Controlling the Expression Level of the Neuronal Reprogramming Factors for a Successful Reprogramming Outcome
Abstract
:1. Introduction
2. Reprogramming Factors during Development
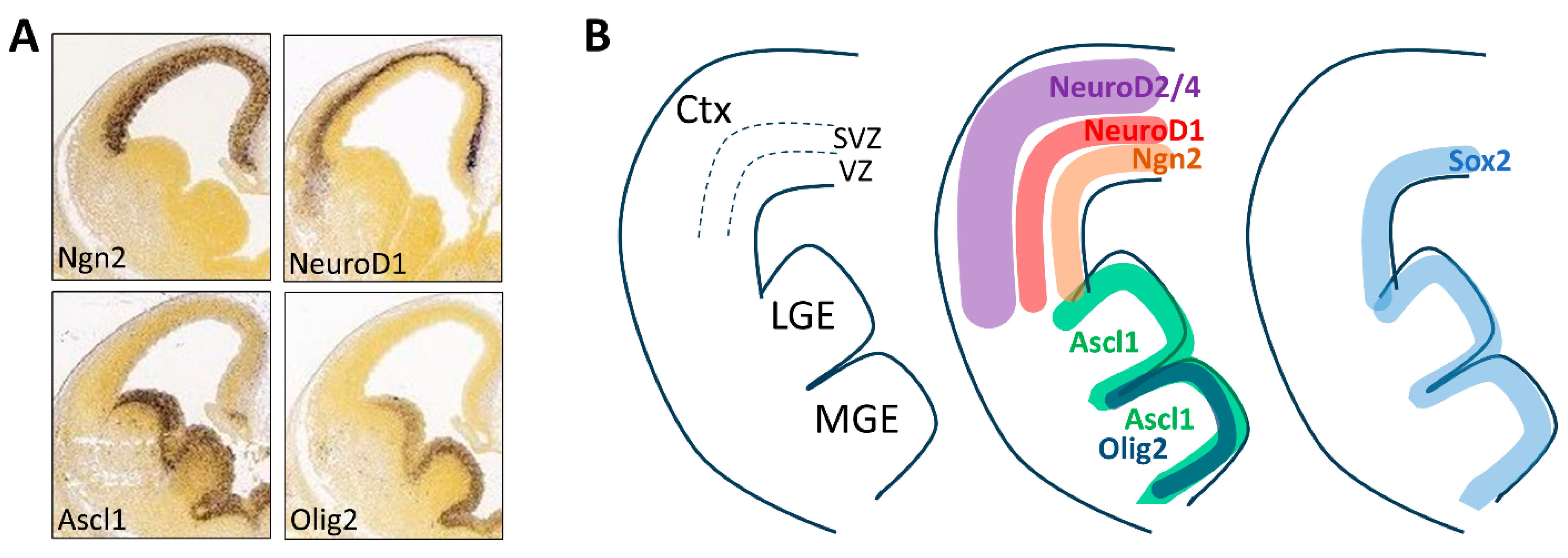
2.1. Expression Pattern and Function
2.2. Expression Regulation
3. Reprogramming Factors during Reprogramming
3.1. Mechanisms of Action
3.2. Expression Level
3.3. Expression Duration
4. Potential Strategies to Control Expression of the Reprogramming Factors
4.1. Cell-Type-Specific Promoters
4.2. Drug-Controllable Promoters
4.3. MiRNA-Mediated Inhibition
5. Additional Notes
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Akamatsu, W.; Nakamura, M.; Okano, H. Regeneration of the damaged central nervous system through reprogramming technology: Basic concepts and potential application for cell replacement therapy. Exp. Neurol. 2014, 260, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, N.; Desplan, C. Neuronal differentiation strategies: Insights from single-cell sequencing and machine learning. Development 2020, 147, dev193631. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pang, Z.P.; Yang, N.; Vierbuchen, T.; Ostermeier, A.; Fuentes, D.R.; Yang, T.Q.; Citri, A.; Sebastiano, V.; Marro, S.; Südhof, T.C.; et al. Induction of human neuronal cells by defined transcription factors. Nature 2011, 476, 220–223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Südhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Yoo, A.S.; Sun, A.X.; Li, L.; Shcheglovitov, A.; Portmann, T.; Li, Y.; Lee-Messer, C.; Dolmetsch, R.E.; Tsien, R.W.; Crabtree, G.R. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 2011, 476, 228–231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Zuo, X.; Jing, J.; Ma, Y.; Wang, J.; Liu, D.; Zhu, J.; Du, X.; Xiong, L.; Du, Y.; et al. Small-Molecule-Driven Direct Reprogramming of Mouse Fibroblasts into Functional Neurons. Cell Stem Cell 2015, 17, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, G. In Vivo Reprogramming for CNS Repair: Regenerating Neurons from Endogenous Glial Cells. Neuron 2016, 91, 728–738. [Google Scholar] [CrossRef]
- Gascón, S.; Masserdotti, G.; Russo, G.L.; Götz, M. Direct Neuronal Reprogramming: Achievements, Hurdles, and New Roads to Success. Cell Stem Cell 2017, 21, 18–34. [Google Scholar] [CrossRef]
- Turinetto, V.; Orlando, L.; Giachino, C. Induced Pluripotent Stem Cells: Advances in the Quest for Genetic Stability during Reprogramming Process. Int. J. Mol. Sci. 2017, 18, 1952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lund, R.J.; Närvä, E.; Lahesmaa, R. Genetic and epigenetic stability of human pluripotent stem cells. Nat. Rev. Genet. 2012, 13, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H. Neuronal reprogramming in treating spinal cord injury. Neural Regen. Res. 2022, 17, 1440–1445. [Google Scholar] [PubMed] [PubMed Central]
- Tai, W.; Wu, W.; Wang, L.L.; Ni, H.; Chen, C.; Yang, J.; Zang, T.; Zou, Y.; Xu, X.M.; Zhang, C.L. In vivo reprogramming of NG2 glia enables adult neurogenesis and functional recovery following spinal cord injury. Cell Stem Cell 2021, 28, 923–937.e4. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Zang, T.; Zou, Y.; Fang, S.; Smith, D.K.; Bachoo, R.; Zhang, C.L. In Vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat. Cell Biol. 2013, 15, 1164–1175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, Z.; Zhang, L.; Wu, Z.; Chen, Y.; Wang, F.; Chen, G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 2014, 14, 188–202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puls, B.; Ding, Y.; Zhang, F.; Pan, M.; Lei, Z.; Pei, Z.; Jiang, M.; Bai, Y.; Forsyth, C.; Metzger, M.; et al. Regeneration of Functional Neurons After Spinal Cord Injury via in situ NeuroD1-Mediated Astrocyte-to-Neuron Conversion. Front. Cell Dev. Biol. 2020, 8, 591883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Todd, L.; Jenkins, W.; Finkbeiner, C.; Hooper, M.J.; Donaldson, P.C.; Pavlou, M.; Wohlschlegel, J.; Ingram, N.; Mu, X.; Rieke, F.; et al. Reprogramming Müller glia to regenerate ganglion-like cells in adult mouse retina with developmental transcription factors. Sci. Adv. 2022, 8, eabq7219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Todd, L.; Hooper, M.J.; Haugan, A.K.; Finkbeiner, C.; Jorstad, N.; Radulovich, N.; Wong, C.K.; Donaldson, P.C.; Jenkins, W.; Chen, Q.; et al. Efficient stimulation of retinal regeneration from Müller glia in adult mice using combinations of proneural bHLH transcription factors. Cell Rep. 2021, 37, 109857. [Google Scholar] [CrossRef] [PubMed Central]
- Livingston, J.M.; Lee, T.T.; Enbar, T.; Daniele, E.; Phillips, C.M.; Krassikova, A.; Bang, K.W.A.; Kortebi, I.; Donville, B.W.; Ibragimov, O.S.; et al. Ectopic Expression of Neurod1 Is Sufficient for Functional Recovery following a Sensory-Motor Cortical Stroke. Biomedicines 2024, 12, 663. [Google Scholar] [CrossRef] [PubMed Central]
- Irie, T.; Matsuda, T.; Hayashi, Y.; Matsuda-Ito, K.; Kamiya, A.; Masuda, T.; Prinz, M.; Isobe, N.; Kira, J.I.; Nakashima, K. Direct neuronal conversion of microglia/macrophages reinstates neurological function after stroke. Proc. Natl. Acad. Sci. USA 2023, 120, e2307972120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.L.; Serrano, C.; Zhong, X.; Ma, S.; Zou, Y.; Zhang, C.L. Revisiting astrocyte to neuron conversion with lineage tracing in vivo. Cell 2021, 184, 5465–5481.e16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiang, Z.; Xu, L.; Liu, M.; Wang, Q.; Li, W.; Lei, W.; Chen, G. Lineage tracing of direct astrocyte-to-neuron conversion in the mouse cortex. Neural Regen. Res. 2021, 16, 750–756. [Google Scholar] [PubMed] [PubMed Central]
- Chen, G. In vivo confusion over in vivo conversion. Mol. Ther. 2021, 29, 3097–3098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.L.; Zhang, C.L. Reply to In vivo confusion over in vivo conversion. Mol. Ther. 2022, 30, 986–987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiang, Z.; He, S.; Chen, R.; Liu, S.; Liu, M.; Xu, L.; Zheng, J.; Jiang, Z.; Ma, L.; Sun, Y.; et al. Two-photon live imaging of direct glia-to-neuron conversion in the mouse cortex. Neural Regen. Res. 2024, 19, 1781–1788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.L.; Zhang, C.L. In vivo glia-to-neuron conversion: Pitfalls and solutions. Dev. Neurobiol. 2022, 82, 367–374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilkinson, G.; Dennis, D.; Schuurmans, C. Proneural genes in neocortical development. Neuroscience 2013, 253, 256–273. [Google Scholar] [CrossRef] [PubMed]
- Bani-Yaghoub, M.; Tremblay, R.G.; Lei, J.X.; Zhang, D.; Zurakowski, B.; Sandhu, J.K.; Smith, B.; Ribecco-Lutkiewicz, M.; Kennedy, J.; Walker, P.R.; et al. Role of Sox2 in the development of the mouse neocortex. Dev. Biol. 2006, 295, 52–66. [Google Scholar] [CrossRef]
- Graham, V.; Khudyakov, J.; Ellis, P.; Pevny, L. SOX2 functions to maintain neural progenitor identity. Neuron 2003, 39, 749–765. [Google Scholar] [CrossRef]
- Fode, C.; Ma, Q.; Casarosa, S.; Ang, S.L.; Anderson, D.J.; Guillemot, F. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 2000, 14, 67–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tutukova, S.; Tarabykin, V.; Hernandez-Miranda, L.R. The Role of Neurod Genes in Brain Development, Function, and Disease. Front. Mol. Neurosci. 2021, 14, 662774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mattar, P.; Langevin, L.M.; Markham, K.; Klenin, N.; Shivji, S.; Zinyk, D.; Schuurmans, C. Basic helix-loop-helix transcription factors cooperate to specify a cortical projection neuron identity. Mol. Cell. Biol. 2008, 28, 1456–1469. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, F.; Joyner, A.L. Dynamic expression of the murine Achaete-Scute homologue Mash-1 in the developing nervous system. Mech. Dev. 1993, 42, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Casarosa, S.; Fode, C.; Guillemot, F. Mash1 regulates neurogenesis in the ventral telencephalon. Development 1999, 126, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.A.; Qiu, M.; Bulfone, A.; Eisenstat, D.D.; Meneses, J.; Pedersen, R.; Rubenstein, J.L. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron 1997, 19, 27–37. [Google Scholar] [CrossRef]
- Anderson, S.A.; Eisenstat, D.D.; Shi, L.; Rubenstein, J.L. Interneuron migration from basal forebrain to neocortex: Dependence on Dlx genes. Science 1997, 278, 474–476. [Google Scholar] [CrossRef]
- Petryniak, M.A.; Potter, G.B.; Rowitch, D.H.; Rubenstein, J.L. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron 2007, 55, 417–433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilson, S.W.; Rubenstein, J.L. Induction and dorsoventral patterning of the telencephalon. Neuron 2000, 28, 641–651. [Google Scholar] [CrossRef]
- Schuurmans, C.; Armant, O.; Nieto, M.; Stenman, J.M.; Britz, O.; Klenin, N.; Brown, C.; Langevin, L.M.; Seibt, J.; Tang, H.; et al. Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. EMBO J. 2004, 23, 2892–2902. [Google Scholar] [CrossRef] [PubMed Central]
- Roybon, L.; Mastracci, T.L.; Ribeiro, D.; Sussel, L.; Brundin, P.; Li, J.Y. GABAergic differentiation induced by Mash1 is compromised by the bHLH proteins Neurogenin2, NeuroD1, and NeuroD2. Cereb. Cortex 2010, 20, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Shimojo, H.; Ohtsuka, T.; Kageyama, R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron 2008, 58, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Vosper, J.M.; Fiore-Heriche, C.S.; Horan, I.; Wilson, K.; Wise, H.; Philpott, A. Regulation of neurogenin stability by ubiquitin-mediated proteolysis. Biochem. J. 2007, 407, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Rajman, M.; Schratt, G. MicroRNAs in neural development: From master regulators to fine-tuners. Development 2017, 144, 2310–2322. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Morrow, E.M.; Cepko, C.L. Misexpression of basic helix-loop-helix genes in the murine cerebral cortex affects cell fate choices and neuronal survival. Development 2000, 127, 3021–3030. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sokirniy, I.; Wang, X.; Jiang, M.; Mseis-Jackson, N.; Williams, C.; Mayes, K.; Jiang, N.; Puls, B.; Du, Q.; et al. MicroRNA-375 Is Induced during Astrocyte-to-Neuron Reprogramming and Promotes Survival of Reprogrammed Neurons when Overexpressed. Cells 2023, 12, 2202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zaret, K.S. Pioneer Transcription Factors Initiating Gene Network Changes. Annu. Rev. Genet. 2020, 54, 367–385. [Google Scholar] [CrossRef] [PubMed Central]
- Iwafuchi-Doi, M.; Zaret, K.S. Cell fate control by pioneer transcription factors. Development 2016, 143, 1833–1837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wapinski, O.L.; Vierbuchen, T.; Qu, K.; Lee, Q.Y.; Chanda, S.; Fuentes, D.R.; Giresi, P.G.; Ng, Y.H.; Marro, S.; Neff, N.F.; et al. Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell 2013, 155, 621–635. [Google Scholar] [CrossRef] [PubMed Central]
- Morris, S.A. Direct lineage reprogramming via pioneer factors; a detour through developmental gene regulatory networks. Development 2016, 143, 2696–2705. [Google Scholar] [CrossRef] [PubMed Central]
- Pataskar, A.; Jung, J.; Smialowski, P.; Noack, F.; Calegari, F.; Straub, T.; Tiwari, V.K. NeuroD1 reprograms chromatin and transcription factor landscapes to induce the neuronal program. EMBO J. 2016, 35, 24–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsuda, T.; Irie, T.; Katsurabayashi, S.; Hayashi, Y.; Nagai, T.; Hamazaki, N.; Adefuin, A.M.D.; Miura, F.; Ito, T.; Kimura, H.; et al. Pioneer Factor NeuroD1 Rearranges Transcriptional and Epigenetic Profiles to Execute Microglia-Neuron Conversion. Neuron 2019, 101, 472–485 e477. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Su, Z.; Tai, W.; Zou, Y.; Xu, X.M.; Zhang, C.L. The p53 Pathway Controls SOX2-Mediated Reprogramming in the Adult Mouse Spinal Cord. Cell Rep. 2016, 17, 891–903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wohlschlegel, J.; Finkbeiner, C.; Hoffer, D.; Kierney, F.; Prieve, A.; Murry, A.D.; Haugan, A.K.; Ortuño-Lizarán, I.; Rieke, F.; Golden, S.A.; et al. ASCL1 induces neurogenesis in human Müller glia. Stem Cell Rep. 2023, 18, 2400–2417. [Google Scholar] [CrossRef] [PubMed Central]
- Pereira, A.; Diwakar, J.; Masserdotti, G.; Beşkardeş, S.; Simon, T.; So, Y.; Martín-Loarte, L.; Bergemann, F.; Vasan, L.; Schauer, T.; et al. Direct neuronal reprogramming of mouse astrocytes is associated with multiscale epigenome remodeling and requires Yy1. Nat. Neurosci. 2024, 27, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Nakashima, K. Clarifying the ability of NeuroD1 to convert mouse microglia into neurons. Neuron 2021, 109, 3912–3913. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Peng, B. Failure of observing NeuroD1-induced microglia-to-neuron conversion in vitro is not attributed to the low NeuroD1 expression level. Mol. Brain 2022, 15, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rao, Y.; Du, S.; Yang, B.; Wang, Y.; Li, Y.; Li, R.; Zhou, T.; Du, X.; He, Y.; Wang, Y.; et al. NeuroD1 induces microglial apoptosis and cannot induce microglia-to-neuron cross-lineage reprogramming. Neuron 2021, 109, 4094–4108.e5. [Google Scholar] [CrossRef]
- Matsuda-Ito, K.; Matsuda, T.; Nakashima, K. Expression level of the reprogramming factor NeuroD1 is critical for neuronal conversion efficiency from different cell types. Sci. Rep. 2022, 12, 17980. [Google Scholar] [CrossRef] [PubMed Central]
- Hersbach, B.A.; Fischer, D.S.; Masserdotti, G.; Deeksha; Mojžišová, K.; Waltzhöni, T.; Rodriguez-Terrones, D.; Heinig, M.; Theis, F.J.; Götz, M.; et al. Probing cell identity hierarchies by fate titration and collision during direct reprogramming. Mol. Syst. Biol. 2022, 18, e11129. [Google Scholar] [CrossRef]
- Ortinski, P.I.; Dong, J.; Mungenast, A.; Yue, C.; Takano, H.; Watson, D.J.; Haydon, P.G.; Coulter, D.A. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat. Neurosci. 2010, 13, 584–591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gotz, M.; Sirko, S.; Beckers, J.; Irmler, M. Reactive astrocytes as neural stem or progenitor cells: In Vivo lineage, In vitro potential, and Genome-wide expression analysis. Glia 2015, 63, 1452–1468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brulet, R.; Matsuda, T.; Zhang, L.; Miranda, C.; Giacca, M.; Kaspar, B.K.; Nakashima, K.; Hsieh, J. NEUROD1 Instructs Neuronal Conversion in Non-Reactive Astrocytes. Stem Cell Rep. 2017, 8, 1506–1515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hevner, R.F.; Hodge, R.D.; Daza, R.A.; Englund, C. Transcription factors in glutamatergic neurogenesis: Conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci. Res. 2006, 55, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Maeda, T.; Lee, J.E. NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes. Dev. 1999, 13, 1647–1652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Su, Z.; Niu, W.; Liu, M.L.; Zou, Y.; Zhang, C.L. In Vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 2014, 5, 3338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heinrich, C.; Bergami, M.; Gascon, S.; Lepier, A.; Vigano, F.; Dimou, L.; Sutor, B.; Berninger, B.; Gotz, M. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Rep. 2014, 3, 1000–1014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brenner, M. Structure and transcriptional regulation of the GFAP gene. Brain Pathol. 1994, 4, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Borodinova, A.A.; Balaban, P.M.; Bezprozvanny, I.B.; Salmina, A.B.; Vlasova, O.L. Genetic Constructs for the Control of Astrocytes’ Activity. Cells 2021, 10, 1600. [Google Scholar] [CrossRef] [PubMed Central]
- Lee, Y.; Messing, A.; Su, M.; Brenner, M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia 2008, 56, 481–493. [Google Scholar] [CrossRef]
- de Leeuw, B.; Su, M.; ter Horst, M.; Iwata, S.; Rodijk, M.; Hoeben, R.C.; Messing, A.; Smitt, P.S.; Brenner, M. Increased glia-specific transgene expression with glial fibrillary acidic protein promoters containing multiple enhancer elements. J. Neurosci. Res. 2006, 83, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Indra, A.K.; Warot, X.; Brocard, J.; Bornert, J.M.; Xiao, J.H.; Chambon, P.; Metzger, D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: Comparison of the recombinase activity of the tamoxifen-inducible Cre-ERT and Cre-ERT2 recombinases. Nucleic Acids Res. 1999, 27, 4324–4327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, N.Y.; Chen, Y.T.; Wang, Q.; Jie, W.; Liu, Y.S.; You, Q.L.; Li, Z.L.; Li, X.W.; Reibel, S.; Pfrieger, F.W.; et al. Expression Patterns of Inducible Cre Recombinase Driven by Differential Astrocyte-Specific Promoters in Transgenic Mouse Lines. Neurosci. Bull. 2020, 36, 530–544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Srinivasan, R.; Lu, T.Y.; Chai, H.; Xu, J.; Huang, B.S.; Golshani, P.; Coppola, G.; Khakh, B.S. New Transgenic Mouse Lines for Selectively Targeting Astrocytes and Studying Calcium Signals in Astrocyte Processes In Situ and In Vivo. Neuron 2016, 92, 1181–1195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koerber, J.T.; Klimczak, R.; Jang, J.H.; Dalkara, D.; Flannery, J.G.; Schaffer, D.V. Molecular evolution of adeno-associated virus for enhanced glial gene delivery. Mol. Ther. 2009, 17, 2088–2095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deverman, B.E.; Pravdo, P.L.; Simpson, B.P.; Kumar, S.R.; Chan, K.Y.; Banerjee, A.; Wu, W.L.; Yang, B.; Huber, N.; Pasca, S.P.; et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 2016, 34, 204–209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Carroll, S.J.; Cook, W.H.; Young, D. AAV Targeting of Glial Cell Types in the Central and Peripheral Nervous System and Relevance to Human Gene Therapy. Front. Mol. Neurosci. 2020, 13, 618020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kallunki, T.; Barisic, M.; Jäättelä, M.; Liu, B. How to Choose the Right Inducible Gene Expression System for Mammalian Studies? Cells 2019, 8, 796. [Google Scholar] [CrossRef] [PubMed Central]
- Gossen, M.; Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 1992, 89, 5547–5551. [Google Scholar] [CrossRef] [PubMed Central]
- Gossen, M.; Freundlieb, S.; Bender, G.; Müller, G.; Hillen, W.; Bujard, H. Transcriptional activation by tetracyclines in mammalian cells. Science 1995, 268, 1766–1769. [Google Scholar] [CrossRef]
- Das, A.T.; Tenenbaum, L.; Berkhout, B. Tet-On Systems For Doxycycline-inducible Gene Expression. Curr. Gene Ther. 2016, 16, 156–167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Knopf, F.; Schnabel, K.; Haase, C.; Pfeifer, K.; Anastassiadis, K.; Weidinger, G. Dually inducible TetON systems for tissue-specific conditional gene expression in zebrafish. Proc. Natl. Acad. Sci. USA 2010, 107, 19933–19938. [Google Scholar] [CrossRef] [PubMed Central]
- Sullivan, K.A.; Vitko, I.; Blair, K.; Gaykema, R.P.; Failor, M.J.; San Pietro, J.M.; Dey, D.; Williamson, J.M.; Stornetta, R.L.; Kapur, J.; et al. Drug-Inducible Gene Therapy Effectively Reduces Spontaneous Seizures in Kindled Rats but Creates Off-Target Side Effects in Inhibitory Neurons. Int. J. Mol. Sci. 2023, 24, 11347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costello, A.; Lao, N.T.; Gallagher, C.; Capella Roca, B.; Julius, L.A.N.; Suda, S.; Ducrée, J.; King, D.; Wagner, R.; Barron, N.; et al. Leaky Expression of the TET-On System Hinders Control of Endogenous miRNA Abundance. Biotechnol. J. 2019, 14, e1800219. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Nicholson, L.F. Optimization of the Tet-On system for inducible expression of RAGE. J. Biomol. Tech. 2006, 17, 283–292. [Google Scholar] [PubMed] [PubMed Central]
- Khor, J.M.; Ettensohn, C.A. An optimized Tet-On system for conditional control of gene expression in sea urchins. Development 2023, 150, dev201373. [Google Scholar] [CrossRef] [PubMed]
- Ali Hosseini Rad, S.M.; Poudel, A.; Tan, G.M.Y.; McLellan, A.D. Optimisation of Tet-On inducible systems for Sleeping Beauty-based chimeric antigen receptor (CAR) applications. Sci. Rep. 2020, 10, 13125. [Google Scholar] [CrossRef]
- Loew, R.; Heinz, N.; Hampf, M.; Bujard, H.; Gossen, M. Improved Tet-responsive promoters with minimized background expression. BMC Biotechnol. 2010, 10, 81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marro, S.; Pang, Z.P.; Yang, N.; Tsai, M.C.; Qu, K.; Chang, H.Y.; Südhof, T.C.; Wernig, M. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell 2011, 9, 374–382. [Google Scholar] [CrossRef] [PubMed Central]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Stefani, G.; Slack, F.J. Small non-coding RNAs in animal development. Nat. Rev. Mol. Cell Biol. 2008, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yeo, G.; Muotri, A.R.; Kuwabara, T.; Gage, F.H. Noncoding RNAs in the mammalian central nervous system. Annu. Rev. Neurosci. 2006, 29, 77–103. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. microRNAs: Tiny regulators with great potential. Cell 2001, 107, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Colin, A.; Faideau, M.; Dufour, N.; Auregan, G.; Hassig, R.; Andrieu, T.; Brouillet, E.; Hantraye, P.; Bonvento, G.; Deglon, N. Engineered lentiviral vector targeting astrocytes in vivo. Glia 2009, 57, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Delzor, A.; Escartin, C.; Deglon, N. Lentiviral vectors: A powerful tool to target astrocytes in vivo. Curr. Drug Targets 2013, 14, 1336–1346. [Google Scholar] [PubMed]
- Taschenberger, G.; Tereshchenko, J.; Kügler, S. A MicroRNA124 Target Sequence Restores Astrocyte Specificity of gfaABC1D-Driven Transgene Expression in AAV-Mediated Gene Transfer. Mol. Ther.-Nucleic Acids 2017, 8, 13–25. [Google Scholar] [CrossRef]
- Gleichman, A.J.; Kawaguchi, R.; Sofroniew, M.V.; Carmichael, S.T. A toolbox of astrocyte-specific, serotype-independent adeno-associated viral vectors using microRNA targeting sequences. Nat. Commun. 2023, 14, 7426. [Google Scholar] [CrossRef]
- Geisler, A.; Jungmann, A.; Kurreck, J.; Poller, W.; Katus, H.A.; Vetter, R.; Fechner, H.; Müller, O.J. microRNA122-regulated transgene expression increases specificity of cardiac gene transfer upon intravenous delivery of AAV9 vectors. Gene Ther. 2011, 18, 199–209. [Google Scholar] [CrossRef]
- Xu, D.; Zhong, L.T.; Cheng, H.Y.; Wang, Z.Q.; Chen, X.M.; Feng, A.Y.; Chen, W.Y.; Chen, G.; Xu, Y. Overexpressing NeuroD1 reprograms Müller cells into various types of retinal neurons. Neural Regen. Res. 2023, 18, 1124–1131. [Google Scholar] [PubMed Central]
- Gascon, S.; Murenu, E.; Masserdotti, G.; Ortega, F.; Russo, G.L.; Petrik, D.; Deshpande, A.; Heinrich, C.; Karow, M.; Robertson, S.P.; et al. Identification and Successful Negotiation of a Metabolic Checkpoint in Direct Neuronal Reprogramming. Cell Stem Cell 2016, 18, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Abernathy, D.G.; Kim, W.K.; McCoy, M.J.; Lake, A.M.; Ouwenga, R.; Lee, S.W.; Xing, X.; Li, D.; Lee, H.J.; Heuckeroth, R.O.; et al. MicroRNAs Induce a Permissive Chromatin Environment that Enables Neuronal Subtype-Specific Reprogramming of Adult Human Fibroblasts. Cell Stem Cell 2017, 21, 332–348 e339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caiazzo, M.; Dell’Anno, M.T.; Dvoretskova, E.; Lazarevic, D.; Taverna, S.; Leo, D.; Sotnikova, T.D.; Menegon, A.; Roncaglia, P.; Colciago, G.; et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 2011, 476, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, C.; Blum, R.; Gascon, S.; Masserdotti, G.; Tripathi, P.; Sanchez, R.; Tiedt, S.; Schroeder, T.; Gotz, M.; Berninger, B. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010, 8, e1000373. [Google Scholar] [CrossRef] [PubMed Central]
- Cabrera, A.; Edelstein, H.I.; Glykofrydis, F.; Love, K.S.; Palacios, S.; Tycko, J.; Zhang, M.; Lensch, S.; Shields, C.E.; Livingston, M.; et al. The sound of silence: Transgene silencing in mammalian cell engineering. Cell Syst. 2022, 13, 950–973. [Google Scholar] [CrossRef]
- Clifford, T.; Finkel, Z.; Rodriguez, B.; Joseph, A.; Cai, L. Current Advancements in Spinal Cord Injury Research-Glial Scar Formation and Neural Regeneration. Cells 2023, 12, 853. [Google Scholar] [CrossRef] [PubMed Central]
- Pavlou, M.; Probst, M.; Blasdel, N.; Prieve, A.R.; Reh, T.A. The impact of timing and injury mode on induced neurogenesis in the adult mammalian retina. Stem Cell Rep. 2024, 19, 239–253. [Google Scholar] [CrossRef] [PubMed Central]
- Zhang, L.; Lei, Z.; Guo, Z.; Pei, Z.; Chen, Y.; Zhang, F.; Cai, A.; Mok, G.; Lee, G.; Swaminathan, V.; et al. Development of Neuroregenerative Gene Therapy to Reverse Glial Scar Tissue Back to Neuron-Enriched Tissue. Front. Cell Neurosci. 2020, 14, 594170. [Google Scholar] [CrossRef] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mseis-Jackson, N.; Sharma, M.; Li, H. Controlling the Expression Level of the Neuronal Reprogramming Factors for a Successful Reprogramming Outcome. Cells 2024, 13, 1223. https://doi.org/10.3390/cells13141223
Mseis-Jackson N, Sharma M, Li H. Controlling the Expression Level of the Neuronal Reprogramming Factors for a Successful Reprogramming Outcome. Cells. 2024; 13(14):1223. https://doi.org/10.3390/cells13141223
Chicago/Turabian StyleMseis-Jackson, Natalie, Mehek Sharma, and Hedong Li. 2024. "Controlling the Expression Level of the Neuronal Reprogramming Factors for a Successful Reprogramming Outcome" Cells 13, no. 14: 1223. https://doi.org/10.3390/cells13141223





