Widespread Distribution of Luteinizing Hormone/Choriogonadotropin Receptor in Human Juvenile Angiofibroma: Implications for a Sex-Specific Nasal Tumor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tumor Specimens
2.2. RNAscope Fluorescence In Situ Hybridization
2.3. Quantification of RNAscope Data
2.4. Immunohistochemistry
2.5. Quantification of Immunohistochemistry Data
2.6. Statistics
3. Results
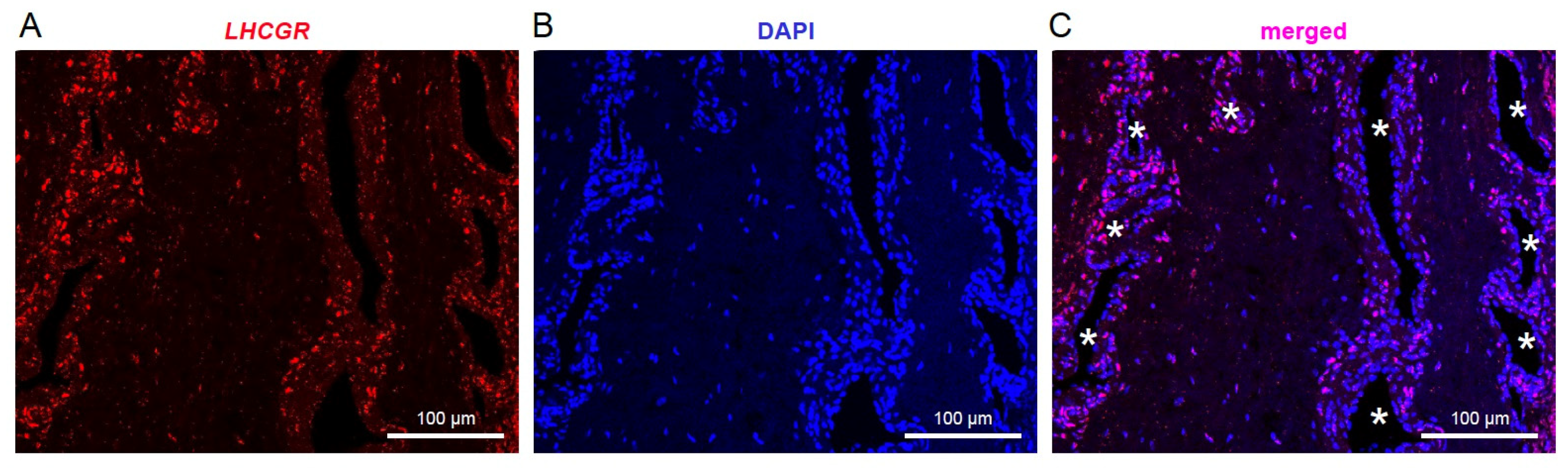
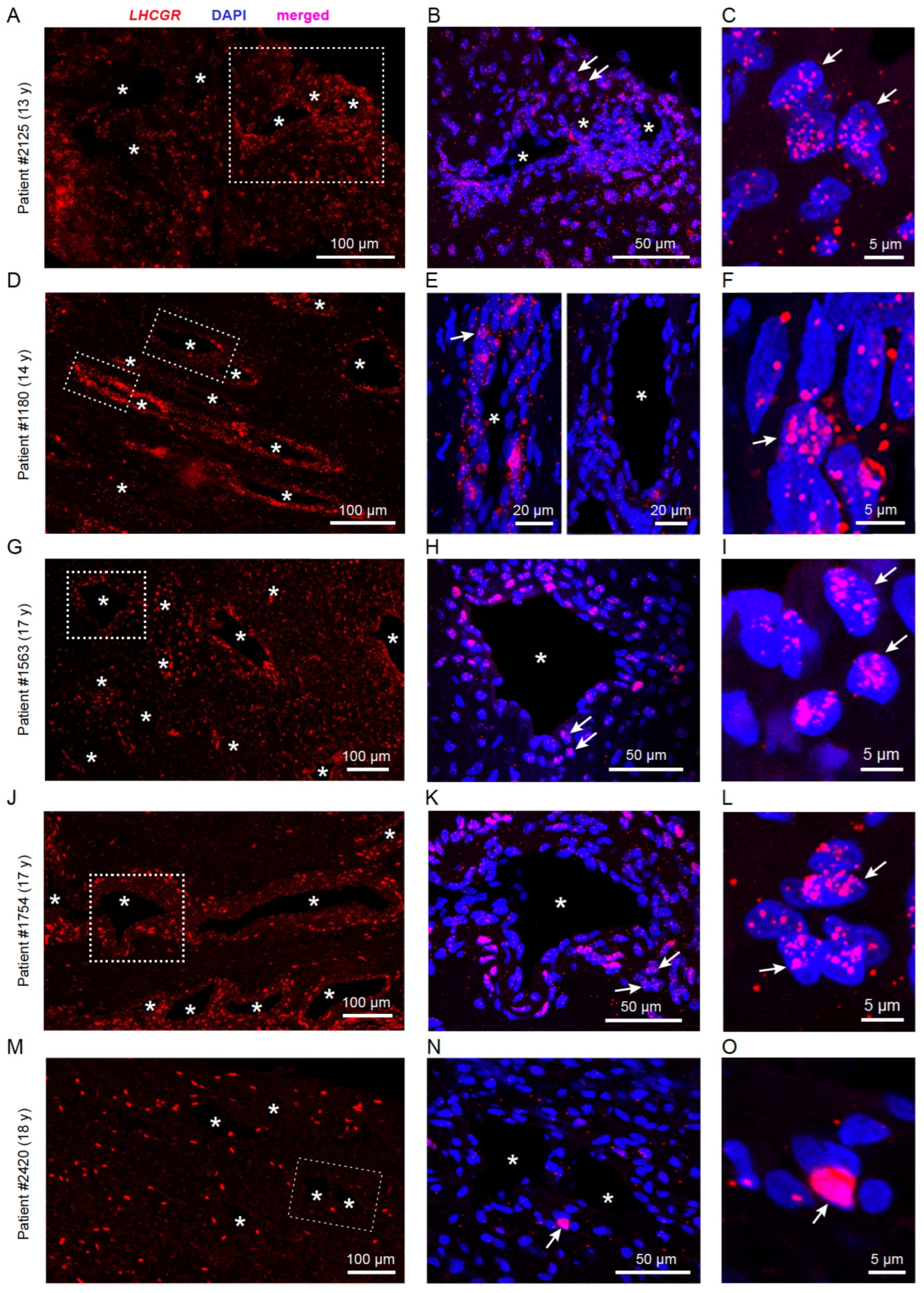
3.1. LHCGR RNA Exists in Individual Cells from JA Tissue
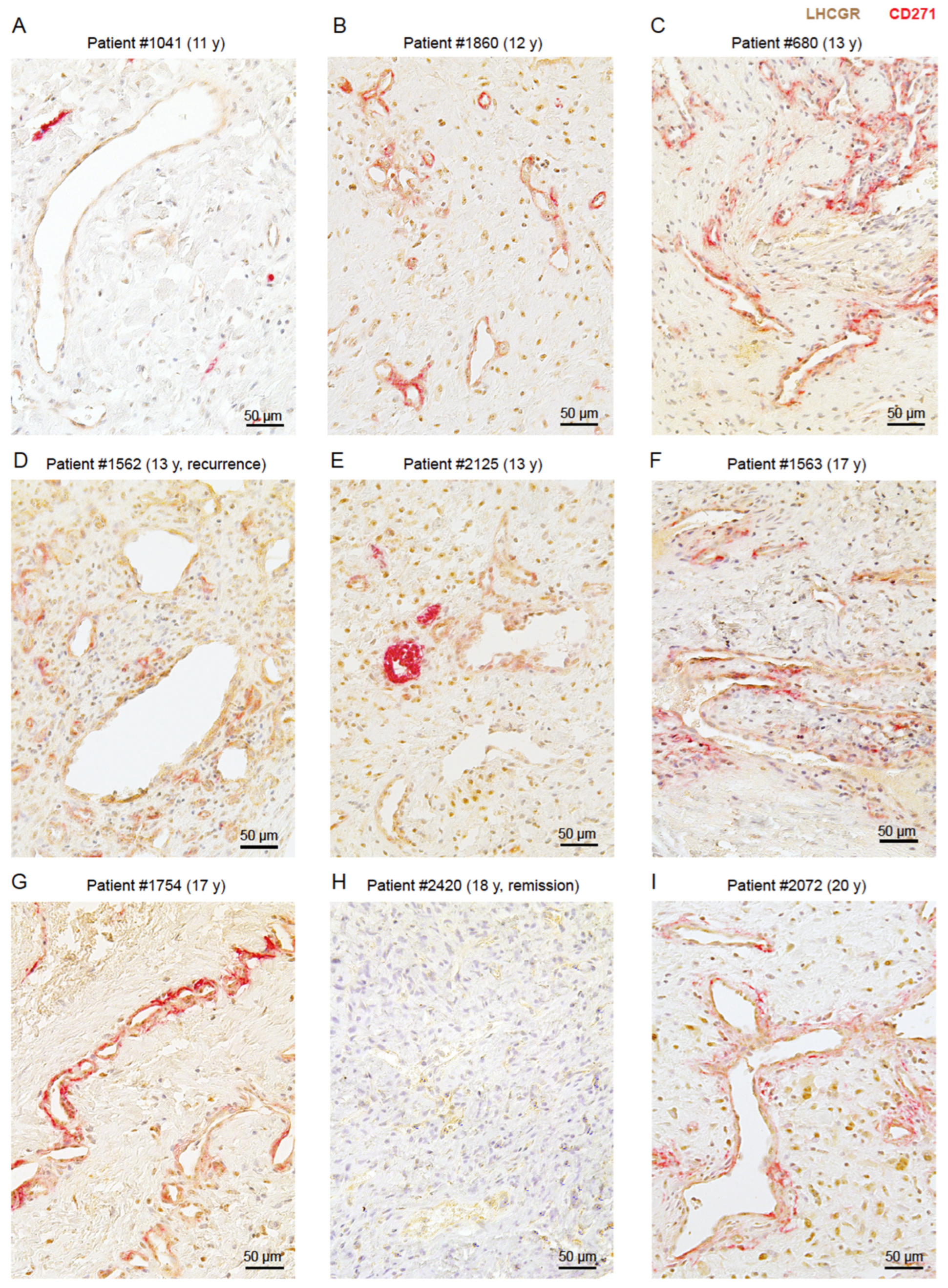
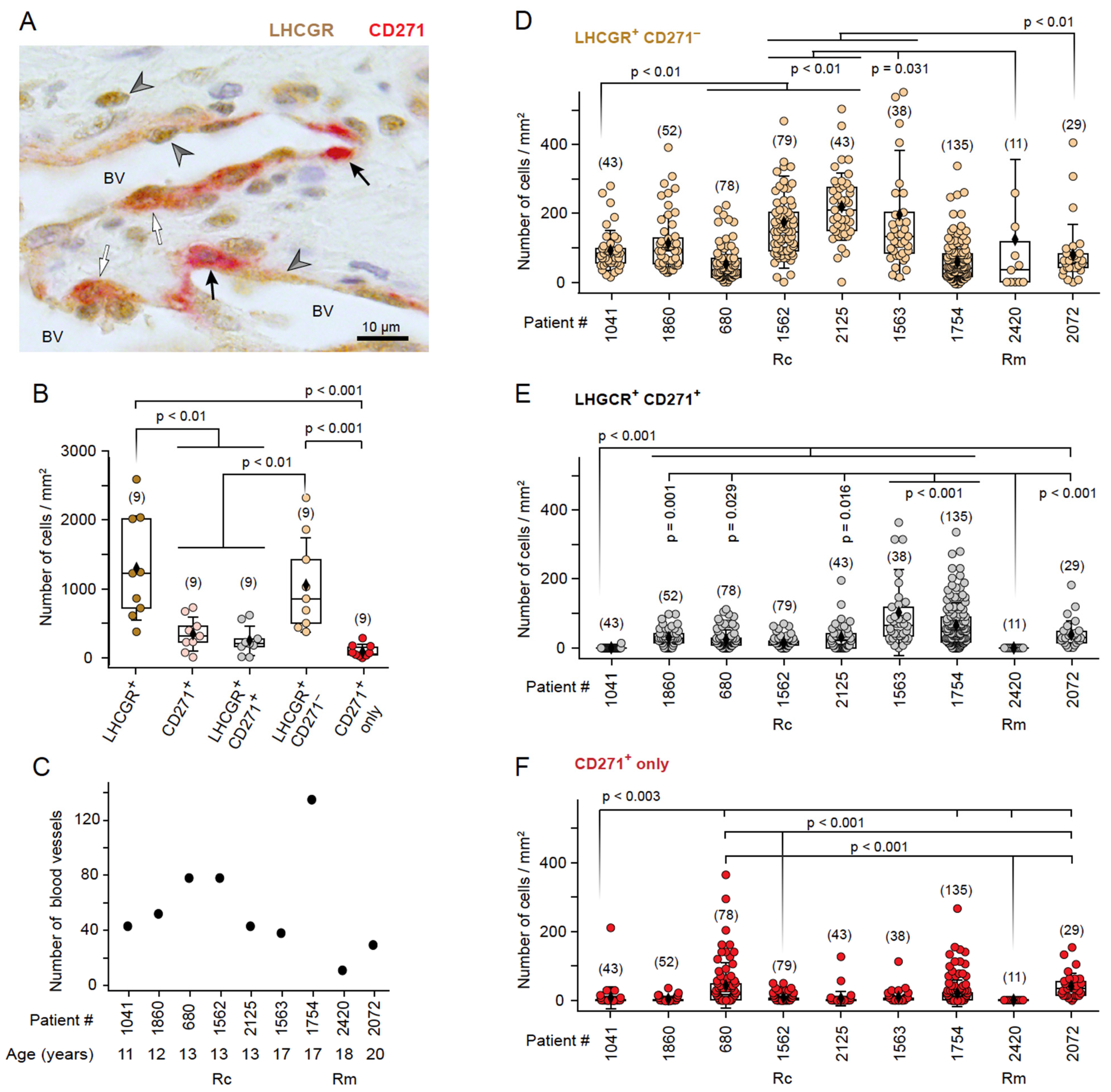
3.2. LHCGR Protein Is Mainly Located near the Vasculature
3.3. The Presence of LHCGR in CD271+ Cells Is Indicative of a Fully Mature JA
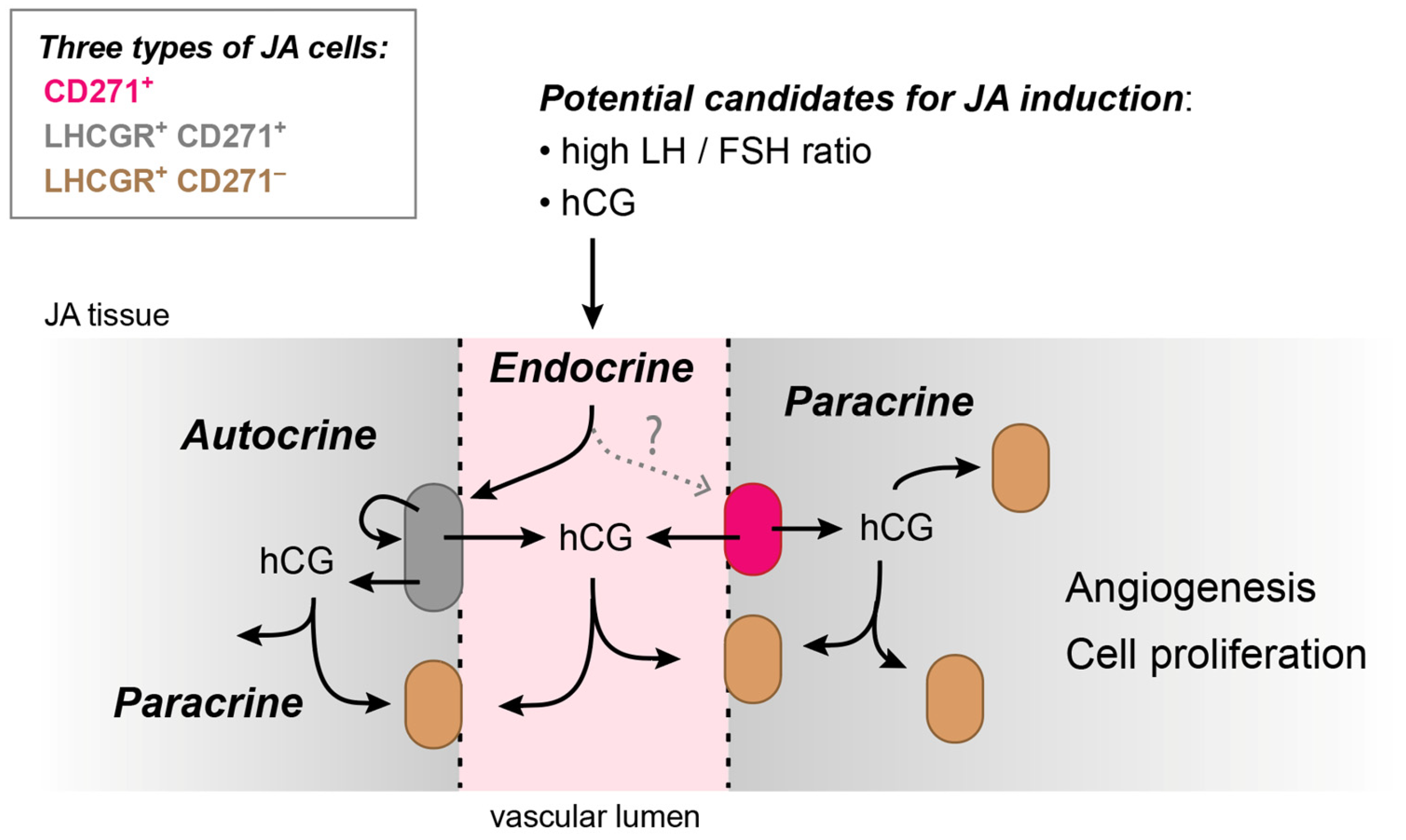
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, M.; Nguyen, T.B.V.; McHugh, T.; Reddy, K.; Sommer, D.D. Early-Onset Juvenile Nasopharyngeal Angiofibroma (Jna): A Systematic Review. J. Otolaryngol. Head Neck Surg. 2023, 52, 85. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Y.; Lai, C.; Wu, L.; Fu, Y. Extranasopharyngeal Angiofibroma in Children: A Case Report. World J. Clin. Cases 2022, 10, 7429–7437. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.F.; Wang, D.H.; Sun, X.C.; Wang, J.J.; Hu, L.; Li, H.; Dai, P.D. The Site of Origin and Expansive Routes of Juvenile Nasopharyngeal Angiofibroma (Jna). Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Sennes, L.U.; Butugan, O.; Sanchez, T.G.; Bento, R.F.; Tsuji, D.H. Juvenile Nasopharyngeal Angiofibroma: The Routes of Invasion. Rhinology 2003, 41, 235–240. [Google Scholar]
- Trivedi, M.; Desai, R.J.; Potdar, N.A.; Shinde, C.A.; Ukirde, V.; Bhuta, M.; Nair, A.G. Vision Loss Due to Central Retinal Artery Occlusion Following Embolization in a Case of a Giant Juvenile Nasopharyngeal Angiofibroma. J. Craniofac. Surg. 2015, 26, e451–e453. [Google Scholar] [CrossRef] [PubMed]
- Schick, B.; Pillong, L.; Wenzel, G.; Wemmert, S. Neural Crest Stem Cells in Juvenile Angiofibromas. Int. J. Mol. Sci. 2022, 23, 1932. [Google Scholar] [CrossRef]
- Lee, D.A.; Rao, B.R.; Meyer, J.S.; Prioleau, P.G.; Bauer, W.C. Hormonal Receptor Determination in Juvenile Nasopharyngeal Angiofibromas. Cancer 1980, 46, 547–551. [Google Scholar] [CrossRef]
- Weprin, L.S.; Siemers, P.T. Spontaneous Regression of Juvenile Nasopharyngeal Angiofibroma. Arch. Otolaryngol. Head Neck Surg. 1991, 117, 796–799. [Google Scholar] [CrossRef]
- Pandey, P.; Mishra, A.; Tripathi, A.M.; Verma, V.; Trivedi, R.; Singh, H.P.; Kumar, S.; Patel, B.; Singh, V.; Pandey, S.; et al. Current Molecular Profile of Juvenile Nasopharyngeal Angiofibroma: First Comprehensive Study from India. Laryngoscope 2017, 127, E100–E106. [Google Scholar] [CrossRef]
- Schick, B.; Dlugaiczyk, J.; Wendler, O. Expression of Sex Hormone Receptors in Juvenile Angiofibromas and Antiproliferative Effects of Receptor Modulators. Head Neck 2014, 36, 1596–1603. [Google Scholar] [CrossRef]
- Schick, B.; Rippel, C.; Brunner, C.; Jung, V.; Plinkert, P.K.; Urbschat, S. Numerical Sex Chromosome Aberrations in Juvenile Angiofibromas: Genetic Evidence for an Androgen-Dependent Tumor? Oncol. Rep. 2003, 10, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Troppmann, B.; Kleinau, G.; Krause, G.; Gromoll, J. Structural and Functional Plasticity of the Luteinizing Hormone/Choriogonadotrophin Receptor. Hum. Reprod. Update 2013, 19, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Casarini, L.; Santi, D.; Brigante, G.; Simoni, M. Two Hormones for One Receptor: Evolution, Biochemistry, Actions, and Pathophysiology of Lh and Hcg. Endocr. Rev. 2018, 39, 549–592. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, C.; Cappa, M. Ontogeny of Hypothalamus-Pituitary Gonadal Axis and Minipuberty: An Ongoing Debate? Front. Endocrinol. 2020, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Renault, C.H.; Aksglaede, L.; Wøjdemann, D.; Hansen, A.B.; Jensen, R.B.; Juul, A. Minipuberty of Human Infancy—A Window of Opportunity to Evaluate Hypogonadism and Differences of Sex Development? Ann. Pediatr. Endocrinol. Metab. 2020, 25, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Hesse, V. Minipuberty: Why Does It Happen? Horm. Res. Paediatr. 2020, 93, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, L.; Alfthan, H.; Stenman, U.H.; Selstam, G.; Rosberg, S.; Albertsson-Wikland, K. Developmental Changes in 24-Hour Profiles of Luteinizing Hormone and Follicle-Stimulating Hormone from Prepuberty to Midstages of Puberty in Boys. J. Clin. Endocrinol. Metab. 1992, 74, 890–897. [Google Scholar] [CrossRef]
- Dunkel, L.; Alfthan, H.; Stenman, U.H.; Tapanainen, P.; Perheentupa, J. Pulsatile Secretion of Lh and Fsh in Prepubertal and Early Pubertal Boys Revealed by Ultrasensitive Time-Resolved Immunofluorometric Assays. Pediatr. Res. 1990, 27, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, T.H.; Main, K.M.; Ljubicic, M.L.; Jensen, T.K.; Andersen, H.R.; Andersen, M.S.; Petersen, J.H.; Andersson, A.M.; Juul, A. Sex Differences in Reproductive Hormones during Mini-Puberty in Infants with Normal and Disordered Sex Development. J. Clin. Endocrinol. Metab. 2018, 103, 3028–3037. [Google Scholar] [CrossRef]
- Io, S.; Kabata, M.; Iemura, Y.; Semi, K.; Morone, N.; Minagawa, A.; Wang, B.; Okamoto, I.; Nakamura, T.; Kojima, Y.; et al. Capturing Human Trophoblast Development with Naive Pluripotent Stem Cells in Vitro. Cell Stem Cell 2021, 28, 1023–1039.e13. [Google Scholar] [CrossRef]
- Cismaru, A.C.; Soritau, O.; Jurj, A.M.; Raduly, L.-Z.; Pop, B.; Bocean, C.; Miclea, D.; Baldasici, O.; Moldovan, C.; Urian, L.; et al. Human Chorionic Gonadotropin Improves the Proliferation and Regenerative Potential of Bone Marrow Adherent Stem Cells and the Immune Tolerance of Fetal Microchimeric Stem Cells In Vitro. Stem Cell Rev. Rep. 2020, 16, 524–540. [Google Scholar] [CrossRef]
- Lenhard, M.; Tsvilina, A.; Schumacher, L.; Kupka, M.; Ditsch, N.; Mayr, D.; Friese, K.; Jeschke, U. Human Chorionic Gonadotropin and Its Relation to Grade, Stage and Patient Survival in Ovarian Cancer. BMC Cancer 2012, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Sisinni, L.; Landriscina, M. The Role of Human Chorionic Gonadotropin as Tumor Marker: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Triozzi, P.L.; Stevens, V.C. Human Chorionic Gonadotropin as a Target for Cancer Vaccines. Oncol. Rep. 1999, 6, 7–17. [Google Scholar] [CrossRef]
- Cole, L.A. Hcg, Five Independent Molecules. Clin. Chim. Acta 2012, 413, 48–65. [Google Scholar] [CrossRef]
- Fournier, T. Human Chorionic Gonadotropin: Different Glycoforms and Biological Activity Depending on Its Source of Production. Ann. Endocrinol. 2016, 77, 75–81. [Google Scholar] [CrossRef]
- Cole, L.A. Hcg, the Wonder of Today’s Science. Reprod. Biol. Endocrinol. 2012, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Nwabuobi, C.; Arlier, S.; Schatz, F.; Guzeloglu-Kayisli, O.; Lockwood, C.J.; Kayisli, U.A. Hcg: Biological Functions and Clinical Applications. Int. J. Mol. Sci. 2017, 18, 2037. [Google Scholar] [CrossRef]
- Perrier d’Hauterive, S.; Close, R.; Gridelet, V.; Mawet, M.; Nisolle, M.; Geenen, V. Human Chorionic Gonadotropin and Early Embryogenesis: Review. Int. J. Mol. Sci. 2022, 23, 1380. [Google Scholar] [CrossRef]
- Shi, Q.J.; Lei, Z.M.; Rao, C.V.; Lin, J. Novel Role of Human Chorionic Gonadotropin in Differentiation of Human Cytotrophoblasts. Endocrinology 1993, 132, 1387–1395. [Google Scholar] [CrossRef]
- Koistinen, H.; Koel, M.; Peters, M.; Rinken, A.; Lundin, K.; Tuuri, T.; Tapanainen, J.S.; Alfthan, H.; Salumets, A.; Stenman, U.H.; et al. Hyperglycosylated Hcg Activates Lh/Hcg-Receptor with Lower Activity Than Hcg. Mol. Cell. Endocrinol. 2019, 479, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.A. Biological Functions of Hcg and Hcg-Related Molecules. Reprod. Biol. Endocrinol. 2010, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Tsampalas, M.; Gridelet, V.; Berndt, S.; Foidart, J.M.; Geenen, V.; Perrier d’Hauterive, S. Human Chorionic Gonadotropin: A Hormone with Immunological and Angiogenic Properties. J. Reprod. Immunol. 2010, 85, 93–98. [Google Scholar] [CrossRef]
- Zygmunt, M.; Herr, F.; Keller-Schoenwetter, S.; Kunzi-Rapp, K.; Munstedt, K.; Rao, C.V.; Lang, U.; Preissner, K.T. Characterization of Human Chorionic Gonadotropin as a Novel Angiogenic Factor. J. Clin. Endocrinol. Metab. 2002, 87, 5290–5296. [Google Scholar] [CrossRef] [PubMed]
- Hota, A.; Sarkar, C.; Gupta, S.D.; Kumar, R.; Bhalla, A.S.; Thakar, A. Expression of Vascular Endothelial Growth Factor in Juvenile Angiofibroma. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 900–902. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Wang, H.; Wang, D.; Hu, L.; Liu, Q.; Sun, X. Hormonal Receptors and Vascular Endothelial Growth Factor in Juvenile Nasopharyngeal Angiofibroma: Immunohistochemical and Tissue Microarray Analysis. Acta Otolaryngol. 2015, 135, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Brouillet, S.; Hoffmann, P.; Chauvet, S.; Salomon, A.; Chamboredon, S.; Sergent, F.; Benharouga, M.; Feige, J.J.; Alfaidy, N. Revisiting the Role of Hcg: New Regulation of the Angiogenic Factor Eg-Vegf and Its Receptors. Cell. Mol. Life Sci. 2012, 69, 1537–1550. [Google Scholar] [CrossRef] [PubMed]
- Jing, G.; Yao, J.; Dang, Y.; Liang, W.; Xie, L.; Chen, J.; Li, Z. The Role of Beta-Hcg and Vegf-Mek/Erk Signaling Pathway in Villi Angiogenesis in Patients with Missed Abortion. Placenta 2021, 103, 16–23. [Google Scholar] [CrossRef]
- Blum, T.; Moreno-Pérez, A.; Pyrski, M.; Bufe, B.; Arifovic, A.; Weissgerber, P.; Freichel, M.; Zufall, F.; Leinders-Zufall, T. Trpc5 Deficiency Causes Hypoprolactinemia and Altered Function of Oscillatory Dopamine Neurons in the Arcuate Nucleus. Proc. Natl. Acad. Sci. USA 2019, 116, 15236–15243. [Google Scholar] [CrossRef]
- Bufe, B.; Teuchert, Y.; Schmid, A.; Pyrski, M.; Pérez-Gómez, A.; Eisenbeis, J.; Timm, T.; Ishii, T.; Lochnit, G.; Bischoff, M.; et al. Bacterial Mgrb Peptide Activates Chemoreceptor Fpr3 in Mouse Accessory Olfactory System and Drives Avoidance Behaviour. Nat. Commun. 2019, 10, 4889. [Google Scholar] [CrossRef]
- Pyrski, M.; Tusty, M.; Eckstein, E.; Oboti, L.; Rodriguez-Gil, D.J.; Greer, C.A.; Zufall, F. P/Q Type Calcium Channel Cav2.1 Defines a Unique Subset of Glomeruli in the Mouse Olfactory Bulb. Front. Cell. Neurosci. 2018, 12, 295. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.T.; Ma, X.J.; Luo, Y. Rnascope: A Novel in Situ Rna Analysis Platform for Formalin-Fixed, Paraffin-Embedded Tissues. J. Mol. Diagn. 2012, 14, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Yoo, S.J.; Clijsters, M.; Backaert, W.; Vanstapel, A.; Speleman, K.; Lietaer, C.; Choi, S.; Hether, T.D.; Marcelis, L.; et al. Visualizing in Deceased COVID-19 Patients How Sars-Cov-2 Attacks the Respiratory and Olfactory Mucosae but Spares the Olfactory Bulb. Cell 2021, 184, 5932–5949.e15. [Google Scholar] [CrossRef]
- Beham, A.; Beham-Schmid, C.; Regauer, S.; Auböck, L.; Stammberger, H. Nasopharyngeal Angiofibroma: True Neoplasm or Vascular Malformation? Adv. Anat. Pathol. 2000, 7, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, A.; Trainor, P.A. Neural Crest Stem Cells: Discovery, Properties and Potential for Therapy. Cell Res. 2012, 22, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Bautch, V.L. Stem Cells and the Vasculature. Nat. Med. 2011, 17, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.; Pearson, A.C.; Sage, M.A.G.; Duffy, D.M. Luteinizing Hormone Receptor Promotes Angiogenesis in Ovarian Endothelial Cells of Macaca fascicularis and Homo sapiens. Biol. Reprod. 2023, 108, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, M.; Nielsen, J.E.; Andreassen, C.H.; Juul, A.; Toft, B.G.; Rajpert-De Meyts, E.; Daugaard, G.; Blomberg Jensen, M. Luteinizing Hormone Receptor Is Expressed in Testicular Germ Cell Tumors: Possible Implications for Tumor Growth and Prognosis. Cancers 2020, 12, 1358. [Google Scholar] [CrossRef] [PubMed]
- Kölbl, A.C.; Birk, A.E.; Kuhn, C.; Jeschke, U.; Andergassen, U. Influence of Vegfr and Lhcgr on Endometrial Adenocarcinoma. Oncol. Lett. 2016, 12, 2092–2098. [Google Scholar] [CrossRef]
- Howard, S.R. Interpretation of Reproductive Hormones before, During and after the Pubertal Transition-Identifying Health and Disordered Puberty. Clin. Endocrinol. 2021, 95, 702–715. [Google Scholar] [CrossRef]
- Pratama, G.; Wiweko, B.; Asmarinah; Widyahening, I.S.; Andraini, T.; Bayuaji, H.; Hestiantoro, A. Mechanism of Elevated Lh/Fsh Ratio in Lean Pcos Revisited: A Path Analysis. Sci. Rep. 2024, 14, 8229. [Google Scholar] [CrossRef] [PubMed]
- Sołek, J.; Kalwas, M.; Sobczak, M.; Dębska-Szmich, S.; Kupnicki, P.; Jesionek-Kupnicka, D. Urothelial Carcinoma of the Prostate with Raised Β-Hcg Levels: A Case Report. J. Med. Case Rep. 2022, 16, 238. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wemmert, S.; Pyrski, M.; Pillong, L.; Linxweiler, M.; Zufall, F.; Leinders-Zufall, T.; Schick, B. Widespread Distribution of Luteinizing Hormone/Choriogonadotropin Receptor in Human Juvenile Angiofibroma: Implications for a Sex-Specific Nasal Tumor. Cells 2024, 13, 1217. https://doi.org/10.3390/cells13141217
Wemmert S, Pyrski M, Pillong L, Linxweiler M, Zufall F, Leinders-Zufall T, Schick B. Widespread Distribution of Luteinizing Hormone/Choriogonadotropin Receptor in Human Juvenile Angiofibroma: Implications for a Sex-Specific Nasal Tumor. Cells. 2024; 13(14):1217. https://doi.org/10.3390/cells13141217
Chicago/Turabian StyleWemmert, Silke, Martina Pyrski, Lukas Pillong, Maximilian Linxweiler, Frank Zufall, Trese Leinders-Zufall, and Bernhard Schick. 2024. "Widespread Distribution of Luteinizing Hormone/Choriogonadotropin Receptor in Human Juvenile Angiofibroma: Implications for a Sex-Specific Nasal Tumor" Cells 13, no. 14: 1217. https://doi.org/10.3390/cells13141217





