Navigating the CRISPR/Cas Landscape for Enhanced Diagnosis and Treatment of Wilson’s Disease
Abstract
1. Introduction
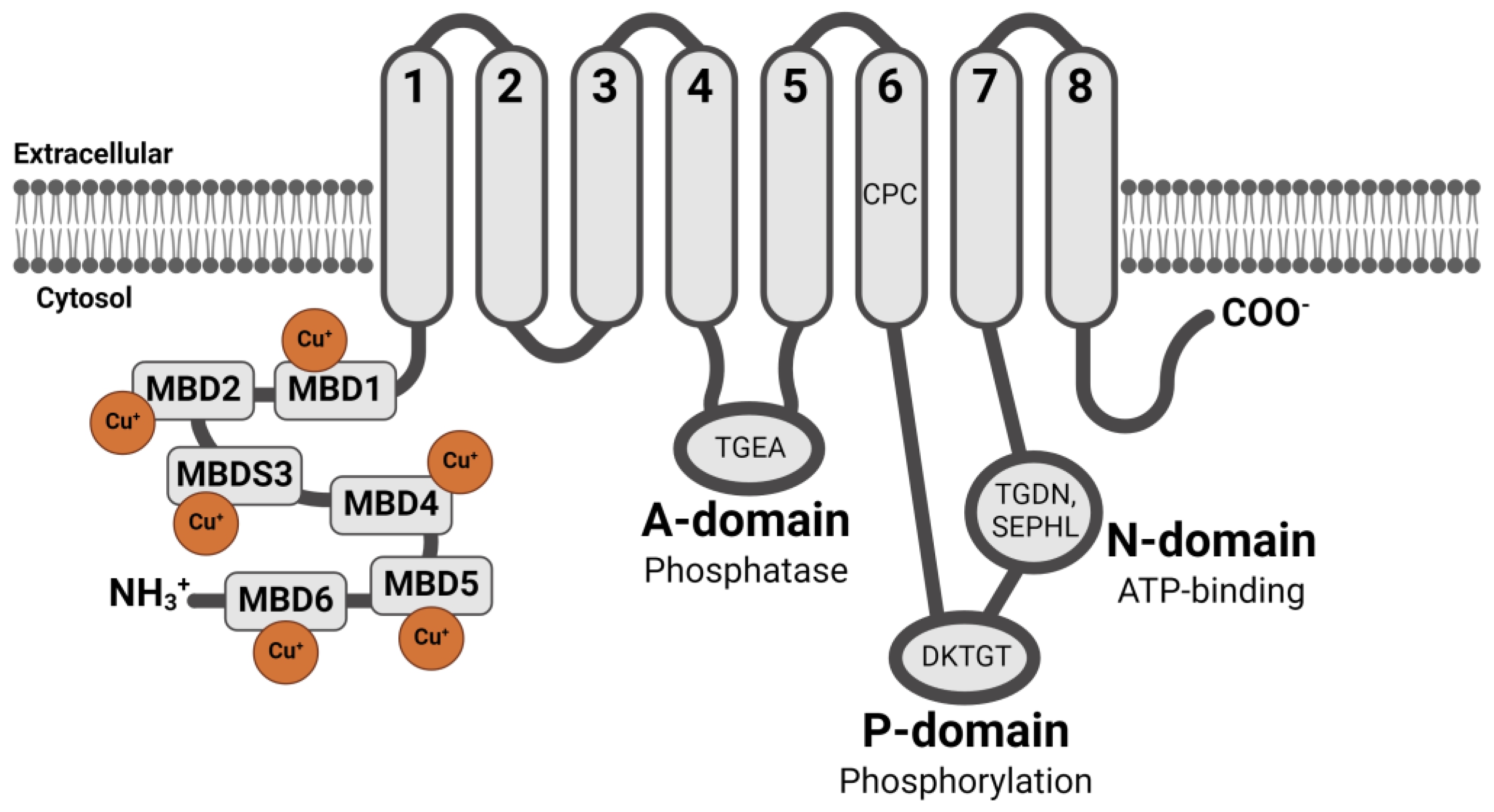
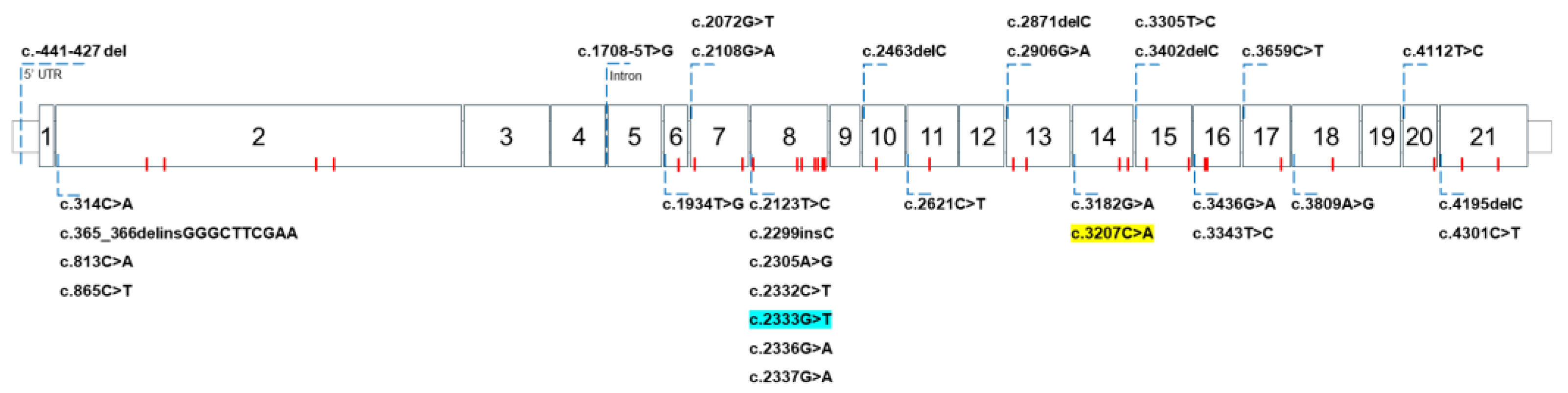
2. WD Caused by ATP7B Gene Mutations
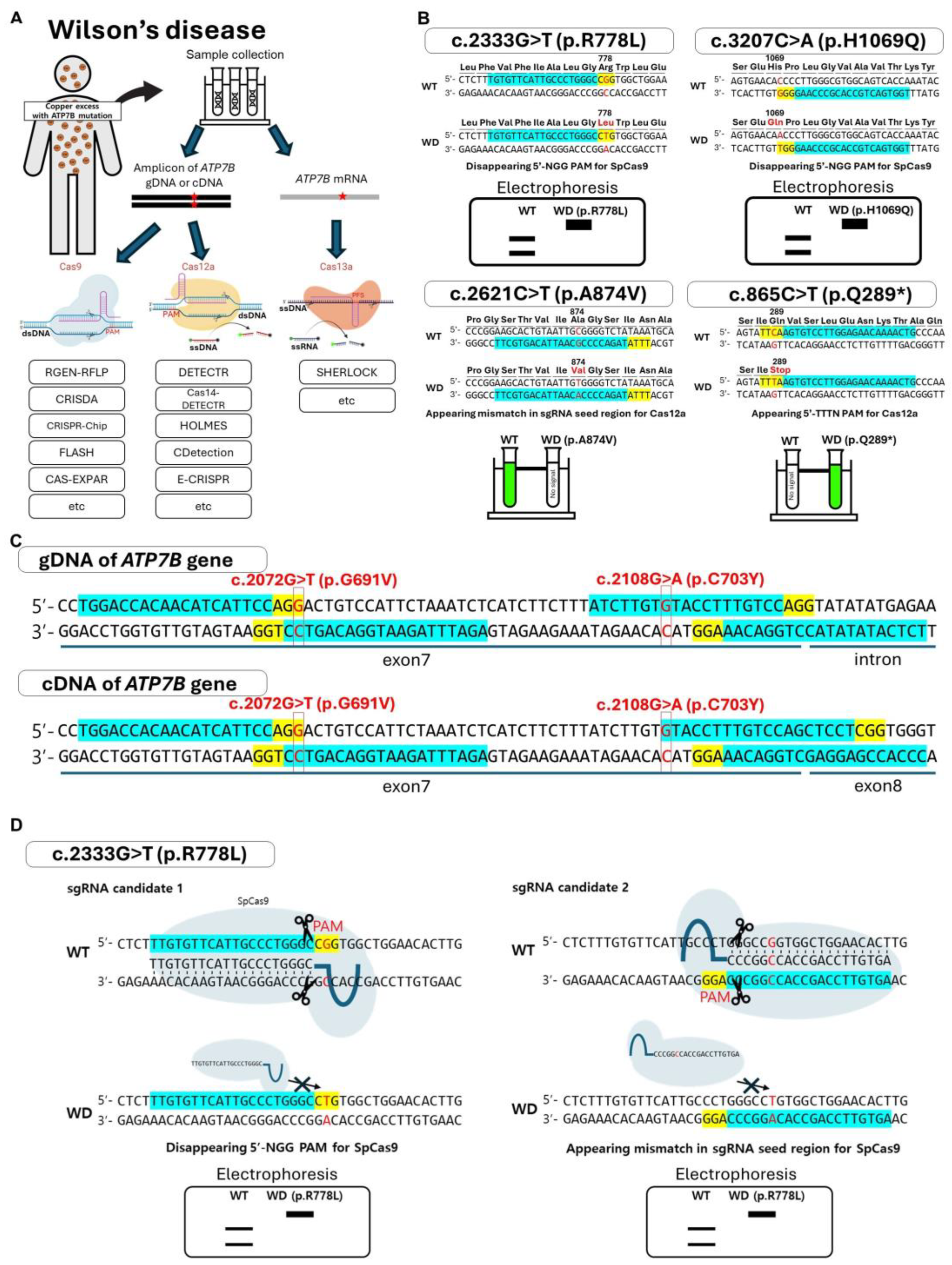
3. Utilizing the CRISPR/Cas System for the Diagnosis of WD
4. Comparison of the Diagnostic Approaches
5. CRISPR/Cas System-Based Gene-Editing Therapy Strategies for WD
6. The Current Stage of Vector Development for Gene Therapy to Treat WD
| Target Locus | CRISPR System | Associated Method | Delivery Method | Purpose | Model | Reference |
|---|---|---|---|---|---|---|
| c.2333G>T (p.R778L) | SpCas9 | HDR with ssODN | Lipofection | Inducement of mutagenesis | Human embryonic stem cells (hESCs) and human-induced pluripotent stem cells (hiPSCs) in vitro | [199] |
| c.2333G>T (p.R778L) | SpCas9 | HDR with ssODN | Electroporation | Repair | Human-induced pluripotent stem cells (hiPSCs) in vitro, ARG mouse ex vivo | [202] |
| c.1184delC (p.E396KfsX11) | SpCas9 | HDR with ssODN | Lipofection | Inducement of mutagenesis, repair | HEK293T cells in vitro | [203] |
| Exon 8 | SpCas9 | HDR with ssODN | Lentivirus: SpCas9 AAV: sgRNA and donor template | Exon8 replacement | Mouse hepatocytes in vivo | [201] |
| c.2333G>T (p.R778L) | SpCas9, eSpCas9(1.1), SpCas9-HF1, and HypaCas9 | HDR with ssODN | Lipofection | Inducement of mutagenesis | HEK293T and HeLa cells in vitro | [198] |
| c.2333G>T (p.R778L) | SpCas9 (WT and nCas), TALEN | HDR with ssODN | Lipofection | Inducement of mutagenesis | HEK293T and HeLa cells in vitro | [200] |
| c.3097G>A (p.T1033A) c.3659C>T (p.T1220M) | xBE3 xABE | – | Lipofection | Repair | HEK293T cells in vitro | [219] |
| c.1288dup (p.S430fs) | PE3 | - | Electroporation | Repair | An organoid grown from a WD patient’s liver cells in vitro | [226] |
| Deletion of 900 bp of the coding region at the 3′ end and ~400 bp of the downstream untranslated region of ATP7B | – | – | Lentivirus | Transgene expression | Lymphatic endothelial cell (LEC) rat in vivo and hepatocytes ex vivo | [291] |
| Exon2 | – | miniATP7B | AAV8 and AAVAnc80 | Transgene expression | Atp7b−/− mice hepatocytes in vivo | [281] |
| Exon2, Exon8 (c.2248G>A) | – | miniATP7B | AAV8 | Transgene expression | Atp7b−/− and Atp7btx-J mice in vivo | [283] |
| Exon2 | – | Split-intein technology | AAV2/8 | Transgene expression | HepG2 ATP7B-KO cells in vitro and Atp7b−/− mice in vivo | [284] |
| MBD 1-6 | – | miniATP7B | Lithium acetate | Eliminating each MBD to assess their respective roles | Yeast Saccharomyces cerevisiae in vitro | [282] |
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of copper on mitochondrial function and metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, Y.; Shi, H.; Peng, Y.; Fan, X.; Li, C. The molecular mechanisms of copper metabolism and its roles in human diseases. Pflug. Arch. 2020, 472, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Kahlson, M.A.; Dixon, S.J. Copper-induced cell death. Science 2022, 375, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Brueggemeyer, M.T.; Transue, W.J.; Park, Y.; Cho, J.; Siegler, M.A.; Solomon, E.I.; Karlin, K.D. Fenton-like chemistry by a Copper(I) complex and H2O2 relevant to enzyme peroxygenase C-H hydroxylation. J. Am. Chem. Soc. 2023, 145, 11735–11744. [Google Scholar] [CrossRef] [PubMed]
- Haidari, M.; Javadi, E.; Kadkhodaee, M.; Sanati, A. Enhanced susceptibility to oxidation and diminished vitamin E content of LDL from patients with stable coronary artery disease. Clin. Chem. 2001, 47, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Oe, S.; Miyagawa, K.; Honma, Y.; Harada, M. Copper induces hepatocyte injury due to the endoplasmic reticulum stress in cultured cells and patients with Wilson disease. Exp. Cell. Res. 2016, 347, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Wooton-Kee, C.R.; Jain, A.K.; Wagner, M.; Grusak, M.A.; Finegold, M.J.; Lutsenko, S.; Moore, D.D. Elevated copper impairs hepatic nuclear receptor function in Wilson’s disease. J. Clin. Investig. 2015, 125, 3449–3460. [Google Scholar] [CrossRef]
- de Bie, P.; Muller, P.; Wijmenga, C.; Klomp, L.W. Molecular pathogenesis of Wilson and Menkes disease: Correlation of mutations with molecular defects and disease phenotypes. J. Med. Genet. 2007, 44, 673–688. [Google Scholar] [CrossRef]
- Kaler, S.G. Inborn errors of copper metabolism. Handb. Clin. Neurol. 2013, 113, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Socha, P.; Czlonkowska, A.; Janczyk, W.; Litwin, T. Wilson’s disease-management and long term outcomes. Best Prac. Res. Clin. Gastroenterol. 2022, 56–57, 101768. [Google Scholar] [CrossRef] [PubMed]
- Nidhi, S.; Anand, U.; Oleksak, P.; Tripathi, P.; Lal, J.A.; Thomas, G.; Kuca, K.; Tripathi, V. Novel CRISPR-Cas systems: An updated review of the current achievements, applications, and future research perspectives. Int. J. Mol. Sci. 2021, 22, 3327. [Google Scholar] [CrossRef] [PubMed]
- Alves-Bezerra, M.; Furey, N.; Johnson, C.G.; Bissig, K.D. Using CRISPR/Cas9 to model human liver disease. JHEP Rep. 2019, 1, 392–402. [Google Scholar] [CrossRef]
- Puig-Serra, P.; Casado-Rosas, M.C.; Martinez-Lage, M.; Olalla-Sastre, B.; Alonso-Yanez, A.; Torres-Ruiz, R.; Rodriguez-Perales, S. CRISPR approaches for the diagnosis of human diseases. Int. J. Mol. Sci. 2022, 23, 1757. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Fujisawa, C.; Bhadhprasit, W. Inherited copper transport disorders: Biochemical mechanisms, diagnosis, and treatment. Curr. Drug Metab. 2012, 13, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Fanni, D.; Pilloni, L.; Orru, S.; Coni, P.; Liguori, C.; Serra, S.; Lai, M.L.; Uccheddu, A.; Contu, L.; Van Eyken, P.; et al. Expression of ATP7B in normal human liver. Eur. J. Histochem. 2005, 49, 371–378. [Google Scholar] [CrossRef]
- Bost, M.; Piguet-Lacroix, G.; Parant, F.; Wilson, C.M. Molecular analysis of Wilson patients: Direct sequencing and MLPA analysis in the ATP7B gene and Atox1 and COMMD1 gene analysis. J. Trace. Elem. Med. Biol. 2012, 26, 97–101. [Google Scholar] [CrossRef]
- Zhang, C.C.; Volkmann, M.; Tuma, S.; Stremmel, W.; Merle, U. Metallothionein is elevated in liver and duodenum of Atp7b(−/−) mice. Biometals 2018, 31, 617–625. [Google Scholar] [CrossRef]
- Lutsenko, S. Dynamic and cell-specific transport networks for intracellular copper ions. J. Cell. Sci. 2021, 134, 240523. [Google Scholar] [CrossRef]
- Prasad, R.; Kumar, S. Biochemistry, Molecular Biology and Molecular Genetics of Wilson Disease; Transworld Research Network: Trivandrum, India, 2013. [Google Scholar]
- Stalke, A.; Behrendt, A.; Hennig, F.; Gohlke, H.; Buhl, N.; Reinkens, T.; Baumann, U.; Schlegelberger, B.; Illig, T.; Pfister, E.D.; et al. Functional characterization of novel or yet uncharacterized ATP7B missense variants detected in patients with clinical Wilson’s disease. Clin. Genet. 2023, 104, 174–185. [Google Scholar] [CrossRef]
- Tsivkovskii, R.; Eisses, J.F.; Kaplan, J.H.; Lutsenko, S. Functional properties of the copper-transporting ATPase ATP7B (the Wilson’s disease protein) expressed in insect cells. J. Biol. Chem. 2002, 277, 976–983. [Google Scholar] [CrossRef]
- Yang, G.M.; Xu, L.; Wang, R.M.; Tao, X.; Zheng, Z.W.; Chang, S.; Ma, D.; Zhao, C.; Dong, Y.; Wu, S.; et al. Structures of the human Wilson disease copper transporter ATP7B. Cell. Rep. 2023, 42, 112417. [Google Scholar] [CrossRef]
- Arioz, C.; Li, Y.; Wittung-Stafshede, P. The six metal binding domains in human copper transporter, ATP7B: Molecular biophysics and disease-causing mutations. Biometals 2017, 30, 823–840. [Google Scholar] [CrossRef]
- Ferenci, P. Regional distribution of mutations of the ATP7B gene in patients with Wilson disease: Impact on genetic testing. Hum. Genet. 2006, 120, 151–159. [Google Scholar] [CrossRef]
- Medici, V.; LaSalle, J.M. Genetics and epigenetic factors of Wilson disease. Ann. Transl. Med. 2019, 7, S58. [Google Scholar] [CrossRef]
- Beyzaei, Z.; Mehrzadeh, A.; Hashemi, N.; Geramizadeh, B. The mutation spectrum and ethnic distribution of Wilson disease, a review. Mol. Genet. Metab. Rep. 2024, 38, 101034. [Google Scholar] [CrossRef]
- Coffey, A.J.; Durkie, M.; Hague, S.; McLay, K.; Emmerson, J.; Lo, C.; Klaffke, S.; Joyce, C.J.; Dhawan, A.; Hadzic, N.; et al. A genetic study of Wilson’s disease in the United Kingdom. Brain 2013, 136, 1476–1487. [Google Scholar] [CrossRef]
- Chang, I.J.; Hahn, S.H. The genetics of Wilson disease. Handb. Clin. Neurol. 2017, 142, 19–34. [Google Scholar] [CrossRef]
- Espinos, C.; Ferenci, P. Are the new genetic tools for diagnosis of Wilson disease helpful in clinical practice? JHEP Rep. 2020, 2, 100114. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, W.; Li, X.; Pei, P.; Dong, T.; Yang, Y.; Zhang, J. Clinical and genetic characterization of a large cohort of patients with Wilson’s disease in China. Transl. Neurodegener. 2022, 11, 13. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Lin, M.T.; Murong, S.X.; Wang, N. Molecular diagnosis and prophylactic therapy for presymptomatic Chinese patients with Wilson disease. Arch. Neurol. 2003, 60, 737–741. [Google Scholar] [CrossRef][Green Version]
- Gu, Y.H.; Kodama, H.; Du, S.L.; Gu, Q.J.; Sun, H.J.; Ushijima, H. Mutation spectrum and polymorphisms in ATP7B identified on direct sequencing of all exons in Chinese Han and Hui ethnic patients with Wilson’s disease. Clin. Genet. 2003, 64, 479–484. [Google Scholar] [CrossRef]
- Ye, S.; Gong, L.; Shui, Q.X.; Zhou, L.F. Wilson disease: Identification of two novel mutations and clinical correlation in Eastern Chinese patients. World. J. Gastroenterol. 2007, 13, 5147–5150. [Google Scholar] [CrossRef]
- Wang, L.H.; Huang, Y.Q.; Shang, X.; Su, Q.X.; Xiong, F.; Yu, Q.Y.; Lin, H.P.; Wei, Z.S.; Hong, M.F.; Xu, X.M. Mutation analysis of 73 southern Chinese Wilson’s disease patients: Identification of 10 novel mutations and its clinical correlation. J. Hum. Genet. 2011, 56, 660–665. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Y.; Liu, A.; Diao, S.; Yu, Q.; Peng, Z.; Hong, M. Mutational characterization of ATP7B gene in 103 Wilson’s disease patients from Southern China: Identification of three novel mutations. Neuroreport 2014, 25, 1075–1080. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, A.; Thapa, B.R.; Modi, M.; Pal, A.; Prasad, R. Characterization of mutation spectrum and identification of novel mutations in ATP7B gene from a cohort of Wilson disease patients: Functional and therapeutic implications. Hum. Mutat. 2018, 39, 1926–1941. [Google Scholar] [CrossRef]
- Kumar, S.; Thapa, B.; Kaur, G.; Prasad, R. Analysis of most common mutations R778G, R778L, R778W, I1102T and H1069Q in Indian Wilson disease patients: Correlation between genotype/phenotype/copper ATPase activity. Mol. Cell. Biochem. 2007, 294, 1–10. [Google Scholar] [CrossRef]
- Gupta, A.; Chattopadhyay, I.; Dey, S.; Nasipuri, P.; Das, S.K.; Gangopadhyay, P.K.; Ray, K. Molecular pathogenesis of Wilson disease among Indians: A perspective on mutation spectrum in ATP7B gene, prevalent defects, clinical heterogeneity and implication towards diagnosis. Cell. Mol. Neurobiol. 2007, 27, 1023–1033. [Google Scholar] [CrossRef]
- Gupta, A.; Aikath, D.; Neogi, R.; Datta, S.; Basu, K.; Maity, B.; Trivedi, R.; Ray, J.; Das, S.K.; Gangopadhyay, P.K.; et al. Molecular pathogenesis of Wilson disease: Haplotype analysis, detection of prevalent mutations and genotype-phenotype correlation in Indian patients. Hum. Genet. 2005, 118, 49–57. [Google Scholar] [CrossRef]
- Santhosh, S.; Shaji, R.V.; Eapen, C.E.; Jayanthi, V.; Malathi, S.; Chandy, M.; Stanley, M.; Selvi, S.; Kurian, G.; Chandy, G.M. ATP7B mutations in families in a predominantly Southern Indian cohort of Wilson’s disease patients. Indian J. Gastroenterol. 2006, 25, 277–282. [Google Scholar]
- Aggarwal, A.; Chandhok, G.; Todorov, T.; Parekh, S.; Tilve, S.; Zibert, A.; Bhatt, M.; Schmidt, H.H.J. Wilson disease mutation pattern with genotype-phenotype correlations from western India: Confirmation of p.C271*as a common Indian mutation and identification of 14 novel mutations. Ann. Hum. Genet. 2013, 77, 299–307. [Google Scholar] [CrossRef]
- Nagral, A.; Mallakmir, S.; Garg, N.; Tiwari, K.; Masih, S.; Nagral, N.; Unavane, O.; Jhaveri, A.; Phadke, S.; ArunKumar, G.; et al. Genomic variations in ATP7B gene in indian Patients with Wilson disease. Indian J. Pediatr. 2023, 90, 240–248. [Google Scholar] [CrossRef]
- Zali, N.; Mohebbi, S.R.; Esteghamat, S.; Chiani, M.; Haghighi, M.M.; Hosseini-Asl, S.M.; Derakhshan, F.; Mohammad-Alizadeh, A.H.; Malek-Hosseini, S.A.; Zali, M.R. Prevalence of ATP7B Gene mutations in Iranian patients with Wilson disease. Hepat. Mon. 2011, 11, 890–894. [Google Scholar] [CrossRef]
- Okada, T.; Shiono, Y.; Hayashi, H.; Satoh, H.; Sawada, T.; Suzuki, A.; Takeda, Y.; Yano, M.; Michitaka, K.; Onji, M.; et al. Mutational analysis of ATP7B and genotype-phenotype correlation in Japanese with Wilson’s disease. Hum. Mutat. 2000, 15, 454–462. [Google Scholar] [CrossRef]
- Tatsumi, Y.; Hattori, A.; Hayashi, H.; Ikoma, J.; Kaito, M.; Imoto, M.; Wakusawa, S.; Yano, M.; Hayashi, K.; Katano, Y.; et al. Current state of Wilson disease patients in central Japan. Intern. Med. 2010, 49, 809–815. [Google Scholar] [CrossRef][Green Version]
- Kim, E.K.; Yoo, O.J.; Song, K.Y.; Yoo, H.W.; Choi, S.Y.; Cho, S.W.; Hahn, S.H. Identification of three novel mutations and a high frequency of the Arg778Leu mutation in Korean patients with Wilson disease. Hum. Mutat. 1998, 11, 275–278. [Google Scholar] [CrossRef]
- Song, M.J.; Lee, S.T.; Lee, M.K.; Ji, Y.; Kim, J.W.; Ki, C.S. Estimation of carrier frequencies of six autosomal-recessive Mendelian disorders in the Korean population. J. Hum. Genet. 2012, 57, 139–144. [Google Scholar] [CrossRef]
- Park, S.; Park, J.; Kim, G.H.; Choi, J.H.; Kim, K.M.; Kim, J.B.; Yoo, H.W. Identification of novel gene mutations and their functional roles in Korean patients with Wilson disease. Hum. Mutat. 2007, 28, 1108–1113. [Google Scholar] [CrossRef]
- Yoo, H.W. Identification of novel mutations and the three most common mutations in the human gene of Korean patients with Wilson disease. Genet. Med. 2002, 4, 43s–48s. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, T.; Bang, S.; Kim, Y.E.; Cho, E.H. Carrier frequency of Wilson’s disease in the Korean population: A DNA-based approach. J. Hum. Genet. 2017, 62, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Yang, J.Y.; Park, J.Y.; Lee, J.J.; Kim, J.H.; Yoo, H.W. Estimation of Wilson’s disease incidence and carrier frequency in the Korean population by screening ATP7B major mutations in newborn filter papers using the SYBR green intercalator method based on the amplification refractory mutation system. Genet. Test. 2008, 12, 395–399. [Google Scholar] [CrossRef]
- Usta, J.; Wehbeh, A.; Rida, K.; El-Rifai, O.; Estiphan, T.A.; Majarian, T.; Barada, K. Phenotype-genotype correlation in Wilson disease in a large Lebanese family: Association of c.2299insC with hepatic and of p. Ala1003Thr with neurologic phenotype. PLoS ONE 2014, 9, e109727. [Google Scholar] [CrossRef]
- Al Jumah, M.; Majumdar, R.; Al Rajeh, S.; Awada, A.; Al Zaben, A.; Al Traif, I.; Al Jumah, A.R.; Rehana, Z. A clinical and genetic study of 56 Saudi Wilson disease patients: Identification of Saudi-specific mutations. Eur. J. Neurol. 2004, 11, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Tsai, C.H.; Tsai, Y.; Hsu, C.M.; Lee, C.C.; Tsai, F.J. Mutation analysis of Taiwanese Wilson disease patients. Biochem. Biophys. Res. Commun. 2006, 345, 734–738. [Google Scholar] [CrossRef]
- Huong, N.T.M.; Hoa, N.P.A.; Ngoc, N.D.; Mai, N.T.P.; Yen, P.H.; Hoa, G.; Dien, T.M.; Van Anh, H.T. Mutation spectrum of ATP7B gene in pediatric patients with Wilson disease in Vietnam. Mol. Genet. Metab. Rep. 2022, 31, 100861. [Google Scholar] [CrossRef]
- Hofer, H.; Willheim-Polli, C.; Knoflach, P.; Gabriel, C.; Vogel, W.; Trauner, M.; Muller, T.; Ferenci, P. Correction: Identification of a novel Wilson disease gene mutation frequent in Upper Austria: A genetic and clinical study. J. Hum. Genet. 2021, 66, 1199. [Google Scholar] [CrossRef] [PubMed]
- Todorov, T.; Savov, A.; Jelev, H.; Panteleeva, E.; Konstantinova, D.; Krustev, Z.; Mihaylova, V.; Tournev, I.; Tankova, L.; Tzolova, N.; et al. Spectrum of mutations in the Wilson disease gene (ATP7B) in the Bulgarian population. Clin. Genet. 2005, 68, 474–476. [Google Scholar] [CrossRef]
- Vrabelova, S.; Letocha, O.; Borsky, M.; Kozak, L. Mutation analysis of the ATP7B gene and genotype/phenotype correlation in 227 patients with Wilson disease. Mol. Genet. Metab. 2005, 86, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Moller, L.B.; Horn, N.; Jeppesen, T.D.; Vissing, J.; Wibrand, F.; Jennum, P.; Ott, P. Clinical presentation and mutations in Danish patients with Wilson disease. Eur. J. Hum. Genet. 2011, 19, 935–941. [Google Scholar] [CrossRef]
- Collet, C.; Laplanche, J.L.; Page, J.; Morel, H.; Woimant, F.; Poujois, A. High genetic carrier frequency of Wilson’s disease in France: Discrepancies with clinical prevalence. BMC Med. Genet. 2018, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- Caca, K.; Ferenci, P.; Kuhn, H.J.; Polli, C.; Willgerodt, H.; Kunath, B.; Hermann, W.; Mossner, J.; Berr, F. High prevalence of the H1069Q mutation in East German patients with Wilson disease: Rapid detection of mutations by limited sequencing and phenotype-genotype analysis. J. Hepatol. 2001, 35, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakaki, E.; Tzetis, M.; Manolaki, N.; Loudianos, G.; Papatheodorou, A.; Manesis, E.; Nousia-Arvanitakis, S.; Syriopoulou, V.; Kanavakis, E. Genotype-phenotype correlations for a wide spectrum of mutations in the Wilson disease gene (ATP7B). Am. J. Med. Genet. A 2004, 131, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Dedoussis, G.V.; Genschel, J.; Sialvera, T.E.; Bochow, B.; Manolaki, N.; Manios, Y.; Tsafantakis, E.; Schmidt, H. Wilson disease: High prevalence in a mountainous area of Crete. Ann. Hum. Genet. 2005, 69, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Firneisz, G.; Lakatos, P.L.; Szalay, F.; Polli, C.; Glant, T.T.; Ferenci, P. Common mutations of ATP7B in Wilson disease patients from Hungary. Am. J. Med. Genet. 2002, 108, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Folhoffer, A.; Ferenci, P.; Csak, T.; Horvath, A.; Hegedus, D.; Firneisz, G.; Osztovits, J.; Kosa, J.P.; Willheim-Polli, C.; Szonyi, L.; et al. Novel mutations of the ATP7B gene among 109 Hungarian patients with Wilson’s disease. Eur. J. Gastroen. Hepat. 2007, 19, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Loudianos, G.; Dessi, V.; Lovicu, M.; Angius, A.; Altuntas, B.; Giacchino, R.; Marazzi, M.; Marcellini, M.; Sartorelli, M.R.; Sturniolo, G.C.; et al. Mutation analysis in patients of Mediterranean descent with Wilson disease: Identification of 19 novel mutations. J. Med. Genet. 1999, 36, 833–836. [Google Scholar]
- Loudianos, G.; Dessi, V.; Lovicu, M.; Angius, A.; Figus, A.; Lilliu, F.; De Virgiliis, S.; Nurchi, A.M.; Deplano, A.; Moi, P.; et al. Molecular characterization of Wilson disease in the Sardinian population—evidence of a founder effect. Hum. Mutat. 1999, 14, 294–303. [Google Scholar] [CrossRef]
- Krumina, A.; Keiss, J.; Sondore, V.; Chernushenko, A.; Cernevska, G.; Zarina, A.; Micule, I.; Piekuse, L.; Kreile, M.; Lace, B.; et al. From clinical and biochemical to molecular genetic diagnosis of Wilson disease in Latvia. Russ. J. Genet. 2008, 44, 1195–1200. [Google Scholar] [CrossRef]
- Stapelbroek, J.M.; Bollen, C.W.; van Amstel, J.K.P.; van Erpecum, K.J.; van Hattum, J.; van den Berg, L.H.; Klomp, L.W.J.; Houwen, R.H.J. The H1069Q mutation in ATP7B is associated with late and neurologic presentation in Wilson disease: Results of a meta-analysis. J. Hepatol. 2004, 41, 758–763. [Google Scholar] [CrossRef]
- Gromadzka, G.; Schmidt, H.H.; Genschel, J.; Bochow, B.; Rodo, M.; Tarnacka, B.; Litwin, T.; Chabik, G.; Czlonkowska, A. Frameshift and nonsense mutations in the gene for ATPase7B are associated with severe impairment of copper metabolism and with an early clinical manifestation of Wilson’s disease. Clin. Genet. 2005, 68, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Kluska, A.; Kulecka, M.; Litwin, T.; Dziezyc, K.; Balabas, A.; Piatkowska, M.; Paziewska, A.; Dabrowska, M.; Mikula, M.; Kaminska, D.; et al. Whole-exome sequencing identifies novel pathogenic variants across the ATP7B gene and some modifiers of Wilson’s disease phenotype. Liver Int. 2019, 39, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Iacob, R.; Iacob, S.; Nastase, A.; Vagu, C.; Ene, A.M.; Constantinescu, A.; Anghel, D.; Banica, C.; Paslaru, L.; Coriu, D.; et al. The His1069Gln mutation in the ATP7B gene in Romanian patients with Wilson’s disease referred to a tertiary gastroenterology center. J. Gastrointestin. Liver Dis. 2012, 21, 181–185. [Google Scholar] [PubMed]
- Shah, A.B.; Chernov, I.; Zhang, H.T.; Ross, B.M.; Das, K.; Lutsenko, S.; Parano, E.; Pavone, L.; Evgrafov, O.; Ivanova-Smolenskaya, I.A.; et al. Identification and analysis of mutations in the Wilson disease gene (ATP7B): Population frequencies, genotype-phenotype correlation, and functional analyses. Am. J. Hum. Genet. 1997, 61, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Tomic, A.; Dobricic, V.; Novakovic, I.; Svetel, M.; Pekmezovic, T.; Kresojevic, N.; Potrebic, A.; Kostic, V.S. Mutational analysis of ATP7B gene and the genotype-phenotype correlation in patients with Wilson’s disease in Serbia. Vojn. Pregl. 2013, 70, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Margarit, E.; Bach, V.; Gomez, D.; Bruguera, M.; Jara, P.; Queralt, R.; Ballesta, F. Mutation analysis of Wilson disease in the Spanish population—Identification of a prevalent substitution and eight novel mutations in the ATP7B gene. Clin. Genet. 2005, 68, 61–68. [Google Scholar] [CrossRef] [PubMed]
- García-Villarreal, L.; Daniels, S.; Shaw, S.H.; Cotton, D.; Galvin, M.; Geskes, J.; Bauer, P.; Sierra-Hernández, A.; Buckler, A.; Tugores, A. High prevalence of the very rare Wilson disease gene mutation Leu708Pro in the island of Gran Canaria (Canary islands, Spain): A genetic and clinical study. Hepatology 2000, 32, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Abdelghaffar, T.Y.; Elsayed, S.M.; Elsobky, E.; Bochow, B.; Buttner, J.; Schmidt, H. Mutational analysis of ATP7B gene in Egyptian children with Wilson disease: 12 novel mutations. J. Hum. Genet. 2008, 53, 681. [Google Scholar] [CrossRef]
- Kuppala, D.; Deng, J.; Brewer, G.J.; Wang, M.M.; Borjigin, J. Wilson disease mutations in the American population: Identification of five novel mutations in ATP7B. Open. Hepatol. J. 2009, 1, 1–4. [Google Scholar] [CrossRef][Green Version]
- Bem, R.S.; Raskin, S.; Muzzillo, D.A.; Deguti, M.M.; Cancado, E.L.; Araujo, T.F.; Nakhle, M.C.; Barbosa, E.R.; Munhoz, R.P.; Teive, H.A. Wilson’s disease in southern Brazil: Genotype-phenotype correlation and description of two novel mutations in ATP7B gene. Arq. Neuropsiquiatr. 2013, 71, 503–507. [Google Scholar] [CrossRef]
- Machado, A.A.; Deguti, M.M.; Genschel, J.; Cancado, E.L.; Bochow, B.; Schmidt, H.; Barbosa, E.R. Neurological manifestations and ATP7B mutations in Wilson’s disease. Park. Relat. Disord. 2008, 14, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Deguti, M.M.; Genschel, J.; Cancado, E.L.; Barbosa, E.R.; Bochow, B.; Mucenic, M.; Porta, G.; Lochs, H.; Carrilho, F.J.; Schmidt, H.H. Wilson disease: Novel mutations in the ATP7B gene and clinical correlation in Brazilian patients. Hum. Mutat. 2004, 23, 398. [Google Scholar] [CrossRef] [PubMed]
- Paradisi, I.; De Freitas, L.; Arias, S. Most frequent mutation c.3402delC (p.Ala1135GlnfsX13) among Wilson disease patients in Venezuela has a wide distribution and two old origins. Eur. J. Med. Genet. 2015, 58, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Haft, D.H.; Selengut, J.; Mongodin, E.F.; Nelson, K.E. A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput. Biol. 2005, 1, e60. [Google Scholar] [CrossRef] [PubMed]
- Horvath, P.; Barrangou, R. CRISPR/Cas, the immune system of bacteria and archaea. Science 2010, 327, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Manghwar, H.; Li, B.; Ding, X.; Hussain, A.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas systems in genome editing: Methodologies and tools for sgRNA design, off-target evaluation, and strategies to mitigate off-target effects. Adv. Sci. 2020, 7, 1902312. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Halder, K.; Datta, A. Classification of CRISPR/Cas system and its application in tomato breeding. Theor. Appl. Genet. 2022, 135, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Makarova, K.S.; Zhang, F. Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 2017, 37, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Z. CRISPR-Cas systems: Overview, innovations and applications in human disease research and gene therapy. Comput. Struct. Biotechnol. J. 2020, 18, 2401–2415. [Google Scholar] [CrossRef]
- Sinkunas, T.; Gasiunas, G.; Fremaux, C.; Barrangou, R.; Horvath, P.; Siksnys, V. Cas3 is a single-stranded DNA nuclease and ATP-dependent helicase in the CRISPR/Cas immune system. Embo J. 2011, 30, 1335–1342. [Google Scholar] [CrossRef]
- Huo, Y.W.; Nam, K.H.; Ding, F.; Lee, H.J.; Wu, L.J.; Xiao, Y.B.; Farchione, M.D.; Zhou, S.; Rajashankar, K.; Kurinov, I.; et al. Structures of CRISPR Cas3 offer mechanistic insights into Cascade-activated DNA unwinding and degradation. Nat. Struct. Mol. Biol. 2014, 21, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Stella, G.; Marraffini, L. Type III CRISPR-Cas: Beyond the Cas10 effector complex. Trends Biochem. Sci. 2024, 49, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.Y.; Yan, H.Q.; Ren, G.X.; Zhao, J.P.; Guo, X.P.; Sun, Y.C. CRISPR-Cas12a-assisted recombineering in bacteria. Appl. Environ. Microbiol. 2017, 83, e00947. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR-Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.B.; Kim, D.Y.; Ko, J.H.; Kim, Y.S. Recent advances in the CRISPR genome editing tool set. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dong, H.; Cui, Y.; Cong, L.; Zhang, D. Application of different types of CRISPR/Cas-based systems in bacteria. Microb. Cell. Fact. 2020, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Wolf, Y.I.; Alkhnbashi, O.S.; Costa, F.; Shah, S.A.; Saunders, S.J.; Barrangou, R.; Brouns, S.J.; Charpentier, E.; Haft, D.H.; et al. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol. 2015, 13, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Zhang, J.T.; Liu, Y.; Liu, Y.; Liu, X.Y.; Wang, C.; Huang, H.; Jia, N. Type IV-A CRISPR-Csf complex: Assembly, dsDNA targeting, and CasDinG recruitment. Mol. Cell 2023, 83, 2493–2508.e2495. [Google Scholar] [CrossRef] [PubMed]
- Hillary, V.E.; Ceasar, S.A. A Review on the mechanism and applications of CRISPR/Cas9/Cas12/Cas13/Cas14 proteins utilized for genome engineering. Mol. Biotechnol. 2023, 65, 311–325. [Google Scholar] [CrossRef]
- Hille, F.; Richter, H.; Wong, S.P.; Bratovic, M.; Ressel, S.; Charpentier, E. The biology of CRISPR-Cas: Backward and forward. Cell 2018, 172, 1239–1259. [Google Scholar] [CrossRef]
- Li, Y.; Peng, N. Endogenous CRISPR-Cas system-based genome editing and antimicrobials: Review and prospects. Front. Microbiol. 2019, 10, 2471. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Doudna, J.A. Chemistry of Class 1 CRISPR-Cas effectors: Binding, editing, and regulation. J. Biol. Chem. 2020, 295, 14473–14487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, G.; Ahmed, F.Y.H.; Yi, T.; Hu, S.; Cai, T.; Liao, Q. In silico method in CRISPR/Cas system: An expedite and powerful booster. Front. Oncol. 2020, 10, 584404. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.M.; Abudayyeh, O.O.; Gootenberg, J.S.; Zhang, F.; Collins, J.J. CRISPR-based diagnostics. Nat. Biomed. Eng. 2021, 5, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ji, S.; Koh, H.R. CRISPR as a diagnostic tool. Biomolecules 2021, 11, 1162. [Google Scholar] [CrossRef] [PubMed]
- Brezgin, S.; Kostyusheva, A.; Kostyushev, D.; Chulanov, V. Dead Cas systems: Types, principles, and applications. Int. J. Mol. Sci. 2019, 20, 6041. [Google Scholar] [CrossRef] [PubMed]
- Courtney, D.G.; Moore, J.E.; Atkinson, S.D.; Maurizi, E.; Allen, E.H.A.; Pedrioli, D.M.L.; McLean, W.H.I.; Nesbit, M.A.; Moore, C.B.T. CRISPR/Cas9 DNA cleavage at SNP-derived PAM enables both and mutation-specific targeting. Gene Ther. 2016, 23, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mendiratta, S.; Ehrhardt, K.; Kashyap, N.; White, M.A.; Bleris, L. Exploiting the CRISPR/Cas9 PAM constraint for single-nucleotide resolution interventions. PLoS ONE 2016, 11, e0144970. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Watanabe, S.; Kamiyoshi, A.; Sato, M.; Shindo, T. A single blastocyst assay optimized for detecting CRISPR/Cas9 system-induced indel mutations in mice. BMC Biotechnol. 2014, 14, 69. [Google Scholar] [CrossRef]
- Sentmanat, M.F.; Peters, S.T.; Florian, C.P.; Connelly, J.P.; Pruett-Miller, S.M. A survey of validation strategies for CRISPR-Cas9 editing. Sci. Rep. 2018, 8, 888. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, Y.; Yu, S.; Lu, L.; Ding, M.; Cheng, J.; Song, G.; Gao, X.; Yao, L.; Fan, D.; et al. An efficient genotyping method for genome-modified animals and human cells generated with CRISPR/Cas9 system. Sci. Rep. 2014, 4, 6420. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Kim, S.; Kim, Y.; Kweon, J.; Kim, H.S.; Bae, S.; Kim, J.S. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 2014, 24, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, D.; Kim, S.; Kim, J.S. Genotyping with CRISPR-Cas-derived RNA-guided endonucleases. Nat. Commun. 2014, 5, 3157. [Google Scholar] [CrossRef] [PubMed]
- Hajian, R.; Balderston, S.; Tran, T.; DeBoer, T.; Etienne, J.; Sandhu, M.; Wauford, N.A.; Chung, J.Y.; Nokes, J.; Athaiya, M.; et al. Detection of unamplified target genes via CRISPR-Cas9 immobilized on a graphene field-effect transistor. Nat. Biomed. Eng. 2019, 3, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Balderston, S.; Taulbee, J.J.; Celaya, E.; Fung, K.; Jiao, A.; Smith, K.; Hajian, R.; Gasiunas, G.; Kutanovas, S.; Kim, D.; et al. Discrimination of single-point mutations in unamplified genomic DNA via Cas9 immobilized on a graphene field-effect transistor. Nat. Biomed. Eng. 2021, 5, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hu, L.; Ying, L.; Zhao, Z.; Chu, P.K.; Yu, X.F. A CRISPR-Cas9-triggered strand displacement amplification method for ultrasensitive DNA detection. Nat. Commun. 2018, 9, 5012. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Langelier, C.; Kuchta, A.; Batson, J.; Teyssier, N.; Lyden, A.; Caldera, S.; McGeever, A.; Dimitrov, B.; King, R.; et al. FLASH: A next-generation CRISPR diagnostic for multiplexed detection of antimicrobial resistance sequences. Nucleic Acids Res. 2019, 47, e83. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhou, X.; Wang, H.; Xing, D. Clustered regularly interspaced short palindromic repeats/Cas9 triggered isothermal amplification for site-specific nucleic acid detection. Anal. Chem. 2018, 90, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, Y.; Sun, H.H.; Yin, B.C.; Ye, B.C. An RNA-guided Cas9 nickase-based method for universal isothermal DNA amplification. Angew. Chem. Int. Ed. Engl. 2019, 58, 5382–5386. [Google Scholar] [CrossRef]
- Gilpatrick, T.; Lee, I.; Graham, J.E.; Raimondeau, E.; Bowen, R.; Heron, A.; Downs, B.; Sukumar, S.; Sedlazeck, F.J.; Timp, W. Targeted nanopore sequencing with Cas9-guided adapter ligation. Nat. Biotechnol. 2020, 38, 433–438. [Google Scholar] [CrossRef]
- Giesselmann, P.; Brändl, B.; Raimondeau, E.; Bowen, R.; Rohrandt, C.; Tandon, R.; Kretzmer, H.; Assum, G.; Galonska, C.; Siebert, R.; et al. Analysis of short tandem repeat expansions and their methylation state with nanopore sequencing. Nat. Biotechnol. 2019, 37, 1478–1481. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Crawford, E.D.; O’Donovan, B.D.; Wilson, M.R.; Chow, E.D.; Retallack, H.; DeRisi, J.L. Depletion of abundant sequences by hybridization (DASH): Using Cas9 to remove unwanted high-abundance species in sequencing libraries and molecular counting applications. Genome Biol. 2016, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Garibyan, L.; Avashia, N. Polymerase chain reaction. J. Investig. Dermatol. 2013, 133, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Shi, X.; Tjian, R.; Lionnet, T.; Singer, R.H. CASFISH: CRISPR/Cas9-mediated in situ labeling of genomic loci in fixed cells. Proc. Natl. Acad. Sci. USA 2015, 112, 11870–11875. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.B.; Burstein, D.; Chen, J.S.; Paez-Espino, D.; Ma, E.; Witte, I.P.; Cofsky, J.C.; Kyrpides, N.C.; Banfield, J.F.; Doudna, J.A. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science 2018, 362, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Cheng, Q.X.; Wang, J.M.; Li, X.Y.; Zhang, Z.L.; Gao, S.; Cao, R.B.; Zhao, G.P.; Wang, J. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, S.; Wu, N.; Wu, J.; Wang, G.; Zhao, G.; Wang, J. HOLMESv2: A CRISPR-Cas12b-assisted platform for nucleic acid detection and DNA methylation quantitation. ACS Synth. Biol. 2019, 8, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Guo, L.; Cui, T.T.; Wang, X.G.; Xu, K.; Gao, Q.Q.; Zhou, Q.; Li, W. CDetection: CRISPR-Cas12b-based DNA detection with sub-attomolar sensitivity and single-base specificity. Genome Biol. 2019, 20, 132. [Google Scholar] [CrossRef]
- Dai, Y.; Somoza, R.A.; Wang, L.; Welter, J.F.; Li, Y.; Caplan, A.I.; Liu, C.C. Exploring the trans-cleavage activity of CRISPR-Cas12a (cpf1) for the development of a universal electrochemical biosensor. Angew. Chem. Int. Ed. Engl. 2019, 58, 17399–17405. [Google Scholar] [CrossRef]
- English, M.A.; Soenksen, L.R.; Gayet, R.V.; de Puig, H.; Angenent-Mari, N.M.; Mao, A.S.; Nguyen, P.Q.; Collins, J.J. Programmable CRISPR-responsive smart materials. Science 2019, 365, 780–785. [Google Scholar] [CrossRef]
- Gayet, R.V.; de Puig, H.; English, M.A.; Soenksen, L.R.; Nguyen, P.Q.; Mao, A.S.; Angenent-Mari, N.M.; Collins, J.J. Creating CRISPR-responsive smart materials for diagnostics and programmable cargo release. Nat. Protoc. 2020, 15, 3030–3063. [Google Scholar] [CrossRef]
- Zhang, H.X.; Zhang, C.; Lu, S.; Tong, X.; Zhang, K.; Yin, H.; Zhang, Y. Cas12a-based one-pot SNP detection with high accuracy. Cell Insight 2023, 2, 100080. [Google Scholar] [CrossRef] [PubMed]
- East-Seletsky, A.; O’Connell, M.R.; Knight, S.C.; Burstein, D.; Cate, J.H.; Tjian, R.; Doudna, J.A. Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature 2016, 538, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Abudayyeh, O.O.; Gootenberg, J.S.; Konermann, S.; Joung, J.; Slaymaker, I.M.; Cox, D.B.T.; Shmakov, S.; Makarova, K.S.; Semenova, E.; Minakhin, L.; et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 2016, 353, aaf5573. [Google Scholar] [CrossRef]
- Kellner, M.J.; Koob, J.G.; Gootenberg, J.S.; Abudayyeh, O.O.; Zhang, F. SHERLOCK: Nucleic acid detection with CRISPR nucleases. Nat. Protoc. 2019, 14, 2986–3012. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Alariqi, M.; Wang, F.; Li, B.; Ding, X.; Rui, H.; Li, Y.; Xu, Z.; Qin, L.; Sun, L.; et al. The application of a heat-inducible CRISPR/Cas12b (C2c1) genome editing system in tetraploid cotton (G. hirsutum) plants. Plant Biotechnol. J. 2020, 18, 2436–2443. [Google Scholar] [CrossRef]
- Sharma, S.; Toppo, A.; Rath, B.; Harbhajanka, A.; Jyotsna, P.L. Hemolytic anemia as a presenting feature of Wilson’s disease: A case report. Indian J. Hematol. Blo. 2010, 26, 101–102. [Google Scholar] [CrossRef]
- Dziezyc-Jaworska, K.; Litwin, T.; Czlonkowska, A. Clinical manifestations of Wilson disease in organs other than the liver and brain. Ann. Transl. Med. 2019, 7, S62. [Google Scholar] [CrossRef] [PubMed]
- Amalnath, D.S.; Subrahmanyam, D.K. Ocular signs in Wilson disease. Ann. Indian Acad. Neurol. 2012, 15, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Grandis, D.J.; Nah, G.; Whitman, I.R.; Vittinghoff, E.; Dewland, T.A.; Olgin, J.E.; Marcus, G.M. Wilson’s disease and cardiac myopathy. Am. J. Cardiol. 2017, 120, 2056–2060. [Google Scholar] [CrossRef]
- Kapoor, N.; Shetty, S.; Thomas, N.; Paul, T.V. Wilson’s disease: An endocrine revelation. Indian J. Endocrinol. Metab. 2014, 18, 855–857. [Google Scholar] [CrossRef]
- Martinez-Morillo, E.; Bauca, J.M. Biochemical diagnosis of Wilson’s disease: An update. Adv. Lab. Med. 2022, 3, 103–125. [Google Scholar] [CrossRef] [PubMed]
- Shribman, S.; Poujois, A.; Bandmann, O.; Czlonkowska, A.; Warner, T.T. Wilson’s disease: Update on pathogenesis, biomarkers and treatments. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1053–1061. [Google Scholar] [CrossRef]
- Gromadzka, G.; Grycan, M.; Przybylkowski, A.M. Monitoring of copper in Wilson disease. Diagnostics 2023, 13, 1830. [Google Scholar] [CrossRef]
- Guillaud, O.; Brunet, A.S.; Mallet, I.; Dumortier, J.; Pelosse, M.; Heissat, S.; Rivet, C.; Lachaux, A.; Bost, M. Relative exchangeable copper: A valuable tool for the diagnosis of Wilson disease. Liver Int. 2018, 38, 350–357. [Google Scholar] [CrossRef]
- El Balkhi, S.; Trocello, J.M.; Poupon, J.; Chappuis, P.; Massicot, F.; Girardot-Tinant, N.; Woimant, F. Relative exchangeable copper: A new highly sensitive and highly specific biomarker for Wilson’s disease diagnosis. Clin. Chim. Acta 2011, 412, 2254–2260. [Google Scholar] [CrossRef]
- Nemeth, D.; Arvai, K.; Horvath, P.; Kosa, J.P.; Tobias, B.; Balla, B.; Folhoffer, A.; Krolopp, A.; Lakatos, P.A.; Szalay, F. Clinical use of next-generation sequencing in the diagnosis of Wilson’s disease. Gastroenterol. Res. Pract. 2016, 2016, 4548039. [Google Scholar] [CrossRef]
- Schwarze, K.; Buchanan, J.; Fermont, J.M.; Dreau, H.; Tilley, M.W.; Taylor, J.M.; Antoniou, P.; Knight, S.J.L.; Camps, C.; Pentony, M.M.; et al. The complete costs of genome sequencing: A microcosting study in cancer and rare diseases from a single center in the United Kingdom. Genet. Med. 2020, 22, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Maiti, S.; Chakraborty, D. Low-cost CRISPR diagnostics for resource-limited settings. Trends Genet. 2021, 37, 776–779. [Google Scholar] [CrossRef] [PubMed]
- de Puig, H.; Lee, R.A.; Najjar, D.; Tan, X.; Soeknsen, L.R.; Angenent-Mari, N.M.; Donghia, N.M.; Weckman, N.E.; Ory, A.; Ng, C.F.; et al. Minimally instrumented SHERLOCK (miSHERLOCK) for CRISPR-based point-of-care diagnosis of SARS-CoV-2 and emerging variants. Sci. Adv. 2021, 7, eabh2944. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Peng, R.; Zhang, R.; Li, J. The applications of CRISPR/Cas system in molecular detection. J. Cell. Mol. Med. 2018, 22, 5807–5815. [Google Scholar] [CrossRef] [PubMed]
- Veluchamy, A.; Teles, K.; Fischle, W. CRISPR-broad: Combined design of multi-targeting gRNAs and broad, multiplex target finding. Sci. Rep. 2023, 13, 19717. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.R.Q.; Cher, W.Y.; Wang, J.; Chen, Y.; Chae, E. Generating minimum set of gRNA to cover multiple targets in multiple genomes with MINORg. Nucleic Acids Res. 2023, 51, e43. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xiao, Q.; Yan, Q. The multiplexed CRISPR targeting platforms. Drug Discov. Today Technol. 2018, 28, 53–61. [Google Scholar] [CrossRef]
- Zetsche, B.; Heidenreich, M.; Mohanraju, P.; Fedorova, I.; Kneppers, J.; DeGennaro, E.M.; Winblad, N.; Choudhury, S.R.; Abudayyeh, O.O.; Gootenberg, J.S.; et al. Multiplex gene editing by CRISPR-Cpf1 using a single crRNA array. Nat. Biotechnol. 2017, 35, 31–34. [Google Scholar] [CrossRef]
- Cao, J.; Wu, L.; Zhang, S.M.; Lu, M.; Cheung, W.K.; Cai, W.; Gale, M.; Xu, Q.; Yan, Q. An easy and efficient inducible CRISPR/Cas9 platform with improved specificity for multiple gene targeting. Nucleic Acids Res. 2016, 44, e149. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Wang, H.; Yang, H.; Shivalila, C.S.; Dawlaty, M.M.; Cheng, A.W.; Zhang, F.; Jaenisch, R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 2013, 153, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mei, Y.; Jiang, X. Universal and high-fidelity DNA single nucleotide polymorphism detection based on a CRISPR/Cas12a biochip. Chem. Sci. 2021, 12, 4455–4462. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, C.M.; Myhrvold, C.; Thakku, S.G.; Freije, C.A.; Metsky, H.C.; Yang, D.K.; Ye, S.H.; Boehm, C.K.; Kosoko-Thoroddsen, T.F.; Kehe, J.; et al. Massively multiplexed nucleic acid detection with Cas13. Nature 2020, 582, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Barber, K.W.; Shrock, E.; Elledge, S.J. CRISPR-based peptide library display and programmable microarray self-assembly for rapid quantitative protein binding assays. Mol. Cell 2021, 81, 3650–3658.e3655. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Gillanders, L.K.; Orr, D.W.; Plank, L.D. Dietary copper restriction in Wilson’s disease. Eur. J. Clin. Nutr. 2018, 72, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Schilsky, M.L.; Roberts, E.A.; Bronstein, J.M.; Dhawan, A.; Hamilton, J.P.; Rivard, A.M.; Washington, M.K.; Weiss, K.H.; Zimbrean, P.C. A multidisciplinary approach to the diagnosis and management of Wilson disease: 2022 practice guidance on Wilson disease from the American association for the study of liver diseases. Hepatology 2022, 77, 1428–1455. [Google Scholar] [CrossRef]
- Li, W.J.; Chen, H.L.; Wang, B.; Yao, L.; Wang, X.P. Wilson’s disease: Food therapy out of trace elements. Front. Cell Dev. Biol. 2022, 10, 1091580. [Google Scholar] [CrossRef]
- Czlonkowska, A.; Gajda, J.; Rodo, M. Effects of long-term treatment in Wilson’s disease with D-penicillamine and zinc sulphate. J. Neurol. 1996, 243, 269–273. [Google Scholar] [CrossRef]
- Grasedyck, K. [D-penicillamine—Side effects, pathogenesis and decreasing the risks]. Z. Rheumatol. 1988, 47 (Suppl. S1), 17–19. [Google Scholar]
- Tang, S.; Bai, L.; Hou, W.; Hu, Z.; Chen, X.; Zhao, J.; Liang, C.; Zhang, W.; Duan, Z.; Zheng, S. Comparison of the effectiveness and safety of d-penicillamine and zinc salt treatment for symptomatic Wilson disease: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 847436. [Google Scholar] [CrossRef]
- Borthwick, T.R.; Benson, G.D.; Schugar, H.J. Copper chelating-agents—A comparison of cupruretic responses to various Tetramines and D-Penicillamine. J. Lab. Clin. Med. 1980, 95, 575–580. [Google Scholar] [PubMed]
- Schilsky, M.L.; Czlonkowska, A.; Zuin, M.; Cassiman, D.; Twardowschy, C.; Poujois, A.; Gondim, F.A.A.; Denk, G.; Cury, R.G.; Ott, P.; et al. Trientine tetrahydrochloride versus penicillamine for maintenance therapy in Wilson disease (CHELATE): A randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol. Hepatol. 2022, 7, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Askari, F.; Lorincz, M.T.; Carlson, M.; Schilsky, M.; Kluin, K.J.; Hedera, P.; Moretti, P.; Fink, J.K.; Tankanow, R.; et al. Treatment of Wilson disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double-blind study of treatment of the neurologic presentation of Wilson disease. Arch. Neurol. 2006, 63, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Kirk, F.T.; Munk, D.E.; Swenson, E.S.; Quicquaro, A.M.; Vendelbo, M.H.; Larsen, A.; Schilsky, M.L.; Ott, P.; Sandahl, T.D. Effects of tetrathiomolybdate on copper metabolism in healthy volunteers and in patients with Wilson disease. J. Hepatol. 2024, 80, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Hedera, P.; Kluin, K.J.; Carlson, M.; Askari, F.; Dick, R.B.; Sitterly, J.; Fink, J.K. Treatment of Wilson disease with ammonium tetrathiomolybdate—III. Initial therapy in a total of 55 neurologically affected patients and follow-up with zinc therapy. Arch. Neurol. 2003, 60, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F. Wilson’s disease. Handb. Clin. Neurol. 2011, 100, 681–709. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, P. Wilson disease. In Metabolic Encephalopathy; Springer: Cham, Switzerland, 2009; pp. 459–482. [Google Scholar]
- Avan, A.; Czlonkowska, A.; Gaskin, S.; Granzotto, A.; Sensi, S.L.; Hoogenraad, T.U. The role of zinc in the treatment of Wilson’s disease. Int. J. Mol. Sci. 2022, 23, 9316. [Google Scholar] [CrossRef]
- Brewer, G.J.; Hill, G.M.; Prasad, A.S.; Cossack, Z.T.; Rabbani, P. Oral zinc therapy for Wilson’s disease. Ann. Intern. Med. 1983, 99, 314–319. [Google Scholar] [CrossRef]
- Ranucci, G.; Di Dato, F.; Spagnuolo, M.I.; Vajro, P.; Iorio, R. Zinc monotherapy is effective in Wilson’s disease patients with mild liver disease diagnosed in childhood: A retrospective study. Orphanet J. Rare. Dis. 2014, 9, 41. [Google Scholar] [CrossRef]
- Lee, E.J.; Woo, M.H.; Moon, J.S.; Ko, J.S. Efficacy and safety of D-penicillamine, trientine, and zinc in pediatric Wilson disease patients. Orphanet J. Rare Dis. 2024, 19, 261. [Google Scholar] [CrossRef]
- Linn, F.H.; Houwen, R.H.; van Hattum, J.; van der Kleij, S.; van Erpecum, K.J. Long-term exclusive zinc monotherapy in symptomatic Wilson disease: Experience in 17 patients. Hepatology 2009, 50, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- European Association For The Study Of The Liver (EASL). Clinical Practice Guidelines: Wilson’s disease. J. Hepatol. 2012, 56, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhou, X.; Hou, H.; Feng, L.; Liu, J.; Liang, Y.; Lin, X.; Zhang, J.; Wu, C.; Liang, X.; et al. Clinical efficacy of combined sodium dimercaptopropanesulfonate and zinc treatment in neurological Wilson’s disease with D-penicillamine treatment failure. Ther. Adv. Neurol. Disord. 2016, 9, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luan, J.; Zhou, X.; Cui, Y.; Han, J. Epidemiology, diagnosis, and treatment of Wilson’s disease. Intract. Rare Dis. Res. 2017, 6, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, C.L.; Fu, D.L.; Lu, L.; Lin, Y.; Dong, Q.Q.; Wang, X.T.; Zheng, G.Q. Clinical efficacy and safety of Chinese herbal medicine for Wilson’s disease: A systematic review of 9 randomized controlled trials. Complement Ther. Med. 2012, 20, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, L.L.; Yang, W.M. Combined sodium Dimercaptopropanesulfonate and zinc versus D-penicillamine as first-line therapy for neurological Wilson’s disease. BMC Neurol. 2020, 20, 255. [Google Scholar] [CrossRef]
- Filippi, C.; Dhawan, A. Current status of human hepatocyte transplantation and its potential for Wilson’s disease. Ann. N. Y. Acad. Sci. 2014, 1315, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Rupp, C.; Stremmel, W.; Weiss, K.H. Novel perspectives on Wilson disease treatment. Handb. Clin. Neurol. 2017, 142, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Sauer, V.; Siaj, R.; Stoppeler, S.; Bahde, R.; Spiegel, H.U.; Kohler, G.; Zibert, A.; Schmidt, H.H. Repeated transplantation of hepatocytes prevents fulminant hepatitis in a rat model of Wilson’s disease. Liver Transpl. 2012, 18, 248–259. [Google Scholar] [CrossRef]
- Teufel-Schafer, U.; Forster, C.; Schaefer, N. Low copper diet—A therapeutic option for Wilson disease? Child 2022, 9, 1132. [Google Scholar] [CrossRef]
- Jiang, F.G.; Doudna, J.A. CRISPR-Cas9 structures and mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef] [PubMed]
- Deltcheva, E.; Chylinski, K.; Sharma, C.M.; Gonzales, K.; Chao, Y.; Pirzada, Z.A.; Eckert, M.R.; Vogel, J.; Charpentier, E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 2011, 471, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Karvelis, T.; Gasiunas, G.; Miksys, A.; Barrangou, R.; Horvath, P.; Siksnys, V. crRNA and tracrRNA guide Cas9-mediated DNA interference in Streptococcus thermophilus. RNA Biol. 2013, 10, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Bibikova, M.; Golic, M.; Golic, K.G.; Carroll, D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics 2002, 161, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Riesenberg, S.; Kanis, P.; Macak, D.; Wollny, D.; Düsterhöft, D.; Kowalewski, J.; Helmbrecht, N.; Maricic, T.; Pääbo, S. Efficient high-precision homology-directed repair-dependent genome editing by HDRobust. Nat. Methods 2023, 20, 1388–1399. [Google Scholar] [CrossRef]
- Kato-Inui, T.; Takahashi, G.; Hsu, S.; Miyaoka, Y. Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 with improved proof-reading enhances homology-directed repair. Nucleic Acids Res. 2018, 46, 4677–4688. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, S.B.; Ryu, J.L.; Hong, H.; Chang, J.H.; Yoo, T.J.; Jin, X.; Park, H.J.; Han, C.; Lee, B.H.; et al. Human Embryonic Stem Cell-Derived Wilson’s Disease Model for Screening Drug Efficacy. Cells 2020, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, Y.; Berman, J.R.; Cooper, S.B.; Mayerl, S.J.; Chan, A.H.; Zhang, B.; Karlin-Neumann, G.A.; Conklin, B.R. Systematic quantification of HDR and NHEJ reveals effects of locus, nuclease, and cell type on genome-editing. Sci. Rep. 2016, 6, 23549. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cao, J.; Chang, Q.; Xing, F.; Yan, G.; Fu, L.; Wang, H.; Ma, Z.; Chen, X.; Li, Y.; et al. In vivo exon replacement in the mouse Atp7b gene by the Cas9 system. Hum. Gene Ther. 2019, 30, 1079–1092. [Google Scholar] [CrossRef]
- Wei, R.; Yang, J.; Cheng, C.W.; Ho, W.I.; Li, N.; Hu, Y.; Hong, X.; Fu, J.; Yang, B.; Liu, Y.; et al. CRISPR-targeted genome editing of human induced pluripotent stem cell-derived hepatocytes for the treatment of Wilson’s disease. JHEP Rep. 2022, 4, 100389. [Google Scholar] [CrossRef]
- Pohler, M.; Guttmann, S.; Nadzemova, O.; Lenders, M.; Brand, E.; Zibert, A.; Schmidt, H.H.; Sandfort, V. CRISPR/Cas9-mediated correction of mutated copper transporter ATP7B. PLoS ONE 2020, 15, e0239411. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, P.; Miccio, A.; Brusson, M. Base and prime editing technologies for blood disorders. Front. Genome Ed. 2021, 3, 618406. [Google Scholar] [CrossRef] [PubMed]
- Testa, L.C.; Musunuru, K. Base editing and prime editing: Potential therapeutic options for rare and common diseases. Biodrugs 2023, 37, 453–462. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 2018, 551, 464–471, Erratum in Nature 2018, 559, E8. [Google Scholar] [CrossRef] [PubMed]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Trevino, A.E.; Zhang, F. Genome editing using Cas9 nickases. Method Enzym. 2014, 546, 161–174. [Google Scholar] [CrossRef]
- Abudayyeh, O.O.; Gootenberg, J.S.; Essletzbichler, P.; Han, S.; Joung, J.; Belanto, J.J.; Verdine, V.; Cox, D.B.T.; Kellner, M.J.; Regev, A.; et al. RNA targeting with CRISPR-Cas13. Nature 2017, 550, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.; Alshareef, S.; Mahfouz, M.M. CRISPR base editors: Genome editing without double-stranded breaks. Biochem. J. 2018, 475, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Conticello, S.G. The AID/APOBEC family of nucleic acid mutators. Genome Biol. 2008, 9, 229. [Google Scholar] [CrossRef]
- Pluciennik, A.; Dzantiev, L.; Iyer, R.R.; Constantin, N.; Kadyrov, F.A.; Modrich, P. PCNA function in the activation and strand direction of MutLalpha endonuclease in mismatch repair. Proc. Natl. Acad. Sci. USA 2010, 107, 16066–16071. [Google Scholar] [CrossRef]
- Kim, J.; Malashkevich, V.; Roday, S.; Lisbin, M.; Schramm, V.L.; Almo, S.C. Structural and kinetic characterization of Escherichia coli TadA, the wobble-specific tRNA deaminase. Biochemistry 2006, 45, 6407–6416. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.P.; Newby, G.A.; Liu, D.V.R. Precision genome editing using cytosine and adenine base editors in mammalian cells. Nat. Protoc. 2021, 16, 1089–1128, Erratum in Nat. Protoc. 2021, 16, 5740. [Google Scholar] [CrossRef] [PubMed]
- Kurt, I.C.; Zhou, R.H.; Iyer, S.; Garcia, S.P.; Miller, B.R.; Langner, L.M.; Grünewald, J.; Joung, J.K. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat. Biotechnol. 2021, 39, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Wang, X.; Liu, Y.; Liu, N.; Li, Y.; Luo, J.; Ma, Q.; Wu, D.; Li, J.; Xu, C.; et al. Programmable A-to-Y base editing by fusing an adenine base editor with an N-methylpurine DNA glycosylase. Nat. Biotechnol. 2023, 41, 1080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.D.; Li, J.; Li, S.W.; Xin, X.Q.; Hu, M.Z.; Price, M.A.; Rosser, S.J.; Bi, C.H.; Zhang, X.L. Glycosylase base editors enable C-to-A and C-to-G base changes. Nat. Biotechnol. 2021, 39, 35–40, Erratum in Nat. Biotechnol. 2021, 39, 115. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.H.; Chen, F.B.; Wang, K.P.; Lai, L.X. Base editors: Development and applications in biomedicine. Front. Med.—PRC 2023, 17, 359–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, G.; Zhou, X.; Qiao, Y.; Wang, R.; Tang, S.; Liu, J.; Wang, L.; Huang, X. Improving editing efficiency for the sequences with NGH PAM using xCas9-Derived base editors. Mol. Ther. Nucleic Acids 2019, 17, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Rees, H.A.; Liu, D.R. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018, 19, 770–788, Erratum in Nat. Rev. Genet. 2018, 19, 801. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Zong, Y.; Gao, Q.; Zhu, Z.; Wang, Y.; Qin, P.; Liang, C.; Wang, D.; Qiu, J.L.; Zhang, F.; et al. Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science 2019, 364, 292–295. [Google Scholar] [CrossRef]
- Zuo, E.W.; Sun, Y.D.; Wei, W.; Yuan, T.L.; Ying, W.Q.; Sun, H.; Yuan, L.Y.; Steinmetz, L.M.; Li, Y.X.; Yang, H. Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science 2019, 364, 289–292. [Google Scholar] [CrossRef]
- Grünewald, J.; Zhou, R.H.; Garcia, S.P.; Iyer, S.; Lareau, C.A.; Aryee, M.J.; Joung, J.K. Transcriptome-wide off-target RNA editing induced by CRISPR-guided DNA base editors. Nature 2019, 569, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.J.; Liu, D.R. Prime editing for precise and highly versatile genome manipulation. Nat. Rev. Genet. 2023, 24, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Schene, I.F.; Joore, I.P.; Oka, R.; Mokry, M.; van Vugt, A.H.M.; van Boxtel, R.; van der Doef, H.P.J.; van der Laan, L.J.W.; Verstegen, M.M.A.; van Hasselt, P.M.; et al. Prime editing for functional repair in patient-derived disease models. Nat. Commun. 2020, 11, 5352. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Miller, S.M.; Geurts, M.H.; Tang, W.X.; Chen, L.W.; Sun, N.; Zeina, C.M.; Gao, X.; Rees, H.A.; Lin, Z.; et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature 2018, 556, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kleinstiver, B.P.; Prew, M.S.; Tsai, S.Q.; Topkar, V.V.; Nguyen, N.T.; Zheng, Z.L.; Gonzales, A.P.W.; Li, Z.Y.; Peterson, R.T.; Yeh, J.R.J.; et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 2015, 523, 481-U249. [Google Scholar] [CrossRef]
- Nishimasu, H.; Shi, X.; Ishiguro, S.; Gao, L.Y.; Hirano, S.; Okazaki, S.; Noda, T.; Abudayyeh, O.O.; Gootenberg, J.S.; Mori, H.; et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science 2018, 361, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.M.; Wang, T.; Randolph, P.B.; Arbab, M.; Shen, M.W.; Huang, T.P.; Matuszek, Z.; Newby, G.A.; Rees, H.A.; Liu, D.R. Continuous evolution of SpCas9 variants compatible with non-G PAMs. Nat. Biotechnol. 2020, 38, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Walton, R.T.; Christie, K.A.; Whittaker, M.N.; Kleinstiver, B.P. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science 2020, 368, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.W.; Randolph, P.B.; Shen, S.P.; Everette, K.A.; Chen, P.J.; Anzalone, A.V.; An, M.R.; Newby, G.A.; Chen, J.C.; Hsu, A.; et al. Engineered pegRNAs improve prime editing efficiency. Nat. Biotechnol. 2022, 40, 402–410, Erratum in Nat. Biotechnol. 2022, 40, 432–432. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Huang, S.H.; Li, X.Y.; Wang, X.; Li, G.L.; Chi, T.; Chen, Y.L.; Huang, X.X.; Wang, X.L. Enhancing prime editing by Csy4-mediated processing of pegRNA. Cell Res. 2021, 31, 1134–1136. [Google Scholar] [CrossRef]
- Li, X.; Zhou, L.; Gao, B.Q.; Li, G.; Wang, X.; Wang, Y.; Wei, J.; Han, W.; Wang, Z.; Li, J.; et al. Highly efficient prime editing by introducing same-sense mutations in pegRNA or stabilizing its structure. Nat. Commun. 2022, 13, 1669. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Kuang, J.Y.; Shao, T.; Xie, S.S.; Li, M.; Zhu, L.Y.; Zhu, Y. Prime editing: An all-rounder for genome editing. Int. J. Mol. Sci. 2022, 23, 9862. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Oyler-Castrillo, P.; Ravisankar, P.; Ward, C.C.; Levesque, S.; Jing, Y.; Simpson, D.; Zhao, A.; Li, H.; Yan, W.; et al. Improving prime editing with an endogenous small RNA-binding protein. Nature 2024, 628, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Foden, J.A.; Khayter, C.; Maeder, M.L.; Reyon, D.; Joung, J.K.; Sander, J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013, 31, 822–826. [Google Scholar] [CrossRef]
- Godbout, K.; Tremblay, J.P. Prime editing for human gene therapy: Where are we now? Cells 2023, 12, 536. [Google Scholar] [CrossRef]
- Nahmad, A.D.; Reuveni, E.; Ella, G.; Asaf, M.; Ben David, U.; Barzel, A. Frequent aneuploidy in primary human T cells after CRISPR-Cas9 cleavage. Mol. Ther. 2023, 31, 402. [Google Scholar] [CrossRef]
- Leibowitz, M.L.; Papathanasiou, S.; Doerfler, P.A.; Blaine, L.J.; Sun, L.L.; Yao, Y.; Zhang, C.Z.; Weiss, M.J.; Pellman, D. Chromothripsis as an on-target consequence of CRISPR-Cas9 genome editing. Nat. Genet. 2021, 53, 895–905. [Google Scholar] [CrossRef]
- Yin, J.H.; Lu, R.S.; Xin, C.C.; Wang, Y.H.; Ling, X.Y.; Li, D.; Zhang, W.W.; Liu, M.Z.; Xie, W.T.; Kong, L.Y.; et al. Cas9 exo-endonuclease eliminates chromosomal translocations during genome editing. Nat. Commun. 2022, 13, 1204. [Google Scholar] [CrossRef]
- Li, A.; Mitsunobu, H.; Yoshioka, S.; Suzuki, T.; Kondo, A.; Nishida, K. Cytosine base editing systems with minimized off-target effect and molecular size. Nat. Commun. 2022, 13, 4531. [Google Scholar] [CrossRef]
- Liang, S.Q.; Liu, P.P.; Ponnienselvan, K.; Suresh, S.; Chen, Z.X.; Kramme, C.; Chatterjee, P.; Zhu, L.J.; Sontheimer, E.J.; Xue, W.; et al. Genome-wide profiling of prime editor off-target sites in vitro and in vivo using PE-tag. Nat. Methods 2023, 20, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Slesarenko, Y.S.; Lavrov, A.V.; Smirnikhina, S.A. Off-target effects of base editors: What we know and how we can reduce it. Curr. Genet. 2022, 68, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Mengstie, M.A.; Azezew, M.T.; Dejenie, T.A.; Teshome, A.A.; Admasu, F.T.; Teklemariam, A.B.; Mulu, A.T.; Agidew, M.M.; Adugna, D.G.; Geremew, H.; et al. Recent advancements in reducing the off-target effect of CRISPR-Cas9 genome editing. Biol.—Targets Ther. 2024, 18, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Koo, T.; Cho, C.S.; Kim, J.H.; Kim, J.S.; Kim, J.H. Long-term effects of in vivo genome editing in the mouse retina using Campylobacter jejuni Cas9 Expressed via Adeno-associated virus. Mol. Ther. 2019, 27, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.X.; Zhai, H.; Shi, Y.; Liu, G.; Lowry, J.; Liu, B.; Ryan, E.B.; Yan, J.; Yang, Y.; Zhang, N.; et al. Efficacy and long-term safety of CRISPR/Cas9 genome editing in the SOD1-linked mouse models of ALS. Commun. Biol. 2021, 4, 396. [Google Scholar] [CrossRef] [PubMed]
- Han, H.A.; Pang, J.K.S.; Soh, B.S. Mitigating off-target effects in CRISPR/Cas9-mediated in vivo gene editing. J. Mol. Med. 2020, 98, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; Gobet, N.; Cruz-Davalos, D.I.; Mounier, N.; Dessimoz, C.; Sedlazeck, F.J. Structural variant calling: The long and the short of it. Genome Biol. 2019, 20, 246. [Google Scholar] [CrossRef]
- Hamdan, A.; Ewing, A. Unravelling the tumour genome: The evolutionary and clinical impacts of structural variants in tumourigenesis. J. Pathol. 2022, 257, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.M.T.; Samson, C.A.; Rand, A.D.; Sheppard, H.M. Unintended CRISPR-Cas9 editing outcomes: A review of the detection and prevalence of structural variants generated by gene-editing in human cells. Hum. Genet. 2023, 142, 705–720. [Google Scholar] [CrossRef]
- Lee, A.B.C.; Tan, M.H.; Chai, C.L.L. Small-molecule enhancers of CRISPR-induced homology-directed repair in gene therapy: A medicinal chemist’s perspective. Drug Discov. Today 2022, 27, 2510–2525. [Google Scholar] [CrossRef]
- Chu, V.T.; Weber, T.; Wefers, B.; Wurst, W.; Sander, S.; Rajewsky, K.; Kuhn, R. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat. Biotechnol. 2015, 33, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Riesenberg, S.; Chintalapati, M.; Macak, D.; Kanis, P.; Maricic, T.; Paabo, S. Simultaneous precise editing of multiple genes in human cells. Nucleic Acids Res. 2019, 47, e116. [Google Scholar] [CrossRef] [PubMed]
- Do, T.U.; Ho, B.; Shih, S.J.; Vaughan, A. Zinc Finger Nuclease induced DNA double stranded breaks and rearrangements in MLL. Mutat. Res. 2012, 740, 34–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, W.; Yin, J.; Zhang-Ding, Z.; Xin, C.; Liu, M.; Wang, Y.; Ai, C.; Hu, J. In-depth assessment of the PAM compatibility and editing activities of Cas9 variants. Nucleic Acids Res. 2021, 49, 8785–8795. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef] [PubMed]
- Edraki, A.; Mir, A.; Ibraheim, R.; Gainetdinov, I.; Yoon, Y.; Song, C.Q.; Cao, Y.Y.; Gallant, J.; Xue, W.; Rivera-Pérez, J.A.; et al. A Compact, high-accuracy Cas9 with a dinucleotide PAM for in vivo genome editing. Mol. Cell 2019, 73, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zhang, M.; Liu, Y.; Sun, X.; Guan, Y.; Chen, X.; Yang, L.; Huo, Y.; Yang, J.; Zhang, X.; et al. Engineering of efficiency-enhanced Cas9 and base editors with improved gene therapy efficacies. Mol. Ther. 2023, 31, 744–759. [Google Scholar] [CrossRef]
- Maeder, M.L.; Linder, S.J.; Cascio, V.M.; Fu, Y.; Ho, Q.H.; Joung, J.K. CRISPR RNA-guided activation of endogenous human genes. Nat. Methods 2013, 10, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Tanenbaum, M.E.; Gilbert, L.A.; Qi, L.S.; Weissman, J.S.; Vale, R.D. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell 2014, 159, 635–646. [Google Scholar] [CrossRef]
- da Silva, J.F.; Oliveira, G.P.; Arasa-Verge, E.A.; Kagiou, C.; Moretton, A.; Timelthaler, G.; Jiricny, J.; Loizou, J.I. Prime editing efficiency and fidelity are enhanced in the absence of mismatch repair. Nat. Commun. 2022, 13, 760. [Google Scholar] [CrossRef]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.S.; Domm, J.; Eustace, B.K.; Foell, J.; de la Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 gene editing for sickle cell disease and beta-thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Baffoe-Bonnie, M.S. A justice-based argument for including sickle cell disease in CRISPR/Cas9 clinical research. Bioethics 2019, 33, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Subica, A.M. CRISPR in public health: The health equity implications and role of community in gene-editing research and applications. Am. J. Public Health 2023, 113, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Witkowsky, L.; Norstad, M.; Glynn, A.R.; Kliegman, M. Towards affordable CRISPR genomic therapies: A task force convened by the Innovative Genomics Institute. Gene Ther. 2023, 30, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Adashi, E.Y.; Gruppuso, P.A.; Cohen, I.G. CRISPR therapy of sickle cell disease: The dawning of the gene editing era. Am. J. Med. 2024, 137, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Mikhalchenko, A.; Ma, H.; Marti Gutierrez, N.; Chen, T.; Lee, Y.; Park, S.W.; Tippner-Hedges, R.; Koski, A.; Darby, H.; et al. Limitations of gene editing assessments in human preimplantation embryos. Nat. Commun. 2023, 14, 1219. [Google Scholar] [CrossRef] [PubMed]
- Alanis-Lobato, G.; Zohren, J.; McCarthy, A.; Fogarty, N.M.E.; Kubikova, N.; Hardman, E.; Greco, M.; Wells, D.; Turner, J.M.A.; Niakan, K.K. Frequent loss of heterozygosity in CRISPR-Cas9-edited early human embryos. Proc. Natl. Acad. Sci. USA 2021, 118, e2004832117. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Baylis, F.; Zhang, F.; Charpentier, E.; Berg, P.; Bourgain, C.; Friedrich, B.; Joung, J.K.; Li, J.; Liu, D.; et al. Adopt a moratorium on heritable genome editing. Nature 2019, 567, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, J.; Li, G.; Huang, S.; Yu, W.; Zhang, Y.; Chen, D.; Chen, J.; Liu, J.; Huang, X. Correction of the Marfan syndrome pathogenic FBN1 mutation by base editing in human cells and heterozygous embryos. Mol. Ther. 2018, 26, 2631–2637. [Google Scholar] [CrossRef]
- Kubikova, N.; Keefe, D.L.; Wells, D.; Oktay, K.H.; Feinberg, E.C. Should we use CRISPR gene editing in human embryos? Fertil. Steril. 2023, 120, 737–744. [Google Scholar] [CrossRef]
- Mays, L.E.; Wang, L.L.; Lin, J.P.; Bell, P.; Crawford, A.; Wherry, E.J.; Wilson, J.M. AAV8 induces tolerance in murine muscle as a result of poor APC transduction, T cell exhaustion, and minimal MHCI upregulation on target cells. Mol. Ther. 2014, 22, 28–41. [Google Scholar] [CrossRef]
- Choi, V.W.; McCarty, D.M.; Samulski, R.J. Host cell DNA repair pathways in adeno-associated viral genome processing. J. Virol. 2006, 80, 10346–10356. [Google Scholar] [CrossRef] [PubMed]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L.; Strohl, W.R. Adeno-associated virus (AAV) as a vector for gene therapy. Biodrugs. 2017, 31, 317–334. [Google Scholar] [CrossRef]
- Grieger, J.C.; Samulski, R.J. Packaging capacity of adeno-associated virus serotypes: Impact of larger genomes on infectivity and postentry steps. J. Virol. 2005, 79, 9933–9944. [Google Scholar] [CrossRef]
- Rabinowitz, J.E.; Soltys, S.; Monahan, P.E.; Samulski, R.J. Direct comparison of AAV serotypes 1-9; onset of gene expression, time to peak expression, and fate of viral genomes in mice after skeletal muscle, and tail vein injection. Circulation 2006, 114, 122. [Google Scholar]
- Su, H.; Huang, Y.; Takagawa, J.; Barcena, A.; Arakawa-Hoyt, J.; Ye, J.; Grossman, W.; Kan, Y.W. AAV serotype-1 mediates early onset of gene expression in mouse hearts and results in better therapeutic effect. Gene Ther. 2006, 13, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- McCarty, D.M. Self-complementary AAV vectors; advances and applications. Mol. Ther. 2008, 16, 1648–1656. [Google Scholar] [CrossRef]
- Wright, J.F. Transient transfection methods for clinical adeno-associated viral vector production. Hum. Gene Ther. 2009, 20, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Murillo, O.; Moreno, D.; Gazquez, C.; Barberia, M.; Cenzano, I.; Navarro, I.; Uriarte, I.; Sebastian, V.; Arruebo, M.; Ferrer, V.; et al. Liver expression of a MiniATP7B gene results in long-term restoration of copper homeostasis in a Wilson disease model in mice. Hepatology 2019, 70, 108–126. [Google Scholar] [CrossRef]
- Ponnandai Shanmugavel, K.; Petranovic, D.; Wittung-Stafshede, P. Probing functional roles of Wilson disease protein (ATP7B) copper-binding domains in yeast. Metallomics 2017, 9, 981–988. [Google Scholar] [CrossRef]
- Padula, A.; Spinelli, M.; Nusco, E.; Cundin, X.B.; Capolongo, F.; Campione, S.; Perna, C.; Bastille, A.; Ericson, M.; Wang, C.C.; et al. Genome editing without nucleases confers proliferative advantage to edited hepatocytes and corrects Wilson disease. JCI Insight 2023, 8, e171281. [Google Scholar] [CrossRef]
- Padula, A.; Petruzzelli, R.; Philbert, S.A.; Church, S.J.; Campione, S.; Monti, M.; Capolongo, F.; Perna, C.; Nusco, E.; Cooper, G.J.; et al. Full-length ATP7B reconstituted through protein trans-splicing corrects Wilson disease in mice. Hum. Gene Ther. 2022, 33, A168–A169. [Google Scholar] [CrossRef]
- Greig, J.A.; Nordin, J.M.L.; Smith, M.K.; Ashley, S.N.; Draper, C.; Zhu, Y.; Bell, P.; Buza, E.L.; Wilson, J.M. A Gene therapy approach to improve copper metabolism and prevent liver damage in a mouse model of Wilson disease. Hum. Gene Ther. Clin. Dev. 2019, 30, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Bhatt, M. Update on Wilson disease. Int. Rev. Neurobiol. 2013, 110, 313–348. [Google Scholar] [CrossRef]
- Cataldo, J.; Allen, J.; Sankoh, S.; Weiss, K.; Askari, F. eP140: A novel, double-blind placebo-controlled seamless phase 1/2/3 AAV9 gene therapy study for Wilson disease. Genet. Med. 2022, 24, S86. [Google Scholar] [CrossRef]
- Nisole, S.; Saib, A. Early steps of retrovirus replicative cycle. Retrovirology 2004, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Palu, G.; Parolin, C.; Takeuchi, Y.; Pizzato, M. Progress with retroviral gene vectors. Rev. Med. Virol. 2000, 10, 185–202. [Google Scholar] [CrossRef]
- Milone, M.C.; O’Doherty, U. Clinical use of lentiviral vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Merle, U.; Encke, J.; Tuma, S.; Volkmann, M.; Naldini, L.; Stremmel, W. Lentiviral gene transfer ameliorates disease progression in Long-Evans cinnamon rats: An animal model for Wilson disease. Scand. J. Gastroenterol. 2006, 41, 974–982. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Bachmann, M.F. Virus-like particle vaccinology, from bench to bedside. Cell Mol. Immunol. 2022, 19, 993–1011. [Google Scholar] [CrossRef]
- Dai, S.; Wang, H.; Deng, F. Advances and challenges in enveloped virus-like particle (VLP)-based vaccines. J. Immunol. Sci. 2018, 2, 1118. [Google Scholar]
- Yang, J.; Zhang, L.; Zhang, C.; Lu, Y. Exploration on the expression and assembly of virus-like particles. Biotechnol. Notes 2021, 2, 51–58. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B.; Kim, J.H.; Hur, W.; Choi, J.E.; Kim, S.M.; Park, D.J.; Kang, B.Y.; Lee, G.W.; Yoon, S.K. Liver-specific gene delivery Using engineered virus-like particles of hepatitis E virus. Sci. Rep. 2019, 9, 1616. [Google Scholar] [CrossRef] [PubMed]
- Mangeot, P.E.; Risson, V.; Fusil, F.; Marnef, A.; Laurent, E.; Blin, J.; Mournetas, V.; Massourides, E.; Sohier, T.J.M.; Corbin, A.; et al. Genome editing in primary cells and in vivo using viral-derived Nanoblades loaded with Cas9-sgRNA ribonucleoproteins. Nat. Commun. 2019, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Banskota, S.; Raguram, A.; Suh, S.; Du, S.W.; Davis, J.R.; Choi, E.H.; Wang, X.; Nielsen, S.C.; Newby, G.A.; Randolph, P.B.; et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell 2022, 185, 250–265.e216. [Google Scholar] [CrossRef] [PubMed]
- Taha, E.A.; Lee, J.; Hotta, A. Delivery of CRISPR-Cas tools for genome editing therapy: Trends and challenges. J. Control. Release 2022, 342, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Ghasemzad, M.; Hashemi, M.; Lavasani, Z.M.; Hossein-Khannazer, N.; Bakhshandeh, H.; Gramignoli, R.; Keshavarz Alikhani, H.; Najimi, M.; Nikeghbalian, S.; Vosough, M. Novel gene-correction-based therapeutic modalities for monogenic liver disorders. Bioengineering 2022, 9, 392. [Google Scholar] [CrossRef]
- Leung, A.K.; Tam, Y.Y.; Chen, S.; Hafez, I.M.; Cullis, P.R. Microfluidic mixing: A general method for encapsulating macromolecules in lipid nanoparticle systems. J. Phys. Chem. B 2015, 119, 8698–8706. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kedmi, R.; Veiga, N.; Ramishetti, S.; Goldsmith, M.; Rosenblum, D.; Dammes, N.; Hazan-Halevy, I.; Nahary, L.; Leviatan-Ben-Arye, S.; Harlev, M.; et al. A modular platform for targeted RNAi therapeutics. Nat. Nanotechnol. 2018, 13, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, P.; Yu, S.Y.; Thomson, S.B.; Birkenshaw, A.; Leavitt, B.R.; Ross, C.J.D. Lipid-nanoparticle-based delivery of CRISPR/Cas9 genome-editing components. Mol. Pharm. 2022, 19, 1669–1686. [Google Scholar] [CrossRef] [PubMed]
- Witzigmann, D.; Kulkarni, J.A.; Leung, J.; Chen, S.; Cullis, P.R.; van der Meel, R. Lipid nanoparticle technology for therapeutic gene regulation in the liver. Adv. Drug Deliv. Rev. 2020, 159, 344–363. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, H.J.; Lindsay, K.; Petersen, R.S.; Fijen, L.M.; Gurugama, P.; Maag, D.; Butler, J.S.; Shah, M.Y.; Golden, A.; Xu, Y.; et al. CRISPR-Cas9 In vivo gene editing of KLKB1 for hereditary angioedema. N. Engl. J. Med. 2024, 390, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.; Wroblewska, L.; Pegman, P.; Amiji, M. Systemic biodistribution and hepatocyte-specific gene editing with CRISPR/Cas9 using hyaluronic acid-based nanoparticles. Nanomedicine 2022, 40, 102488. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, W.S.; Seo, S.J.; Jung, C.W.; Kim, E.H. Effects of gold nanoparticles on normal hepatocytes in radiation therapy. Transl. Cancer Res. 2022, 11, 2572–2581. [Google Scholar] [CrossRef]



| Continent | Country [Reference] | Mutation Variant 1 | Allele Frequency (%) | Exon | Nucleotide Change | dbSNP 2 |
|---|---|---|---|---|---|---|
| Asia | China [33,34] | p.R778L | 34.6–55.9 | 8 | c.2333G>T | rs28942074 |
| Eastern China [35] | p.R778L | 50 | 8 | c.2333G>T | rs28942074 | |
| Southern China [36,37] | p.R778L p.I1148T | 18.93–23.29 8.74 | 8 16 | c.2333G>T c.3443T>C | rs28942074 rs60431989 | |
| India [38,39,40,41] | p.C271* p.R778W p.G1061E p.I1102T | 10–19 16 12.1 12 | 2 8 14 19 | c.813C>A c.2332C>T c.3182G>A c.3305T>C | rs572147914 rs137853284 rs764131178 rs560952220 | |
| Southern India [42] | p.C271* p.G1061E | 12 16 | 2 14 | c.813C>A c.3182G>A | rs572147914 rs764131178 | |
| Western India [43,44] | p.C271* p.E122fs | 20.2 10.6 | 2 2 | c.813C>A c.365_366 delinsGGGCTTCGAA 3 | rs572147914 - | |
| Iran [45] | p.H1069Q | 19 | 14 | c.3207C>A | rs76151636 | |
| Japan [46,47] | - p.R778L p.N958fs | 11.0 13.4–20 15.9–20 | Intron 4 8 13 | c.1708-5T>G c.2333G>T c.2871delC | rs770829226 rs28942074 rs1957668488 | |
| South Korea [48,49,50,51,52,53] | p.R778L p.A874V p.N1270S | 37.5–49.3 8.3–19.4 12.1–14.9 | 8 11 18 | c.2333G>T c.2621C>T c.3809A>G | rs28942074 rs121907994 rs121907990 | |
| Lebanon [54] | p.M769fs | 77.8 | 8 | c.2304dup | rs137853287 | |
| Saudi Arabia [55] | p.Q1399R | 32 | 21 | c.4195delC | rs886041336 | |
| Taiwan [56] | p.R778L | 43.1 | 8 | c.2333G>T | rs28942074 | |
| Vietnam [57] | p.S105* p.L1371P | 32.27 9.09 | 2 20 | c.314C>A c.4112T>C | rs753236073 rs1444841250 | |
| Europe | Austria [58] | p.H1069Q | 36 | 14 | c.3207C>A | rs76151636 |
| Bulgaria [59] | p.H1069Q | 58.75 | 14 | c.3207C>A | rs76151636 | |
| Czech and Slovakia [60] | p.H1069Q | 57 | 14 | c.3207C>A | rs76151636 | |
| Denmark [61] | p.W779X p.H1069Q | 16 18 | 8 14 | c.2336G>A or c.2337G>A c.3207C>A | rs137853282-3 rs76151636 | |
| France [18,62] | p.H1069Q p.T1434M | 14.8 24.32 | 14 21 | c.3207C>A c.4301C>T | rs76151636 rs60986317 | |
| East Germany [63] | p.H1069Q | 63 | 14 | c.3207C>A | rs76151636 | |
| Greece [64] | p.R969Q p.H1069Q | 12 35 | 13 14 | c.2906G>A c.3207C>A | rs121907996 rs76151636 | |
| Greece–Crete [65] | p.Q289* | 88.8 | 2 | c.865C>T | rs121907999 | |
| Hungary [66,67] | p.H1069Q | 46.8–64.3 | 14 | c.3207C>A | rs76151636 | |
| Continental Italy [68] | p.H1069Q | 17.5 | 14 | c.3207C>A | rs76151636 | |
| Italy–Sardinia [68,69] | - p.M822fs p.V1146M | 60.5 8.5 7.9 | 5′ UTR 10 16 | c.-441_-427del c.2463delC c.3436G>A | rs879255499 - rs1213481140 | |
| Latvia [70] | p.H1069Q | 52.5 | 14 | c.3207C>A | rs76151636 | |
| Netherland [71] | p.H1069Q | 32.9 | 14 | c.3207C>A | rs76151636 | |
| Poland [72,73] | p.H1069Q | 66–71.5 | 14 | c.3207C>A | rs76151636 | |
| Romanian [74] | p.H1069Q | 38.1 | 14 | c.3207C>A | rs76151636 | |
| Russia [75] | p.H1069Q p.A1135fs | 39 19 | 14 15 | c.3207C>A c.3402delC | rs76151636 rs137853281 | |
| Serbia [76] | p.H1069Q | 38.4 | 14 | c.3207C>A | rs76151636 | |
| Spain [77] | p.M645R | 27.5 | 6 | c.1934T>G | rs121907998 | |
| Spain–Canary Islands [78] | p.L708P | 64.6 | 8 | c.2123T>C | rs121908000 | |
| Sweden [75] | p.W779X p.H1069Q | 16 38 | 8 14 | c.2336G>A or c.2337G>A c.3207C>A | rs137853282-3 rs76151636 | |
| Turkey [68] | p.T1220M | 10 | 17 | c.3659C>T | rs193922107 | |
| United Kingdom [29] | p.M769V p.H1069Q | 6 19 | 8 14 | c.2305A>G c.3207C>A | rs193922103 rs76151636 | |
| Africa | Egypt [79] | p.C703Y p.N1270S | 6.2 7.8 | 7 18 | c.2108G>A c.3809A>G | rs767218895 rs121907990 |
| North America | North American [75] | p.H1069Q | 38 | 14 | c.3207C>A | rs76151636 |
| United States [80] | p.H1069Q | 40.3 | 14 | c.3207C>A | rs76151636 | |
| South America | Brazil [81,82,83] | p.L708P p.H1069Q p.A1135fs | 16.7 37.1 30.8–31.7 | 8 14 15 | c.2123T>C c.3207C>A c.3402delC | rs121908000 rs76151636 rs137853281 |
| Venezuela [84] | p.G691V p.H1069Q p.A1135fs | 9.6 7.7 26.9 | 7 14 15 | c.2072G>T c.3207C>A c.3402delC | rs1555291801 rs76151636 rs137853281 |
| Class | Type | Subtype | Cleavage Domain | Substrate | Typical PAM/PFS 1 |
|---|---|---|---|---|---|
| 1 | I | A, B, C, D, E, F, U | HD domain of Cas3 or Cas3” | dsDNA | 3′-nt |
| III | A, B, C, D | HD domain of Cas10, different from type I | ssRNA | Without PAM | |
| IV | Variants 1 and 2 | Functionally uncharacterized | dsDNA | 3′-DWN | |
| 2 | II | A, B, C | RuvC, HNH | dsDNA | 5′-NGG |
| V | A, B, C | RuvC, NUC | dsDNA | 5′-TTTN | |
| VI | A, B, C, D | HEPN | ssRNA | A given single base or without PFS |
| Treatments | Mechanism of Action | Advantages | Disadvantages |
|---|---|---|---|
| D-penicillamine (DPA) [169,170,171] | A copper chelator inducing cupruria. Upregulation of metallothionein. | The drug of choice for WD. Effectively alleviates hepatic symptoms. | Slow mitigation of neurologic symptoms can cause initial deterioration. |
| Trientine [172,173,174,175] | A copper chelator that induces fecal secretion and cupruria. | Alternative for DPA-intolerant patients. Used both for reversing symptoms or as a maintenance drug after withdrawal of DPA. Milder adverse side effects with lower incidence than DPA, including headache, arthralgias, myalgias, and abdominal pain. | Humidity control is required to store Trientine dihydrochloride salt (TETA 2HCL). The possibility of neurological worsening due to the rapid mobilization of large amounts of copper. |
| Bis-choline tetrathiomolybdate [174,175,176,177,178] | A decoppering agent inhibiting gastrointestinal absorption. Forms a complex with plasma copper and prevents intracellular uptake. | Prompt symptom reversal and alleviation. Useful for initial treatment of patients with neurologic symptoms Received orphan designation in the US and EU as a potential therapy for WD. | Infrequent but serious major adverse events, such as bone marrow suppression, aminotransferase elevation, and neurologic deterioration. There are currently no efforts to make the product commercially available. |
| Zinc [179,180,181,182,183] | Upregulates metallothionein in gastrointestinal epithelial cells, resulting in inhibition of gastrointestinal absorption and increase through fecal secretion. | Low cost in comparison to other treatments. In pediatric WD patients, zinc shows more efficient therapeutical effects than DPA and trientine. | There is a possibility of liver deterioration. Negative copper balance may be caused by inhibition of resorption of copper secreted in saliva, gastric juice, and intestinal secretions Exhibits mostly mild adverse events, but gastric irritation may be common (33.3%) |
| Sodium dimercaptopropane sulfate (DMPS) and Dimercaptosuccinic acid (DMSA) [184,185,186,187,188] | Forms complexes with heavy metals. Eliminates renal copper. | Decent neurologic symptom reversal in combination with zinc therapy. Relatively safe. | Should be aware of renal and dermatologic deterioration. Amount and quality of studies are limited. |
| Liver transplantation [189,190,191] | Replaces decompensated hepatic tissue. | Emergency treatment for acute liver failure. | Low engraftment efficiency in chronic liver disease. Requires lifelong immunosuppression. Not permanently effective. |
| The Available CRIPSR-Based Approaches | Advantages | Disadvantages | Current CRISPR-Based Trials for WD |
|---|---|---|---|
| Homology-directed repair (HDR) with Cas9 |
|
| [198,199,200,201,202,203] |
| Base editing (BE) |
|
| [219] |
| Prime editing (PE) |
|
| [226] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.; Cha, S.; Kim, K. Navigating the CRISPR/Cas Landscape for Enhanced Diagnosis and Treatment of Wilson’s Disease. Cells 2024, 13, 1214. https://doi.org/10.3390/cells13141214
Choi W, Cha S, Kim K. Navigating the CRISPR/Cas Landscape for Enhanced Diagnosis and Treatment of Wilson’s Disease. Cells. 2024; 13(14):1214. https://doi.org/10.3390/cells13141214
Chicago/Turabian StyleChoi, Woong, Seongkwang Cha, and Kyoungmi Kim. 2024. "Navigating the CRISPR/Cas Landscape for Enhanced Diagnosis and Treatment of Wilson’s Disease" Cells 13, no. 14: 1214. https://doi.org/10.3390/cells13141214
APA StyleChoi, W., Cha, S., & Kim, K. (2024). Navigating the CRISPR/Cas Landscape for Enhanced Diagnosis and Treatment of Wilson’s Disease. Cells, 13(14), 1214. https://doi.org/10.3390/cells13141214







