Novel Technologies to Address the Lower Motor Neuron Injury and Augment Reconstruction in Spinal Cord Injury
Abstract
:1. Introduction
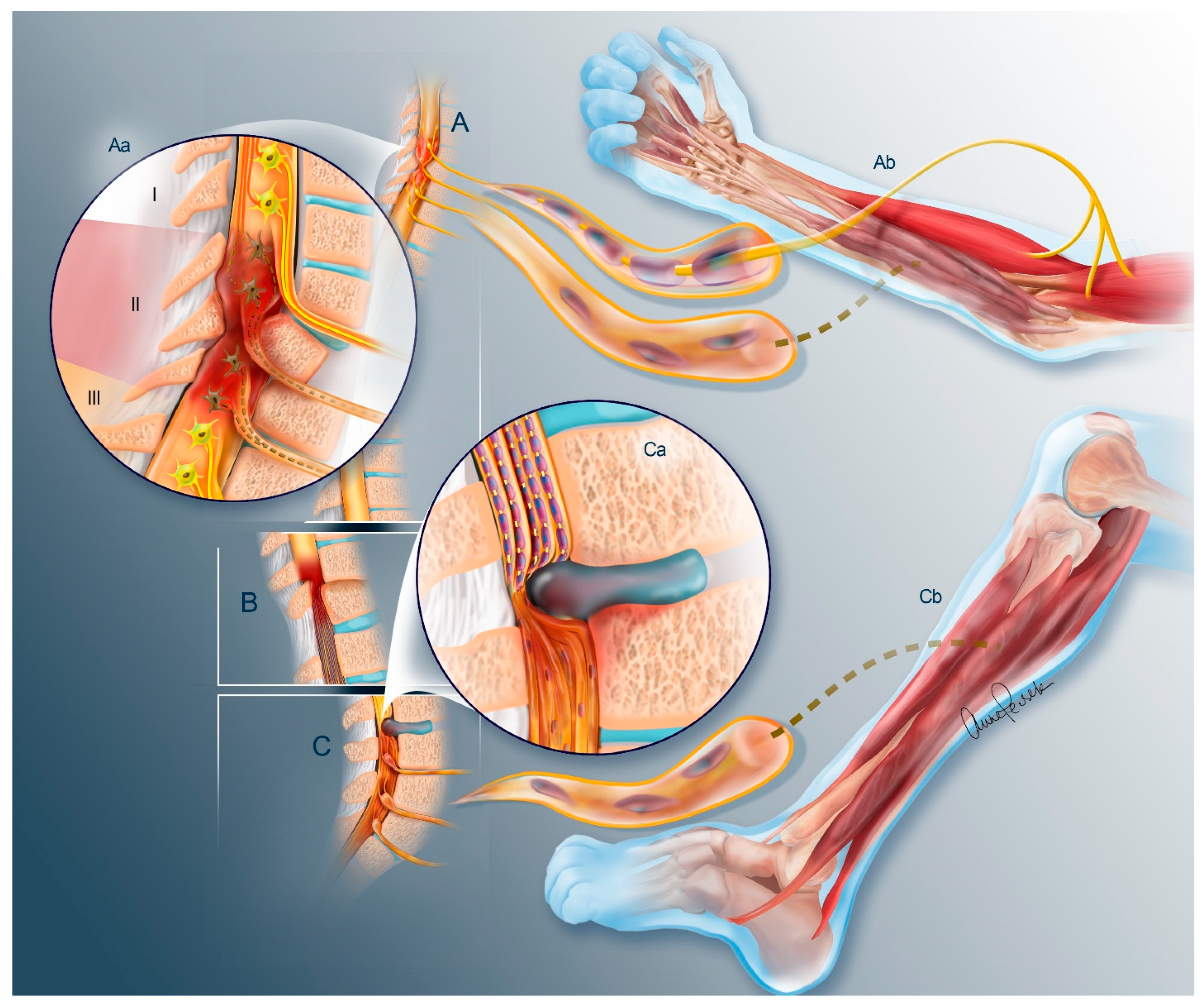
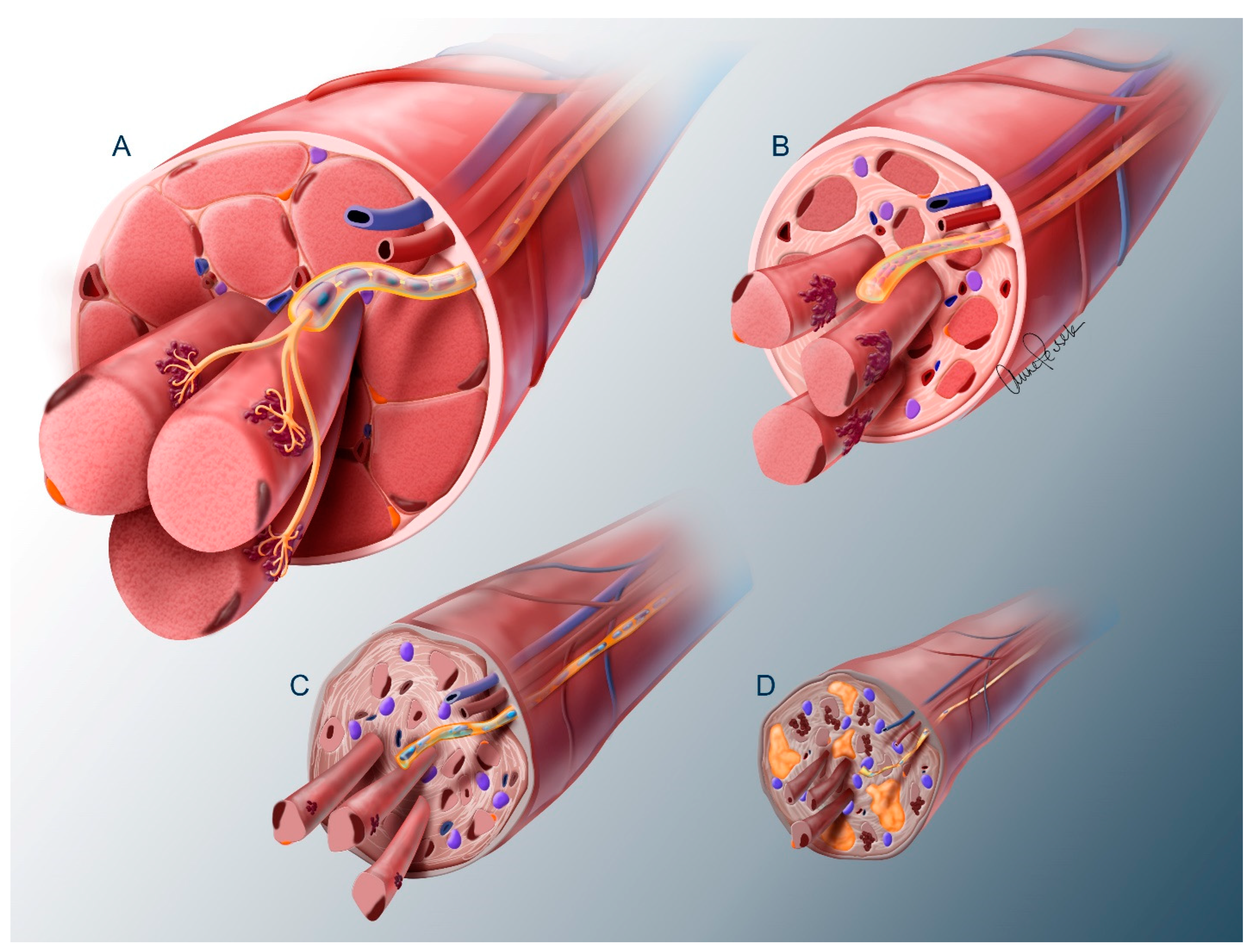
2. The Problem of Denervation Atrophy
2.1. Nerve Trunk
2.2. Neuromuscular Junction
2.3. Muscle
3. Strategies to Preserve Neuromuscular Viability Following Denervation
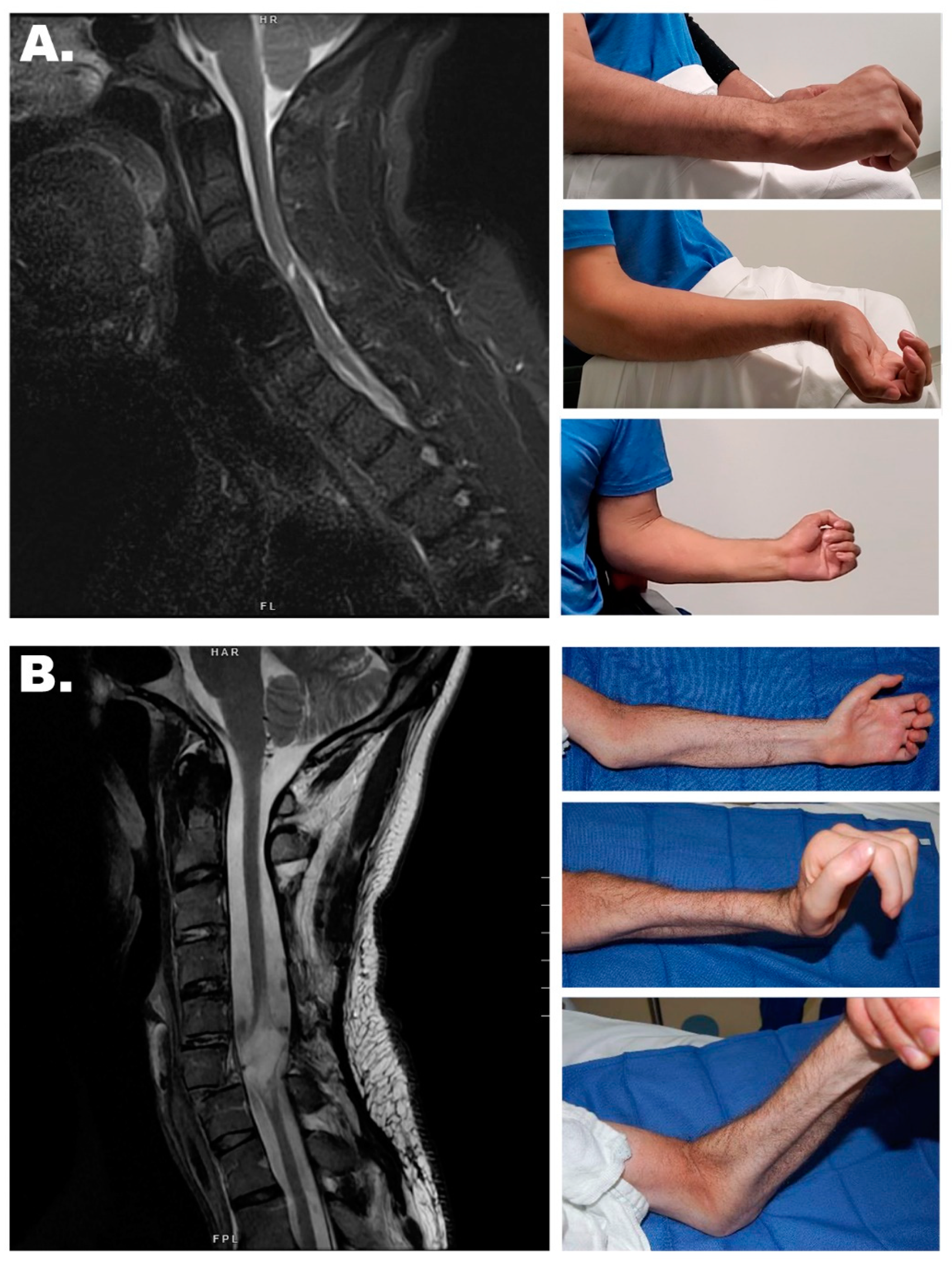
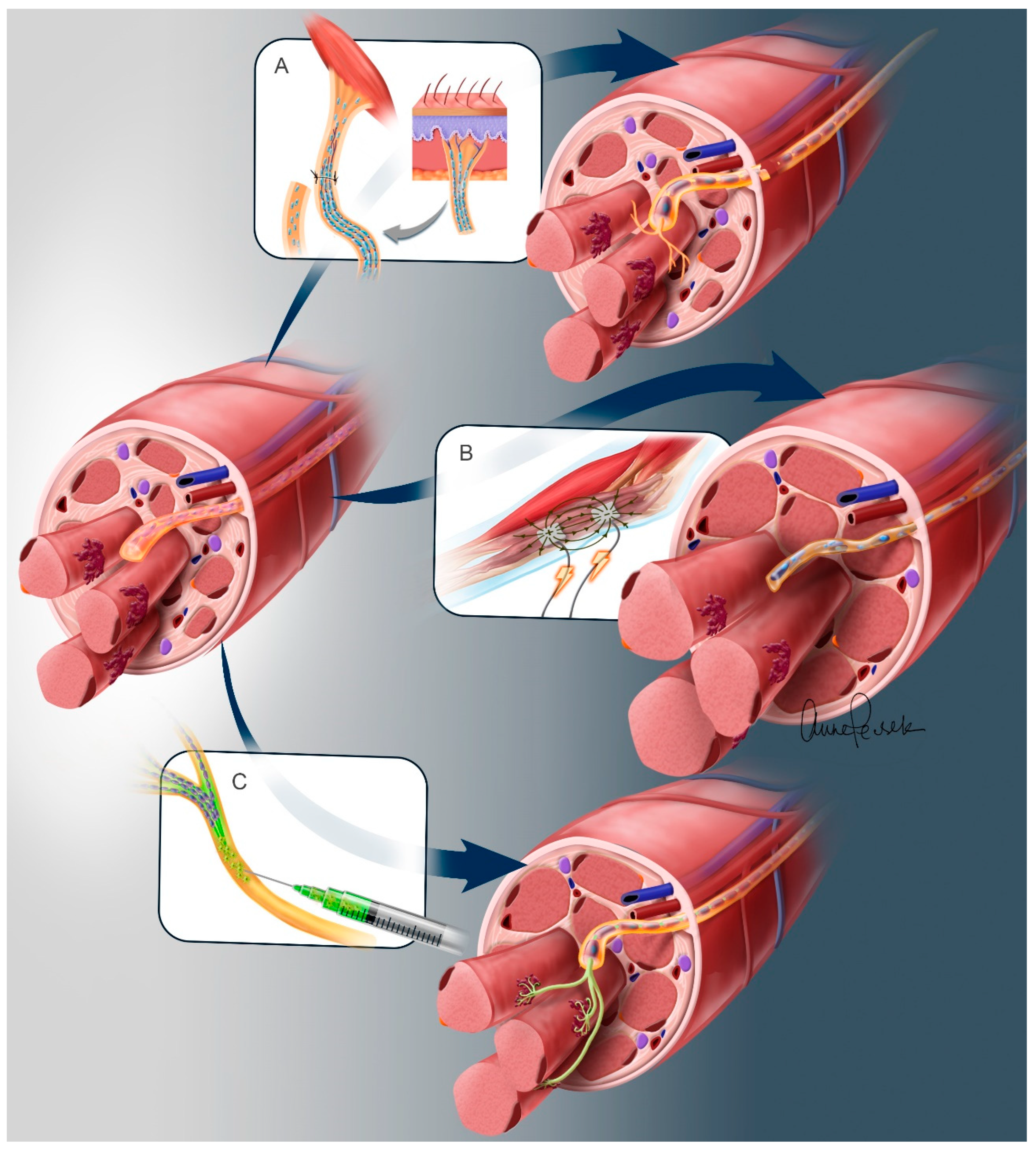
3.1. Nerve Transfers
Sensory Preservation
3.2. Spinal Motor Neuron Transplantation
3.3. Electrical Stimulation of Denervated Muscles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BCI | Brain–computer interface |
| CNS | Central nervous system |
| EMS | High-energy muscle stimulation |
| FAP | Fibro-adipogenic progenitor |
| FES | Functional electrical stimulation |
| IPSC | Induced pluripotent stem cell |
| LMN | Lower motor neuron |
| NMES | Neuromuscular electrical stimulation |
| NMJ | Neuromuscular junction |
| PNS | Peripheral nervous system |
| SC | Schwann cell |
| SCI | Spinal cord injury |
| SMN | Spinal motor neuron |
| UMN | Upper motor neuron |
References
- Rolls, A.; Shechter, R.; Schwartz, M. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 2009, 10, 235–241. [Google Scholar] [CrossRef]
- Varadarajan, S.G.; Hunyara, J.L.; Hamilton, N.R.; Kolodkin, A.L.; Huberman, A.D. Central nervous system regeneration. Cell 2022, 185, 77–94. [Google Scholar] [CrossRef]
- Slutzky, M.W. Brain-Machine Interfaces: Powerful Tools for Clinical Treatment and Neuroscientific Investigations. Neuroscientist 2019, 25, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Bednar, M.S.; Woodside, J.C. Management of Upper Extremities in Tetraplegia: Current Concepts. J. Am. Acad. Orthop. Surg. 2018, 26, e333–e341. [Google Scholar] [CrossRef]
- Dunn, J.A.; Sinnott, K.A.; Rothwell, A.G.; Mohammed, K.D.; Simcock, J.W. Tendon Transfer Surgery for People With Tetraplegia: An Overview. Arch. Phys. Med. Rehabil. 2016, 97, S75–S80. [Google Scholar] [CrossRef] [PubMed]
- Bazarek, S.; Brown, J.M. The evolution of nerve transfers for spinal cord injury. Exp. Neurol. 2020, 333, 113426. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; McMahon, S.B.; Field-Fote, E.C.; Bradbury, E.J. Neuromodulation in the restoration of function after spinal cord injury. Lancet Neurol. 2018, 17, 905–917. [Google Scholar] [CrossRef]
- Herring, E.Z.; Graczyk, E.L.; Memberg, W.D.; Adams, R.D.; Baca-Vaca, G.F.; Hutchison, B.C.; Krall, J.T.; Alexander, B.J.; Conlan, E.C.; Alfaro, K.E.; et al. Reconnecting the Hand and Arm to the Brain: Efficacy of Neural Interfaces for Sensorimotor Restoration after Tetraplegia. medRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, A.B.; Willett, F.R.; Young, D.R.; Memberg, W.D.; Murphy, B.A.; Miller, J.P.; Walter, B.L.; Sweet, J.A.; Hoyen, H.A.; Keith, M.W.; et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: A proof-of-concept demonstration. Lancet 2017, 389, 1821–1830. [Google Scholar] [CrossRef]
- Gordon, T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef]
- Carlson, B.M. The Biology of Long-Term Denervated Skeletal Muscle. Eur. J. Transl. Myol. 2014, 24, 3293. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Tyreman, N.; Raji, M.A. The basis for diminished functional recovery after delayed peripheral nerve repair. J. Neurosci. 2011, 31, 5325–5334. [Google Scholar] [CrossRef] [PubMed]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. Biomed. Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M. Nerve transfers in tetraplegia I: Background and technique. Surg. Neurol. Int. 2011, 2, 121. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; Kern, H.; Rossini, K.; Hofer, C.; Mayr, W.; Carraro, U.; Protasi, F. Structural differentiation of skeletal muscle fibers in the absence of innervation in humans. Proc. Natl. Acad. Sci. USA 2007, 104, 19339–19344. [Google Scholar] [CrossRef] [PubMed]
- Chepla, K.J.; Perkins, B.; Bryden, A.M.; Keith, M.W. Clinical Outcomes of “Paralyzed” Nerve Transfer for Treating Spinal Cord Injury: A Proof of Concept in a Human Model. Cureus 2024, 16, e52447. [Google Scholar] [CrossRef]
- Bazarek, S.; Sten, M.; Thum, J.; Mandeville, R.; Magee, G.; Brown, J.M. Supinator to Posterior Interosseous Nerve Transfer for Recovery of Hand Opening in the Tetraplegic Patient: A Case Series. Neurosurgery 2024, 94, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Bazarek, S.; Sten, M.; Nin, D.; Brown, J.M. Supinator to Posterior Interosseous Nerve Transfer for Restoration of Finger Extension. Oper. Neurosurg. 2021, 21, E408–E413. [Google Scholar] [CrossRef]
- Coleman, M.P.; Höke, A. Programmed axon degeneration: From mouse to mechanism to medicine. Nat. Rev. Neurosci. 2020, 21, 183–196. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef]
- Jonsson, S.; Wiberg, R.; McGrath, A.M.; Novikov, L.N.; Wiberg, M.; Novikova, L.N.; Kingham, P.J. Effect of delayed peripheral nerve repair on nerve regeneration, Schwann cell function and target muscle recovery. PLoS ONE 2013, 8, e56484. [Google Scholar] [CrossRef] [PubMed]
- Sarhane, K.A.; Slavin, B.R.; Hricz, N.; Malapati, H.; Guo, Y.N.; Grzelak, M.; Chang, I.A.; Shappell, H.; von Guionneau, N.; Wong, A.L.; et al. Defining the relative impact of muscle versus Schwann cell denervation on functional recovery after delayed nerve repair. Exp. Neurol. 2021, 339, 113650. [Google Scholar] [CrossRef] [PubMed]
- Rönkkö, H.; Göransson, H.; Siironen, P.; Taskinen, H.S.; Vuorinen, V.; Röyttä, M. The capacity of the distal stump of peripheral nerve to receive growing axons after two and six months denervation. Scand. J. Surg. 2011, 100, 223–229. [Google Scholar] [CrossRef]
- Gordon, T.; Tyreman, N. Sprouting capacity of lumbar motoneurons in normal and hemisected spinal cords of the rat. J. Physiol. 2010, 588, 2745–2768. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The Role of c-Jun and Autocrine Signaling Loops in the Control of Repair Schwann Cells and Regeneration. Front. Cell. Neurosci. 2021, 15, 820216. [Google Scholar] [CrossRef]
- Wagstaff, L.J.; Gomez-Sanchez, J.A.; Fazal, S.V.; Otto, G.W.; Kilpatrick, A.M.; Michael, K.; Wong, L.Y.N.; Ma, K.H.; Turmaine, M.; Svaren, J.; et al. Failures of nerve regeneration caused by aging or chronic denervation are rescued by restoring Schwann cell c-Jun. eLife 2021, 10, e62232. [Google Scholar] [CrossRef] [PubMed]
- Rueger, M.A.; Aras, S.; Guntinas-Lichius, O.; Neiss, W.F. Re-activation of atrophic motor Schwann cells after hypoglossal-facial nerve anastomosis. Neurosci. Lett. 2008, 434, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Wood, P.; Sulaiman, O.A.R. Long-Term Denervated Rat Schwann Cells Retain Their Capacity to Proliferate and to Myelinate Axons in vitro. Front. Cell. Neurosci. 2019, 12, 511. [Google Scholar] [CrossRef]
- Gause Ii, T.M.; Sivak, W.N.; Marra, K.G. The role of chondroitinase as an adjuvant to peripheral nerve repair. Cells Tissues Organs 2014, 200, 59–68. [Google Scholar] [CrossRef]
- Muir, E.; De Winter, F.; Verhaagen, J.; Fawcett, J. Recent advances in the therapeutic uses of chondroitinase ABC. Exp. Neurol. 2019, 321, 113032. [Google Scholar] [CrossRef]
- Yin, X.; Yu, T.; Chen, B.; Xu, J.; Chen, W.; Qi, Y.; Zhang, P.; Li, Y.; Kou, Y.; Ma, Y.; et al. Spatial Distribution of Motor Endplates and its Adaptive Change in Skeletal Muscle. Theranostics 2019, 9, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yokoyama, H.; Kaburagi, H.; Hirai, T.; Tsuji, K.; Enomoto, M.; Wakabayashi, Y.; Okawa, A. Remnant neuromuscular junctions in denervated muscles contribute to functional recovery in delayed peripheral nerve repair. Neural Regen. Res. 2020, 15, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, M.; Gorski, G.; Sheu, S.H.; Lee, S.; Barrett, L.B.; Singh, B.; Omura, T.; Latremoliere, A.; Woolf, C.J.; Gaspar, P. Lack of motor recovery after prolonged denervation of the neuromuscular junction is not due to regenerative failure. Eur. J. Neurosci. 2015, 43, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Chan, J.P.; Uong, J.; Palispis, W.A.; Wright, D.J.; Shah, S.B.; Ward, S.R.; Lee, T.Q.; Steward, O. Human motor endplate remodeling after traumatic nerve injury. J. Neurosurg. 2020, 135, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, G.A.; Brunelli, G.R. Direct muscle neurotization. J. Reconstr. Microsurg. 1993, 9, 81–90, discussion 89–90. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.; Harrison, C.; Eaton, S.L.; Llavero Hurtado, M.; Graham, L.C.; Alkhammash, L.; Oladiran, O.A.; Gale, A.; Lamont, D.J.; Simpson, H.; et al. Cellular and Molecular Anatomy of the Human Neuromuscular Junction. Cell Rep. 2017, 21, 2348–2356. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.-X.; Huang, S.-K.; Carlson, B.M. Electron microscopic study of long-term denervated rat skeletal muscle. Anat. Rec. 1997, 248, 355–365. [Google Scholar] [CrossRef]
- Viguie, C.A.; Lu, D.-X.; Huang, S.-K.; Rengen, H.; Carlson, B.M. Quantitative study of the effects of long-term denervation on the extensor digitorum longus muscle of the rat. Anat. Rec. 1997, 248, 346–354. [Google Scholar] [CrossRef]
- Kern, H.; Boncompagni, S.; Rossini, K.; Mayr, W.; Fanò, G.; Zanin, M.E.; Podhorska-Okolow, M.; Protasi, F.; Carraro, U. Long-term denervation in humans causes degeneration of both contractile and excitation-contraction coupling apparatus, which is reversible by functional electrical stimulation (FES): A role for myofiber regeneration? J. Neuropathol. Exp. Neurol. 2004, 63, 919–931. [Google Scholar] [CrossRef]
- Carraro, U.; Kern, H. Severely Atrophic Human Muscle Fibers with Nuclear Misplacement Survive Many Years of Permanent Denervation. Eur. J. Transl. Myol. 2016, 26, 5894. [Google Scholar] [CrossRef]
- Carraro, U.; Kern, H.; Zampieri, S.; Gargiulo, P.; Pond, A.; Piccione, F.; Masiero, S.; Bassetto, F.; Vindigni, V. Muscle Fiber Regeneration in Long-Term Denervated Muscles: Basics and Clinical Perspectives. In Regenerative Medicine and Plastic Surgery; Springer: Cham, Switzerland, 2019; pp. 301–309. [Google Scholar]
- Carraro, U.; Boncompagni, S.; Gobbo, V.; Rossini, K.; Zampieri, S.; Mosole, S.; Ravara, B.; Nori, A.; Stramare, R.; Ambrosio, F.; et al. Persistent Muscle Fiber Regeneration in Long Term Denervation. Past, Present, Future. Eur. J. Transl. Myol. 2015, 25, 4832. [Google Scholar] [CrossRef]
- Wong, A.; Garcia, S.M.; Tamaki, S.; Striedinger, K.; Barruet, E.; Hansen, S.L.; Young, D.M.; Pomerantz, J.H. Satellite cell activation and retention of muscle regenerative potential after long-term denervation. Stem Cells 2021, 39, 331–344. [Google Scholar] [CrossRef]
- Wong, A.; Pomerantz, J.H. The Role of Muscle Stem Cells in Regeneration and Recovery after Denervation: A Review. Plast. Reconstr. Surg. 2019, 143, 779–788. [Google Scholar] [CrossRef]
- Mussini, I.; Favaro, G.; Carraro, U. Maturation, dystrophic changes and the continuous production of fibers in skeletal muscle regenerating in the absence of nerve. J. Neuropathol. Exp. Neurol. 1987, 46, 315–331. [Google Scholar] [CrossRef]
- Madaro, L.; Passafaro, M.; Sala, D.; Etxaniz, U.; Lugarini, F.; Proietti, D.; Alfonsi, M.V.; Nicoletti, C.; Gatto, S.; De Bardi, M.; et al. Denervation-activated STAT3-IL-6 signalling in fibro-adipogenic progenitors promotes myofibres atrophy and fibrosis. Nat. Cell Biol. 2018, 20, 917–927. [Google Scholar] [CrossRef]
- Joe, A.W.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010, 12, 153–163. [Google Scholar] [CrossRef]
- Contreras, O.; Rossi, F.M.V.; Theret, M. Origins, potency, and heterogeneity of skeletal muscle fibro-adipogenic progenitors-time for new definitions. Skelet. Muscle 2021, 11, 16. [Google Scholar] [CrossRef]
- Garg, K.; Corona, B.T.; Walters, T.J. Therapeutic strategies for preventing skeletal muscle fibrosis after injury. Front. Pharmacol. 2015, 6, 87. [Google Scholar] [CrossRef]
- Bersini, S.; Gilardi, M.; Mora, M.; Krol, S.; Arrigoni, C.; Candrian, C.; Zanotti, S.; Moretti, M. Tackling muscle fibrosis: From molecular mechanisms to next generation engineered models to predict drug delivery. Adv. Drug Deliv. Rev. 2018, 129, 64–77. [Google Scholar] [CrossRef]
- Bain, J.R.; Veltri, K.L.; Chamberlain, D.; Fahnestock, M. Improved functional recovery of denervated skeletal muscle after temporary sensory nerve innervation. Neuroscience 2001, 103, 503–510. [Google Scholar] [CrossRef]
- Fu, S.Y.; Gordon, T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged denervation. J. Neurosci. 1995, 15, 3886–3895. [Google Scholar] [CrossRef] [PubMed]
- Barbour, J.; Yee, A.; Kahn, L.C.; Mackinnon, S.E. Supercharged end-to-side anterior interosseous to ulnar motor nerve transfer for intrinsic musculature reinnervation. J. Hand Surg. Am. 2012, 37, 2150–2159. [Google Scholar] [CrossRef]
- Terzis, J.K.; Tzafetta, K. The “babysitter” procedure: Minihypoglossal to facial nerve transfer and cross-facial nerve grafting. Plast. Reconstr. Surg. 2009, 123, 865–876. [Google Scholar] [CrossRef]
- Adidharma, W.; Khouri, A.N.; Lee, J.C.; Vanderboll, K.; Kung, T.A.; Cederna, P.S.; Kemp, S.W.P. Sensory nerve regeneration and reinnervation in muscle following peripheral nerve injury. Muscle Nerve 2022, 66, 384–396. [Google Scholar] [CrossRef]
- Veltri, K.; Kwiecien, J.M.; Minet, W.; Fahnestock, M.; Bain, J.R. Contribution of the distal nerve sheath to nerve and muscle preservation following denervation and sensory protection. J. Reconstr. Microsurg. 2005, 21, 57–70, discussion 71–54. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, K.C.; Kamin, E.; Terzis, J.K. Muscle preservation by prolonged sensory protection. J. Reconstr. Microsurg. 2002, 18, 173–182, discussion 183–174. [Google Scholar] [CrossRef]
- Li, Q.T.; Zhang, P.X.; Yin, X.F.; Han, N.; Kou, Y.H.; Deng, J.X.; Jiang, B.G. Functional recovery of denervated skeletal muscle with sensory or mixed nerve protection: A pilot study. PLoS ONE 2013, 8, e79746. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.R.; Hason, Y.; Veltri, K.; Fahnestock, M.; Quartly, C. Clinical application of sensory protection of denervated muscle. J. Neurosurg. 2008, 109, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, M.R.; Braga-Silva, J.; Gehlen, D.; Pereira-Filho Gde, A.; Zettler, C.G.; de Souza, M.A.; Veas, J.R.; Sebben, A. End-to-end versus end-to-side motor and sensory neurorrhaphy in the repair of the acute muscle denervation. Ann. Plast. Surg. 2011, 67, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Bazarek, S.; Johnston, B.R.; Sten, M.; Mandeville, R.; Eggan, K.; Wainger, B.J.; Brown, J.M. Spinal motor neuron transplantation to enhance nerve reconstruction strategies: Towards a cell therapy. Exp. Neurol. 2022, 353, 114054. [Google Scholar] [CrossRef]
- Kurimoto, S.; Kato, S.; Nakano, T.; Yamamoto, M.; Takanobu, N.; Hirata, H. Transplantation of embryonic motor neurons into peripheral nerve combined with functional electrical stimulation restores functional muscle activity in the rat sciatic nerve transection model. J. Tissue Eng. Regen. Med. 2016, 10, E477–E484. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Tsunemoto, R.K.; Eade, K.T.; Blanchard, J.W.; Baldwin, K.K. Forward engineering neuronal diversity using direct reprogramming. EMBO J. 2015, 34, 1445–1455. [Google Scholar] [CrossRef]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Nam, Y.; Rim, Y.A.; Ju, J.H. Review of the Current Trends in Clinical Trials Involving Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 2022, 18, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, M.W.; Croft, G.F.; Williams, D.J.; O’Keeffe, S.; Carrasco, M.A.; Davis, A.R.; Roybon, L.; Oakley, D.H.; Maniatis, T.; Henderson, C.E.; et al. Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. J. Neurosci. 2013, 33, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, H.; Lieberam, I.; Porter, J.A.; Jessell, T.M. Directed differentiation of embryonic stem cells into motor neurons. Cell 2002, 110, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; White, K.; Olroyd, A.G.; DeJesus, R.; Dominguez, A.A.; Dowdle, W.E.; Friera, A.M.; Young, C.; Wells, F.; Chu, E.Y.; et al. Hypoimmune induced pluripotent stem cells survive long term in fully immunocompetent, allogeneic rhesus macaques. Nat. Biotechnol. 2024, 42, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Pizzato, H.A.; Alonso-Guallart, P.; Woods, J.; Connelly, J.P.; Fehniger, T.A.; Atkinson, J.P.; Pruett-Miller, S.M.; Monsma, F.J., Jr.; Bhattacharya, D. Engineering human pluripotent stem cell lines to evade xenogeneic transplantation barriers. Stem Cell Rep. 2024, 19, 299–313. [Google Scholar] [CrossRef]
- Fernandopulle, M.S.; Prestil, R.; Grunseich, C.; Wang, C.; Gan, L.; Ward, M.E. Transcription Factor-Mediated Differentiation of Human iPSCs into Neurons. Curr. Protoc. Cell Biol. 2018, 79, e51. [Google Scholar] [CrossRef]
- Bryson, J.B.; Machado, C.B.; Crossley, M.; Stevenson, D.; Bros-Facer, V.; Burrone, J.; Greensmith, L.; Lieberam, I. Optical control of muscle function by transplantation of stem cell-derived motor neurons in mice. Science 2014, 344, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Toma, J.S.; Shettar, B.C.; Chipman, P.H.; Pinto, D.M.; Borowska, J.P.; Ichida, J.K.; Fawcett, J.P.; Zhang, Y.; Eggan, K.; Rafuse, V.F. Motoneurons derived from induced pluripotent stem cells develop mature phenotypes typical of endogenous spinal motoneurons. J. Neurosci. 2015, 35, 1291–1306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fang, X.; Zhang, C.; Li, W.; Wong, W.M.; Xu, Y.; Wu, W.; Lin, J. Transplantation of embryonic spinal cord neurons to the injured distal nerve promotes axonal regeneration after delayed nerve repair. Eur. J. Neurosci. 2017, 45, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Burrell, J.C.; Das, S.; Laimo, F.A.; Katiyar, K.S.; Browne, K.D.; Shultz, R.B.; Tien, V.J.; Vu, P.T.; Petrov, D.; Ali, Z.S.; et al. Engineered neuronal microtissue provides exogenous axons for delayed nerve fusion and rapid neuromuscular recovery in rats. Bioact. Mater. 2022, 18, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Yohn, D.C.; Miles, G.B.; Rafuse, V.F.; Brownstone, R.M. Transplanted mouse embryonic stem-cell-derived motoneurons form functional motor units and reduce muscle atrophy. J. Neurosci. 2008, 28, 12409–12418. [Google Scholar] [CrossRef] [PubMed]
- Pepper, J.P.; Wang, T.V.; Hennes, V.; Sun, S.Y.; Ichida, J.K. Human Induced Pluripotent Stem Cell-Derived Motor Neuron Transplant for Neuromuscular Atrophy in a Mouse Model of Sciatic Nerve Injury. JAMA Facial Plast. Surg. 2017, 19, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wang, L.; Cai, J.; Yuan, Q.; Yang, X.; Yao, X.; Wong, W.M.; Huang, W.; Li, Z.; Wan, J.B.; et al. Transplanted motoneurons derived from human induced pluripotent stem cells form functional connections with target muscle. Stem Cell Res. 2013, 11, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Bersch, I.; Fridén, J. Electrical stimulation alters muscle morphological properties in denervated upper limb muscles. EBioMedicine 2021, 74, 103737. [Google Scholar] [CrossRef] [PubMed]
- Nix, W.A. Effects of intermittent high frequency electrical stimulation on denervated EDL muscle of rabbit. Muscle Nerve 1990, 13, 580–585. [Google Scholar] [CrossRef]
- Piccinini, G.; Cuccagna, C.; Caliandro, P.; Coraci, D.; Germanotta, M.; Pecchioli, C.; Padua, L. Efficacy of electrical stimulation of denervated muscle: A multicenter, double-blind, randomized clinical trial. Muscle Nerve 2020, 61, 773–778. [Google Scholar] [CrossRef]
- Woodcock, A.H.; Taylor, P.N.; Ewins, D.J. Long pulse biphasic electrical stimulation of denervated muscle. Artif. Organs 1999, 23, 457–459. [Google Scholar] [CrossRef]
- Ashley, Z.; Sutherland, H.; Lanmuller, H.; Unger, E.; Li, F.; Mayr, W.; Kern, H.; Jarvis, J.C.; Salmons, S. Determination of the chronaxie and rheobase of denervated limb muscles in conscious rabbits. Artif. Organs 2005, 29, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Ashley, Z.; Sutherland, H.; Russold, M.F.; Lanmüller, H.; Mayr, W.; Jarvis, J.C.; Salmons, S. Therapeutic stimulation of denervated muscles: The influence of pattern. Muscle Nerve 2008, 38, 875–886. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Davis, J.; Bersch, I.; Goldberg, G.; Gorgey, A.S. Electrical stimulation and denervated muscles after spinal cord injury. Neural Regen. Res. 2020, 15, 1397–1407. [Google Scholar] [CrossRef]
- Mödlin, M.; Forstner, C.; Hofer, C.; Mayr, W.; Richter, W.; Carraro, U.; Protasi, F.; Kern, H. Electrical stimulation of denervated muscles: First results of a clinical study. Artif. Organs 2005, 29, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Kern, H.; Carraro, U. Home-Based Functional Electrical Stimulation of Human Permanent Denervated Muscles: A Narrative Review on Diagnostics, Managements, Results and Byproducts Revisited 2020. Diagnostics 2020, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Bersch, I.; Mayr, W. Electrical stimulation in lower motoneuron lesions, from scientific evidence to clinical practice: A successful transition. Eur. J. Transl. Myol. 2023, 33, 11230. [Google Scholar] [CrossRef]
- Ashley, Z.; Salmons, S.; Boncompagni, S.; Protasi, F.; Russold, M.; Lanmuller, H.; Mayr, W.; Sutherland, H.; Jarvis, J.C. Effects of chronic electrical stimulation on long-term denervated muscles of the rabbit hind limb. J. Muscle Res. Cell Motil. 2007, 28, 203–217. [Google Scholar] [CrossRef]
- Bueno, C.R.S.; Pereira, M.; Favaretto, I.A.J.; Bortoluci, C.H.F.; Santos, T.; Dias, D.V.; Daré, L.R.; Rosa, G.M.J. Electrical stimulation attenuates morphological alterations and prevents atrophy of the denervated cranial tibial muscle. Einstein 2017, 15, 71–76. [Google Scholar] [CrossRef]
- Kern, H.; Hofer, C.; Mayr, W.; Carraro, U.; Löfler, S.; Vogelauer, M.; Mödlin, M.; Forstner, C.; Bijak, M.; Rafolt, D.; et al. European Project RISE: Partners, protocols, demography. Basic Appl Myol/Eur. J. Transl. Myol. 2009, 19, 211–216. [Google Scholar]
- Kern, H.; Carraro, U.; Adami, N.; Biral, D.; Hofer, C.; Forstner, C.; Mödlin, M.; Vogelauer, M.; Pond, A.; Boncompagni, S.; et al. Home-based functional electrical stimulation rescues permanently denervated muscles in paraplegic patients with complete lower motor neuron lesion. Neurorehabil. Neural Repair 2010, 24, 709–721. [Google Scholar] [CrossRef]
- Carraro, U.; Rossini, K.; Mayr, W.; Kern, H. Muscle fiber regeneration in human permanent lower motoneuron denervation: Relevance to safety and effectiveness of FES-training, which induces muscle recovery in SCI subjects. Artif. Organs 2005, 29, 187–191. [Google Scholar] [CrossRef]
- Tam, S.L.; Archibald, V.; Jassar, B.; Tyreman, N.; Gordon, T. Increased neuromuscular activity reduces sprouting in partially denervated muscles. J. Neurosci. 2001, 21, 654–667. [Google Scholar] [CrossRef]
- Jaweed, M.M.; Herbison, G.J.; Ditunno, J.F. Direct electrical stimulation of rat soleus during denervation-reinnervation. Exp. Neurol. 1982, 75, 589–599. [Google Scholar] [CrossRef]
- Hennig, R. Late reinnervation of the rat soleus muscle is differentially suppressed by chronic stimulation and by ectopic innervation. Acta Physiol. Scand. 1987, 130, 153–160. [Google Scholar] [CrossRef]
- Williams, H.B. The value of continuous electrical muscle stimulation using a completely implantable system in the preservation of muscle function following motor nerve injury and repair: An experimental study. Microsurgery 1996, 17, 589–596. [Google Scholar] [CrossRef]
- Zealear, D.L.; Rodriguez, R.J.; Kenny, T.; Billante, M.J.; Cho, Y.; Billante, C.R.; Garren, K.C. Electrical stimulation of a denervated muscle promotes selective reinnervation by native over foreign motoneurons. J. Neurophysiol. 2002, 87, 2195–2199. [Google Scholar] [CrossRef]
- Marqueste, T.; Decherchi, P.; Desplanches, D.; Favier, R.; Grelot, L.; Jammes, Y. Chronic electrostimulation after nerve repair by self-anastomosis: Effects on the size, the mechanical, histochemical and biochemical muscle properties. Acta Neuropathol. 2006, 111, 589–600. [Google Scholar] [CrossRef]
- Willand, M.P.; Holmes, M.; Bain, J.R.; Fahnestock, M.; de Bruin, H. Determining the effects of electrical stimulation on functional recovery of denervated rat gastrocnemius muscle using motor unit number estimation. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2011, 2011, 1977–1980. [Google Scholar] [CrossRef] [PubMed]
- Willand, M.P.; Chiang, C.D.; Zhang, J.J.; Kemp, S.W.; Borschel, G.H.; Gordon, T. Daily Electrical Muscle Stimulation Enhances Functional Recovery Following Nerve Transection and Repair in Rats. Neurorehabil. Neural Repair 2015, 29, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Cercone, M.; Jarvis, J.C.; Ducharme, N.G.; Perkins, J.; Piercy, R.J.; Willand, M.P.; Mitchell, L.M.; Sledziona, M.; Soderholm, L.; Cheetham, J. Functional electrical stimulation following nerve injury in a large animal model. Muscle Nerve 2019, 59, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Jiang, L.; Peng, Y.; Li, Z.; Liu, S.; Lu, J.; Zhang, F.; Zhang, J. Electrical Muscle Stimulation Accelerates Functional Recovery After Nerve Injury. Neuroscience 2020, 426, 179–188. [Google Scholar] [CrossRef]
- Puls, W.C.; Jarvis, J.C.; Ruck, A.; Lehmann, T.; Guntinas-Lichius, O.; Volk, G.F. Surface electrical stimulation for facial paralysis is not harmful. Muscle Nerve 2020, 61, 347–353. [Google Scholar] [CrossRef]
- Sommerauer, L.; Engelmann, S.; Ruewe, M.; Anker, A.; Prantl, L.; Kehrer, A. Effects of electrostimulation therapy in facial nerve palsy. Arch. Plast. Surg. 2021, 48, 278–281. [Google Scholar] [CrossRef]
- Scholz, T.; Pharaon, M.; Evans, G.R. Peripheral nerve anatomy for regeneration studies in pigs: Feasibility of large animal models. Ann. Plast. Surg. 2010, 65, 43–47. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazarek, S.F.; Krenn, M.J.; Shah, S.B.; Mandeville, R.M.; Brown, J.M. Novel Technologies to Address the Lower Motor Neuron Injury and Augment Reconstruction in Spinal Cord Injury. Cells 2024, 13, 1231. https://doi.org/10.3390/cells13141231
Bazarek SF, Krenn MJ, Shah SB, Mandeville RM, Brown JM. Novel Technologies to Address the Lower Motor Neuron Injury and Augment Reconstruction in Spinal Cord Injury. Cells. 2024; 13(14):1231. https://doi.org/10.3390/cells13141231
Chicago/Turabian StyleBazarek, Stanley F., Matthias J. Krenn, Sameer B. Shah, Ross M. Mandeville, and Justin M. Brown. 2024. "Novel Technologies to Address the Lower Motor Neuron Injury and Augment Reconstruction in Spinal Cord Injury" Cells 13, no. 14: 1231. https://doi.org/10.3390/cells13141231
APA StyleBazarek, S. F., Krenn, M. J., Shah, S. B., Mandeville, R. M., & Brown, J. M. (2024). Novel Technologies to Address the Lower Motor Neuron Injury and Augment Reconstruction in Spinal Cord Injury. Cells, 13(14), 1231. https://doi.org/10.3390/cells13141231





