Human-Induced Pluripotent Stem Cell (iPSC)-Derived GABAergic Neuron Differentiation in Bipolar Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Neuronal Differentiation
2.2. Immunocytochemistry
2.3. RNA Sequencing
2.4. qRT-PCR
2.5. Statistical Analysish
2.6. Scanning Electron Microscopy
2.7. Live Cell Calcium Imaging
2.8. Multi-Electrode Array Analysis
3. Results
3.1. Generation and Validation of CRISPR-Corrected Isogenic Bipolar Disorder Cell Lines
3.2. GABAergic Neuron Differentiation
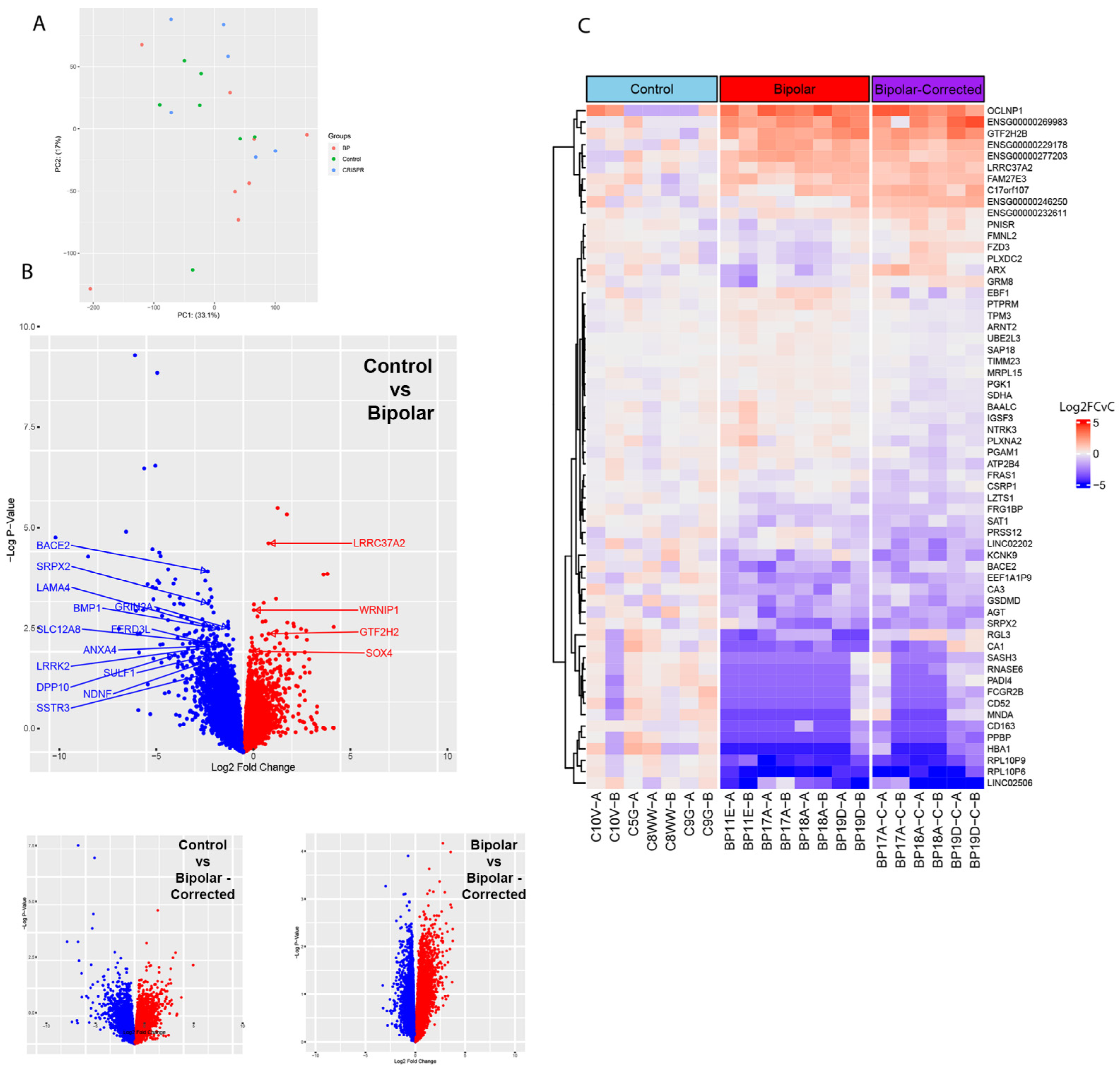
3.3. Bulk RNA Sequencing Analysis of GABAergic Neurons
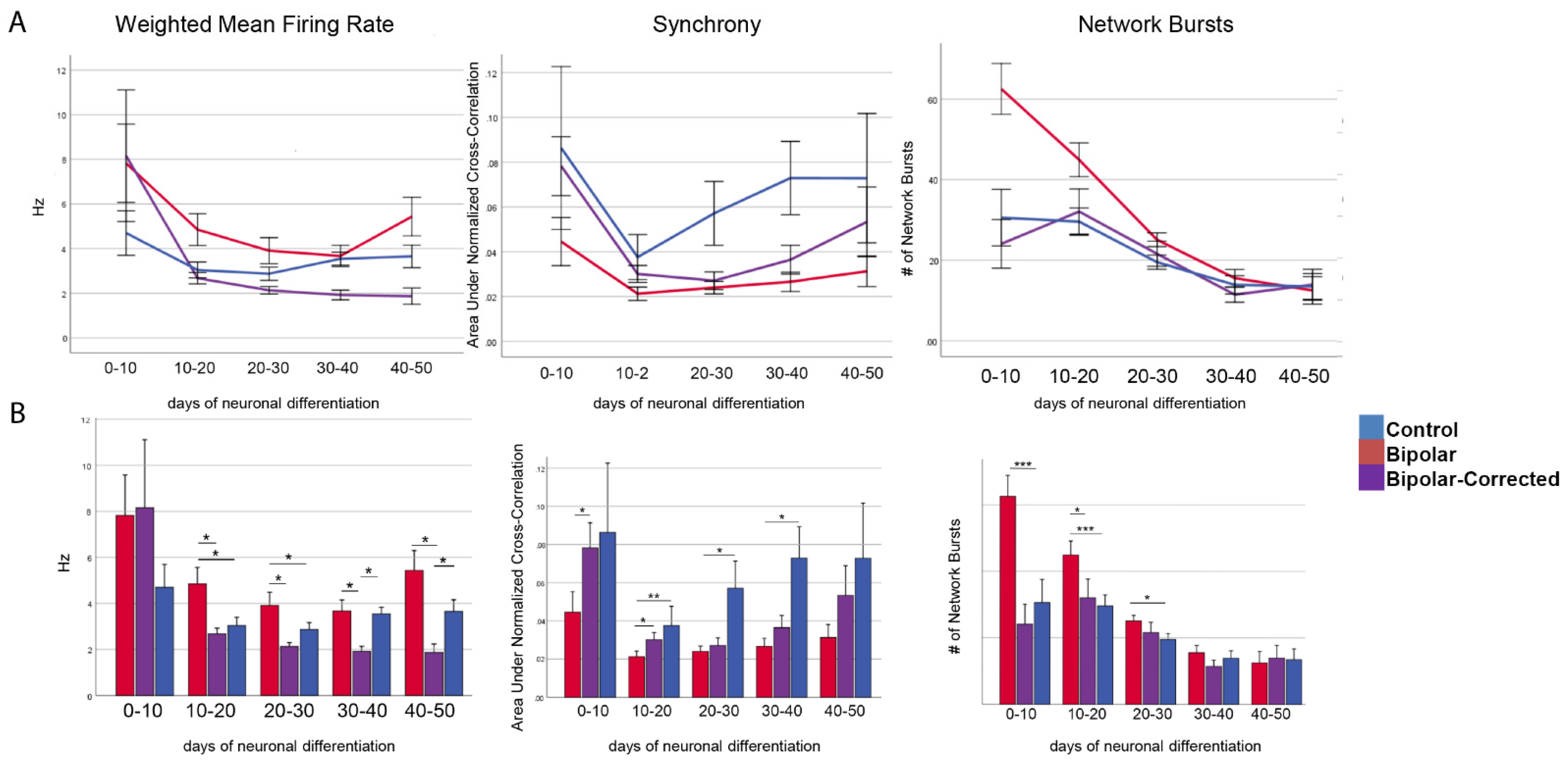
3.4. Multi-Electrode Array Analysis of GABAergic Neurons
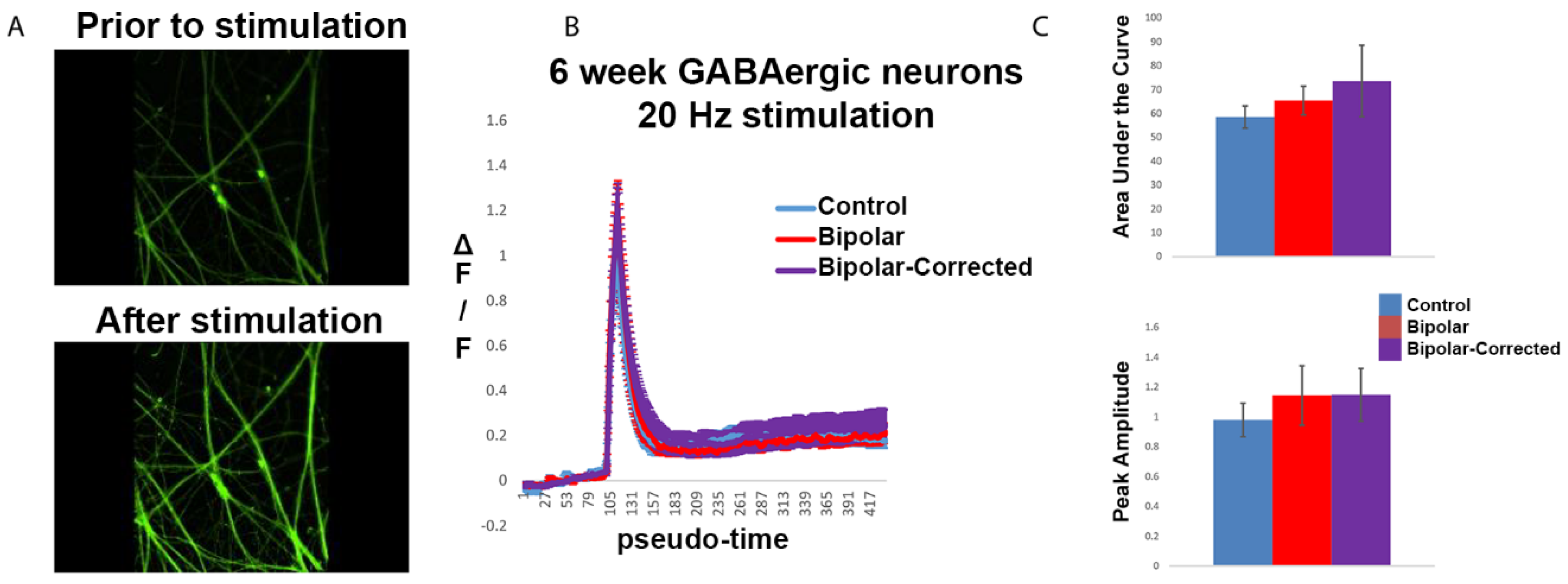
3.5. Live Cell Calcium Imaging of GABAergic Neurons
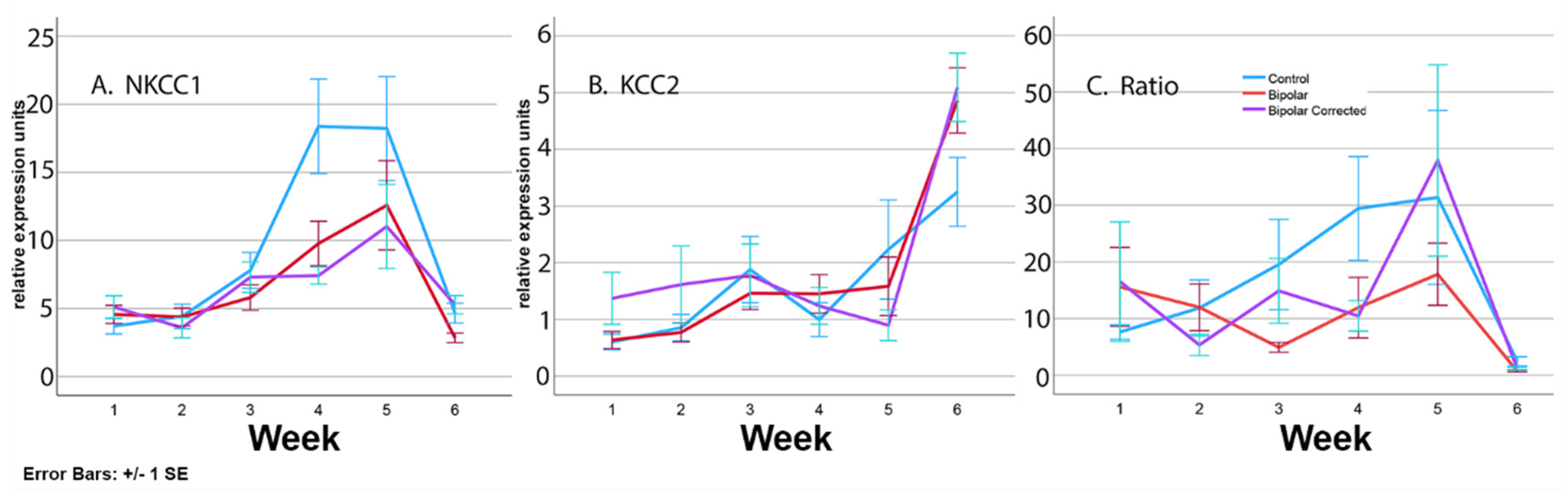
3.6. Expression of Transporters during Differentiation of BP Patient GABAergic Neurons
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quadrato, G.; Brown, J.; Arlotta, P. The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat. Med. 2016, 22, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Eichmuller, O.L.; Knoblich, J.A. Human cerebral organoids-a new tool for clinical neurology research. Nat. Rev. Neurol. 2022, 18, 661–680. [Google Scholar] [CrossRef] [PubMed]
- Moore, Y.E.; Kelley, M.R.; Brandon, N.J.; Deeb, T.Z.; Moss, S.J. Seizing control of KCC2: A new therapeutic target for epilepsy. Trends Neurosci. 2017, 40, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.O., Jr.; McCarthy, J.M.; Prescot, A.P.; Jensen, J.E.; Cooper, A.J.; Cohen, B.M.; Renshaw, P.F.; Ongur, D. Brain gamma-aminobutyric acid (GABA) abnormalities in bipolar disorder. Bipolar Disord. 2013, 15, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, P.; Perez, J.; Barale, F.; Schettini, G.; Soares, J.C. GABAergic dysfunction in mood disorders. Mol. Psychiatry 2003, 8, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Emrich, H.M.; von Zerssen, D.; Kissling, W.; Moller, J.H.; Windorfer, A. Effect of sodium valproate on mania. The GABA-hypothesis of affective disorders. Arch. Psychiatr. Nervenkrankh. 1980, 229, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, J.-P.; Viou, L.; Launay, P.-S.; Luccardini, C.; Gil, S.E.; Kiyasova, V.; Irinopoulou, T.; Alvarez, C.; Rio, J.-P.; Boudier, T.; et al. Tangentially migrating neurons assemble a primary cilium that promotes their reorientation to the cortical plate. Neuron 2012, 76, 1108–1122. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.; Voipio, J.; Payne, J.A.; Ruusuvuori, E.; Lahtinen, H.; Lamsa, K.; Pirvola, U.; Saarma, M.; Kaila, K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 1999, 397, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, M.; Tsukahara, T.; Tomito, K.; Iwai, H.; Sonomuro, T.; Miyawaki, S.; Sato, T. Neonatal maternal separation delays the GABA excitatory-to-inhibitory functional switch by inhibiting KCC2 expression. Biochem. Bioiophys. Res. Commun. 2017, 493, 1243–1249. [Google Scholar] [CrossRef]
- Veerawatananan, B.; Surakul, P.; Chutabhakdikul, N. Maternal restraint stress delays maturation of cation-chloride cotransporters and GABAA receptor subunits in the hippocampus of rat pups at puberty. Neurobiol. Stress 2015, 14, 1–7. [Google Scholar] [CrossRef]
- Marin, O. Developmental timing and critical windows for the treatment of psychiatric disorders. Nat. Med. 2016, 22, 1229–1238. [Google Scholar] [CrossRef]
- Chao, H.-T.; Chen, H.; Samaco, R.C.; Xue, M.; Chahrour, M.; Yoo, J.; Neul, J.L.; Gong, S.; Lu, H.-C.; Heintz, N.; et al. GABAergic dysfunction mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 2010, 468, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sokolova, I.; Domissy, A.; Davis, J.; Rao, L.; Utami, K.H.; Wang, Y.; Hagerman, R.J.; Pouladi, M.A.; Sanna, P.; et al. Maturation delay of human GABAergic neurogenesis in Fragile X syndrome pluripotent stem cells. Stem Cells Transl. Med. 2022, 11, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Drotar, J.; Li, K.; Clairmont, C.D.; Brumm, A.S.; Sullins, A.J.; Wu, H.; Liu, X.S.; Wang, J.; Gray, N.S.; et al. Pharmacological enhancement of KCC2 gene expression exerts therapeutic effects on human Rett syndrome neurons and Mecp2 mutant mice. Sci. Transl. Med. 2019, 11, eaau0164. [Google Scholar] [CrossRef]
- Lysenko, L.V.; Kim, J.; Madamba, F.; Tyrtyshnaia, A.A.; Ruparelia, A.; Kleschevnikov, A.M. Developmental excitatory-to- inhibitory GABA polarity switch is delayed in Ts65Dn mice, a genetic model of Down syndrome. Neurobiol. Dis. 2018, 115, 1–8. [Google Scholar] [CrossRef]
- Kim, J.Y.; Liu, C.Y.; Zhang, F.; Duan, X.; Wen, Z.; Song, J.; Feighery, E.; Lu, B.; Rujescu, D.; St Clair, D.; et al. Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell 2012, 148, 1051–1064. [Google Scholar] [CrossRef]
- Moon, A.L.; Haan, N.; Wilkinson, L.S.; Thomas, K.L.; Hall, J. CACNA1C: Association with Psychiatric Disorders, Behavior, and Neurogenesis. Schizophr. Bull. 2018, 44, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Sklar, P.; Smoller, J.W.; Fan, J.; Ferreira, M.A.; Perlis, R.H.; Chambert, K.; Nimgaonkar, V.L.; McQueen, M.B.; Faraone, S.V.; Kirby, A.; et al. Whole-genome association study of bipolar disorder. Mol. Psychiatry 2008, 13, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.A.; O’Donovan, M.C.; Meng, Y.A. Wellcome Trust Case Control consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat. Genet. 2008, 40, 11056–11058. [Google Scholar] [CrossRef]
- Green, E.K.; Grozeva, D.; Jones, I.; Jones, L.; Kirov, G.; Caesar, S.; Gordon-Smith, K.; Fraser, C.; Forty, L.; Russell, E.; et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol. Psychiatry 2010, 15, 1016–1022. [Google Scholar] [CrossRef]
- Dedic, N.; Pohlmann, M.L.; Richter, J.S.; Mehta, D.; Czamara, D.; Metzer, M.W.; Dine, J.; Bedenk, B.T.; Hartman, J.; Wagner, K.V.; et al. Cross-disorder risk gene CACNA1C differentially modulates susceptibility to psychiatric disorders during development and adulthood. Mol. Psychiatry 2018, 23, 533–543. [Google Scholar] [CrossRef]
- Zhu, D.; Yin, J.; Liang, C.; Luo, X.; Lv, D.; Dai, Z.; Xiong, S.; Fu, J.; Li, Y.; Lin, J.; et al. CACNA1C (rs1006737) may be a susceptibility gene for schizophrenia: An updated meta-analysis. Brain Behav. 2019, 9, e01292. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Matsumura, Y.; Sato, Y.; Okada, A.; Morizane, A.; Okamoto, S.; Hong, H.; Nakagawa, M.; Tanabe, K.; Tezuka, K.I.; et al. A more efficient method to generate integration-free human iPS cells. Nat. Methods 2011, 8, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Sauvey, C.; Yao, L.; Zarnowska, E.D.; Zhang, S.C. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nat. Protoc. 2013, 8, 1670–1679. [Google Scholar] [CrossRef]
- 2Tukey, J.W. Exploratory Data Analysis; Addison-Wesley: Reading, MA, USA, 1977. [Google Scholar]
- Hoaglin, D.C.; Iglewicz, B.; Tukey, J.W. Performance of some resistant rules for outlier labeling. J. Am. Stat. Assoc. 1986, 81, 991–999. [Google Scholar] [CrossRef]
- Bragina, O.; Sergejeva, S.; Serg, M.; Zarkovsky, T.; Malovwerjan, A.; Koerman, P.; Zarkovsky, A. Smoothened agonist augments proliferation and survival of neural cells. Neurosci. Lett. 2010, 27, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Mak, S.K.; Huang, S.; Iranmanesh, S.; Vangipuram, M.; Sundararajaana, R.; Mguyen, L.; Lanagston, J.W.; Schule, B. Small molecules greatly improve coversion of human-induced pluripotent stem cells to the neuronal lineage. Stem Cells Int. 2012, 2012, 140427. [Google Scholar] [CrossRef] [PubMed]
- Peerboom, C.; Wierenga, C.J.; Bruining, H. The postnatal GABA shift: A developmental perspective. Neurosci. Biobehav. Rev. 2021, 124, 179–192. [Google Scholar] [CrossRef]
- Wang, D.D.; Kriegstein, A.R. Defining the role of GABA in cortical development. J. Physiol. 2009, 587, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Schulte, J.T.; Wierenga, C.J.; Bruining, H. Chloride transporters and GABA polarity in Develop. Neurolog. Psychiat Cond. Neurosci. Biobehav. Rev. 2018, 90, 260–271. [Google Scholar] [CrossRef]
- Savardi, A.; Borggogo, M.; Narducci, R.; La Sala, G.; Ortega, J.A.; Summa, M.; Armirotti, A.; Bertorelli, R.; Contestabile, A.; De Vivo, M.; et al. Discovery of a small molecule drug candidate for selective NKCC1 inhbition in brain disorders. Chemistry 2020, 6, 2073–2096. [Google Scholar] [CrossRef] [PubMed]
- Savardi, A.; Bogogno, M.; De Vivo, M.; Cancedda, L. Pharmacological tools to target NKCC1 in brain disorders. Trend Pharm. Sci. 2021, 42, 1009–1034. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Uvarov, P.; Soni, S.; Thomas-Crusells, J.; Airaksinen, M.S.; Rivera, C. Early growth response 4 mediates BDNF induction of potassium chloride cotransporter 2 transcription. J. Neurosci. 2011, 31, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Emamghoreishi, M.; Schlichter, L.; Li, P.P.; Parikh, S.; Sen, J.; Kamble, A.; Warsh, J.J. High intracellular calcium concentrations in transformed lymphoblasts from subjects with bipolar I disorder. Am. J. Psychiatry 1997, 154, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Yung, S.Y.; Gokhan, S.; Jurcsak, J.; Molero, A.E.; Abrajano, J.J.; Mehler, M.F. Differential modulation of BMP signaling promotes the elaboration of cerebral cortical GABAergic neurons or oligodendrocytes from a common sonic hedgehog-responsive ventral forebrain progenitor species. Proc. Natl. Acad. Sci. USA 2002, 99, 16273–16278. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; Shaw, A.D.; Allcock RJ, N.; Heath, A.; Pierce, K.D.; Mitchell, P.B.; Schofield, P.R.; Fullerton, J.M. An examination of multiple classes of rare variants in extended families with bipolar disorder. Transl. Psychiatry 2018, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, N.; Kasai, S.; Suzuki, N.; Nishi, N.; Oishi, S.; Fujii, N.; Kadoya, Y.; Hatori, K.; Mizuno, Y.; Nomizu, M.; et al. Identification of neurite outgrowth active sites on the laminin alpha4 chain G domain. Biochemistry 2005, 44, 5755–5762. [Google Scholar] [CrossRef] [PubMed]
- Goes, F.S.; Pirooznia, M.; Parla, J.S.; Kramer, M.; Ghiban, E.; Mavruk, S.; Chen, Y.C.; Monson, E.T.; Willour, V.L.; Karchin, R.; et al. Exome Sequencing of Familial Bipolar Disorder. JAMA Psychiatry 2016, 73, 590–597. [Google Scholar] [CrossRef]
- Niemsiri, V.; Rosenthal, S.B.; Nievergelt, C.M.; Maihofer, A.X.; Marchetto, M.C.; Santos, R.; Shekhtman, T.; Alliey-Rodriguez, N.; Anand, A.; Balaraman, Y.; et al. Focal adhesion is associated with lithium response in bipolar disorder: Evidence from a network-based multi-omics analysis. Mol. Psychiatry 2023, 29, 6–19. [Google Scholar] [CrossRef]
- Kuang, X.L.; Zhao, X.M.; Xu, H.F.; Shi, Y.Y.; Deng, J.B.; Sun, G.T. Spatio-temporal expression of a novel neuron-derived neurotrophic factor (NDNF) in mouse brains during development. BMC Neurosci. 2010, 11, 137. [Google Scholar] [CrossRef]
- Kamimura, K.; Maeda, N. Glypicans and Heparan Sulfate in Synaptic Development, Neural Plasticity, and Neurological Disorders. Front. Neural Circuits 2021, 15, 595596. [Google Scholar] [CrossRef] [PubMed]
- Cong, Q.; Soteros, B.M.; Wollet, M.; Kim, J.H.; Sia, G.M. The endogenous neuronal complement inhibitor SRPX2 protects against complement-mediated synapse elimination during development. Nat. Neurosci. 2020, 23, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Soteros, B.M.; Cong, Q.; Palmer, C.R.; Sia, G.M. Sociability and synapse subtype-specific defects in mice lacking SRPX2, a language-associated gene. PLoS ONE 2018, 13, e0199399. [Google Scholar] [CrossRef] [PubMed]
- Zach, S.; Felk, S.; Gillardon, F. Signal transduction protein array analysis links LRRK2 to Ste20 kinases and PKC zeta that modulate neuronal plasticity. PLoS ONE 2010, 5, e13191. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.; Onofri, F.; Cirnaru, M.D.; Kaiser, C.J.; Jagtap, P.; Kastenmüller, A.; Pischedda, F.; Marte, A.; von Zweydorf, F.; Vogt, A.; et al. Leucine-rich repeat kinase 2 binds to neuronal vesicles through protein interactions mediated by its C-terminal WD40 domain. Mol. Cell. Biol. 2014, 34, 2147–2161. [Google Scholar] [CrossRef] [PubMed]
- Steger, M.; Diez, F.; Dhekne, H.S.; Lis, P.; Nirujogi, R.S.; Karayel, O.; Tonelli, F.; Martinez, T.N.; Lorentzen, E.; Pfeffer, S.R.; et al. Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. eLife 2017, 6, e31012. [Google Scholar] [CrossRef] [PubMed]
- Shanker, V.; Groves, M.; Heiman, G.; Palmese, C.; Saunders-Pullman, R.; Ozelius, L.; Raymond, D.; Bressman, S. Mood and cognition in leucine-rich repeat kinase 2 G2019S Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Alhassen, W.; Chen, S.; Vawter, M.; Robbins, B.K.; Nguyen, H.; Myint, T.N.; Saito, Y.; Schulmann, A.; Nauli, S.M.; Civelli, O.; et al. Patterns of cilia gene dysregulations in major psychiatric disorders. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 109, 110255. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Yoon, J.; Lee, S.H. The role of neuropeptide somatostatin in the brain and its application in treating neurological disorders. Exp. Mol. Med. 2021, 53, 328–338. [Google Scholar] [CrossRef]
- Lukomska, A.; Dobrzanski, G.; Liguz-Lecznar, M.; Kossut, M. Somatostatin receptors (SSTR1-5) on inhibitory interneurons in the barrel cortex. Brain Struct. Funct. 2020, 225, 387–401. [Google Scholar] [CrossRef]
- Akkouh, I.A.; Skrede, S.; Holmgren, A.; Ersland, K.M.; Hansson, L.; Bahrami, S.; Andreassen, O.A.; Steen, V.M.; Djurovic, S.; Hughes, T. Exploring lithium’s transcriptional mechanisms of action in bipolar disorder: A multi-step study. Neuropsychopharmacology 2020, 45, 947–955. [Google Scholar] [CrossRef]
- Jerng, H.H.; Lauver, A.D.; Pfaffinger, P.J. DPP10 splice variants are localized in distinct neuronal populations and act to differentially regulate the inactivation properties of Kv4-based ion channels. Mol. Cell. Neurosci. 2007, 35, 604–624. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Villa, E.; Rodriguez, M.; Ramirez, M.; Zavala, J.; Armas, R.; Dassori, A.; Contreras, J.; Raventós, H.; Flores, D.; et al. Fine-mapping scan of bipolar disorder susceptibility loci in Latino pedigrees. Am. J. Med. Genetics. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2019, 180, 213–222. [Google Scholar] [CrossRef]
- Lachman, H.M. Copy variations in schizophrenia and bipolar disorder. Cytogenet. Genome Res. 2008, 123, 27–35. [Google Scholar] [CrossRef]
- Zhuo, C.; Tian, H.; Chen, J.; Li, Q.; Yang, L.; Zhang, Q.; Chen, G.; Cheng, L.; Zhou, C.; Song, X. Associations of cognitive impairment in patients with schizophrenia with genetic features and with schizophrenia-related structural and functional brain changes. Front. Genet. 2022, 13, 880027. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Q.; Cai, F.; Liu, X.; Wu, Y.; Song, W. BACE2, a conditional β-secretase, contributes to Alzheimer’s disease pathogenesis. JCI Insight 2019, 4, e123431. [Google Scholar] [CrossRef]
- Alić, I.; Goh, P.A.; Murray, A.; Portelius, E.; Gkanatsiou, E.; Gough, G.; Mok, K.Y.; Koschut, D.; Brunmeir, R.; Yeap, Y.J.; et al. Patient-specific Alzheimer-like pathology in trisomy 21 cerebral organoids reveals BACE2 as a gene dose-sensitive AD suppressor in human brain. Mol. Psychiatry 2021, 26, 5766–5788. [Google Scholar] [CrossRef]
- Verzi, M.P.; Anderson, J.P.; Dodou, E.; Kelly, K.K.; Greene, S.B.; North, B.J.; Cripps, R.M.; Black, B.L. N-Twist, an Evolutionarily Conserved bHLH Protein Expressed in the Developing CNS, Functions as a Transcriptional Inhibitor. Dev. Biol. 2002, 249, 174–190. [Google Scholar] [CrossRef]
- Ono, Y.; Nakatani, T.; Minaki, Y.; Kumai, M. The basic helix-loop-helix transcription factor Nato3 controls neurogenic activity in mesencephalic floor plate cells. Development 2010, 137, 1897–1906. [Google Scholar] [CrossRef]
- Jukic, M.M.; Carrillo-Roa, T.; Bar, M.; Becker, G.; Jovanovic, V.M.; Zega, K.; Binder, E.B.; Brodski, C. Abnormal development of monoaminergic neurons is implicated in mood fluctuations and bipolar disorder. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015, 40, 839–848. [Google Scholar] [CrossRef]
- Smith, E.N.; Bloss, C.S.; Badner, J.A.; Barrett, T.; Belmonte, P.L.; Berrettini, W.; Byerley, W.; Coryell, W.; Craig, D.; Edenberg, H.J.; et al. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol. Psychiatry 2009, 14, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Kazuno, A.A.; Ohtawa, K.; Otsuki, K.; Usui, M.; Sugawara, H.; Okazaki, Y.; Kato, T. Proteomic analysis of lymphoblastoid cells derived from monozygotic twins discordant for bipolar disorder: A preliminary study. PLoS ONE 2013, 8, e53855. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gedik, H.; Nguyen, T.H.; Peterson, R.E.; Chatzinakos, C.; Vladimirov, V.I.; Riley, B.P.; Bacanu, S.A. Identifying potential risk genes and pathways for neuropsychiatric and substance use disorders using intermediate molecular mediator information. Front. Genet. 2023, 14, 1191264. [Google Scholar] [CrossRef] [PubMed]
- Merikangas, A.K.; Shelly, M.; Knighton, A.; Kotler, N.; Tanenbaum, N.; Almasy, L. What genes are differentially expressed in individuals with schizophrenia? A systematic review. Mol. Psychiatry 2022, 27, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Johansen, N.; Somasundaram, S.; Travaglini, K.J.; Yanny, A.M.; Shumyatcher, M.; Casper, T.; Cobbs, C.; Dee, N.; Ellenbogen, R.; Ferreira, M.; et al. Interindividual variation in human cortical cell type abundance and expression. Science 2023, 382, eadf2359. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, Y.; Seki, M.; Yoshimura, A.; Nishino, K.; Hayashi, T.; Takeuchi, T.; Iguchi, S.; Kusa, Y.; Ohtsuki, M.; Tsuyama, T.; et al. Analyses of the interaction of WRNIP1 with Werner syndrome protein (WRN) in vitro and in the cell. DNA Repair 2006, 5, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.T.; Jiang, X.; Akula, N.; Shugart, Y.Y.; Wendland, J.R.; Steele, C.J.M.; Kassem, L.; Park, J.H.; Chatterjee, N.; Jamain, S.; et al. Genome-wide association study meta-analysis of European and Asian-ancestry samples identifies three novel loci associated with bipolar disorder. Mol. Psychiatry 2013, 18, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.; Abu-Elmagd, M.; Clevers, H.; Scotting, P.J. Roles of Sox4 in central nervous system development. Brain Res. Mol. Brain Res. 2000, 79, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Berti, L.; Masserdotti, G.; Covic, M.; Michaelidis, T.M.; Doberauer, K.; Merz, K.; Rehfeld, F.; Haslinger, A.; Wegner, M.; et al. SoxC transcription factors are required for neuronal differentiation in adult hippocampal neurogenesis. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 3067–3080. [Google Scholar] [CrossRef] [PubMed]
- Bergsland, M.; Werme, M.; Malewicz, M.; Perlmann, T.; Muhr, J. The establishment of neuronal properties is controlled by Sox4 and Sox11. Genes. Dev. 2006, 20, 3475–3486. [Google Scholar] [CrossRef]


| Lab Number | M/F | Age of Donor | rs1006737 |
|---|---|---|---|
| C5 | M | Newborn | GG |
| C8 | M | 59 | GG |
| C9 | F | 49 | GG |
| C10 | F | 32 | GG |
| BP11 | M | 39 | AA |
| BP17 | F | 26 | AA |
| BP18 | F | 33 | AA |
| BP19 | M | 59 | AA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schill, D.J.; Attili, D.; DeLong, C.J.; McInnis, M.G.; Johnson, C.N.; Murphy, G.G.; O’Shea, K.S. Human-Induced Pluripotent Stem Cell (iPSC)-Derived GABAergic Neuron Differentiation in Bipolar Disorder. Cells 2024, 13, 1194. https://doi.org/10.3390/cells13141194
Schill DJ, Attili D, DeLong CJ, McInnis MG, Johnson CN, Murphy GG, O’Shea KS. Human-Induced Pluripotent Stem Cell (iPSC)-Derived GABAergic Neuron Differentiation in Bipolar Disorder. Cells. 2024; 13(14):1194. https://doi.org/10.3390/cells13141194
Chicago/Turabian StyleSchill, Daniel J., Durga Attili, Cynthia J. DeLong, Melvin G. McInnis, Craig N. Johnson, Geoffrey G. Murphy, and K. Sue O’Shea. 2024. "Human-Induced Pluripotent Stem Cell (iPSC)-Derived GABAergic Neuron Differentiation in Bipolar Disorder" Cells 13, no. 14: 1194. https://doi.org/10.3390/cells13141194
APA StyleSchill, D. J., Attili, D., DeLong, C. J., McInnis, M. G., Johnson, C. N., Murphy, G. G., & O’Shea, K. S. (2024). Human-Induced Pluripotent Stem Cell (iPSC)-Derived GABAergic Neuron Differentiation in Bipolar Disorder. Cells, 13(14), 1194. https://doi.org/10.3390/cells13141194







