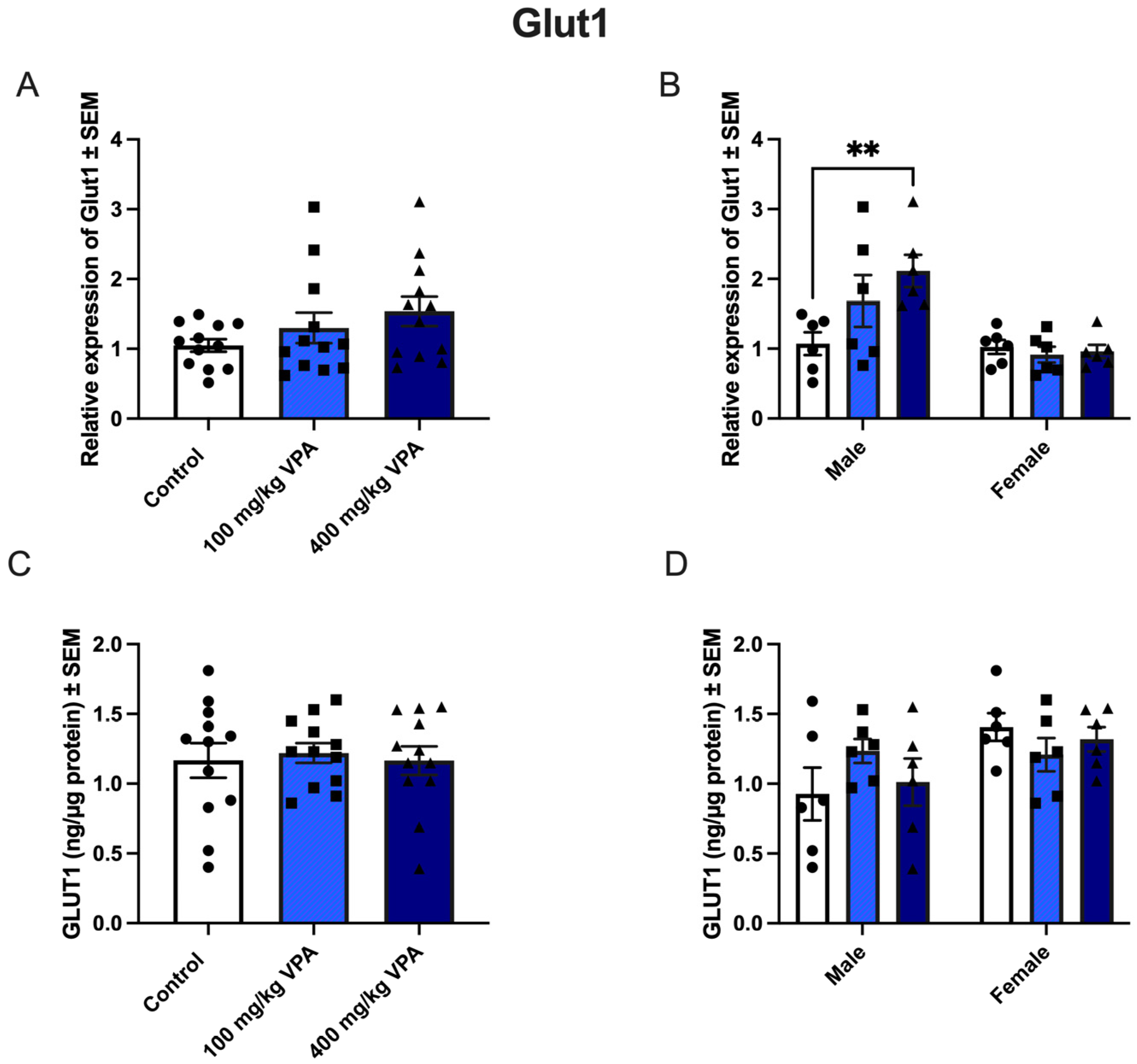
3.4. VPA Increases Glut1 mRNA in Male but Not in Female Mice
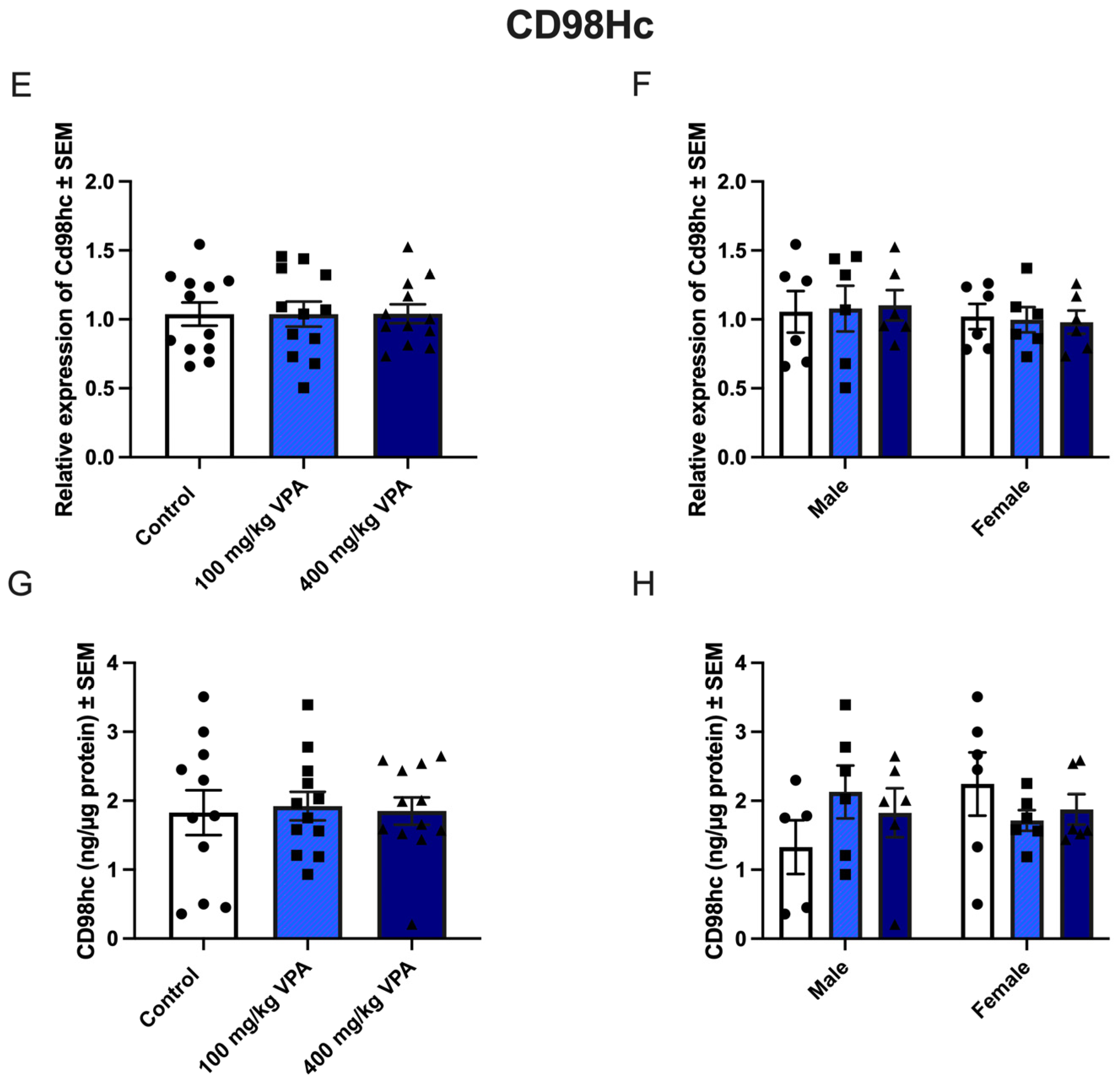
3.5. CD98hc Expression Is Not Affected by VPA Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbott, N.J. Blood-Brain Barrier Structure and Function and the Challenges for CNS Drug Delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2020, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; Dong, X.-P.; Wang, F.; Xu, H. Mechanisms of Brain Iron Transport: Insight into Neurodegeneration and CNS Disorders. Future Med. Chem. 2010, 2, 51–64. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-Endothelial Interactions at the Blood-Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood-Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The Blood-Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Hanley, N.; Campbell, M. Claudin-5: Gatekeeper of Neurological Function. Fluids Barriers CNS 2019, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Reese, T.S.; Karnovsky, M.J. Fine Structural Localization of a Blood-Brain Barrier to Exogenous Peroxidase. J. Cell Biol. 1967, 34, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef]
- Kevadiya, B.D.; Ottemann, B.M.; Thomas, M.B.; Mukadam, I.; Nigam, S.; McMillan, J.E.; Gorantla, S.; Bronich, T.K.; Edagwa, B.; Gendelman, H.E. Neurotheranostics as Personalized Medicines. Adv. Drug Deliv. Rev. 2019, 148, 252–289. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood-Brain Barrier: Structure, Regulation, and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Moura, R.P.; Martins, C.; Pinto, S.; Sousa, F.; Sarmento, B. Blood-Brain Barrier Receptors and Transporters: An Insight on Their Function and How to Exploit Them through Nanotechnology. Expert Opin. Drug Deliv. 2019, 16, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Pornnoppadol, G.; Bond, L.G.; Lucas, M.J.; Zupancic, J.M.; Kuo, Y.H.; Zhang, B.; Greineder, C.F.; Tessier, P.M. Bispecific Antibody Shuttles Targeting CD98hc Mediate Efficient and Long-Lived Brain Delivery of IgGs. Cell Chem. Biol. 2024, 31, 361–372.e8. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H. Glucose Transporters in Brain in Health and Disease. Pflug. Arch. Eur. J. Physiol. 2020, 472, 1299–1343. [Google Scholar] [CrossRef] [PubMed]
- Haqqani, A.S.; Bélanger, K.; Stanimirovic, D.B. Receptor-Mediated Transcytosis for Brain Delivery of Therapeutics: Receptor Classes and Criteria. Front. Drug Deliv. 2024, 4, 1360302. [Google Scholar] [CrossRef]
- Bhatti, U.F.; Williams, A.M.; Georgoff, P.E.; Alam, H.B. The ‘Omics’ of Epigenetic Modulation by Valproic Acid Treatment in Traumatic Brain Injury—What We Know and What the Future Holds. Proteom. Clin. Appl. 2019, 13, e1900068. [Google Scholar] [CrossRef] [PubMed]
- Helgudottir, S.S.; Routhe, L.J.; Burkhart, A.; Jønsson, K.; Pedersen, I.S.; Lichota, J.; Moos, T. Epigenetic Regulation of Ferroportin in Primary Cultures of the Rat Blood-Brain Barrier. Mol. Neurobiol. 2020, 57, 3526–3539. [Google Scholar] [CrossRef]
- Zuchero, Y.J.Y.; Chen, X.; Bien-Ly, N.; Bumbaca, D.; Tong, R.K.; Gao, X.; Zhang, S.; Hoyte, K.; Luk, W.; Huntley, M.A.; et al. Discovery of Novel Blood-Brain Barrier Targets to Enhance Brain Uptake of Therapeutic Antibodies. Neuron 2016, 89, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Birkelund, S.; Burkhart, A.; Stensballe, A.; Moos, T. Synthesis and Deposition of Basement Membrane Proteins by Primary Brain Capillary Endothelial Cells in a Murine Model of the Blood-Brain Barrier. J. Neurochem. 2017, 140, 741–754. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Humle, N.; Hede, E.; Moos, T.; Burkhart, A.; Thomsen, L.B. The Blood-Brain Barrier Studied in Vitro across Species. PLoS ONE 2021, 16, e0236770. [Google Scholar] [CrossRef]
- Perrière, N.; Demeuse, P.H.; Garcia, E.; Regina, A.; Debray, M.; Andreux, J.P.; Couvreur, P.; Scherrmann, J.M.; Temsamani, J.; Couraud, P.O.; et al. Puromycin-Based Purification of Rat Brain Capillary Endothelial Cell Cultures. Effect on the Expression of Blood-Brain Barrier-Specific Properties. J. Neurochem. 2005, 93, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Calabria, A.R.; Weidenfeller, C.; Jones, A.R.; De Vries, H.E.; Shusta, E.V. Puromycin-Purified Rat Brain Microvascular Endothelial Cell Cultures Exhibit Improved Barrier Properties in Response to Glucocorticoid Induction. J. Neurochem. 2006, 97, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Zeng, Q.; Wang, K.; Sharma, A.; He, G. Gender Difference in Valproic Acid-Induced Neuroprotective Effects on APP/PS1 Double Transgenic Mice Modeling Alzheimer’s Disease. Acta Biochim. Et Biophys. Sin. 2016, 48, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, M.; Vázquez, M.; Fagiolino, P.; Derendorf, H. Sex Related Differences on Valproic Acid Pharmacokinetics after Oral Single Dose. J. Pharmacokinet. Pharmacodyn. 2013, 40, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Kucharz, K.; Kristensen, K.; Johnsen, K.B.; Lund, M.A.; Lønstrup, M.; Moos, T.; Andresen, T.L.; Lauritzen, M.J. Post-Capillary Venules Are the Key Locus for Transcytosis-Mediated Brain Delivery of Therapeutic Nanoparticles. Nat. Commun. 2021, 12, 4121. [Google Scholar] [CrossRef] [PubMed]
- Chaliha, D.; Albrecht, M.; Vaccarezza, M.; Takechi, R.; Lam, V.; Al-Salami, H.; Mamo, J. A Systematic Review of the Valproic-Acid-Induced Rodent Model of Autism. Dev. Neurosci. 2020, 42, 12–48. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Bak, M.; Kempen, P.J.; Melander, F.; Burkhart, A.; Thomsen, M.S.; Nielsen, M.S.; Moos, T.; Andresen, T.L. Antibody Affinity and Valency Impact Brain Uptake of Transferrin Receptor-Targeted Gold Nanoparticles. Theranostics 2018, 8, 3416–3436. [Google Scholar] [CrossRef]
- Hill, J.J.; Haqqani, A.S.; Stanimirovic, D.B. Proteome of the Luminal Surface of the Blood-Brain Barrier. Proteomes 2021, 9, 45. [Google Scholar] [CrossRef]
- Moos, T.; Thomsen, M.S.; Burkhart, A.; Hede, E.; Laczek, B. Targeted Transport of Biotherapeutics at the Blood-Brain Barrier. Expert Opin. Drug Deliv. 2023, 20, 1823–1838. [Google Scholar] [CrossRef]
- Ying, G.; Jing, C.; Li, J.; Wu, C.; Yan, F.; Chen, J.; Wang, L.; Dixon, B.J.; Chen, G. Neuroprotective Effects of Valproic Acid on Blood-Brain Barrier Disruption and Apoptosis-Related Early Brain Injury in Rats Subjected to Subarachnoid Hemorrhage Are Modulated by Heat Shock Protein 70/Matrix Metalloproteinases and Heat Shock Protein 70/AKT Pathways. Neurosurgery 2016, 79, 286–295. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, L.; Guo, Z.; Hou, Y.; Jiang, J.; Song, Y. Valproate Sodium Protects Blood Brain Barrier Integrity in Intracerebral Hemorrhage Mice. Oxidative Med. Cell. Longev. 2020, 2020, 8884320. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Aromolaran, K.A.; Zukin, R.S. The Emerging Field of Epigenetics in Neurodegeneration and Neuroprotection. Nat. Rev. Neurosci. 2017, 18, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.D.; Poirier, M.G. Post-Translational Modifications of Histones That Influence Nucleosome Dynamics. Chem. Rev. 2015, 115, 2274–2295. [Google Scholar] [CrossRef]
- Ornoy, A.; Becker, M.; Weinstein-Fudim, L.; Ergaz, Z. S-Adenosine Methionine (SAME) and Valproic Acid (VPA) as Epigenetic Modulators: Special Emphasis on Their Interactions Affecting Nervous Tissue during Pregnancy. Int. J. Mol. Sci. 2020, 21, 3721. [Google Scholar] [CrossRef]
- Jefferies, W.A.; Brandon, M.R.; Hunt, S.V.; Williams, A.F.; Gatter, K.C.; Mason, D.Y. Transferrin Receptor on Endothelium of Brain Capillaries. Nature 1984, 312, 162–163. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Q.Y.; Haqqani, A.S.; Leclerc, S.; Liu, Z.; Fauteux, F.; Baumann, E.; Delaney, C.E.; Ly, D.; Star, A.T.; et al. Differential Expression of Receptors Mediating Receptor-Mediated Transcytosis (RMT) in Brain Microvessels, Brain Parenchyma and Peripheral Tissues of the Mouse and the Human. Fluids Barriers CNS 2020, 17, 47. [Google Scholar] [CrossRef]
- Crielaard, B.J.; Lammers, T.; Rivella, S. Targeting Iron Metabolism in Drug Discovery and Delivery. Nat. Rev. Drug Discov. 2017, 16, 400–423. [Google Scholar] [CrossRef]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out Ferroportin. Cell Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Gulec, S.; Anderson, G.J.; Collins, J.F. Mechanistic and Regulatory Aspects of Intestinal Iron Absorption. AJP: Gastrointest. Liver Physiol. 2014, 307, G397–G409. [Google Scholar] [CrossRef]
- Belaidi, A.A.; Bush, A.I. Iron Neurochemistry in Alzheimer’s Disease and Parkinson’s Disease: Targets for Therapeutics. J. Neurochem. 2016, 139, 179–197. [Google Scholar] [CrossRef]
- Silva, B.; Faustino, P. An Overview of Molecular Basis of Iron Metabolism Regulation and the Associatefd Pathologies. Biochim. Et Biophys. Acta 2015, 1852, 1347–1359. [Google Scholar] [CrossRef] [PubMed]
- Helgudottir, S.S. Expressional Prerequisites for Targeted Drug Delivery to the Pathological Brain. Ph.D. Thesis, Aalborg Universitetsforlag, Aalborg, Denmark, 2021. [Google Scholar] [CrossRef]
- Rouault, T.A. The Role of Iron Regulatory Proteins in Mammalian Iron Homeostasis and Disease. Nat. Chem. Biol. 2006, 2, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Moos, T.; Morgan, E.H. A morphological study of the developmentally regulated transport of iron into the brain. Devl. Neurosci. 2002, 24, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.M.; Crowe, A.; Morgan, E.H. Transferrin and Iron Uptake by the Brain: Effects of Altered Iron Status. J. Neurochem. 1991, 57, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Moos, T.; Morgan, E.H. Restricted Transport of Anti-Transferrin Receptor Antibody (OX26) through the Blood-Brain Barrier in the Rat. J. Neurochem. 2001, 79, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, W.; Huijskes-Heins, M.I.E.; Van Dijk, J.P.; Cleton-Soeteman, M.I.; Van Eijk, H.G. Quantification of Different Transferrin Receptor Pools in Primary Cultures of Porcine Blood-Brain Barrier Endothelial Cells. J. Neurochem. 1995, 64, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Friden, P.M.; Walus, L.R.; Musso, G.F.; Taylor, M.A.; Malfroy, B.; Starzyk, R.M. Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc. Natl. Acad. Sci. USA 1991, 88, 4771–4775. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Kempen, P.J.; Vejlebo, J.B.; Siupka, P.; Nielsen, M.S.; Andresen, T.L.; Moos, T. Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma. Sci. Rep. 2017, 7, 10396. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Zhou, L.; Agalliu, D.; Cahoy, J.D.; Kaushal, A.; Barres, B.A. The Mouse Blood-Brain Barrier Transcriptome: A New Resource for Understanding the Development and Function of Brain Endothelial Cells. PLoS ONE 2010, 5, e13741. [Google Scholar] [CrossRef]
- Simpson, I.A.; Carruthers, A.; Vannucci, S.J. Supply and Demand in Cerebral Energy Metabolism: The Role of Nutrient Transporters. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2007, 27, 1766–1791. [Google Scholar] [CrossRef]
- Simpson, I.A.; Vannucci, S.J.; DeJoseph, M.R.; Hawkins, R.A. Glucose Transporter Asymmetries in the Bovine Blood-Brain Barrier. J. Biol. Chem. 2001, 276, 12725–12729. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.A.; Nishida, Y.; Sagare, A.P.; Rege, S.V.; Bell, R.D.; Perlmutter, D.; Sengillo, J.D.; Hillman, S.; Kong, P.; Nelson, A.R.; et al. GLUT1 Reductions Exacerbate Alzheimer’s Disease Vasculo-Neuronal Dysfunction and Degeneration. Nat. Neurosci. 2015, 18, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Geng, M.; Du, G. Glucose Transporter 1, Distribution in the Brain and in Neural Disorders: Its Relationship with Transport of Neuroactive Drugs through the Blood-Brain Barrier. Biochem. Genet. 2005, 43, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, M.; Tremblay, C.; Bourassa, P.; Schneider, J.A.; Bennett, D.A.; Calon, F. Lower GLUT1 and Unchanged MCT1 in Alzheimer’s Disease Cerebrovasculature. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2024. [Google Scholar] [CrossRef] [PubMed]
- Protas, H.D.; Chen, K.; Langbaum, J.B.S.; Fleisher, A.S.; Alexander, G.E.; Lee, W.; Bandy, D.; De Leon, M.J.; Mosconi, L.; Buckley, S.; et al. Posterior Cingulate Glucose Metabolism, Hippocampal Glucose Metabolism, and Hippocampal Volume in Cognitively Normal, Late-Middle-Aged Persons at 3 Levels of Genetic Risk for Alzheimer Disease. JAMA Neurol. 2013, 70, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; Van Der Flier, W.M.; Zwan, M.D.; Adriaanse, S.F.; Boellaard, R.; Windhorst, A.D.; Barkhof, F.; Lammertsma, A.A.; Scheltens, P.; Van Berckel, B.N.M. Differential Effect of APOE Genotype on Amyloid Load and Glucose Metabolism in AD Dementia. Neurology 2013, 80, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Ohinata, J.; Suzuki, N.; Amamiya, S.; Kajihama, A.; Sugai, R.; Araki, A.; Fujieda, K.; Tanaka, H. Molecular Analysis and Anticonvulsant Therapy in Two Patients with Glucose Transporter 1 Deficiency Syndrome: A Successful Use of Zonisamide for Controlling the Seizures. Epilepsy Res. 2008, 80, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Brukner, A.M.; Ben-Hur, T.; Honig, A.; Ekstein, D.; Eyal, S. Effects of Valproic Acid on Cerebral Nutrient Carriers’ Expression in the Rat. Front. Pharmacol. 2018, 9, 1054. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.Y.; Chu, T.S.; Lai, J.C.; Fung, K.P.; Fok, T.F.; Fujii, T.; Ho, Y.Y. Sodium Valproate Inhibits Glucose Transport and Exacerbates Glut1-Deficiency in Vitro. J. Cell. Biochem. 2005, 96, 775–785. [Google Scholar] [CrossRef]
- Chew, K.S.; Wells, R.C.; Moshkforoush, A.; Chan, D.; Lechtenberg, K.J.; Tran, H.L.; Chow, J.; Kim, D.J.; Robles-Colmenares, Y.; Srivastava, D.B.; et al. CD98hc Is a Target for Brain Delivery of Biotherapeutics. Nat. Commun. 2023, 14, 5053. [Google Scholar] [CrossRef]
- Uchida, Y.; Ohtsuki, S.; Katsukura, Y.; Ikeda, C.; Suzuki, T.; Kamiie, J.; Terasaki, T. Quantitative Targeted Absolute Proteomics of Human Blood-Brain Barrier Transporters and Receptors. J. Neurochem. 2011, 117, 333–345. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helgudóttir, S.S.; Johnsen, K.B.; Routhe, L.G.; Rasmussen, C.L.M.; Thomsen, M.S.; Moos, T. Upregulation of Transferrin Receptor 1 (TfR1) but Not Glucose Transporter 1 (GLUT1) or CD98hc at the Blood–Brain Barrier in Response to Valproic Acid. Cells 2024, 13, 1181. https://doi.org/10.3390/cells13141181
Helgudóttir SS, Johnsen KB, Routhe LG, Rasmussen CLM, Thomsen MS, Moos T. Upregulation of Transferrin Receptor 1 (TfR1) but Not Glucose Transporter 1 (GLUT1) or CD98hc at the Blood–Brain Barrier in Response to Valproic Acid. Cells. 2024; 13(14):1181. https://doi.org/10.3390/cells13141181
Chicago/Turabian StyleHelgudóttir, Steinunn Sara, Kasper Bendix Johnsen, Lisa Greve Routhe, Charlotte Laurfelt Munch Rasmussen, Maj Schneider Thomsen, and Torben Moos. 2024. "Upregulation of Transferrin Receptor 1 (TfR1) but Not Glucose Transporter 1 (GLUT1) or CD98hc at the Blood–Brain Barrier in Response to Valproic Acid" Cells 13, no. 14: 1181. https://doi.org/10.3390/cells13141181
APA StyleHelgudóttir, S. S., Johnsen, K. B., Routhe, L. G., Rasmussen, C. L. M., Thomsen, M. S., & Moos, T. (2024). Upregulation of Transferrin Receptor 1 (TfR1) but Not Glucose Transporter 1 (GLUT1) or CD98hc at the Blood–Brain Barrier in Response to Valproic Acid. Cells, 13(14), 1181. https://doi.org/10.3390/cells13141181





