An Introductory Guide to Using Bloomington Drosophila Stock Center and FlyBase for Aging Research
Abstract
:1. Introduction
2. Conserved Molecular Mechanisms Involved in Aging
2.1. The Insulin/Insulin-Like Growth Factor Signaling and mTOR Pathway
2.2. Mitochondrial Dysfunction
2.3. Inflammation
2.4. Microbiome
2.5. Circadian Rhythms
3. Age-Associated Diseases and Drug Discovery
4. Drosophila as a Model Organism for Aging Studies
5. Navigating the BDSC Website
5.1. The Overall Organization of the BDSC Website
5.2. Browsing Tables
5.3. Searches
5.3.1. Stock Search
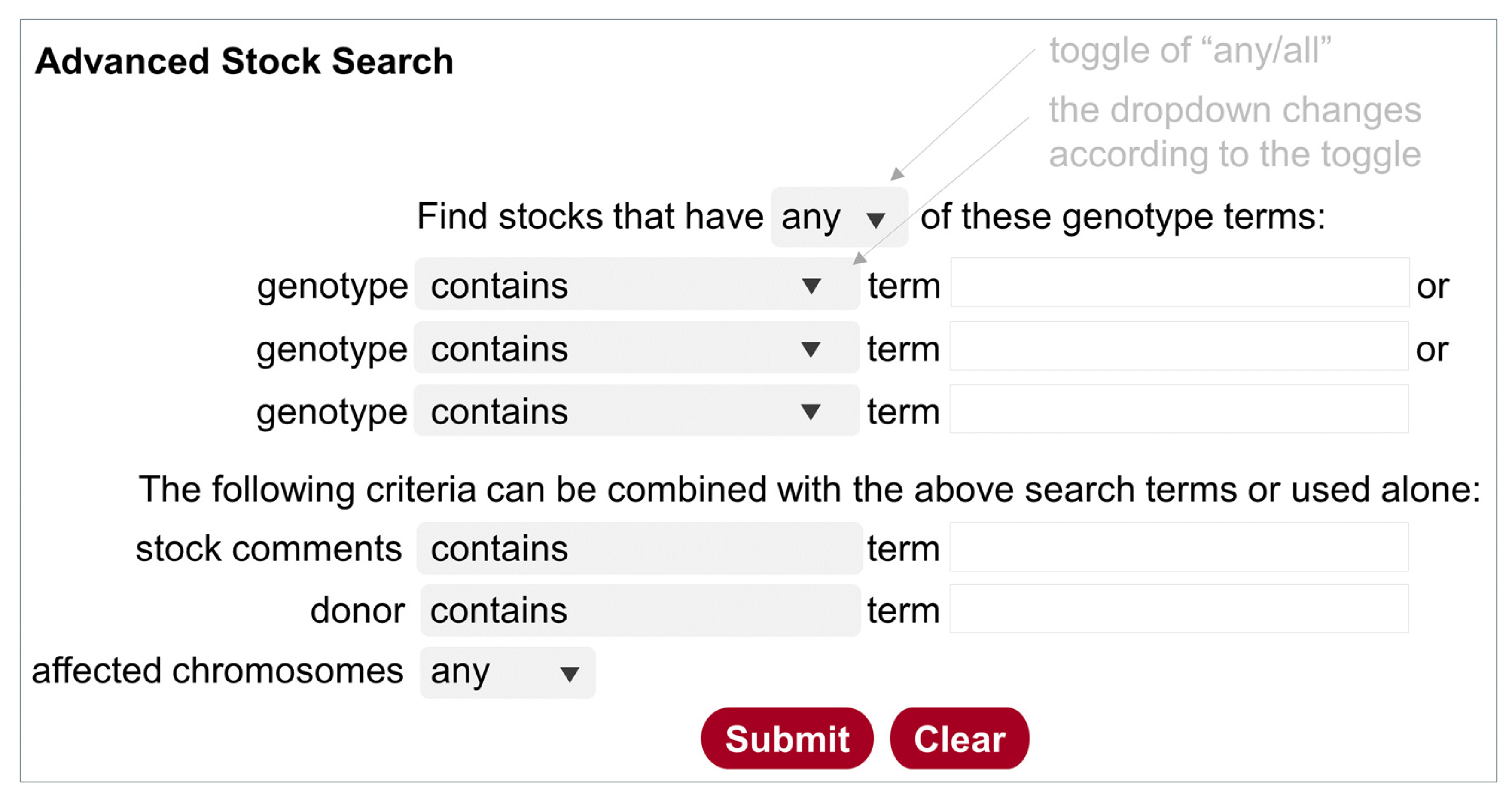
5.3.2. Advanced Stock Search
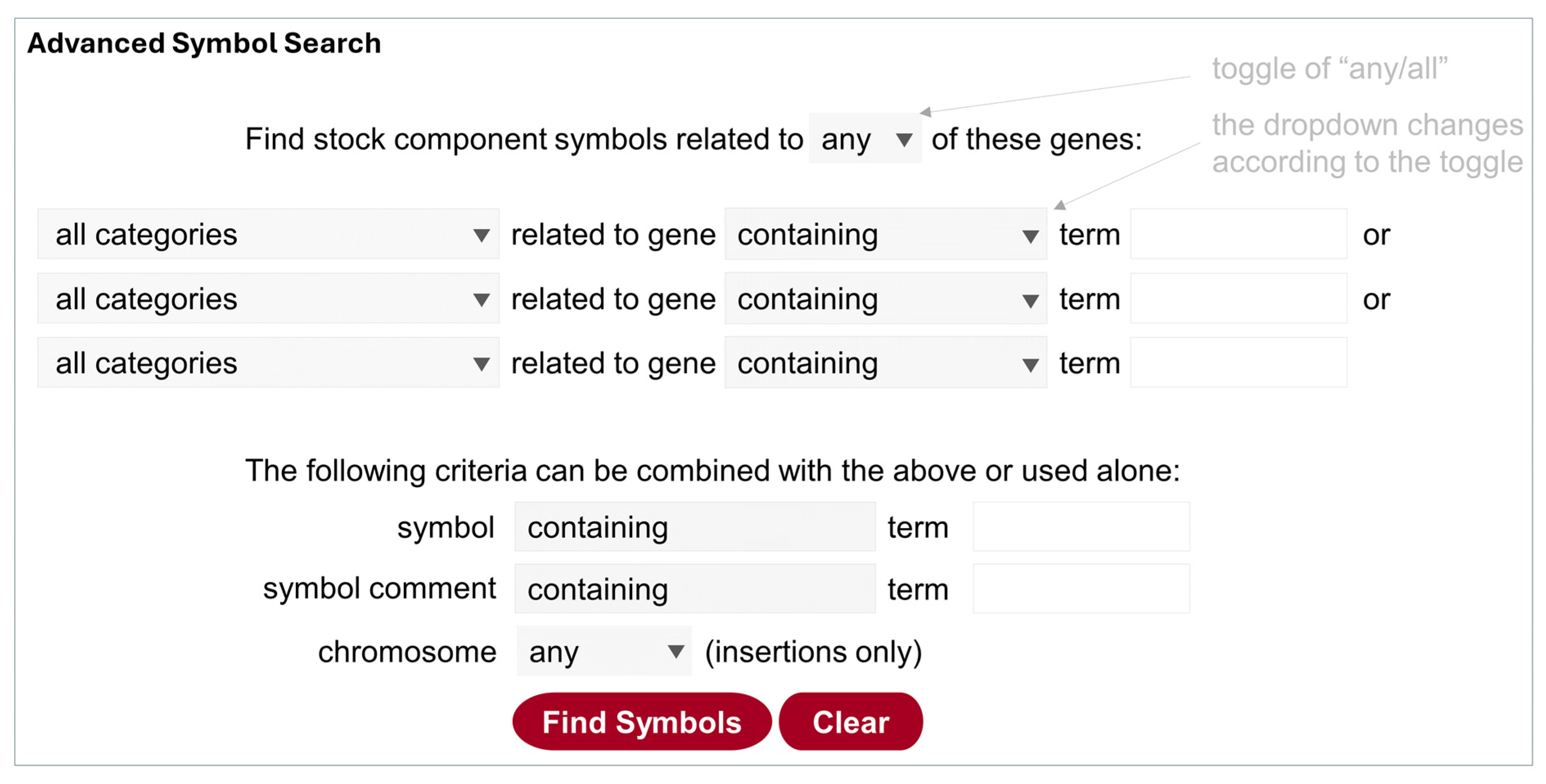
5.3.3. Advanced Symbol Search
5.4. Useful Tips for Searches
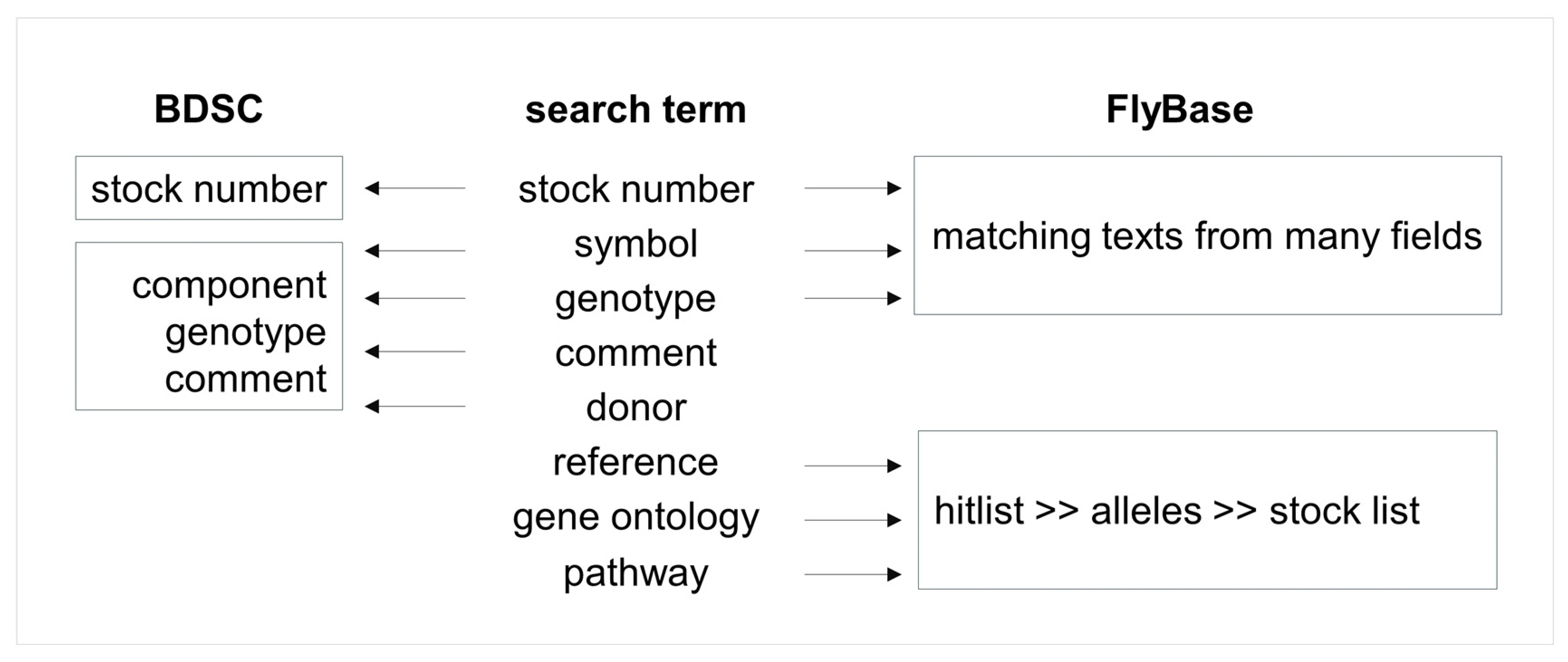
5.5. Sharing Information between BDSC and FlyBase
5.6. Ordering Stocks
6. Finding Stocks for Studying Conserved Mechanisms
6.1. IIS and mTOR
6.2. Autophagy
6.3. Inflammation
6.4. Oxidative Stress
6.5. Microbiome and External Stressors
6.6. Circadian Rhythms
7. Screening for Novel Mechanisms of Aging
8. Concluding Remarks
Funding
Data Availability Statement
Conflicts of Interest
References
- Mirisola, M.G.; Longo, V.D. Yeast Chronological Lifespan: Longevity Regulatory Genes and Mechanisms. Cells 2022, 11, 1714. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, M. The Evolution of the Hallmarks of Aging. Front. Genet. 2021, 12, 693071. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Neve, I.A.A.; Wang, M.C. Neuronal regulation of longevity by staying cool. Genes Dev. 2018, 32, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Soltany, A.; Visavadiya, N.P.; Burtscher, M.; Millet, G.P.; Khoramipour, K.; Khamoui, A.V. Mitochondrial stress and mitokines in aging. Aging Cell 2023, 22, e13770. [Google Scholar] [CrossRef]
- Dinić, M.; Herholz, M.; Kačarević, U.; Radojević, D.; Novović, K.; Đokić, J.; Trifunović, A.; Golić, N. Host-commensal interaction promotes health and lifespan in Caenorhabditis elegans through the activation of HLH-30/TFEB-mediated autophagy. Aging 2021, 13, 8040–8054. [Google Scholar] [CrossRef]
- Grenier, T.; Leulier, F. How commensal microbes shape the physiology of Drosophila melanogaster. Curr. Opin. Insect Sci. 2020, 41, 92–99. [Google Scholar] [CrossRef]
- Zavala, D.V.; Dzikowski, N.; Gopalan, S.; Harrington, K.D.; Pasquini, G.; Mogle, J.; Reid, K.; Sliwinski, M.; Graham-Engeland, J.E.; Engeland, C.G.; et al. Epigenetic Age Acceleration and Chronological Age: Associations With Cognitive Performance in Daily Life. J. Gerontol. Ser. A 2024, 79, glad242. [Google Scholar] [CrossRef]
- Bell, C.G.; Lowe, R.; Adams, P.D.; Baccarelli, A.A.; Beck, S.; Bell, J.T.; Christensen, B.C.; Gladyshev, V.N.; Heijmans, B.T.; Horvath, S.; et al. DNA methylation aging clocks: Challenges and recommendations. Genome Biol. 2019, 20, 249. [Google Scholar] [CrossRef]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef]
- Smulders, L.; Deelen, J. Genetics of human longevity: From variants to genes to pathways. J. Intern. Med. 2024, 295, 416–435. [Google Scholar] [CrossRef]
- Kenyon, C. A conserved regulatory system for aging. Cell 2001, 105, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Tatar, M. Unraveling the Molecular Mechanism of Immunosenescence in Drosophila. Int. J. Mol. Sci. 2018, 19, 2472. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, P.; Pozza, F.; Pletcher, S.D.; Gendron, C.M.; Longo, V.D. Regulation of longevity and stress resistance by Sch9 in yeast. Science 2001, 292, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 1993, 366, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Tatar, M.; Kopelman, A.; Epstein, D.; Tu, M.P.; Yin, C.M.; Garofalo, R.S. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 2001, 292, 107–110. [Google Scholar] [CrossRef]
- Clancy, D.J.; Gems, D.; Harshman, L.G.; Oldham, S.; Stocker, H.; Hafen, E.; Leevers, S.J.; Partridge, L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 2001, 292, 104–106. [Google Scholar] [CrossRef]
- Nelson, J.F.; Strong, R.; Bokov, A.; Diaz, V.; Ward, W. Probing the Relationship Between Insulin Sensitivity and Longevity Using Genetically Modified Mice. J. Gerontol. Ser. A 2012, 67, 1332–1338. [Google Scholar] [CrossRef]
- Cao, G.; Lin, M.; Gu, W.; Su, Z.; Duan, Y.; Song, W.; Liu, H.; Zhang, F. The rules and regulatory mechanisms of FOXO3 on inflammation, metabolism, cell death and aging in hosts. Life Sci. 2023, 328, 121877. [Google Scholar] [CrossRef]
- Curtis, R.; O’Connor, G.; DiStefano, P.S. Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (aak-2) links multiple aging and metabolism pathways. Aging Cell 2006, 5, 119–126. [Google Scholar] [CrossRef]
- Martins, R.; Lithgow, G.J.; Link, W. Long live FOXO: Unraveling the role of FOXO proteins in aging and longevity. Aging Cell 2016, 15, 196–207. [Google Scholar] [CrossRef]
- Lin, K.; Dorman, J.B.; Rodan, A.; Kenyon, C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 1997, 278, 1319–1322. [Google Scholar] [CrossRef] [PubMed]
- Giannakou, M.E.; Goss, M.; Jünger, M.A.; Hafen, E.; Leevers, S.J.; Partridge, L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science 2004, 305, 361. [Google Scholar] [CrossRef]
- Clancy, D.J.; Gems, D.; Hafen, E.; Leevers, S.J.; Partridge, L. Dietary restriction in long-lived dwarf flies. Science 2002, 296, 319. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.; Shah Bana, M.A.F.; Tan, J.K.; Goon, J.A. Effect of dietary restriction on health span in Caenorhabditis elegans: A systematic review. Exp. Gerontol. 2023, 182, 112294. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J.H.; Seo, H.D.; Jung, C.H.; Ahn, J. Longevity through diet restriction and immunity. BMB Rep. 2023, 56, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Grande de França, N.A.; Rolland, Y.; Guyonnet, S.; de Souto Barreto, P. The role of dietary strategies in the modulation of hallmarks of aging. Ageing Res. Rev. 2023, 87, 101908. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Dowlatshahi, D.; Banko, M.R.; Villen, J.; Hoang, K.; Blanchard, D.; Gygi, S.P.; Brunet, A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol. 2007, 17, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Novelle, M.G.; Ali, A.; Diéguez, C.; Bernier, M.; de Cabo, R. Metformin: A Hopeful Promise in Aging Research. Cold Spring Harb. Perspect. Med. 2016, 6, a025932. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab. 2020, 32, 15–30. [Google Scholar] [CrossRef]
- Lee, D.J.W.; Hodzic Kuerec, A.; Maier, A.B. Targeting ageing with rapamycin and its derivatives in humans: A systematic review. Lancet Healthy Longev. 2024, 5, e152–e162. [Google Scholar] [CrossRef]
- Yang, F.; Liu, X.; Li, Y.; Yu, Z.; Huang, X.; Yang, G.; Xu, S. Evolutionary analysis of the mTOR pathway provide insights into lifespan extension across mammals. BMC Genom. 2023, 24, 456. [Google Scholar] [CrossRef] [PubMed]
- Cornu, M.; Albert, V.; Hall, M.N. mTOR in aging, metabolism, and cancer. Curr. Opin. Genet. Dev. 2013, 23, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A.; List, E.O.; Kopchick, J.J. The somatotropic axis and aging: Benefits of endocrine defects. Growth Horm. IGF Res. 2016, 27, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Poloz, Y.; Stambolic, V. Obesity and cancer, a case for insulin signaling. Cell Death Dis. 2015, 6, e2037. [Google Scholar] [CrossRef] [PubMed]
- Bjedov, I.; Rallis, C. The Target of Rapamycin Signalling Pathway in Ageing and Lifespan Regulation. Genes 2020, 11, 1043. [Google Scholar] [CrossRef] [PubMed]
- Sun-Wang, J.L.; Ivanova, S.; Zorzano, A. The dialogue between the ubiquitin-proteasome system and autophagy: Implications in ageing. Ageing Res. Rev. 2020, 64, 101203. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Taguchi, K.; Tanaka, M. Ubiquitin, Autophagy and Neurodegenerative Diseases. Cells 2020, 9, 2022. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Strong, R. Rapamycin, the only drug that has been consistently demonstrated to increase mammalian longevity. An update. Exp. Gerontol. 2023, 176, 112166. [Google Scholar] [CrossRef]
- Maruzs, T.; Simon-Vecsei, Z.; Kiss, V.; Csizmadia, T.; Juhász, G. On the Fly: Recent Progress on Autophagy and Aging in Drosophila. Front. Cell Dev. Biol. 2019, 7, 140. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Picca, A.; Mankowski, R.T.; Burman, J.L.; Donisi, L.; Kim, J.-S.; Marzetti, E.; Leeuwenburgh, C. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat. Rev. Cardiol. 2018, 15, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guan, T.; Shafiq, K.; Yu, Q.; Jiao, X.; Na, D.; Li, M.; Zhang, G.; Kong, J. Mitochondrial dysfunction in aging. Ageing Res. Rev. 2023, 88, 101955. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Faitg, J.; Auwerx, J.; Ferrucci, L.; D’Amico, D. Mitophagy in human health, ageing and disease. Nat. Metab. 2023, 5, 2047–2061. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef] [PubMed]
- Weir, H.J.; Yao, P.; Huynh, F.K.; Escoubas, C.C.; Goncalves, R.L.; Burkewitz, K.; Laboy, R.; Hirschey, M.D.; Mair, W.B. Dietary Restriction and AMPK Increase Lifespan via Mitochondrial Network and Peroxisome Remodeling. Cell Metab. 2017, 26, 884–896.e5. [Google Scholar] [CrossRef]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Trefts, E.; Shaw, R.J. AMPK: Restoring metabolic homeostasis over space and time. Mol. Cell 2021, 81, 3677–3690. [Google Scholar] [CrossRef]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-Dit-Félix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef]
- Chamoli, M.; Rane, A.; Foulger, A.; Chinta, S.J.; Shahmirzadi, A.A.; Kumsta, C.; Nambiar, D.K.; Hall, D.; Holcom, A.; Angeli, S.; et al. A drug-like molecule engages nuclear hormone receptor DAF-12/FXR to regulate mitophagy and extend lifespan. Nat. Aging 2023, 3, 1529–1543. [Google Scholar] [CrossRef]
- Guo, J.; Chiang, W.C. Mitophagy in aging and longevity. IUBMB Life 2022, 74, 296–316. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Fülöp, T.; Pawelec, G. Immunosenescence in vertebrates and invertebrates. Immun. Ageing 2013, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Pradeu, T.; Thomma, B.; Girardin, S.E.; Lemaitre, B. The conceptual foundations of innate immunity: Taking stock 30 years later. Immunity 2024, 57, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Sciambra, N.; Chtarbanova, S. The Impact of Age on Response to Infection in Drosophila. Microorganisms 2021, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef] [PubMed]
- Garschall, K.; Flatt, T. The interplay between immunity and aging in Drosophila. F1000Res 2018, 7, 160. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Kaeberlein, M. How healthy is the healthspan concept? Geroscience 2018, 40, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qu, Z.; Chu, J.; Hun, S. Aging Gut Microbiome in Healthy and Unhealthy Aging. Aging Dis. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Pereira, Q.C.; Fortunato, I.M.; Oliveira, F.S.; Alvarez, M.C.; Santos, T.W.D.; Ribeiro, M.L. Polyphenolic Compounds: Orchestrating Intestinal Microbiota Harmony during Aging. Nutrients 2024, 16, 1066. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Awe, T.; Fasawe, A.; Sawe, C.; Ogunware, A.; Jamiu, A.T.; Allen, M. The modulatory role of gut microbiota on host behavior: Exploring the interaction between the brain-gut axis and the neuroendocrine system. AIMS Neurosci. 2024, 11, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.H.P.; Shamsuddin, S.; Zainuddin, A. Ageing and the gut-brain axis: Lessons from the Drosophila model. Benef. Microbes 2023, 14, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tang, X.; Mao, B.; Zhang, Q.; Tian, F.; Zhao, J.; Cui, S.; Chen, W. Anti-aging effects and mechanisms of anthocyanins and their intestinal microflora metabolites. Crit. Rev. Food Sci. Nutr. 2024, 64, 2358–2374. [Google Scholar] [CrossRef] [PubMed]
- Mattis, J.; Sehgal, A. Circadian Rhythms, Sleep, and Disorders of Aging. Trends Endocrinol. Metab. 2016, 27, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Allada, R.; Bass, J. Circadian Mechanisms in Medicine. N. Engl. J. Med. 2021, 384, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of circadian rhythms in health and disease. Genome Med. 2019, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Katewa, S.D.; Akagi, K.; Bose, N.; Rakshit, K.; Camarella, T.; Zheng, X.; Hall, D.; Davis, S.; Nelson, C.S.; Brem, R.B.; et al. Peripheral Circadian Clocks Mediate Dietary Restriction-Dependent Changes in Lifespan and Fat Metabolism in Drosophila. Cell Metab. 2016, 23, 143–154. [Google Scholar] [CrossRef]
- Lavallee, C.M.; Bruno, A.; Ma, C.; Raman, M. The Role of Intermittent Fasting in the Management of Nonalcoholic Fatty Liver Disease: A Narrative Review. Nutrients 2022, 14, 4655. [Google Scholar] [CrossRef]
- Hu, X.; Peng, J.; Tang, W.; Xia, Y.; Song, P. A circadian rhythm-restricted diet regulates autophagy to improve cognitive function and prolong lifespan. Biosci. Trends 2023, 17, 356–368. [Google Scholar] [CrossRef]
- Yin, Z.; Klionsky, D.J. Intermittent time-restricted feeding promotes longevity through circadian autophagy. Autophagy 2022, 18, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Ulgherait, M.; Midoun, A.M.; Park, S.J.; Gatto, J.A.; Tener, S.J.; Siewert, J.; Klickstein, N.; Canman, J.C.; Ja, W.W.; Shirasu-Hiza, M. Circadian autophagy drives iTRF-mediated longevity. Nature 2021, 598, 353–358. [Google Scholar] [CrossRef]
- Nathan, P.; Gibbs, J.E.; Rainger, G.E.; Chimen, M. Changes in Circadian Rhythms Dysregulate Inflammation in Ageing: Focus on Leukocyte Trafficking. Front. Immunol. 2021, 12, 673405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Warman, G.R.; Cheeseman, J.F. The functional changes of the circadian system organization in aging. Ageing Res. Rev. 2019, 52, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Froy, O. Circadian rhythms, aging, and life span in mammals. Physiology 2011, 26, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich-Nikitin, I.; Kirshenbaum, E.; Kirshenbaum, L.A. Autophagy, Clock Genes, and Cardiovascular Disease. Can. J. Cardiol. 2023, 39, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.A.; Polino, A.J.; King, M.W.; Musiek, E.S. Circadian clock protein BMAL1 broadly influences autophagy and endolysosomal function in astrocytes. Proc. Natl. Acad. Sci. USA 2023, 120, e2220551120. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Z.; Cai, Y.; Zeng, S.; Peng, B.; Ren, X.; Yan, Y.; Gong, Z. Rheostatic Balance of Circadian Rhythm and Autophagy in Metabolism and Disease. Front. Cell Dev. Biol. 2020, 8, 616434. [Google Scholar] [CrossRef]
- Ryzhikov, M.; Ehlers, A.; Steinberg, D.; Xie, W.; Oberlander, E.; Brown, S.; Gilmore, P.E.; Townsend, R.R.; Lane, W.S.; Dolinay, T.; et al. Diurnal Rhythms Spatially and Temporally Organize Autophagy. Cell Rep. 2019, 26, 1880–1892.e6. [Google Scholar] [CrossRef]
- Kalfalah, F.; Janke, L.; Schiavi, A.; Tigges, J.; Ix, A.; Ventura, N.; Boege, F.; Reinke, H. Crosstalk of clock gene expression and autophagy in aging. Aging 2016, 8, 1876–1895. [Google Scholar] [CrossRef]
- Mauri, S.; Favaro, M.; Bernardo, G.; Mazzotta, G.M.; Ziviani, E. Mitochondrial autophagy in the sleeping brain. Front. Cell Dev. Biol. 2022, 10, 956394. [Google Scholar] [CrossRef]
- Bedont, J.L.; Toda, H.; Shi, M.; Park, C.H.; Quake, C.; Stein, C.; Kolesnik, A.; Sehgal, A. Short and long sleeping mutants reveal links between sleep and macroautophagy. eLife 2021, 10, e64140. [Google Scholar] [CrossRef]
- Mejía, S.T.; Su, T.T.; Washington, F.C.; Golinski, S.; Sosnoff, J.J. Everyday Experiences of Physical Function and Awareness of Fall Risk in Older Adulthood. Innov. Aging 2023, 7, igad037. [Google Scholar] [CrossRef]
- Atella, V.; Piano Mortari, A.; Kopinska, J.; Belotti, F.; Lapi, F.; Cricelli, C.; Fontana, L. Trends in age-related disease burden and healthcare utilization. Aging Cell 2019, 18, e12861. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Lee, Y.R.; Ong, L.; Gold, M.; Kalali, A.; Sarkar, J. Alzheimer’s Disease: Key Insights from Two Decades of Clinical Trial Failures. J. Alzheimers Dis. 2022, 87, 83–100. [Google Scholar] [CrossRef]
- Liu, W.S.; You, J.; Ge, Y.J.; Wu, B.S.; Zhang, Y.; Chen, S.D.; Zhang, Y.R.; Huang, S.Y.; Ma, L.Z.; Feng, J.F.; et al. Association of biological age with health outcomes and its modifiable factors. Aging Cell 2023, 22, e13995. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.; Guvatova, Z.; Lopes, I.A.; Beckett, C.W.; Kennedy, B.K.; De Magalhaes, J.P.; Makarov, A.A. Targeting aging mechanisms: Pharmacological perspectives. Trends Endocrinol. Metab. 2022, 33, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Bakula, D.; Ablasser, A.; Aguzzi, A.; Antebi, A.; Barzilai, N.; Bittner, M.I.; Jensen, M.B.; Calkhoven, C.F.; Chen, D.; Grey, A.; et al. Latest advances in aging research and drug discovery. Aging 2019, 11, 9971–9981. [Google Scholar] [CrossRef]
- Meron, E.; Thaysen, M.; Angeli, S.; Antebi, A.; Barzilai, N.; Baur, J.A.; Bekker-Jensen, S.; Birkisdottir, M.; Bischof, E.; Bruening, J.; et al. Meeting Report: Aging Research and Drug Discovery. Aging 2022, 14, 530–543. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Pennypacker, J.K. Drugs that modulate aging: The promising yet difficult path ahead. Transl. Res. 2014, 163, 456–465. [Google Scholar] [CrossRef]
- Ogienko, A.A.; Omelina, E.S.; Bylino, O.V.; Batin, M.A.; Georgiev, P.G.; Pindyurin, A.V. Drosophila as a Model Organism to Study Basic Mechanisms of Longevity. Int. J. Mol. Sci. 2022, 23, 11244. [Google Scholar] [CrossRef] [PubMed]
- Nainu, F.; Bahar, M.A.; Sartini, S.; Rosa, R.A.; Rahmah, N.; Kamri, R.A.; Rumata, N.R.; Yulianty, R.; Wahyudin, E. Proof-of-Concept Preclinical Use of Drosophila melanogaster in the Initial Screening of Immunomodulators. Sci. Pharm. 2022, 90, 11. [Google Scholar] [CrossRef]
- Gasque, G.; Conway, S.; Huang, J.; Rao, Y.; Vosshall, L.B. Small molecule drug screening in Drosophila identifies the 5HT2A receptor as a feeding modulation target. Sci. Rep. 2013, 3, srep02120. [Google Scholar] [CrossRef]
- Munnik, C.; Xaba, M.P.; Malindisa, S.T.; Russell, B.L.; Sooklal, S.A. Drosophila melanogaster: A platform for anticancer drug discovery and personalized therapies. Front. Genet. 2022, 13, 949241. [Google Scholar] [CrossRef] [PubMed]
- Su, T.T. Drug screening in Drosophila; why, when, and when not? Wiley Interdiscip. Rev. Dev. Biol. 2019, 8, e346. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Lim, C.X.; Shaukat, Z.; Islam, A.; Caseley, E.A.; Lippiat, J.D.; Rychkov, G.Y.; Ricos, M.G.; Dibbens, L.M. Drosophila expressing mutant human KCNT1 transgenes make an effective tool for targeted drug screening in a whole animal model of KCNT1-epilepsy. Sci. Rep. 2024, 14, 3357. [Google Scholar] [CrossRef]
- DeLoriea, J.; Millet-Boureima, C.; Gamberi, C. Protocol to build a drug-testing pipeline using large populations of Drosophila melanogaster. STAR Protoc. 2023, 4, 102747. [Google Scholar] [CrossRef] [PubMed]
- Hohman, L.S.; Osborne, L.C. A gut-centric view of aging: Do intestinal epithelial cells contribute to age-associated microbiota changes, inflammaging, and immunosenescence? Aging Cell 2022, 21, e13700. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.Q.; Markstein, M.; Binari, R.; Pfeiffer, B.; Liu, L.P.; Villalta, C.; Booker, M.; Perkins, L.; Perrimon, N. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat. Methods 2008, 5, 49–51. [Google Scholar] [CrossRef]
- Öztürk-Çolak, A.; Marygold, S.J.; Antonazzo, G.; Attrill, H.; Goutte-Gattat, D.; Jenkins, V.K.; Matthews, B.B.; Millburn, G.; dos Santos, G.; Tabone, C.J.; et al. FlyBase: Updates to the Drosophila genes and genomes database. Genetics 2024, 227, iyad211. [Google Scholar] [CrossRef]
- Hu, Y.; Comjean, A.; Mohr, S.E.; Perrimon, N. Gene2Function: An Integrated Online Resource for Gene Function Discovery. G3 2017, 7, 2855–2858. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chung, V.; Comjean, A.; Rodiger, J.; Nipun, F.; Perrimon, N.; Mohr, S.E. BioLitMine: Advanced Mining of Biomedical and Biological Literature About Human Genes and Genes from Major Model Organisms. G3 2020, 10, 4531–4539. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, V.K.; Larkin, A.; Thurmond, J. Using FlyBase: A Database of Drosophila Genes and Genetics. In Drosophila: Methods and Protocols; Dahmann, C., Ed.; Springer: New York, NY, USA, 2022; pp. 1–34. [Google Scholar]
- Zhang, H. The Genetics of Autophagy in Multicellular Organisms. Annu. Rev. Genet. 2022, 56, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Juhász, G.; Erdi, B.; Sass, M.; Neufeld, T.P. Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Genes Dev. 2007, 21, 3061–3066. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, A.; Cumming, R.C.; Brech, A.; Isakson, P.; Schubert, D.R.; Finley, K.D. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy 2008, 4, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Ulgherait, M.; Rana, A.; Rera, M.; Graniel, J.; Walker, D.W. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 2014, 8, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.-O.; Yoo, S.-M.; Ahn, H.-H.; Nah, J.; Hong, S.-H.; Kam, T.-I.; Jung, S.; Jung, Y.-K. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat. Commun. 2013, 4, 2300. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, Z.; Liu, D.; Gregory, S. Sterile inflammation in Drosophila. Mediat. Inflamm. 2015, 2015, 369286. [Google Scholar] [CrossRef]
- Arch, M.; Vidal, M.; Koiffman, R.; Melkie, S.T.; Cardona, P.-J. Drosophila melanogaster as a model to study innate immune memory. Front. Microbiol. 2022, 13, 991678. [Google Scholar] [CrossRef]
- García-García, V.A.; Alameda, J.P.; Page, A.; Casanova, M.L. Role of NF-κB in Ageing and Age-Related Diseases: Lessons from Genetically Modified Mouse Models. Cells 2021, 10, 1906. [Google Scholar] [CrossRef]
- Kounatidis, I.; Chtarbanova, S. Role of Glial Immunity in Lifespan Determination: A Drosophila Perspective. Front. Immunol. 2018, 9, 1362. [Google Scholar] [CrossRef] [PubMed]
- Khor, S.; Cai, D. Control of lifespan and survival by Drosophila NF-κB signaling through neuroendocrine cells and neuroblasts. Aging 2020, 12, 24604–24622. [Google Scholar] [CrossRef] [PubMed]
- Ehrenreich, I.M.; Torabi, N.; Jia, Y.; Kent, J.; Martis, S.; Shapiro, J.A.; Gresham, D.; Caudy, A.A.; Kruglyak, L. Dissection of genetically complex traits with extremely large pools of yeast segregants. Nature 2010, 464, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- King, E.G.; Merkes, C.M.; McNeil, C.L.; Hoofer, S.R.; Sen, S.; Broman, K.W.; Long, A.D.; Macdonald, S.J. Genetic dissection of a model complex trait using the Drosophila Synthetic Population Resource. Genome Res. 2012, 22, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, S.J.; Long, A.D. Discovery of malathion resistance QTL in Drosophila melanogaster using a bulked phenotyping approach. G3 Genes|Genomes|Genet. 2022, 12, jkac279. [Google Scholar] [CrossRef] [PubMed]
- Mackay, T.F.; Richards, S.; Stone, E.A.; Barbadilla, A.; Ayroles, J.F.; Zhu, D.; Casillas, S.; Han, Y.; Magwire, M.M.; Cridland, J.M.; et al. The Drosophila melanogaster Genetic Reference Panel. Nature 2012, 482, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Ariyapala, I.S.; Holsopple, J.M.; Popodi, E.M.; Hartwick, D.G.; Kahsai, L.; Cook, K.R.; Sokol, N.S. Identification of Split-GAL4 Drivers and Enhancers That Allow Regional Cell Type Manipulations of the Drosophila melanogaster Intestine. Genetics 2020, 216, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Newby, L.M.; Jackson, F.R. Drosophila ebony mutants have altered circadian activity rhythms but normal eclosion rhythms. J. Neurogenet. 1991, 7, 85–101. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jasper, H. Studying aging in Drosophila. Methods 2014, 68, 129–133. [Google Scholar] [CrossRef]
- Martin, I.; Grotewiel, M.S. Distinct genetic influences on locomotor senescence in Drosophila revealed by a series of metrical analyses. Exp. Gerontol. 2006, 41, 877–881. [Google Scholar] [CrossRef]


| Dropdown Item | Description |
|---|---|
| all categories | No filter for any category |
| allele | Classical gene alleles, insertions associated with altered genomic DNA |
| antibody fragment against | Gene product targeted by antibody expressed from the transgene |
| coding | Insertions carrying coding sequence for the gene |
| coding and regulatory | Insertions carrying both coding and regulatory sequences for the gene |
| deficiency or putative deficiency | Deficiencies that delete or may delete the gene |
| gene trap | Insertion sequences may be transcribed with the gene |
| guideRNA | Guide RNAs for the gene |
| inserted or swapped in | Insertions in the gene |
| noncoding RNA | Insertions carrying a noncoding RNA gene |
| potential misexpression | Insertions carrying UAS sequences that might direct expression of nearby genes |
| protein binding site (e.g., FRT or loxP site) | Transgenes harboring non-regulatory DNA sequences (e.g., recombinase target sites) |
| protein trap | Insertion sequences (usually reporter) may be incorporated into the protein |
| regulatory or putative regulatory | Insertions carrying regulatory or putative regulatory sequences from the gene |
| RNA sponge | Insertions carrying RNA for sequestering the gene transcript |
| RNAi | Insertions carrying sequences for RNAi against the gene |
| wildtype allele | Wild-type alleles of the gene |
| Zn finger nuclease | Insertions carrying Zn finger nuclease that targets the gene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X. An Introductory Guide to Using Bloomington Drosophila Stock Center and FlyBase for Aging Research. Cells 2024, 13, 1192. https://doi.org/10.3390/cells13141192
Zheng X. An Introductory Guide to Using Bloomington Drosophila Stock Center and FlyBase for Aging Research. Cells. 2024; 13(14):1192. https://doi.org/10.3390/cells13141192
Chicago/Turabian StyleZheng, Xiangzhong. 2024. "An Introductory Guide to Using Bloomington Drosophila Stock Center and FlyBase for Aging Research" Cells 13, no. 14: 1192. https://doi.org/10.3390/cells13141192






