The EphA2 Receptor Regulates Invasiveness and Drug Sensitivity in Canine and Human Osteosarcoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Cell Culture
2.3. Transduction with shRNA
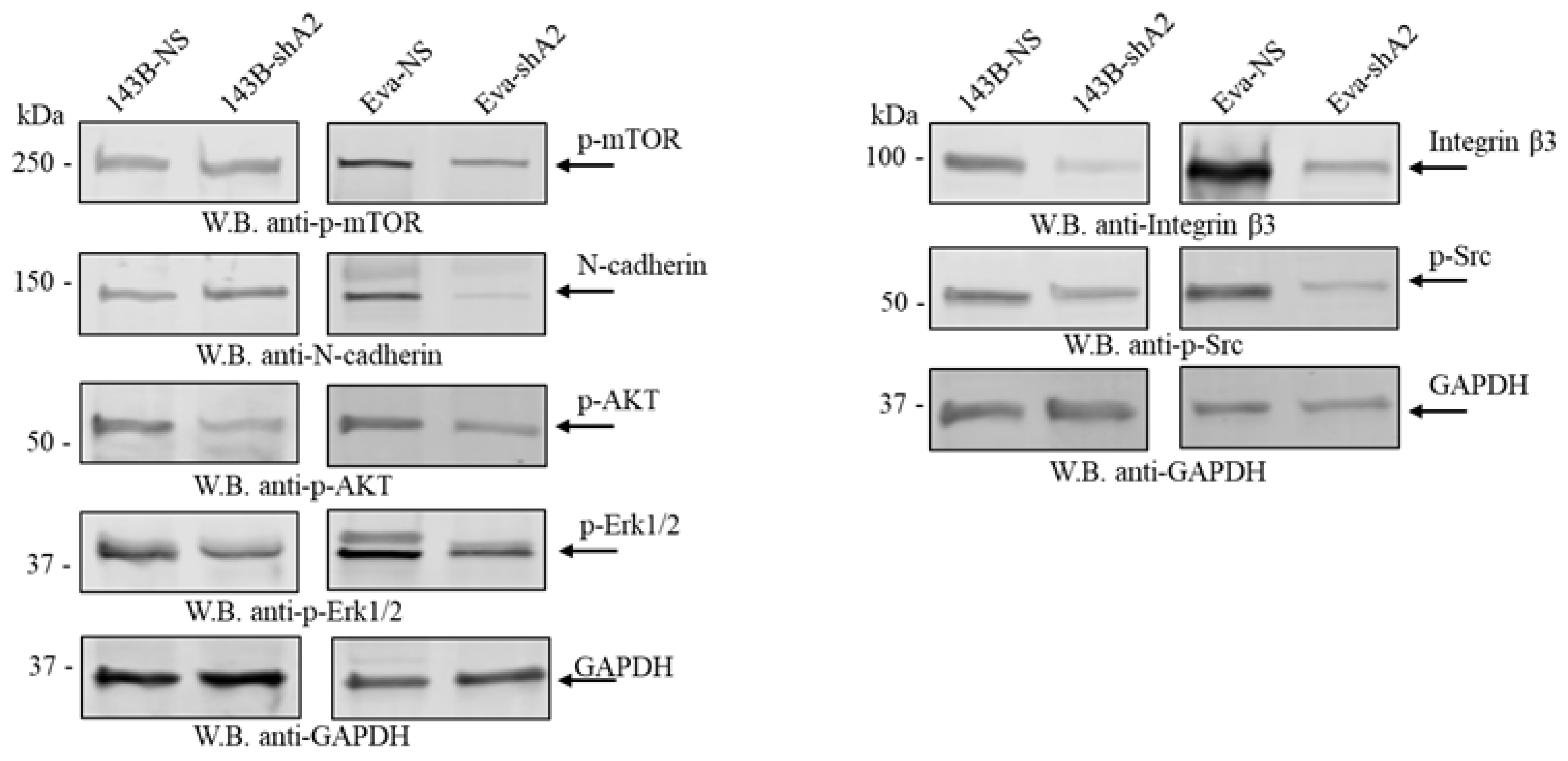
2.4. Western Blot Analysis
2.5. Immunofluorescence
2.6. Cell Proliferation
2.7. Transwell Migration and Invasion Assay
2.8. Wound-Healing Assay
2.9. Drug Sensitivity
2.10. Colony Formation
2.11. Animal Studies
2.12. Immunohistochemistry
2.13. Statistical Analysis
3. Results
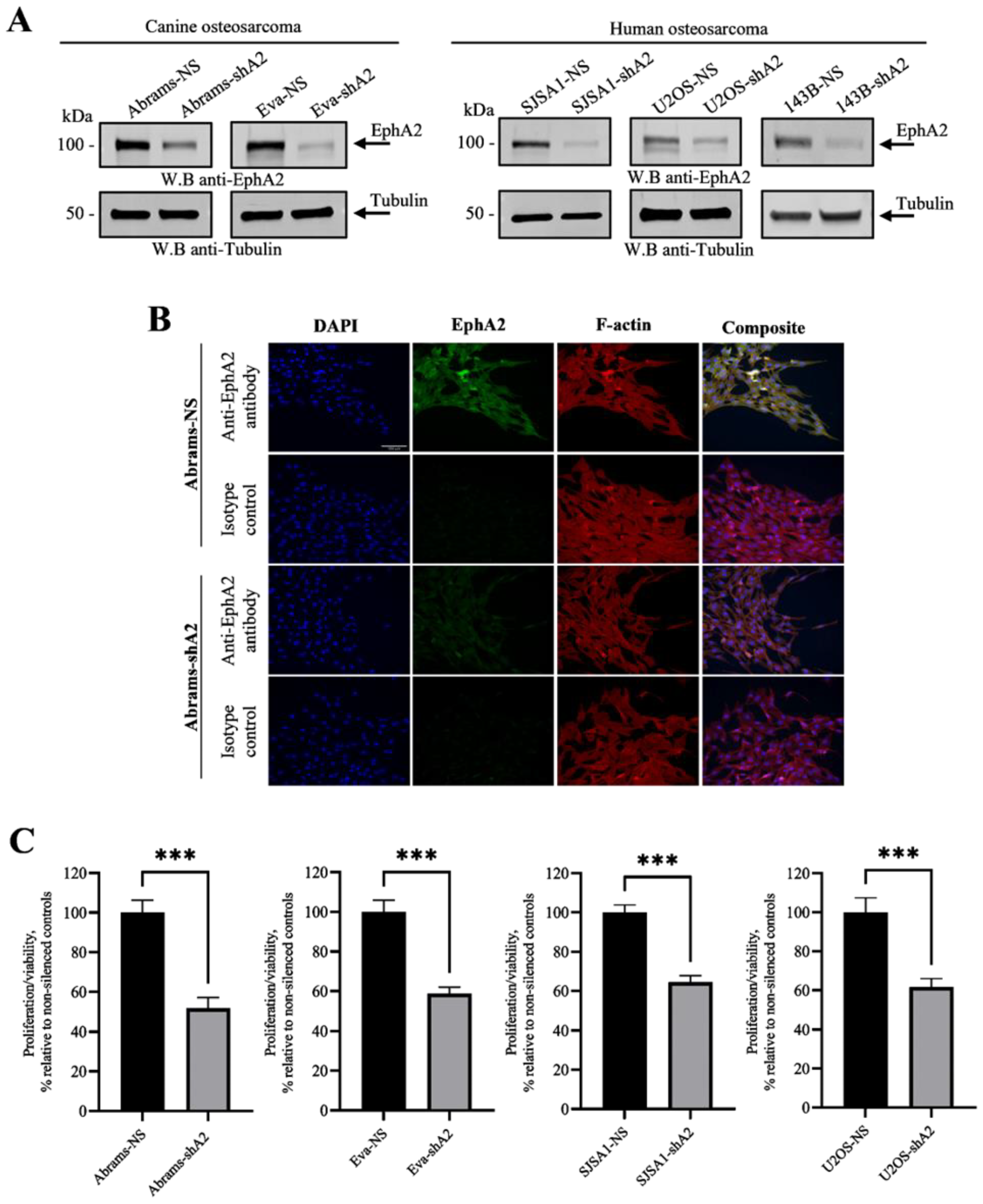
3.1. EphA2 Is Overexpressed in Canine and Human Osteosarcoma
3.2. EphA2 Enhances Viability of OS Cells
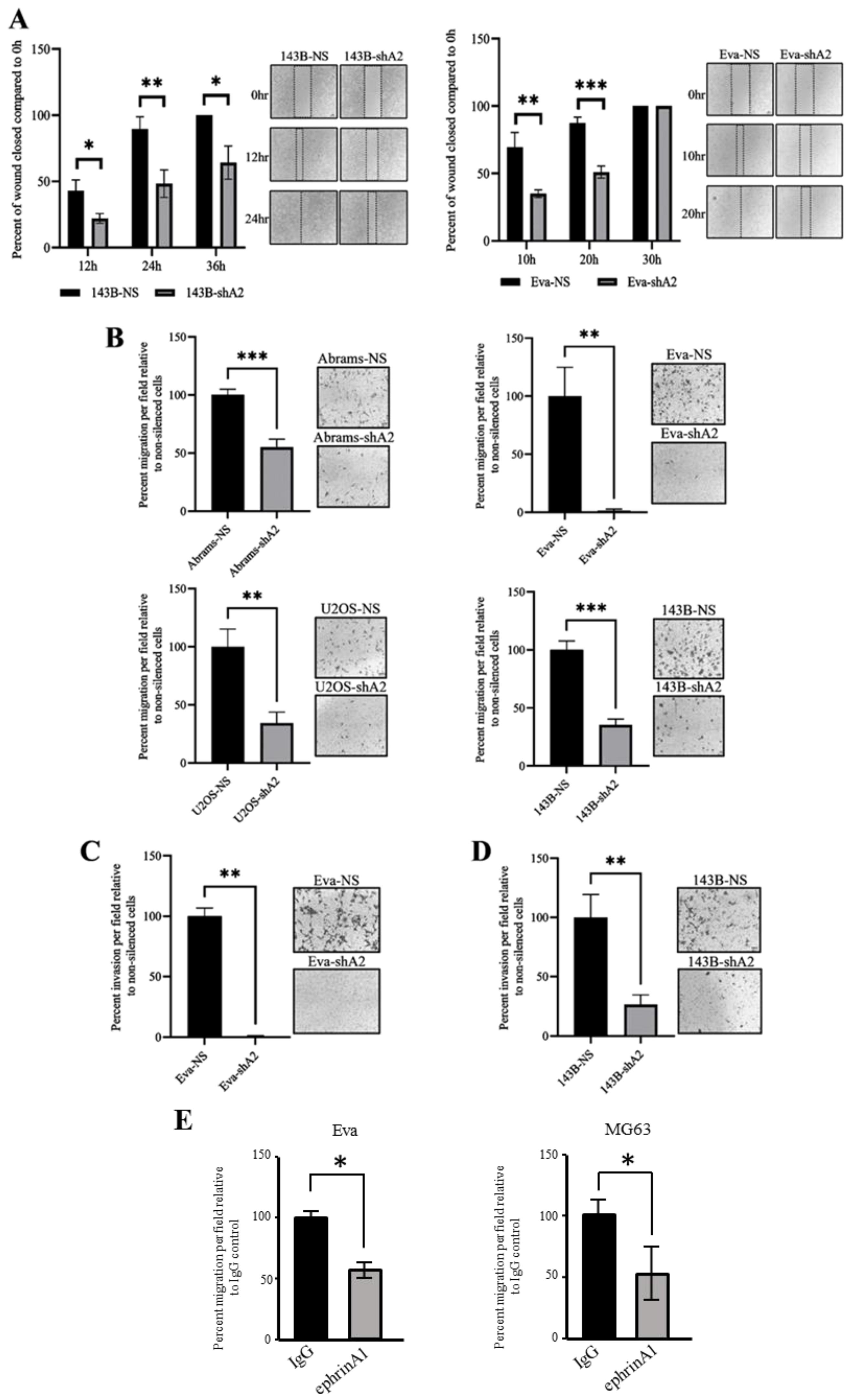
3.3. EphA2 Supports OS Cell Migration and Invasion
3.4. EphA2 Enhances Resistance of OS Cells to Cisplatin
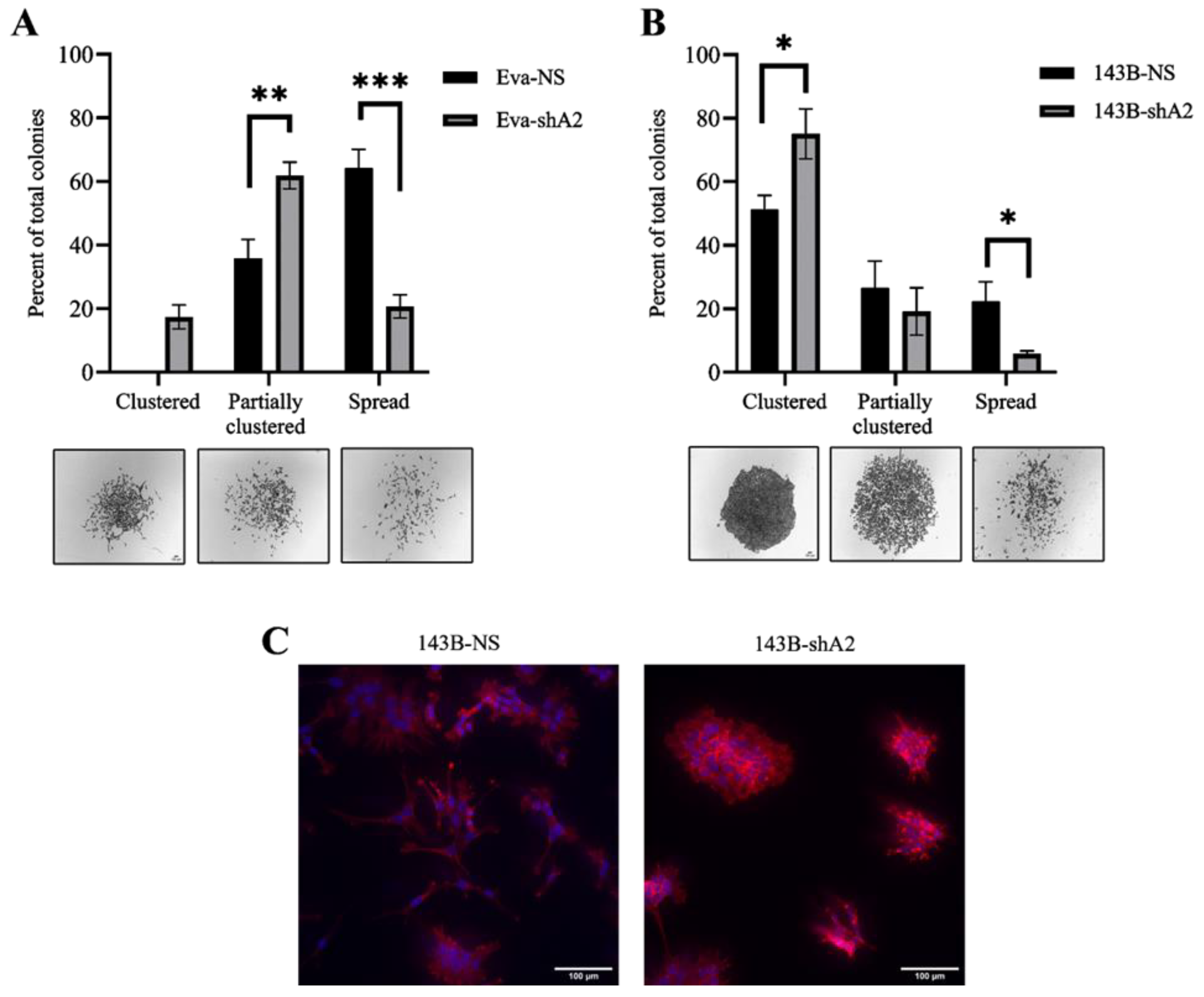
3.5. EphA2 Supports a More Invasive Phenotype in OS Cells
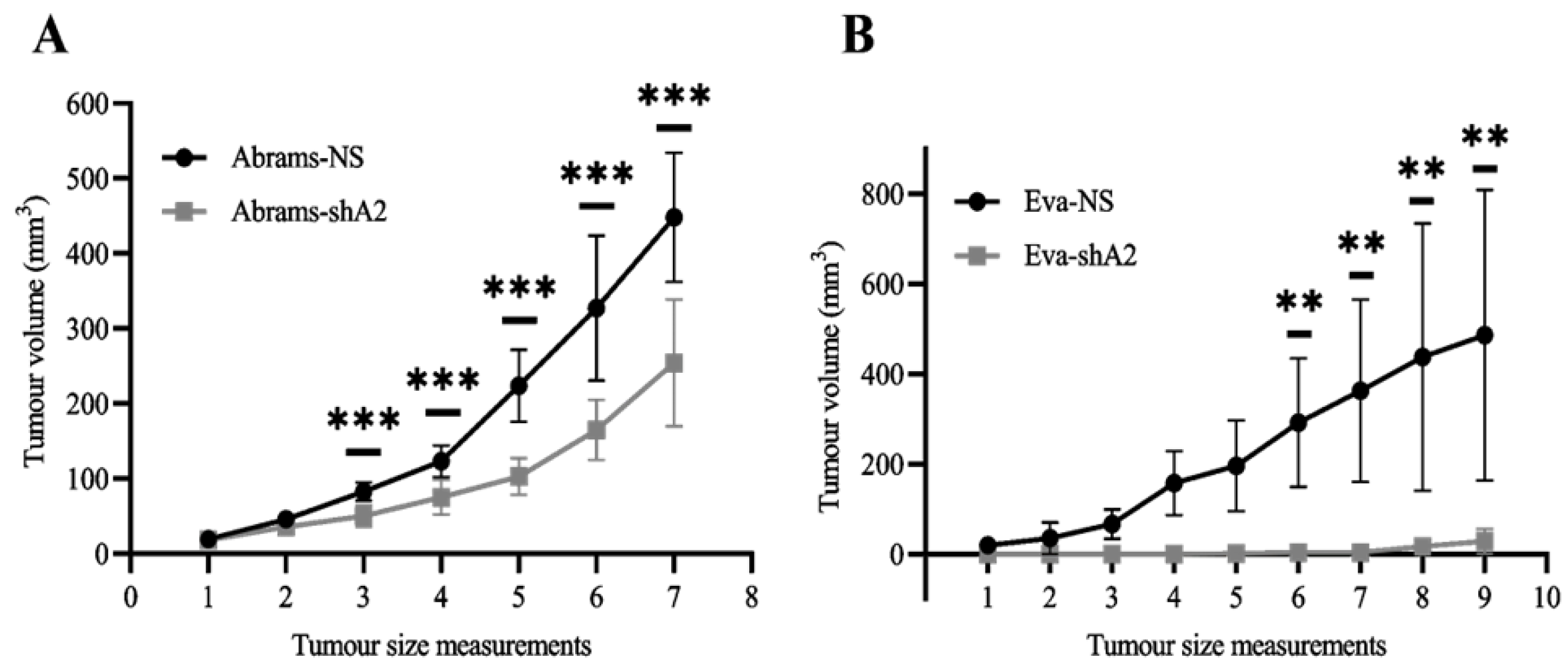
3.6. EphA2 Supports OS Tumor Development in Xenograft Models
3.7. EphA2 Is Associated with the Activation of Various Signaling Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anfinsen, K.P.; Grotmol, T.; Bruland, O.S.; Jonasdottir, T.J. Breed-specific incidence rates of canine primary bone tumors—A population based survey of dogs in Norway. Can. J. Vet. Res. 2011, 75, 209–215. [Google Scholar] [PubMed]
- Withrow, S.J.; Wilkins, R.M. Cross talk from pets to people: Translational osteosarcoma treatments. ILAR J. 2010, 51, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Morello, E.; Martano, M.; Buracco, P. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differences with human osteosarcoma. Vet. J. 2011, 189, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Varshney, J.; Scott, M.C.; Largaespada, D.A.; Subramanian, S. Understanding the osteosarcoma pathobiology: A comparative oncology approach. Vet. Sci. 2016, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.; Dunning, M.D.; de Brot, S.; Grau-Roma, L.; Mongan, N.P.; Rutland, C.S. Comparative review of human and canine osteosarcoma: Morphology, epidemiology, prognosis, treatment and genetics. Acta Vet. Scand. 2017, 59, 71. [Google Scholar] [CrossRef] [PubMed]
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Osteosarcoma. Nat. Rev. Dis. Primers 2022, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Kansara, M.; Teng, M.W.; Smyth, M.J.; Thomas, D.M. Translational biology of osteosarcoma. Nat. Rev. Cancer 2014, 14, 722–735. [Google Scholar] [CrossRef]
- Garden, O.; Volk, S.; Mason, N.; Perry, J. Companion animals in comparative oncology: One Medicine in action. Vet. J. 2018, 240, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Eph receptors and ephrins in cancer: Bidirectional signalling and beyond. Nat. Rev. Cancer 2010, 10, 165–180. [Google Scholar] [CrossRef]
- Lisabeth, E.M.; Falivelli, G.; Pasquale, E.B. Eph receptor signaling and ephrins. Cold Spring Harb. Perspect. Biol. 2013, 5, a009159. [Google Scholar] [CrossRef]
- Kou, C.-T.J.; Kandpal, R.P. Differential Expression Patterns of Eph Receptors and Ephrin Ligands in Human Cancers. BioMed Res. Int. 2018, 2018, 7390104. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.D.; Klein-Nulend, J. Osteoblast isolation from murine calvaria and long bones. Bone Res. Protoc. 2012, 816, 19–29. [Google Scholar]
- Meeson, R.L.; Perpétuo, I.P.; Parsons, K.; Orriss, I.R.; Shah, M.; Pitsillides, A.A.; Doube, M. The in vitro behaviour of canine osteoblasts derived from different bone types. BMC Vet. Res. 2019, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- El Zawily, A.; McEwen, E.; Toosi, B.; Vizeacoumar, F.S.; Freywald, T.; Vizeacoumar, F.J.; Freywald, A. The EphB6 receptor is overexpressed in pediatric T cell acute lymphoblastic leukemia and increases its sensitivity to doxorubicin treatment. Sci. Rep. 2017, 7, 14767. [Google Scholar] [CrossRef] [PubMed]
- Udayakumar, D.; Zhang, G.; Ji, Z.; Njauw, C.-N.; Mroz, P.; Tsao, H. EphA2 is a critical oncogene in melanoma. Oncogene 2011, 30, 4921–4929. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Hwang, Y.; Youngblood, V.M.; Cook, R.S.; Balko, J.M.; Chen, J.; Brantley-Sieders, D.M. Targeting EphA2 impairs cell cycle progression and growth of basal-like/triple-negative breast cancers. Oncogene 2017, 36, 5620–5630. [Google Scholar] [CrossRef] [PubMed]
- Brannan, J.M.; Sen, B.; Saigal, B.; Prudkin, L.; Behrens, C.; Solis, L.; Dong, W.; Bekele, B.N.; Wistuba, I.; Johnson, F.M. EphA2 in the early pathogenesis and progression of non-small cell lung cancer. Cancer Prev. Res. 2009, 2, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, Y.; Negishi, M.; Katoh, H. EphA2 is a key effector of the MEK/ERK/RSK pathway regulating glioblastoma cell proliferation. Cell. Signal. 2016, 28, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Hecker-Nolting, S.; Blattmann, C.; Kager, L. Advances in the management of osteosarcoma. F1000Research 2016, 5, 2767. [Google Scholar] [CrossRef]
- Friedl, P.; Alexander, S. Cancer Invasion and the Microenvironment: Plasticity and Reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Xiao, Y.; Wang, W.; Tang, Y.Y.; Xiao, Z.; Su, M. Targeting EphA2 in cancer. J. Hematol. Oncol. 2020, 13, 114. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, Z.; Wu, S.; Zang, X.; Liu, M.; Shi, J. miR-33a is up-regulated in chemoresistant osteosarcoma and promotes osteosarcoma cell resistance to cisplatin by down-regulating TWIST. J. Exp. Clin. Cancer Res. 2014, 33, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-T.; Liao, L.-Z.; Lu, C.-H.; Huang, Y.-H.; Lin, Y.-K.; Lin, J.-H.; Chow, L.-P. Quantitative phosphoproteomic analysis identifies the potential therapeutic target EphA2 for overcoming sorafenib resistance in hepatocellular carcinoma cells. Exp. Mol. Med. 2020, 52, 497–513. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, G.; Brantley-Sieders, D.M.; Vaught, D.; Yu, J.; Xie, L.; Wells, S.; Jackson, D.; Muraoka-Cook, R.; Arteaga, C.; Chen, J. Elevation of Receptor Tyrosine Kinase EphA2 Mediates Resistance to Trastuzumab Therapy. Cancer Res. 2010, 70, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Miao, B.; Ji, Z.; Tan, L.; Taylor, M.; Zhang, J.; Choi, H.G.; Frederick, D.T.; Kumar, R.; Wargo, J.A.; Flaherty, K.T.; et al. EPHA2 is a mediator of vemurafenib resistance and a novel therapeutic target in melanoma. Cancer Discov. 2015, 5, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Moyano-Galceran, L.; Pietilä, E.A.; Turunen, S.P.; Corvigno, S.; Hjerpe, E.; Bulanova, D.; Joneborg, U.; Alkasalias, T.; Miki, Y.; Yashiro, M.; et al. Adaptive RSK-EphA2-GPRC5A signaling switch triggers chemotherapy resistance in ovarian cancer. EMBO Mol. Med. 2020, 12, e11177. [Google Scholar] [CrossRef] [PubMed]
- Kenny, P.A.; Lee, G.Y.; Myers, C.A.; Neve, R.M.; Semeiks, J.R.; Spellman, P.T.; Lorenz, K.; Lee, E.H.; Barcellos-Hoff, M.H.; Petersen, O.W.; et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol. 2007, 1, 84–96. [Google Scholar] [CrossRef]
- Bielack, S.S.; Carrle, D.; Hardes, J.; Schuck, A.; Paulussen, M. Bone tumors in adolescents and young adults. Curr. Treat. Options Oncol. 2008, 9, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Mueller, F.; Fuchs, B.; Kaser-Hotz, B. Comparative Biology of Human and Canine Osteosarcoma. Anticancer Res. 2007, 27, 155–164. [Google Scholar]
- Nordberg, J.; Mpindi, J.P.; Iljin, K.; Pulliainen, A.T.; Kallajoki, M.; Kallioniemi, O.; Elenius, K.; Elenius, V. Systemic analysis of gene expression profiles identifies ErbB3 as a potential drug target in pediatric alveolar rhabdomyosarcoma. PLoS ONE 2012, 7, e50819. [Google Scholar] [CrossRef] [PubMed]
- Fritsche-Guenther, R.; Noske, A.; Ungethüm, U.; Kuban, R.; Schlag, P.M.; Tunn, P.; Karle, J.; Krenn, V.; Dietel, M.; Sers, C. De novo expression of EphA2 in osteosarcoma modulates activation of the mitogenic signalling pathway. Histopathology 2010, 57, 836–850. [Google Scholar] [CrossRef] [PubMed]
- PosthumaDeBoer, J.; Piersma, S.R.; Pham, T.V.; van Egmond, P.W.; Knol, J.C.; Cleton-Jansen, A.M.; A van Geer, M.; van Beusechem, V.W.; Kaspers, G.J.L.; van Royen, B.J.; et al. Surface proteomic analysis of osteosarcoma identifies EPHA2 as receptor for targeted drug delivery. Br. J. Cancer 2013, 109, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Cioce, M.; Fazio, V.M. EphA2 and EGFR: Friends in Life, Partners in Crime. Can EphA2 Be a Predictive Biomarker of Response to Anti-EGFR Agents? Cancers 2021, 13, 700. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sakurai, H. Emerging and Diverse Functions of the EphA2 Noncanonical Pathway in Cancer Progression. Biol. Pharm. Bull. 2017, 40, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.-D.; Lee, M.-J.; Kim, J.-H.; Hao, P.-P.; Liu, L.; Yu, G.-R.; Kim, D.-G. Activation of mammalian target of rapamycin complex 1 (mTORC1) and Raf/Pyk2 by growth factor-mediated Eph receptor 2 (EphA2) is required for cholangiocarcinoma growth and metastasis. Hepatology 2013, 57, 2248–2260. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Li, D.-Q.; Mukherjee, A.; Guo, H.; Petty, A.; Cutter, J.; Basilion, J.P.; Sedor, J.; Wu, J.; Danielpour, D.; et al. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell 2009, 16, 9–20. [Google Scholar] [CrossRef]
- Barquilla, A.; Lamberto, I.; Noberini, R.; Heynen-Genel, S.; Brill, L.M.; Pasquale, E.B. Protein kinase A can block EphA2 receptor–mediated cell repulsion by increasing EphA2 S897 phosphorylation. Mol. Biol. Cell 2016, 27, 2757–2770. [Google Scholar] [CrossRef]
- Gehring, M.P.; Pasquale, E.B. Protein kinase C phosphorylates the EphA2 receptor on serine 892 in the regulatory linker connecting the kinase and SAM domains. Cell. Signal. 2020, 73, 109668. [Google Scholar] [CrossRef]
- Hasegawa, J.; Sue, M.; Yamato, M.; Ichikawa, J.; Ishida, S.; Shibutani, T.; Kitamura, M.; Wada, T.; Agatsuma, T. Novel anti-EPHA2 antibody, DS-8895a for cancer treatment. Cancer Biol. Ther. 2016, 17, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Mohammed, K.A.; Goldberg, E.P.; Kaye, F.; Nasreen, N. Silencing Receptor EphA2 Enhanced Sensitivity to Lipoplatin™ in Lung Tumor and MPM Cells. Cancer Investig. 2016, 34, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ju, Q.; Zhang, J.; Gu, W.; Du, J. MiR-302a-3p reduces cisplatin resistance of esophageal squamous cell carcinoma cells by targeting EphA2. J. Chemother. 2023, 36, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Monclús, S.; López-Alemany, R.; Almacellas-Rabaiget, O.; Herrero-Martín, D.; Huertas-Martinez, J.; Lagares-Tena, L.; Alba-Pavón, P.; Hontecillas-Prieto, L.; Mora, J.; de Álava, E.; et al. EphA2 receptor is a key player in the metastatic onset of Ewing sarcoma. Int. J. Cancer 2018, 143, 1188–1201. [Google Scholar] [CrossRef] [PubMed]
- Dunne, P.D.; Dasgupta, S.; Blayney, J.K.; McArt, D.G.; Redmond, K.L.; Weir, J.-A.; Bradley, C.A.; Sasazuki, T.; Shirasawa, S.; Wang, T.; et al. EphA2 Expression Is a Key Driver of Migration and Invasion and a Poor Prognostic Marker in Colorectal Cancer. Clin. Cancer Res. 2016, 22, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ma, Y.; Wang, J.; Brantley-Sieders, D.; Chen, J. JNK signaling mediates EPHA2-dependent tumor cell proliferation, motility, and cancer stem cell-like properties in non-small cell lung cancer. Cancer Res. 2014, 74, 2444–2454. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Guo, J.; Liao, Q.; Zhao, Y. β1 and β3 integrins in breast, prostate and pancreatic cancer: A novel implication. Oncol. Lett. 2018, 15, 5412–5416. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.C.; Lai, Y.Y.; Hsu, H.C.; Fong, Y.C.; Lien, M.Y.; Tang, C.H. CCL4 Stimulates Cell Migration in Human Osteosarcoma via the mir-3927-3p/Integrin alphavbeta3 Axis. Int. J. Mol. Sci. 2021, 22, 12737. [Google Scholar] [CrossRef]
- Tome, Y.; Kimura, H.; Kiyuna, T.; Sugimoto, N.; Tsuchiya, H.; Kanaya, F.; Bouvet, M.; Hoffman, R.M. Disintegrin targeting of an alphavbeta3 integrin-over-expressing high-metastatic human osteosarcoma with echistatin inhibits cell proliferation, migration, invasion and adhesion in vitro. Oncotarget 2016, 7, 46315–46320. [Google Scholar]
- Kawai, H.; Kobayashi, M.; Hiramoto-Yamaki, N.; Harada, K.; Negishi, M.; Katoh, H. Ephexin4-mediated promotion of cell migration and anoikis resistance is regulated by serine 897 phosphorylation of EphA2. FEBS Open Bio 2013, 3, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto-Yamaki, N.; Takeuchi, S.; Ueda, S.; Harada, K.; Fujimoto, S.; Negishi, M.; Katoh, H. Ephexin4 and EphA2 mediate cell migration through a RhoG-dependent mechanism. J. Cell Biol. 2010, 190, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, Z.; He, Y.; He, Z.; Ban, Z.; Zhu, Y.; Ding, L.; Yang, C.; Jeong, J.; Yuan, W.; et al. EphA2 promotes tumorigenicity of cervical cancer by up-regulating CDK6. J. Cell. Mol. Med. 2021, 25, 2967–2975. [Google Scholar] [CrossRef] [PubMed]
- Ludwig Institute for Cancer Research. Safety and Bioimaging Trial of DS-8895a in Patients with Advanced EphA2 Positive Cancers. 2014. Available online: https://ClinicalTrials.gov/show/NCT02252211 (accessed on 10 November 2022).
- Shitara, K.; Satoh, T.; Iwasa, S.; Yamaguchi, K.; Muro, K.; Komatsu, Y.; Nishina, T.; Esaki, T.; Hasegawa, J.; Kakurai, Y.; et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the afucosylated, humanized anti-EPHA2 antibody DS-8895a: A first-in-human phase I dose escalation and dose expansion study in patients with advanced solid tumors. J. Immunother. Cancer 2019, 7, 219. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, E.D.; Sharpe, J.C.; Strozen, T.; Abdi, S.; Kliewer, M.; Sanchez, M.G.; Hogan, N.S.; MacDonald-Dickinson, V.; Vizeacoumar, F.J.; Toosi, B.M. The EphA2 Receptor Regulates Invasiveness and Drug Sensitivity in Canine and Human Osteosarcoma Cells. Cells 2024, 13, 1201. https://doi.org/10.3390/cells13141201
Harris ED, Sharpe JC, Strozen T, Abdi S, Kliewer M, Sanchez MG, Hogan NS, MacDonald-Dickinson V, Vizeacoumar FJ, Toosi BM. The EphA2 Receptor Regulates Invasiveness and Drug Sensitivity in Canine and Human Osteosarcoma Cells. Cells. 2024; 13(14):1201. https://doi.org/10.3390/cells13141201
Chicago/Turabian StyleHarris, Evelyn D., Jessica C. Sharpe, Timothy Strozen, Shabnam Abdi, Maya Kliewer, Malkon G. Sanchez, Natacha S. Hogan, Valerie MacDonald-Dickinson, Franco J. Vizeacoumar, and Behzad M. Toosi. 2024. "The EphA2 Receptor Regulates Invasiveness and Drug Sensitivity in Canine and Human Osteosarcoma Cells" Cells 13, no. 14: 1201. https://doi.org/10.3390/cells13141201
APA StyleHarris, E. D., Sharpe, J. C., Strozen, T., Abdi, S., Kliewer, M., Sanchez, M. G., Hogan, N. S., MacDonald-Dickinson, V., Vizeacoumar, F. J., & Toosi, B. M. (2024). The EphA2 Receptor Regulates Invasiveness and Drug Sensitivity in Canine and Human Osteosarcoma Cells. Cells, 13(14), 1201. https://doi.org/10.3390/cells13141201






