Achyranthis radix Extract Enhances Antioxidant Effect of Placenta-Derived Mesenchymal Stem Cell on Injured Human Ocular Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Achyranthis radix Extract (ARE)
2.2. HPLC Analysis of ARE
2.3. Cell Culture
2.4. In Vitro Coculture System
2.5. Cell Culture Immunophenotypes by Flow Cytometry
2.6. Multilineage Differentiation
2.7. Quantitative Real-Time PCR (qRT-PCR)
2.8. Western Blot
2.9. Population Doubling Time (PDT)
2.10. ROS Measurement
2.11. SA-β-Gal Staining
2.12. Statistical Analysis
3. Results
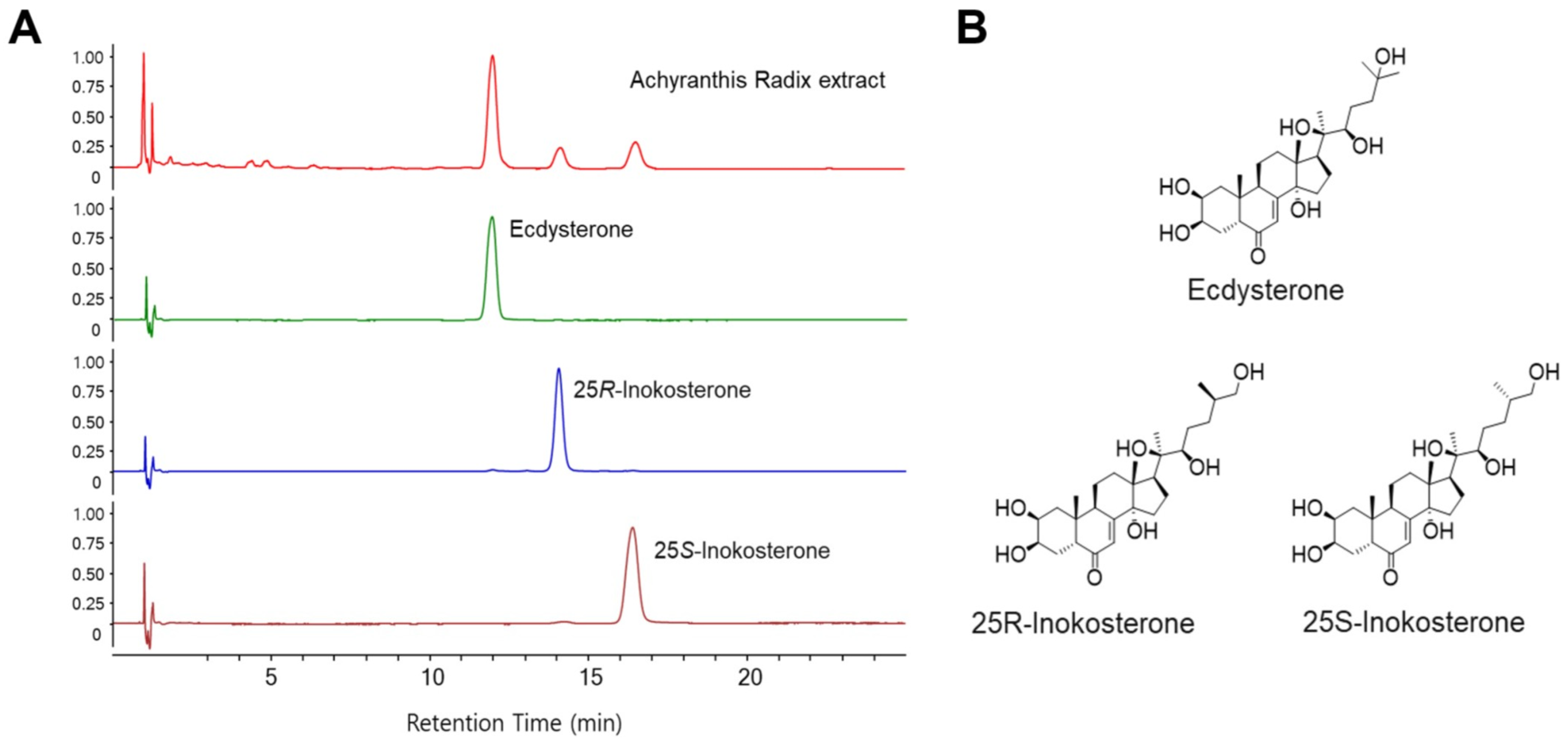
3.1. HPLC Analysis of Compounds in ARE
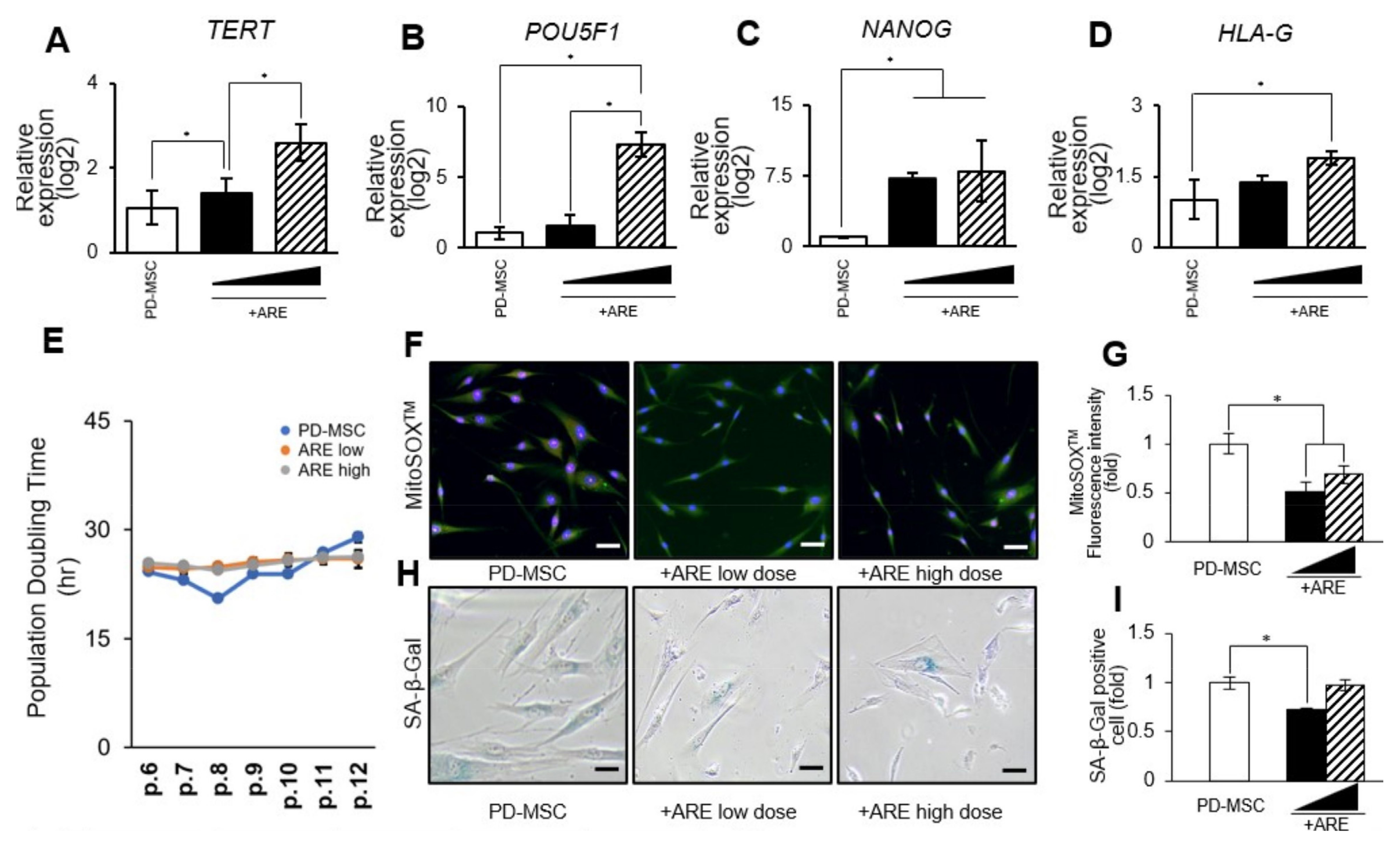
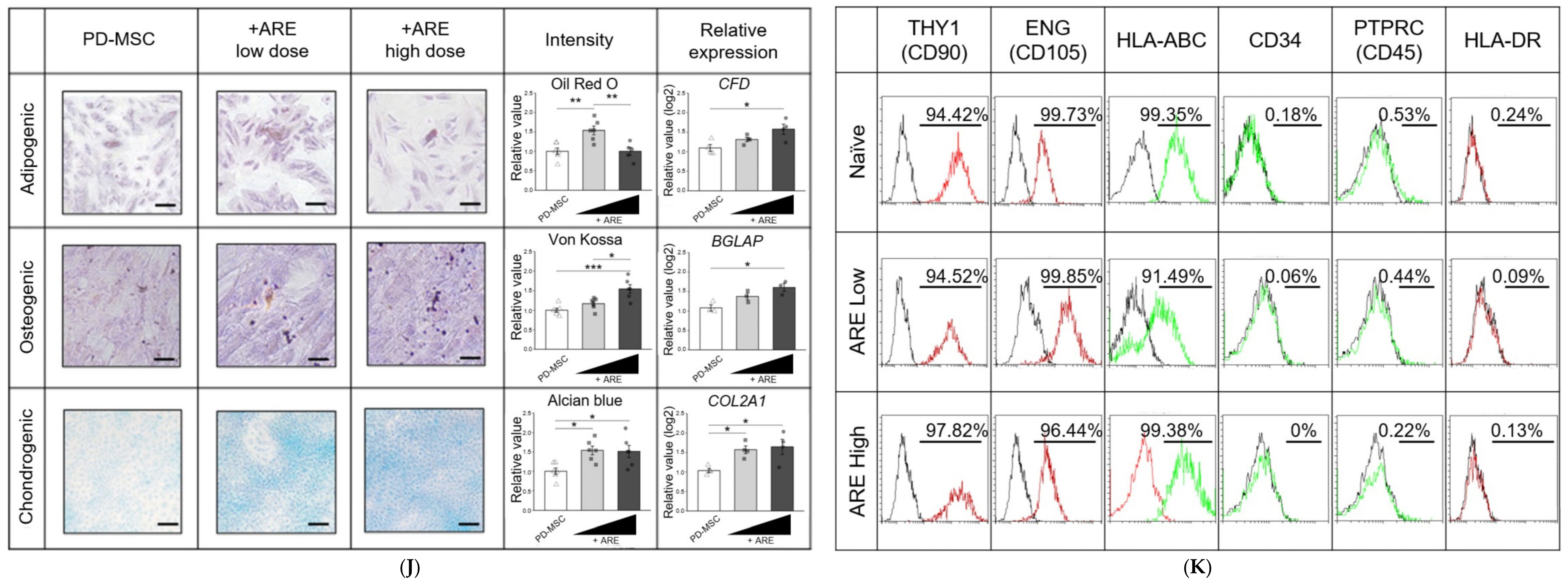
3.2. ARE Enhanced Function of PD-MSC
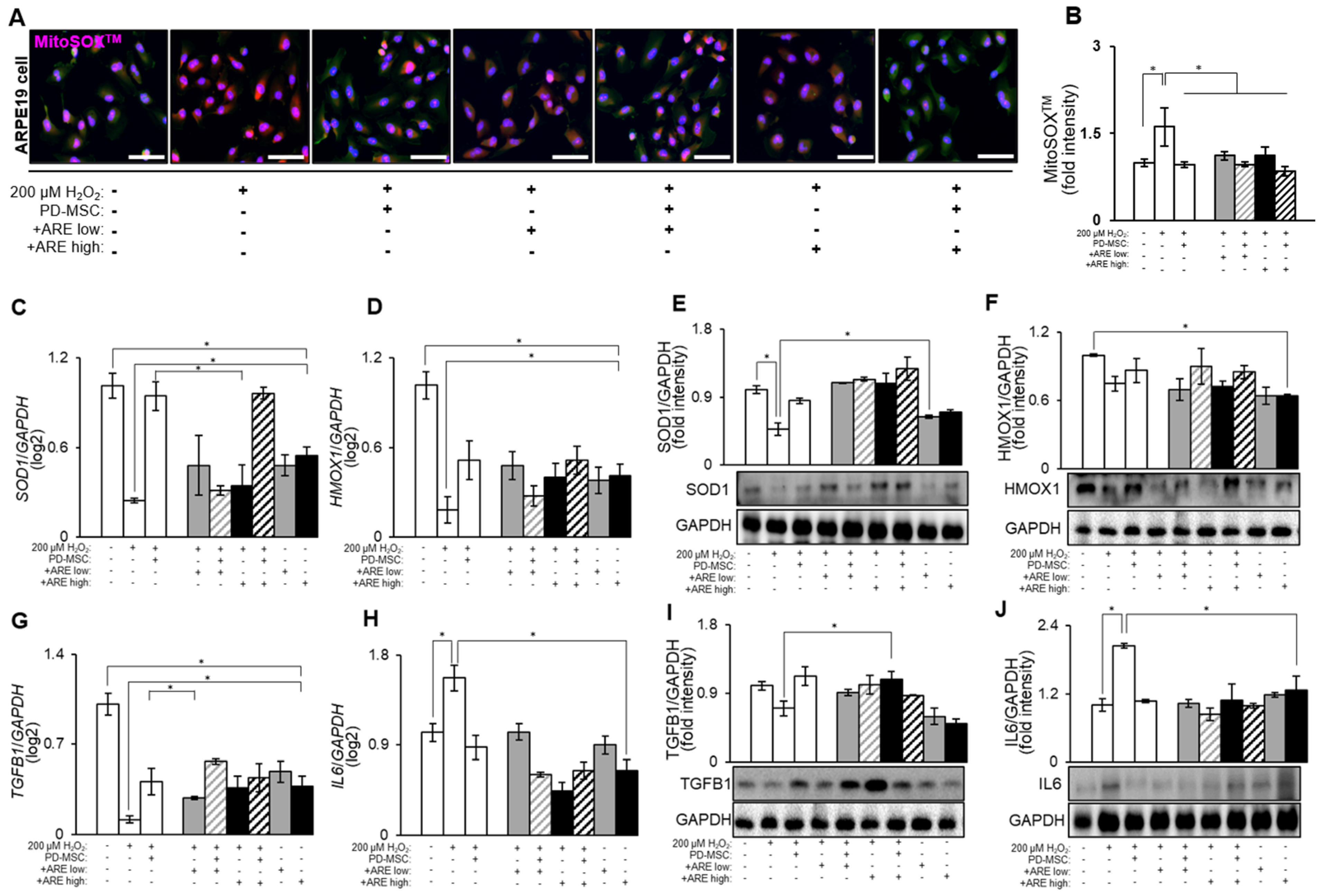
3.3. ARE Increased Antioxidant Factors and Modulate Inflammatory Response in ARPE19 Cell
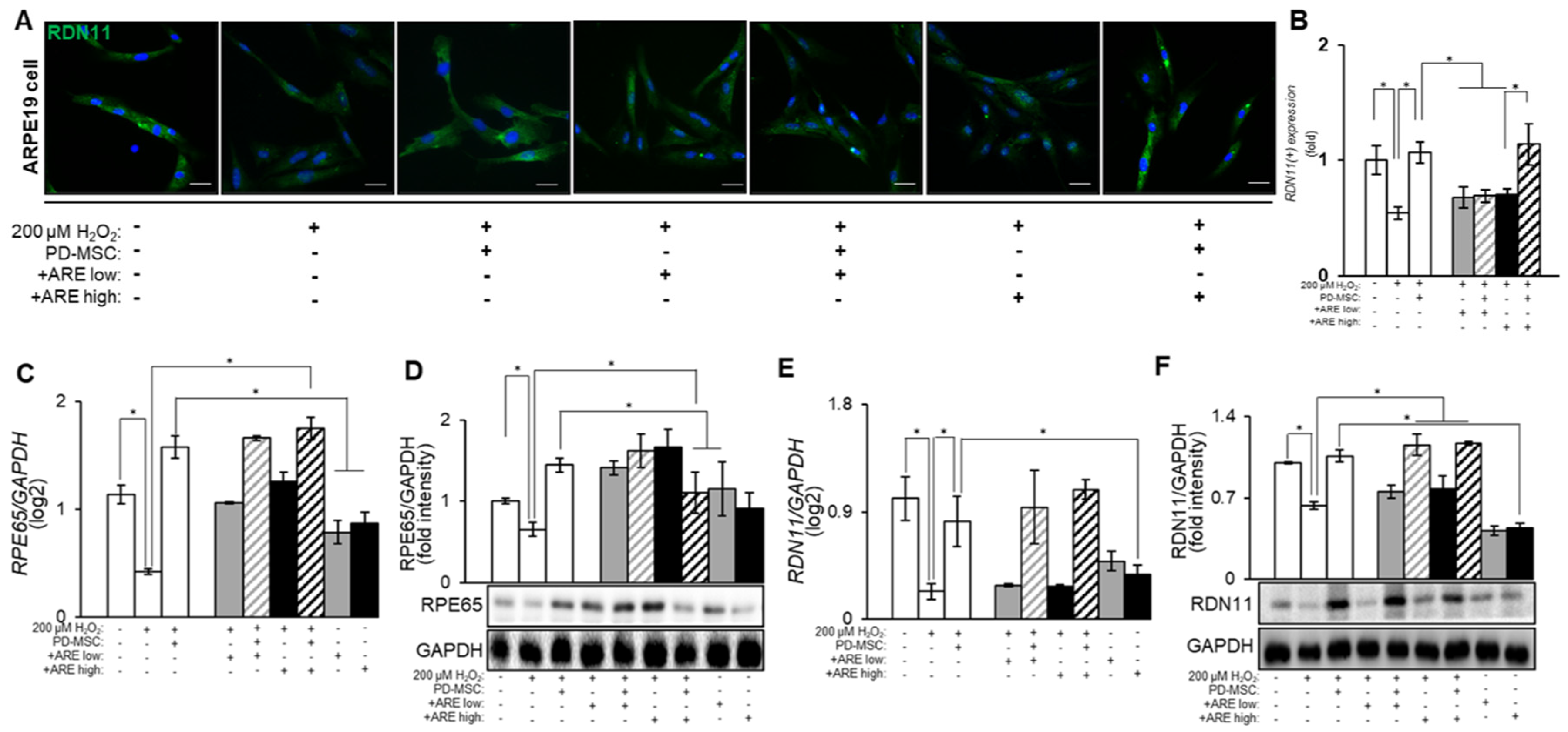
3.4. ARE-Primed PD-MSC Increased RPE65 and RDN11 in ARPE19 Cell
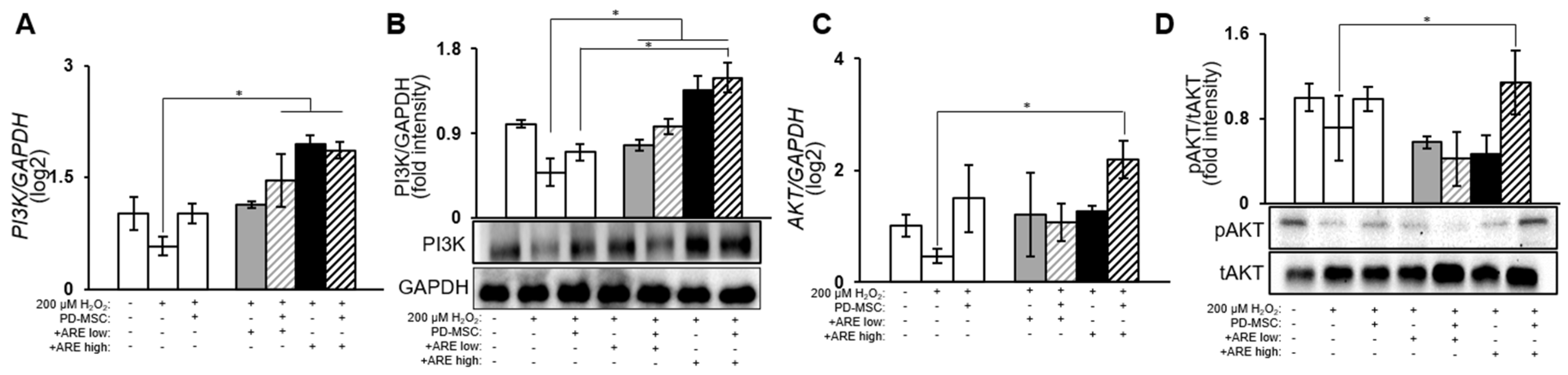
3.5. ARE-Activated PI3K/Akt Signaling Pathway in Dry Eye Model
4. Discussion
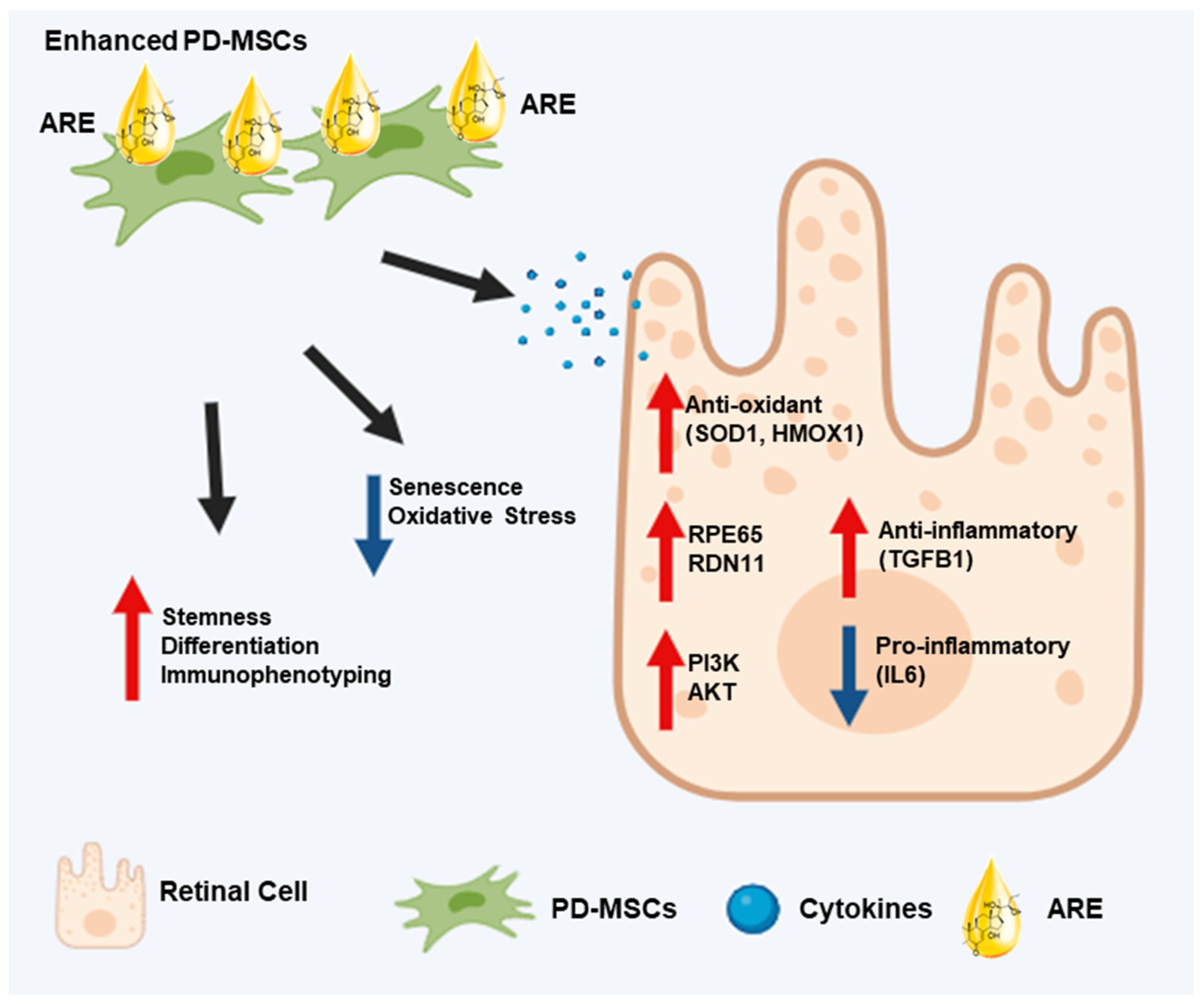
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Ah Tong, B.A.M.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Wei, Y.; Zhang, X.; Guan, J.; Mao, S. Challenges and strategies for ocular posterior diseases therapy via non-invasive advanced drug delivery. J. Control Release 2023, 361, 191–211. [Google Scholar] [CrossRef] [PubMed]
- Vyawahare, H.; Shinde, P. Age-Related Macular Degeneration: Epidemiology, Pathophysiology, Diagnosis, and Treatment. Cureus 2022, 14, e29583. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Barber, A.J.; Bronson, S.K.; Freeman, W.M.; Gardner, T.W.; Jefferson, L.S.; Kester, M.; Kimball, S.R.; Krady, J.K.; LaNoue, K.F.; et al. Diabetic retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes 2006, 55, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.; Catalani, E.; Dal Monte, M.; Cammalleri, M.; Cervia, D.; Casini, G. Morpho-functional analysis of the early changes induced in retinal ganglion cells by the onset of diabetic retinopathy: The effects of a neuroprotective strategy. Pharmacol. Res. 2022, 185, 106516. [Google Scholar] [CrossRef] [PubMed]
- Bohm, E.W.; Buonfiglio, F.; Voigt, A.M.; Bachmann, P.; Safi, T.; Pfeiffer, N.; Gericke, A. Oxidative stress in the eye and its role in the pathophysiology of ocular diseases. Redox Biol. 2023, 68, 102967. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Geng, Z.; Khattak, S.; Ji, X.; Wu, D.; Dang, Y. Role of Oxidative Stress in Retinal Disease and the Early Intervention Strategies: A Review. Oxid. Med. Cell Longev. 2022, 2022, 7836828. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.J.; Chen, Y.N.; Tsao, Y.T.; Cheng, C.M.; Wu, W.C.; Chen, H.C. The Pathomechanism, Antioxidant Biomarkers, and Treatment of Oxidative Stress-Related Eye Diseases. Int. J. Mol. Sci. 2022, 23, 1255. [Google Scholar] [CrossRef]
- Bang, S.Y.; Kim, J.H.; Kim, H.Y.; Lee, Y.J.; Park, S.Y.; Lee, S.J.; Kim, Y. Achyranthes japonica exhibits anti-inflammatory effect via NF-kappaB suppression and HO-1 induction in macrophages. J. Ethnopharmacol. 2012, 144, 109–117. [Google Scholar] [CrossRef]
- Choi, B.R.; Kang, S.J.; Kim, J.L.; Lee, Y.J.; Ku, S.K. Anti-Osteoarthritic Effects of a Mixture of Dried Pomegranate Concentrate Powder, Eucommiae Cortex, and Achyranthis radix 5:4:1 (g/g) in a Surgically Induced Osteoarthritic Rabbit Model. Nutrients 2020, 12, 852. [Google Scholar] [CrossRef]
- Hyun, S.W.; Lee, T.G.; Song, S.J.; Kim, C.S. Evaluation of oral toxicity and genotoxicity of Achyranthis radix extract. J. Ethnopharmacol. 2021, 274, 113944. [Google Scholar] [CrossRef]
- Siu, W.S.; Zhou, X.; Fung, C.H.; Shum, W.T.; Lau, C.B.; Leung, P.C.; Ko, C.H.; Hung, L.K. Preclinical evaluations on the efficacy of a topical Chinese herbal formula for swelling control and pain relief. J. Ethnopharmacol. 2015, 162, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Salari, V.; Mengoni, F.; Del Gallo, F.; Bertini, G.; Fabene, P.F. The Anti-Inflammatory Properties of Mesenchymal Stem Cells in Epilepsy: Possible Treatments and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 9683. [Google Scholar] [CrossRef]
- Venkatakrishnan, J.; Saeed, Y.; Kao, W.W. Trends in using mesenchymal stromal/stem cells (MSCs) in treating corneal diseases. Ocul. Surf. 2022, 26, 255–267. [Google Scholar] [CrossRef]
- Paulus, Y.M.; Sodhi, A. Anti-angiogenic Therapy for Retinal Disease. Handb. Exp. Pharmacol. 2017, 242, 271–307. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ng, T.K.; Chen, C.B.; Huang, B.; Liang, J.; Pang, C.P.; Zhang, M. Notch Signaling Activation Enhances Human Adipose-Derived Stem Cell Retinal Differentiation. Stem Cells Int. 2018, 2018, 9201374. [Google Scholar] [CrossRef]
- Amirpour, N.; Amirizade, S.; Hashemibeni, B.; Kazemi, M.; Hadian, M.; Salehi, H. Differentiation of eye field neuroectoderm from human adipose-derived stem cells by using small-molecules and hADSC-conditioned medium. Ann. Anat. 2019, 221, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Kim, J.J.; Seo, M.S.; Park, S.B.; Shin, T.H.; Shin, J.H.; Seo, Y.; Kim, H.S.; Kang, K.S. Inhibition by miR-410 facilitates direct retinal pigment epithelium differentiation of umbilical cord blood-derived mesenchymal stem cells. J. Vet. Sci. 2017, 18, 59–65. [Google Scholar] [CrossRef]
- Song, W.K.; Park, K.M.; Kim, H.J.; Lee, J.H.; Choi, J.; Chong, S.Y.; Shim, S.H.; Del Priore, L.V.; Lanza, R. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: Preliminary results in Asian patients. Stem Cell Rep. 2015, 4, 860–872. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.S.; Kumar, S.; Mok, P.L. Cellular Reparative Mechanisms of Mesenchymal Stem Cells for Retinal Diseases. Int. J. Mol. Sci. 2017, 18, 1406. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Levy, S.; Benes, S.C. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: A case report of improvement in relapsing auto-immune optic neuropathy. Neural Regen. Res. 2015, 10, 1507–1515. [Google Scholar] [CrossRef]
- Satarian, L.; Nourinia, R.; Safi, S.; Kanavi, M.R.; Jarughi, N.; Daftarian, N.; Arab, L.; Aghdami, N.; Ahmadieh, H.; Baharvand, H. Intravitreal Injection of Bone Marrow Mesenchymal Stem Cells in Patients with Advanced Retinitis Pigmentosa; a Safety Study. J. Ophthalmic Vis. Res. 2017, 12, 58–64. [Google Scholar] [CrossRef]
- Oner, A.; Gonen, Z.B.; Sinim, N.; Cetin, M.; Ozkul, Y. Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: A phase I clinical safety study. Stem Cell Res. Ther. 2016, 7, 178. [Google Scholar] [CrossRef]
- Moller-Hansen, M. Mesenchymal stem cell therapy in aqueous deficient dry eye disease. Acta Ophthalmol. 2023, 101 (Suppl. 277), 3–27. [Google Scholar] [CrossRef]
- Jackson, C.J.; Naqvi, M.; Gundersen, K.G.; Utheim, T.P. Role of stem cells in regenerative treatment of dry eye disease caused by lacrimal gland dysfunction. Acta Ophthalmol. 2023, 101, 360–375. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, K.S.; Jeon, J.H.; Lee, D.R.; Shim, S.H.; Kim, J.K.; Cha, D.H.; Yoon, T.K.; Kim, G.J. Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: A comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011, 346, 53–64. [Google Scholar] [CrossRef]
- Lee, J.M.; Jung, J.; Lee, H.J.; Jeong, S.J.; Cho, K.J.; Hwang, S.G.; Kim, G.J. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int. Immunopharmacol. 2012, 13, 219–224. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.; Park, S.H.; Lee, D.; Kim, G.H.; Noh, J.E.; Lee, K.J.; Kim, G.J. Overexpression of pigment epithelium-derived factor in placenta-derived mesenchymal stem cells promotes mitochondrial biogenesis in retinal cells. Lab. Investig. 2021, 101, 51–69. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.; Park, H.J.; Kim, S.H.; Lew, H.; Kim, G.J. PEDF-Mediated Mitophagy Triggers the Visual Cycle by Enhancing Mitochondrial Functions in a H2O2-Injured Rat Model. Cells 2021, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, S.; Lee, H.J.; Lew, H.; Kim, G.J. Functionally enhanced placenta-derived mesenchymal stem cells inhibit adipogenesis in orbital fibroblasts with Graves’ ophthalmopathy. Stem Cell Res. Ther. 2020, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.S.L.; Subbiah, S.K.; Khan, M.S.A.; Farhana, A.; Mok, P.L. Empowering Mesenchymal Stem Cells for Ocular Degenerative Disorders. Int. J. Mol. Sci. 2019, 20, 1784. [Google Scholar] [CrossRef]
- Jiang, N.; Tian, X.; Wang, Q.; Hao, J.; Jiang, J.; Wang, H. Regulation Mechanisms and Maintenance Strategies of Stemness in Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2023, 20, 455–483. [Google Scholar] [CrossRef]
- Noronha, N.C.; Mizukami, A.; Caliari-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Baldari, S.; Di Rocco, G.; Piccoli, M.; Pozzobon, M.; Muraca, M.; Toietta, G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int. J. Mol. Sci. 2017, 18, 2087. [Google Scholar] [CrossRef]
- Lee, T.G.; Hyun, S.W.; Jo, K.; Park, B.; Lee, I.S.; Song, S.J.; Kim, C.S. Achyranthis radix Extract Improves Urban Particulate Matter-Induced Dry Eye Disease. Int. J. Environ. Res. Public Health 2019, 16, 3229. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Jung, J.; Na, K.H.; Moon, J.S.; Lee, H.J.; Kim, J.H.; Kim, G.I.; Kwon, S.W.; Hwang, S.G.; Kim, G.J. Anti-fibrotic effect of chorionic plate-derived mesenchymal stem cells isolated from human placenta in a rat model of CCl4-injured liver: Potential application to the treatment of hepatic diseases. J. Cell Biochem. 2010, 111, 1453–1463. [Google Scholar] [CrossRef]
- Hung, Y.W.; Ouyang, C.; Ping, X.; Qi, Y.; Wang, Y.C.; Kung, H.J.; Ann, D.K. Extracellular arginine availability modulates eIF2alpha O-GlcNAcylation and heme oxygenase 1 translation for cellular homeostasis. J. Biomed. Sci. 2023, 30, 32. [Google Scholar] [CrossRef]
- Hofmann, A.; Mishra, J.S.; Yadav, P.; Dangudubiyyam, S.V.; Blesson, C.S.; Kumar, S. PFOS Impairs Mitochondrial Biogenesis and Dynamics and Reduces Oxygen Consumption in Human Trophoblasts. J. Environ. Sci. Public Health 2023, 7, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Jia, Q.; Shen, H.; Xu, X.; Ling, J.; Jing, C.; Zhang, B. Treatment with the herbal formula Songyou Yin inhibits epithelial-mesenchymal transition in hepatocellular carcinoma through downregulation of TGF-beta1 expression and inhibition of the SMAD2/3 signaling pathway. Oncol. Lett. 2017, 13, 2309–2315. [Google Scholar] [CrossRef]
- Innamorato, N.G.; Rojo, A.I.; Garcia-Yague, A.J.; Yamamoto, M.; de Ceballos, M.L.; Cuadrado, A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J. Immunol. 2008, 181, 680–689. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, T.; Wu, T.; Ye, W.; Wang, Y.; Dou, G.; Du, H.; Hui, Y.; Guo, C. Connective tissue growth factor promotes retinal pigment epithelium mesenchymal transition via the PI3K/AKT signaling pathway. Mol. Med. Rep. 2021, 23, 389. [Google Scholar] [CrossRef]
- Suomalainen, L.; Pentikainen, V.; Dunkel, L. Sphingosine-1-phosphate inhibits nuclear factor kappaB activation and germ cell apoptosis in the human testis independently of its receptors. Am. J. Pathol. 2005, 166, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Chuang, W.W.; Sun, Z. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation. J. Biol. Chem. 2002, 277, 30935–30941. [Google Scholar] [CrossRef] [PubMed]
- Shaiken, T.E.; Opekun, A.R. Dissecting the cell to nucleus, perinucleus and cytosol. Sci. Rep. 2014, 4, 4923. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.Y.; Myung, S.; Jung, N.; Choi, Y.; Jung, S.C. Differentiation of Motor Neuron-Like Cells from Tonsil-Derived Mesenchymal Stem Cells and Their Possible Application to Neuromuscular Junction Formation. Int. J. Mol. Sci. 2019, 20, 2702. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.X.; Shen, Y.H.; Burks, J.K.; Li, X.N.; LeMaire, S.A.; Yoshimura, K.; Aoki, H.; Matsuzaki, M.; An, F.S.; et al. Role of NonO-histone interaction in TNFalpha-suppressed prolyl-4-hydroxylase alpha1. Biochim. Biophys. Acta 2008, 1783, 1517–1528. [Google Scholar] [CrossRef]
- Sheng, X.; Sheng, Y.; Liu, Y.; Li, X.; Shu, B.; Li, D. Effects of FSS on the expression and localization of the core proteins in two Wnt signaling pathways, and their association with ciliogenesis. Int. J. Mol. Med. 2018, 42, 1809–1818. [Google Scholar] [CrossRef]
- Zhao, B.T.; Jeong, S.Y.; Moon, D.C.; Son, K.H.; Son, J.K.; Woo, M.H. High performance liquid chromatography used for quality control of Achyranthis radix. Arch. Pharm. Res. 2012, 35, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Fan, B.; Li, Y.L.; Zuo, Z.Y.; Li, G.Y. Oxidative Stress-Involved Mitophagy of Retinal Pigment Epithelium and Retinal Degenerative Diseases. Cell Mol. Neurobiol. 2023, 43, 3265–3276. [Google Scholar] [CrossRef] [PubMed]
- Kiser, P.D. Retinal pigment epithelium 65 kDa protein (RPE65): An update. Prog. Retin. Eye Res. 2022, 88, 101013. [Google Scholar] [CrossRef] [PubMed]
- Kedishvili, N.Y.; Chumakova, O.V.; Chetyrkin, S.V.; Belyaeva, O.V.; Lapshina, E.A.; Lin, D.W.; Matsumura, M.; Nelson, P.S. Evidence that the human gene for prostate short-chain dehydrogenase/reductase (PSDR1) encodes a novel retinal reductase (RalR1). J. Biol. Chem. 2002, 277, 28909–28915. [Google Scholar] [CrossRef]
- Nakazawa, T.; Shimura, M.; Tomita, H.; Akiyama, H.; Yoshioka, Y.; Kudou, H.; Tamai, M. Intrinsic activation of PI3K/Akt signaling pathway and its neuroprotective effect against retinal injury. Curr. Eye Res. 2003, 26, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Shu, D.Y.; Chaudhary, S.; Cho, K.S.; Lennikov, A.; Miller, W.P.; Thorn, D.C.; Yang, M.; McKay, T.B. Role of Oxidative Stress in Ocular Diseases: A Balancing Act. Metabolites 2023, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Choi, J.H.; Jun, J.H.; Park, S.; Jung, J.; Bae, S.H.; Kim, G.J. Enhanced PRL-1 expression in placenta-derived mesenchymal stem cells accelerates hepatic function via mitochondrial dynamics in a cirrhotic rat model. Stem Cell Res. Ther. 2020, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Alagesan, S.; Brady, J.; Byrnes, D.; Fandino, J.; Masterson, C.; McCarthy, S.; Laffey, J.; O’Toole, D. Enhancement strategies for mesenchymal stem cells and related therapies. Stem Cell Res. Ther. 2022, 13, 75. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: The dose-response revolution. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 175–197. [Google Scholar] [CrossRef]
- Gavanji, S.; Bakhtari, A.; Famurewa, A.C.; Othman, E.M. Cytotoxic Activity of Herbal Medicines as Assessed in Vitro: A Review. Chem. Biodivers. 2023, 20, e202201098. [Google Scholar] [CrossRef]
- Karin, M.; Hunter, T. Transcriptional control by protein phosphorylation: Signal transmission from the cell surface to the nucleus. Curr. Biol. 1995, 5, 747–757. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer | Annealing Temperature (°C) |
|---|---|---|
| POU5F1 (OCT4) | F: 5′-AGTGAGAGGCAACCTGGAGA-3′ | 52 |
| R: 5′-GTGAAGTGAGGGCTCCCATA-3′ | ||
| NANOG | F: 5′-TTCTTGACTGGGACCTTGTC-3′ | 52 |
| R: 5′-GCTTGCCTTGCTTTGAAGCA-3′ | ||
| TERT | F: 5′-GAGCTGACGTGGAAGATGAG-3′ | 55 |
| R: 5′-CTTCAAGTGCTGTCTGATTCCAATG-3′ | ||
| HLA-G | F: 5′-GCGGCTACTACAACCAGAGC-3′ | 58 |
| R: 5′-GCACATGGCACGTGTATCTC-3′ | ||
| ACTB (β-actin) | F: 5′-TCCTTCTGCATCCTGTCAGCA-3′ | 58 |
| R: 5′-CAGGAGATGGCCACTGCCGCA-3′ | ||
| CFD (Adipsin) | F: 5′-GGTCACCCAAGCAACAAAGT-3′ | 57.8 |
| R: 5′-CCTCCTGCGTTCAAGTCATC-3′ | ||
| BGLAP (Osteocalcin) | F: 5′-TGAATCGGAACAACCTGACTGA-3′ | 59.1 |
| R: 5′-TTCCACTAGCAAGAAGAAGCCTTT-3′ | ||
| COL2A1 (Col II) | F: 5′-CTCGTGGCAGAGATGGAGAA-3′ | 58 |
| R: 5′-CACCAGGTTCACCAGGATTG-3′ | ||
| RPE65 | F: 5′-CGCCAGAACCGTGCAGA-3′ | 59.1 |
| R: 5′-TCAGGCTGCTGGCTGAC-3′ | ||
| RDN11 | F: 5′-AGCAGGTGTTGGTGCGGAAACT-3′ | 62 |
| R: 5′-CGGACACATCATCACTCCTGCA-3′ | ||
| SOD1 | F: 5′-GCTGTACCAGTGCAGGTCCTCA-3′ | 60 |
| R: 5′-CATTTCCACCTTTGCCCAAGTC-3′ | ||
| HMOX1 (HO-1) | F: 5′-GCGAAACAAGCAGAACCCA-3′ | 58.5 |
| R: 5′-GCGAAACAAGCAGAACCCA-3′ | ||
| TGFB1 | F: 5′-TACCAGAAATACAGCAACAATTCC-3′ | 57 |
| R: 5′-AAAGCCCTCAATTTCCCCTCC-3′ | ||
| IL6 | F: 5′-TCCACAAGCGCCTTCGGTCCAGTTG-3′ | 67 |
| R: 5′-AGAGGTGAGTGGCTGTCTGTGTGGG-3′ | ||
| PI3K | F: 5′-GGAGCCTGGAAGAGCCC-3′ | 57.8 |
| R: 5′-CGTGGAGGCATTGTTCTGAT-3′ | ||
| AKT | F: 5′-GTCGCCTGCCCTTCTACAAC-3′ | 59.5 |
| R: 5′-CACACGATACCGGCAAAGAA-3′ | ||
| GAPDH | F: 5′-GCACCGTCAAGGCTGAGAAC-3′ | 60 |
| R: 5′-GTGGTGAAGACGCCAGTGGA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-H.; Han, J.W.; Park, H.; Hong, S.J.; Kim, C.-S.; Kim, Y.S.; Lee, I.S.; Kim, G.J. Achyranthis radix Extract Enhances Antioxidant Effect of Placenta-Derived Mesenchymal Stem Cell on Injured Human Ocular Cells. Cells 2024, 13, 1229. https://doi.org/10.3390/cells13141229
Lee D-H, Han JW, Park H, Hong SJ, Kim C-S, Kim YS, Lee IS, Kim GJ. Achyranthis radix Extract Enhances Antioxidant Effect of Placenta-Derived Mesenchymal Stem Cell on Injured Human Ocular Cells. Cells. 2024; 13(14):1229. https://doi.org/10.3390/cells13141229
Chicago/Turabian StyleLee, Dae-Hyun, Ji Woong Han, Hyeri Park, Se Jin Hong, Chan-Sik Kim, Young Sook Kim, Ik Soo Lee, and Gi Jin Kim. 2024. "Achyranthis radix Extract Enhances Antioxidant Effect of Placenta-Derived Mesenchymal Stem Cell on Injured Human Ocular Cells" Cells 13, no. 14: 1229. https://doi.org/10.3390/cells13141229





