Synovium-Derived and Bone-Derived Mesenchymal Stem/Stromal Cells from Early OA Patients Show Comparable In Vitro Properties to Those of Non-OA Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Inclusion and Tissue Sampling
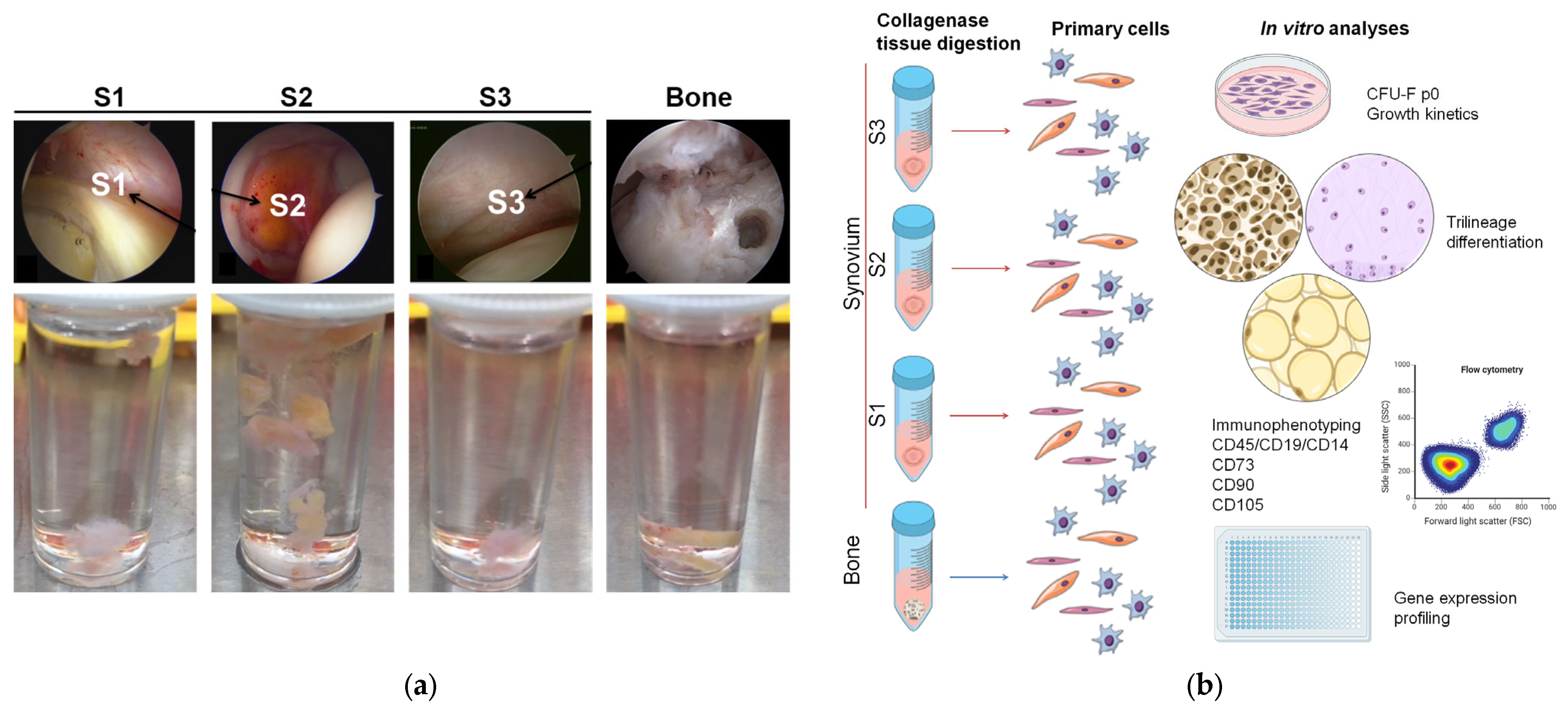
2.2. Primary Cell Isolation
2.3. Colony-Forming Unit Fibroblast Assay
2.4. Cell Expansion
2.5. Multilineage Differentiation
2.6. Immunophenotyping
2.7. Gene Expression
2.8. Statistical Analysis
3. Results
3.1. Patients Characteristics
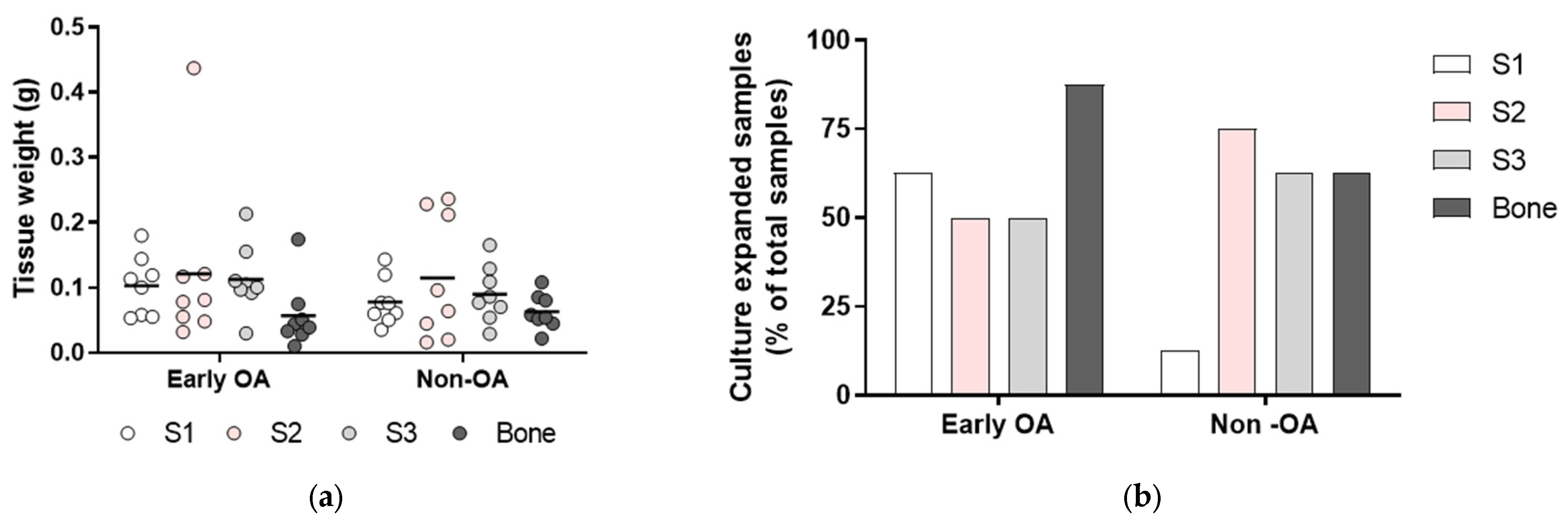
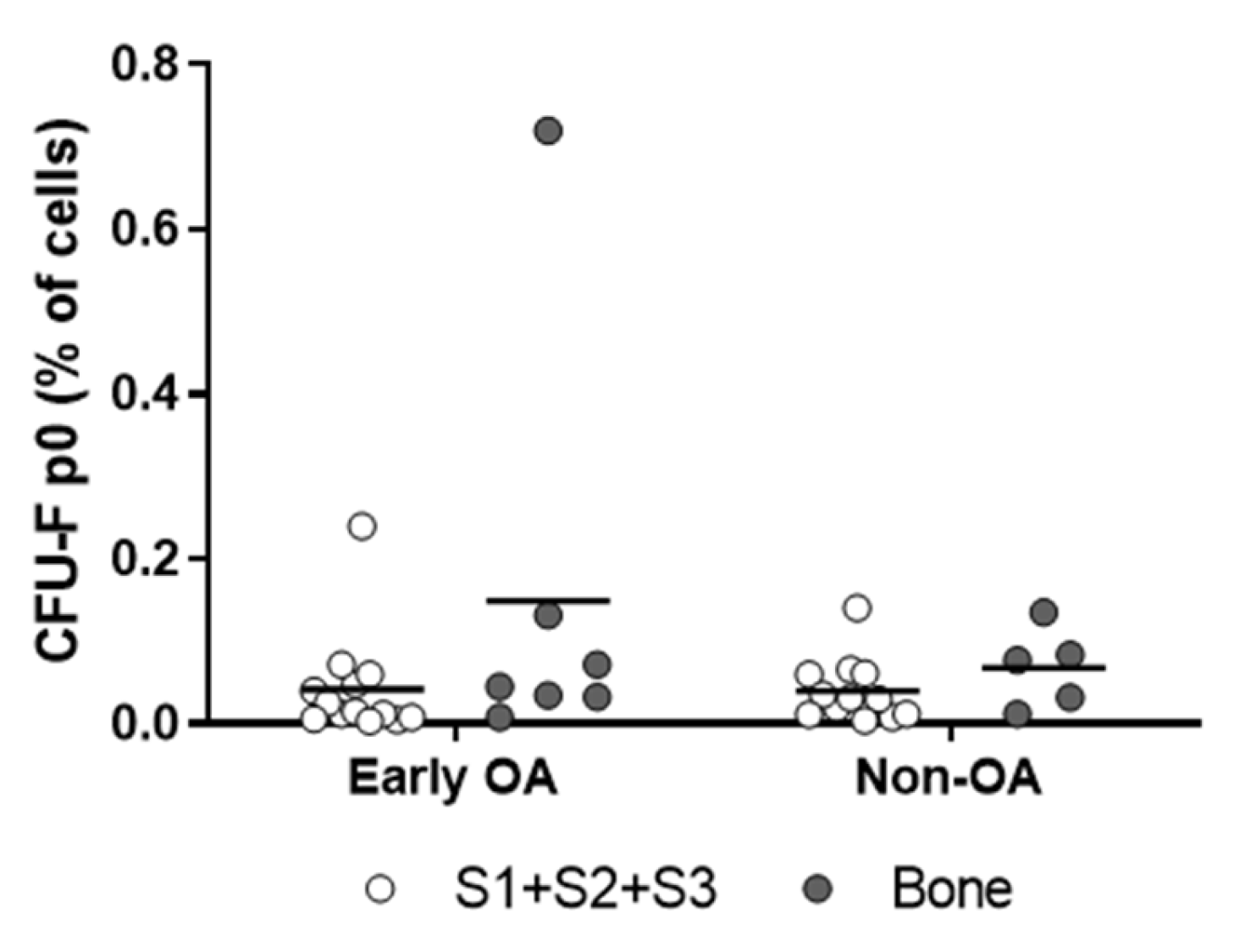
3.2. Tissue Samples and Isolation Efficacy of the Primary Cells
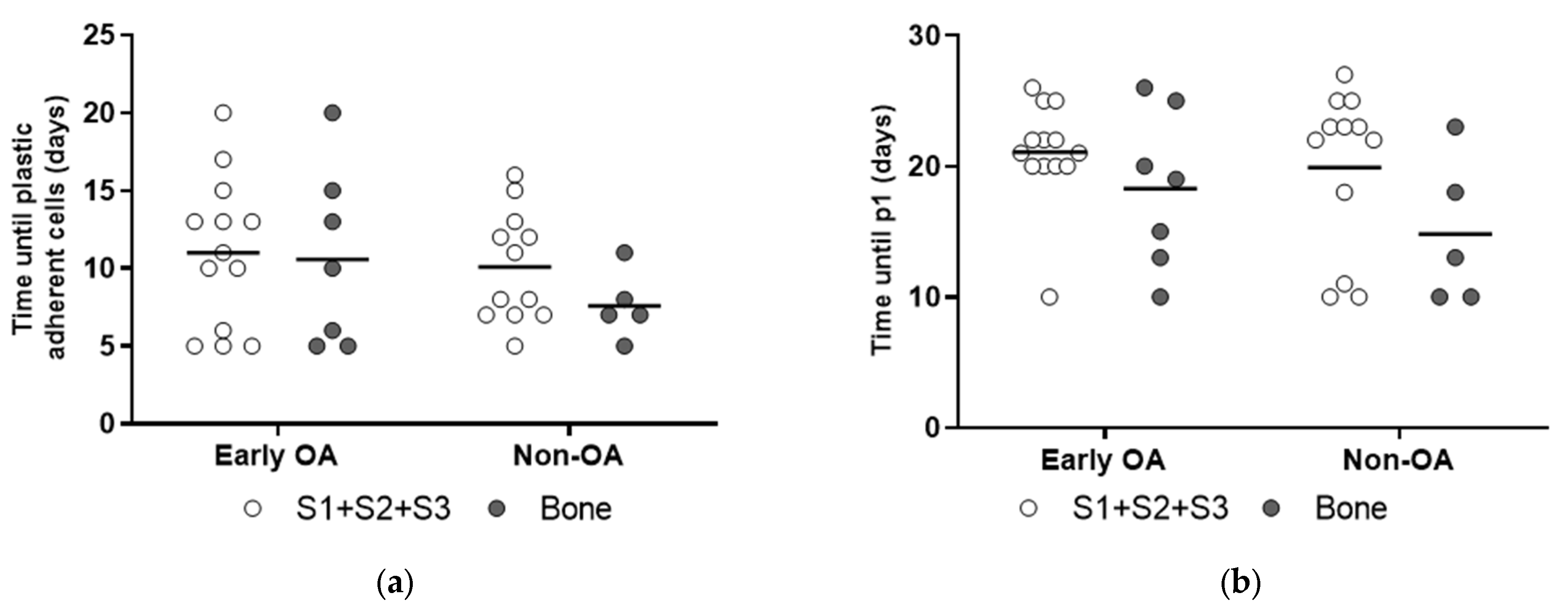
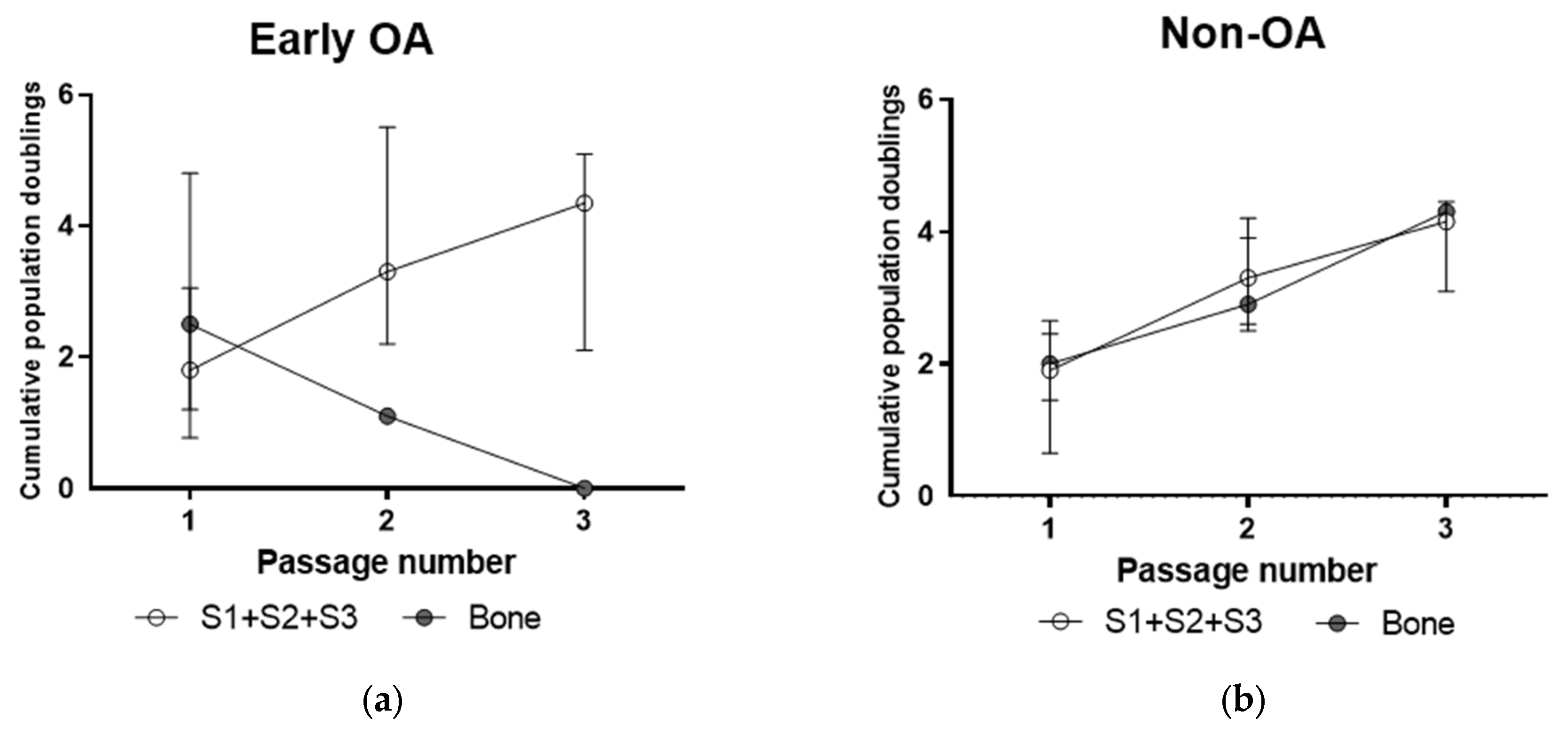
3.3. Culture Expansion and Growth Kinetics
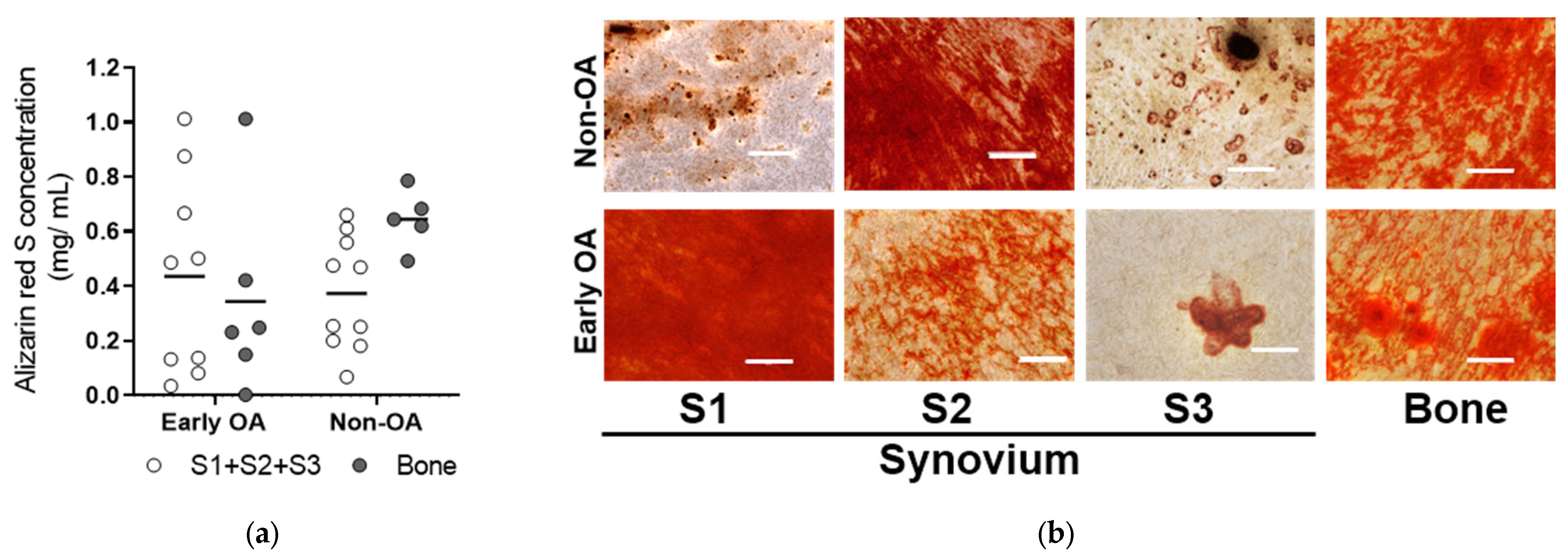
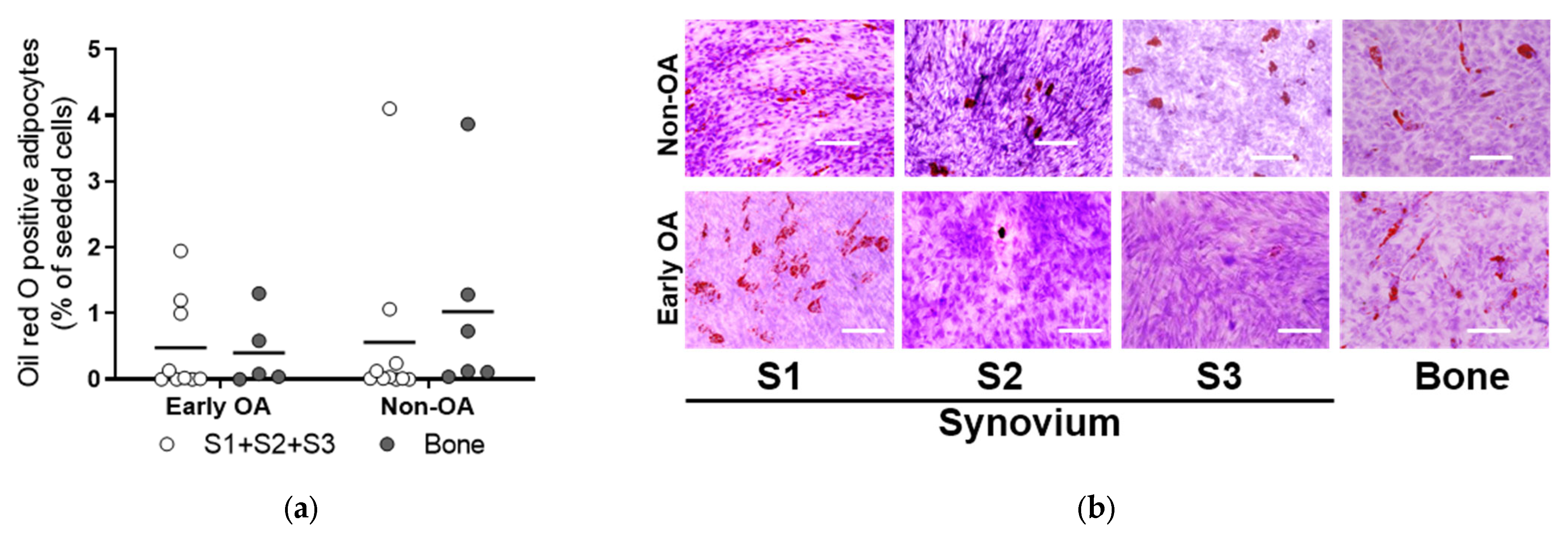
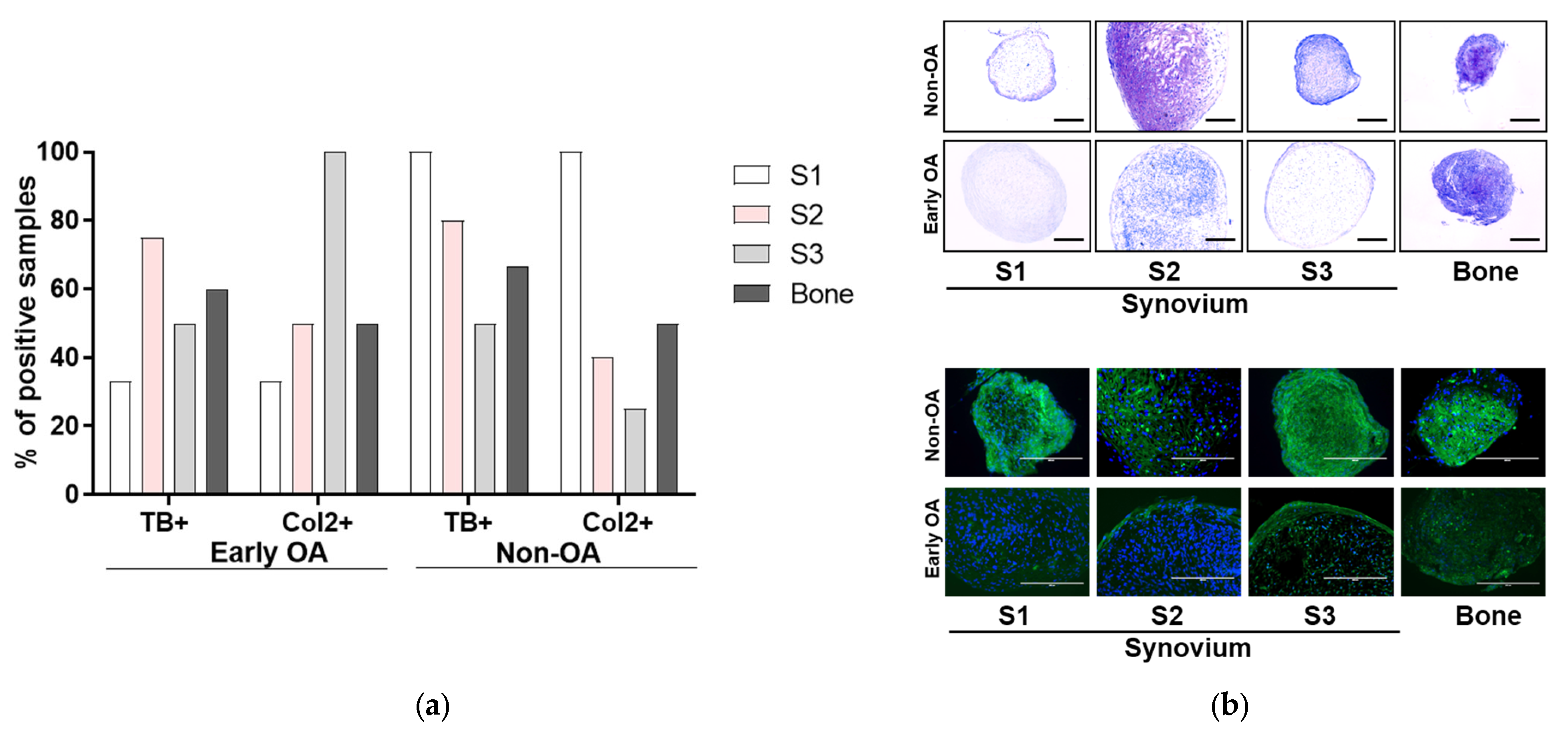
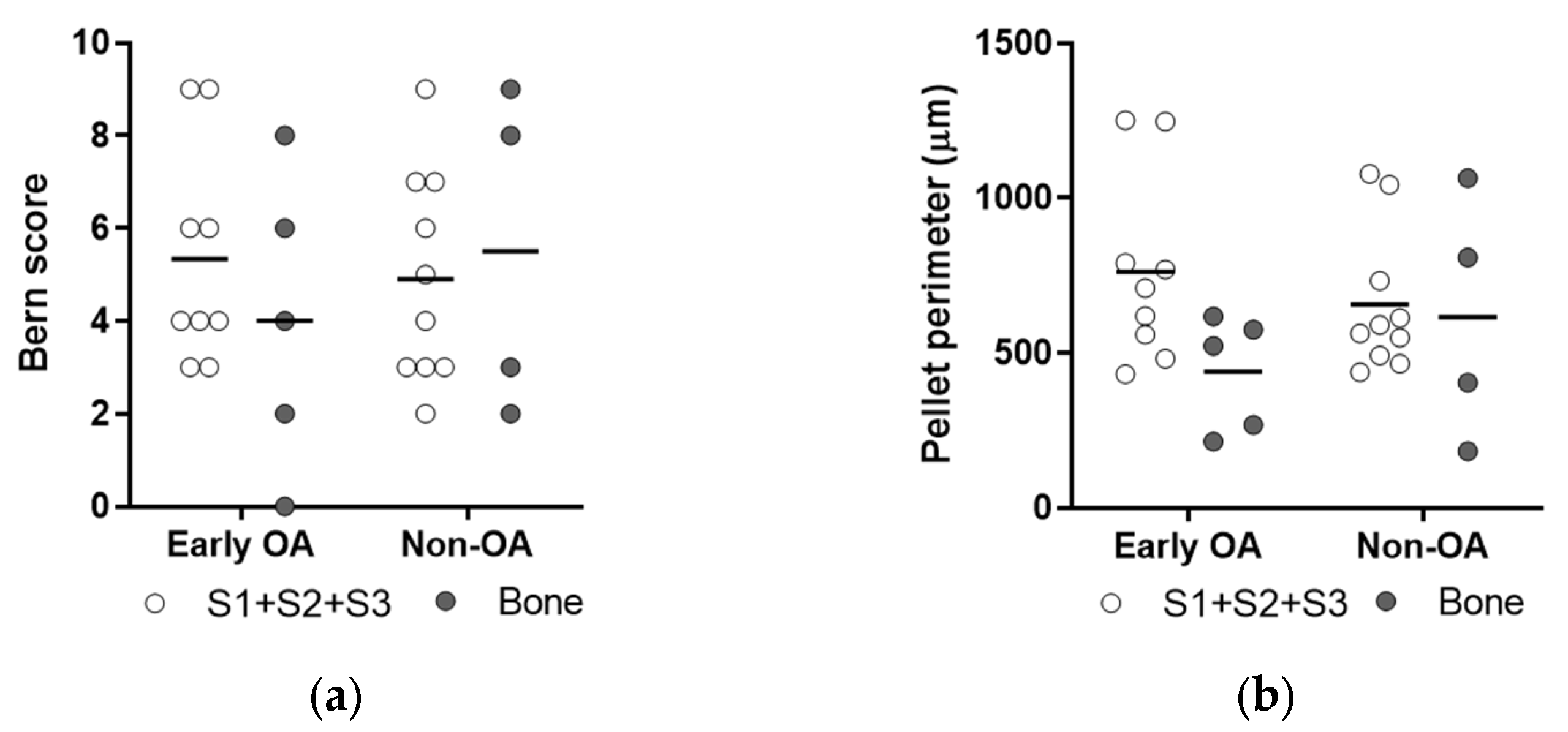
3.4. Multilineage Differentiation
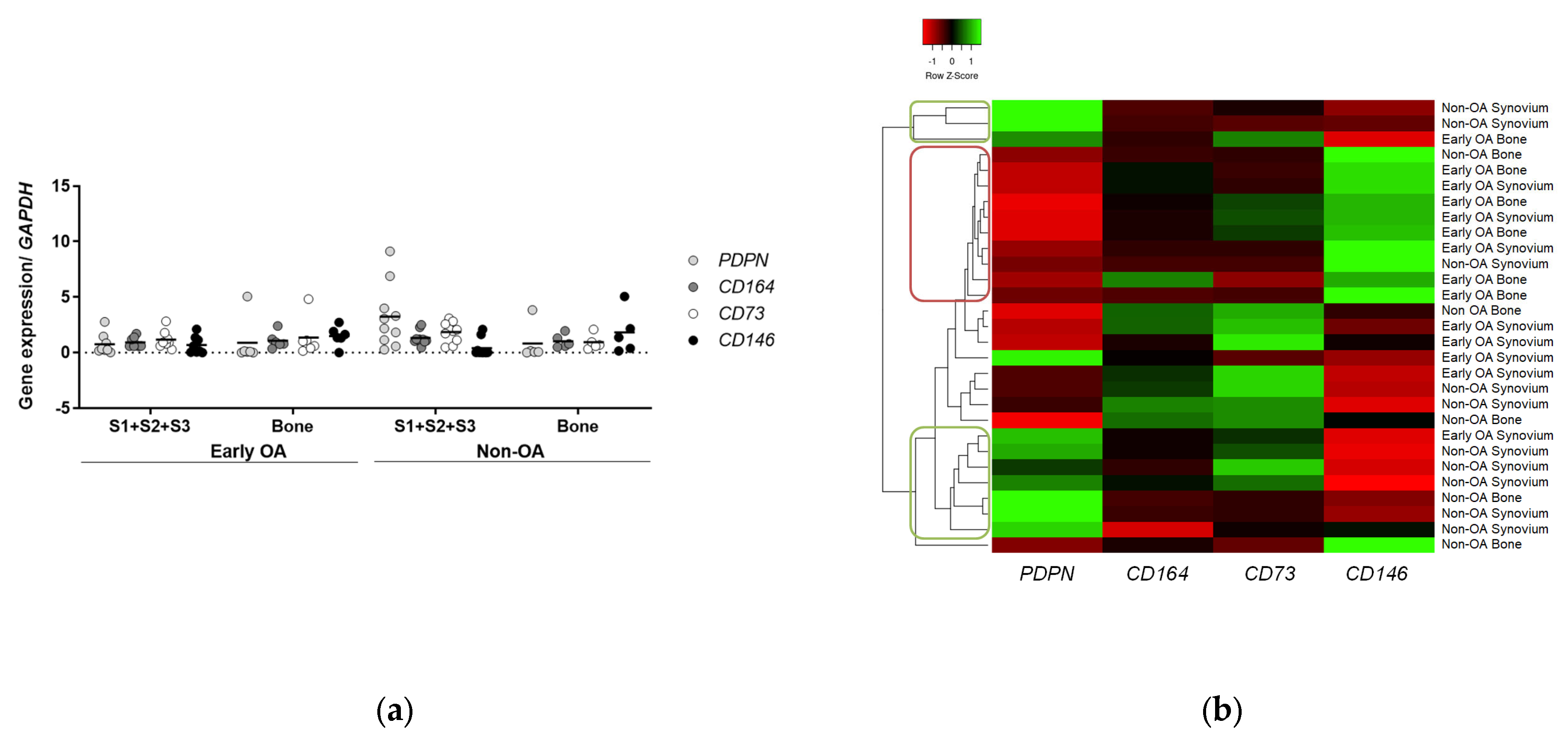
3.5. Immunophenotyping
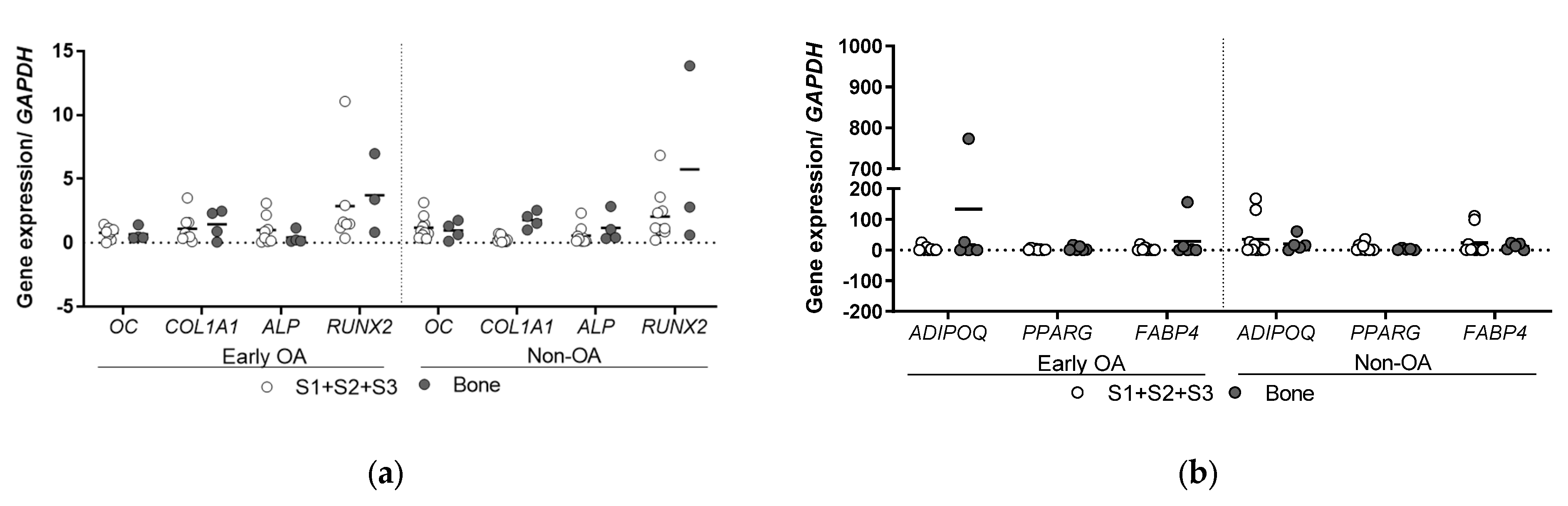
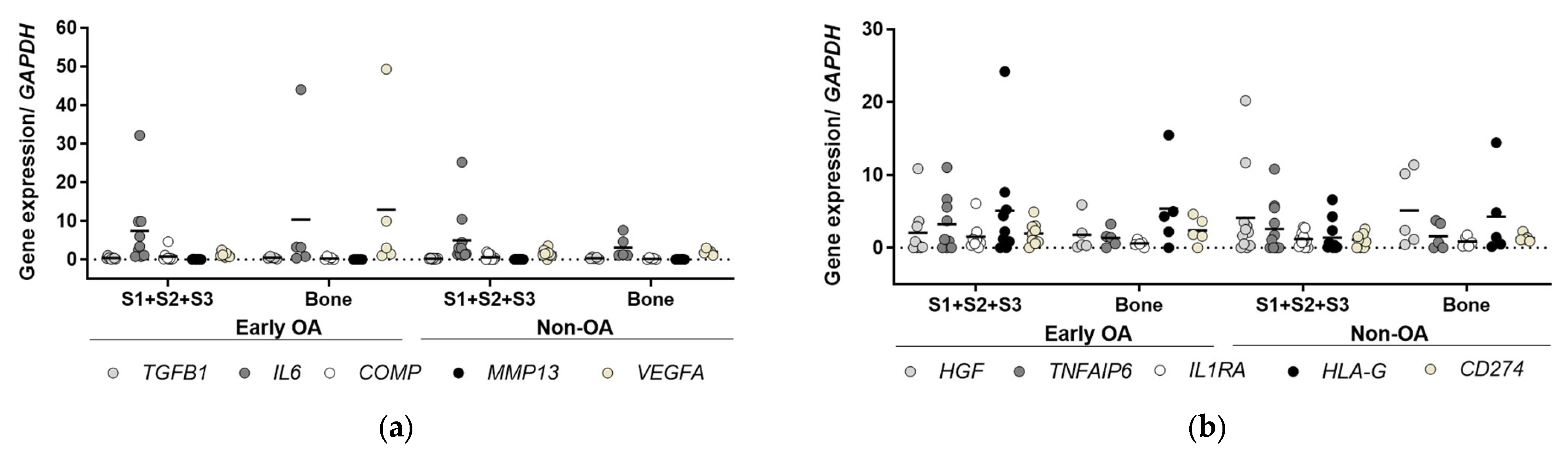
3.6. Gene Expression Profiling
3.6.1. Clusters of Cells from Non-OA Patients Express Similar Profiles of PDPN and CD146 Markers to Human Skeletal Stem Cells
3.6.2. Inflammatory and Immunomodulatory Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roelofs, A.J.; Zupan, J.; Riemen, A.H.K.; Kania, K.; Ansboro, S.; White, N.; Clark, S.M.; De Bari, C. Joint morphogenetic cells in the adult mammalian synovium. Nat. Commun. 2017, 8, 15040. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Akpancar, S.; Tatar, O.; Turgut, H.; Akyildiz, F.; Ekinci, S. The current perspectives of stem cell therapy in orthopedic surgery. Arch. Trauma Res. 2016, 5, e37976. [Google Scholar] [CrossRef] [PubMed]
- Cotter, E.J.; Wang, K.C.; Yanke, A.B.; Chubinskaya, S. Bone marrow aspirate concentrate for cartilage defects of the knee: From bench to bedside evidence. Cartilage 2018, 9, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Čamernik, K.; Mihelič, A.; Mihalič, R.; Haring, G.; Herman, S.; Marolt Presen, D.; Janež, A.; Trebše, R.; Marc, J.; Zupan, J. Comprehensive analysis of skeletal muscle- and bone-derived mesenchymal stem/stromal cells in patients with osteoarthritis and femoral neck fracture. Stem Cell Res. Ther. 2020, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Dixon, K.; Beck, S.; Fabian, D.; Feldman, A.; Barry, F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002, 46, 704–713. [Google Scholar] [CrossRef]
- Campbell, T.M.; Churchman, S.M.; Gomez, A.; Mcgonagle, D.; Conaghan, P.G.; Ponchel, F.; Jones, E. Mesenchymal stem cell alterations in bone marrow lesions in patients with hip osteoarthritis. Arthritis Rheum. 2016, 68, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, C.T.; Hu, N.; Li, J.; Lemme, N.; Terek, R.; Ehrlich, M.G.; Chen, Q. Molecular characterization of mesenchymal stem cells in human osteoarthritis cartilage reveals contribution to the OA phenotype. Sci. Rep. 2018, 8, 7044. [Google Scholar] [CrossRef] [PubMed]
- Brady, K.; Dickinson, S.C.; Hollander, A.P. Changes in chondrogenic progenitor populations associated with aging and osteoarthritis. Cartilage 2015, 6, 30–35. [Google Scholar] [CrossRef]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef]
- Lories, R.J.; Luyten, F.P. The bone–cartilage unit in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Čamernik, K.; Mihelič, A.; Mihalič, R.; Marolt Presen, D.; Janež, A.; Trebše, R.; Marc, J.; Zupan, J. Increased exhaustion of the subchondral bone-derived mesenchymal stem/ stromal cells in primary versus dysplastic osteoarthritis. Stem Cell Rev. Reports 2020, 16, 742–754. [Google Scholar] [CrossRef]
- Murphy, M.P.; Koepke, L.S.; Lopez, M.T.; Tong, X.; Ambrosi, T.H.; Gulati, G.S.; Marecic, O.; Wang, Y.; Ransom, R.C.; Hoover, M.Y.; et al. Articular cartilage regeneration by activated skeletal stem cells. Nat. Med. 2020, 26, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the human skeletal stem cell. Cell 2018, 175, 43–56.e21. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Roelofs, A.J. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr. Opin. Pharmacol. 2018, 40, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.; Pei, M.; Campbell, D.D.; Pei, M. Surface markers for chondrogenic determination: A highlight of synovium-derived stem cells. Cells 2012, 1, 1107–1120. [Google Scholar] [CrossRef]
- Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular Cartilage Regeneration in Osteoarthritis. Cells 2019, 8, 1305. [Google Scholar] [CrossRef] [PubMed]
- Vasanthan, J.; Gurusamy, N.; Rajasingh, S.; Sigamani, V.; Kirankumar, S.; Thomas, E.L.; Rajasingh, J. Role of Human Mesenchymal Stem Cells in Regenerative Therapy. Cells 2021, 10, 54. [Google Scholar] [CrossRef]
- Murata, Y.; Uchida, S.; Utsunomiya, H.; Hatakeyama, A.; Nakashima, H.; Chang, A.; Sekiya, I.; Sakai, A. Synovial mesenchymal stem cells derived from the cotyloid fossa synovium have higher self-renewal and differentiation potential than those from the paralabral synovium in the hip joint. Am. J. Sports Med. 2018, 46, 2942–2953. [Google Scholar] [CrossRef]
- Li, N.; Gao, J.; Mi, L.; Zhang, G.; Zhang, L.; Zhang, N.; Huo, R.; Hu, J.; Xu, K. Synovial membrane mesenchymal stem cells: Past life, current situation, and application in bone and joint diseases. Stem Cell Res. Ther. 2020, 11, 381. [Google Scholar] [CrossRef]
- Parolini, O.; Pei, M.; Khan, W.; To, K.; Zhang, B.; Romain, K.; Mak, C. Synovium-Derived Mesenchymal Stem Cell Transplantation in Cartilage Regeneration: A PRISMA Review of in vivo Studies. Front. Bioeng. Biotechnol. 2019, 1, 314. [Google Scholar] [CrossRef]
- Kurth, T.B.; Dell’Accio, F.; Crouch, V.; Augello, A.; Sharpe, P.T.; De Bari, C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011, 63, 1289–1300. [Google Scholar] [CrossRef]
- Kohno, Y.; Mizuno, M.; Ozeki, N.; Katano, H.; Komori, K.; Fujii, S.; Otabe, K.; Horie, M.; Koga, H.; Tsuji, K.; et al. Yields and chondrogenic potential of primary synovial mesenchymal stem cells are comparable between rheumatoid arthritis and osteoarthritis patients. Stem Cell Res. Ther. 2017, 8, 115. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Muneta, T.; Horie, M.; Koga, H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin. Orthop. Relat. Res. 2015, 473, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Ichinose, S.; Shinomiya, K.; Muneta, T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Stem Cells 2009, 104, 2728–2735. [Google Scholar] [CrossRef]
- Haring, G.; Zupan, J. Knee and peri-knee tissues of post mortem donors are strategic sources of mesenchymal stem/stromal cells for regenerative procedures. Int. J. Mol. Sci. 2022, 23, 3170. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Grogan, S.P.; Barbero, A.; Winkelmann, V.; Rieser, F.; Fitzsimmons, J.S.; O’Driscoll, S.; Martin, I.; Mainil-Varlet, P. Visual histological grading system for the evaluation of in vitro-generated neocartilage. Tissue Eng. 2006, 12, 2141–2149. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Čamernik, K.; Mihelič, A.; Mihalič, R.; Marolt Presen, D.; Janež, A.; Trebše, R.; Marc, J.; Zupan, J. Skeletal-muscle-derived mesenchymal stem/stromal cells from patients with osteoarthritis show superior biological properties compared to bone-derived cells. Stem Cell Res. 2019, 38, 101465. [Google Scholar] [CrossRef] [PubMed]
- Jasenc, L.; Stražar, K.; Mihelič, A.; Mihalič, R.; Trebše, R.; Haring, G.; Jeras, M.; Zupan, J. In vitro Characterization of the Human Skeletal Stem Cell-like Properties of Primary Bone-Derived Mesenchymal Stem/Stromal Cells in Patients with Late and Early Hip Osteoarthritis. Life 2022, 12, 899. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, K.; Kucia, M.; Wysoczynski, M.; Reca, R.; Zhao, D.; Trzyna, E.; Trent, J.; Peiper, S.; Zembala, M.; Ratajczak, J.; et al. Both hepatocyte growth factor (HGF) and stromal-derived factor-1 regulate the metastatic behavior of human rhabdomyosarcoma cells, but only HGF enhances their resistance to radiochemotherapy. Cancer Res. 2003, 63, 7926–7935. [Google Scholar] [PubMed]
- Kui, L.; Chan, G.C.; Lee, P.P.W. TSG-6 Downregulates IFN-Alpha and TNF-Alpha Expression by Suppressing IRF7 Phosphorylation in Human Plasmacytoid Dendritic Cells. Mediators Inflamm. 2017, 2017, 7462945. [Google Scholar] [CrossRef] [PubMed]
- Zeeuwen, P.L.J.M.; de Jongh, G.J.; Rodijk-Olthuis, D.; Kamsteeg, M.; Verhoosel, R.M.; van Rossum, M.M.; Hiemstra, P.S.; Schalkwijk, J. Genetically Programmed Differences in Epidermal Host Defense between Psoriasis and Atopic Dermatitis Patients. PLoS ONE 2008, 3, e2301. [Google Scholar] [CrossRef]
- Barsoum, I.B.; Smallwood, C.A.; Siemens, D.R.; Graham, C.H. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014, 74, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef] [PubMed]
- Tönnis, D.; Heinecke, A. Acetabular and femoral anteversion: Relationship with osteoarthritis of the hip. J. Bone Jt. Surg. Ser. A 1999, 81, 1747–1770. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Koga, H.; Muneta, T.; Nagase, T.; Nimura, A.; Ju, Y.-J.; Mochizuki, T.; Sekiya, I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: Suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008, 333, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, L.; Iselin, L.; Berkelaar, M.H.M.; Salzmann, G.M.; Wolf, F.; Feliciano, S.; Vogel, N.; Pagenstert, G.; Martin, I.; Pelttari, K.; et al. Comparison of Human Articular Cartilage Tissue and Chondrocytes Isolated from Peripheral versus Central Regions of Traumatic Lesions. Cartilage 2020, 13 (Suppl. 2), 68S–81S. [Google Scholar] [PubMed]
- Najar, M.; Fahmi, H. Of mesenchymal stem/stromal cells and osteoarthritis: Time to merge the latest breakthroughs. Stem Cell Rev. Reports 2020, 16, 1016–1018. [Google Scholar] [CrossRef]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Dell’Accio, F.; Vandenabeele, F.; Vermeesch, J.R.; Raymackers, J.-M.; Luyten, F.P. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J. Cell Biol. 2003, 160, 909–918. [Google Scholar] [CrossRef]
- Djouad, F.; Bony, C.; Häupl, T.; Uzé, G.; Lahlou, N.; Louis-Plence, P.; Apparailly, F.; Canovas, F.; Rème, T.; Sany, J.; et al. Transcriptional profiles discriminate bone marrow-derived and synovium-derived mesenchymal stem cells. Arthritis Res. Ther. 2005, 7, 1304–1315. [Google Scholar] [CrossRef]
- Mizuno, M.; Katano, H.; Mabuchi, Y.; Ogata, Y.; Ichinose, S.; Fujii, S.; Otabe, K.; Komori, K.; Ozeki, N.; Koga, H.; et al. Specific markers and properties of synovial mesenchymal stem cells in the surface, stromal, and perivascular regions. Stem Cell Res. Ther. 2018, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Mendicino, M.; Bailey, A.M.; Wonnacott, K.; Puri, R.K.; Bauer, S.R. MSC-based product characterization for clinical trials: An FDA perspective. Cell Stem Cell 2014, 14, 141–145. [Google Scholar] [PubMed]
- McGonagle, D.; Baboolal, T.G.; Jones, E. Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 719–730. [Google Scholar] [CrossRef]
- Nakamura, T.; Sekiya, I.; Muneta, T.; Hatsushika, D.; Horie, M.; Tsuji, K.; Kawarasaki, T.; Watanabe, A.; Hishikawa, S.; Fujimoto, Y.; et al. Arthroscopic, histological and MRI analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy 2012, 14, 327–338. [Google Scholar] [CrossRef]

| No. | Patient Group | Age (years) | Sex (M/F) | BMI (kg/m2) | Side (L/R) | Diagnosis | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | Early OA | 36 | F | 21.3 | L | Degenerative labrum changes with rupture | Labrum resection, acetabuloplasty |
| 5 | 46 | F | 24.6 | L | Labrum lesion | Labrum resection | |
| 6 | 47 | F | 22.4 | R | Degenerative labrum changes with rupture | Labrum resection, acetabuloplasty | |
| 8 | 60 | M | 28.9 | L | Degenerative labrum changes with rupture | Labrum resection, acetabuloplasty | |
| 11 | 49 | M | 26.2 | R | Degenerative labrum changes with rupture | Labrum refixation, minimal acetabuloplasty, femoral head-neck osteoplasty | |
| 12 | 54 | F | 29.7 | L | Degenerative labrum changes with rupture, pincer impingement | Labrum resection, acetabuloplasty | |
| 14 | 45 | F | 23.6 | R | Early degenerative changes | Labrum refixation | |
| 16 | 48 | F | 24.8 | L | Early degenerative changes | Labrum refixation | |
| 2 | Non-OA | 44 | F | 22.7 | L | Labrum lesion | Labrum refixation |
| 3 | 18 | M | 23.0 | R | Mild acetabular dysplasia, hypertrophic labrum changes with rupture | Labrum refixation | |
| 4 | 15 | F | 18.8 | R | Mild acetabular dysplasia | Diagnostic arthroscopy | |
| 7 | 42 | M | 23.5 | L | Femoroacetabular impingement syndrome | Labrum refixation, femoral head-neck osteoplasty | |
| 9 | 45 | F | 25.0 | R | Revision after arthroscopic labrum refixation | Resection of adhesions | |
| 10 | 24 | F | 22.1 | L | Suspected labrum lesion | Diagnostic arthroscopy | |
| 13 | 23 | F | 25.6 | L | Labrum lesion | Diagnostic arthroscopy | |
| 15 | 23 | F | 20.0 | L | Labrum lesion | Labrum refixation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zupan, J.; Stražar, K. Synovium-Derived and Bone-Derived Mesenchymal Stem/Stromal Cells from Early OA Patients Show Comparable In Vitro Properties to Those of Non-OA Patients. Cells 2024, 13, 1238. https://doi.org/10.3390/cells13151238
Zupan J, Stražar K. Synovium-Derived and Bone-Derived Mesenchymal Stem/Stromal Cells from Early OA Patients Show Comparable In Vitro Properties to Those of Non-OA Patients. Cells. 2024; 13(15):1238. https://doi.org/10.3390/cells13151238
Chicago/Turabian StyleZupan, Janja, and Klemen Stražar. 2024. "Synovium-Derived and Bone-Derived Mesenchymal Stem/Stromal Cells from Early OA Patients Show Comparable In Vitro Properties to Those of Non-OA Patients" Cells 13, no. 15: 1238. https://doi.org/10.3390/cells13151238
APA StyleZupan, J., & Stražar, K. (2024). Synovium-Derived and Bone-Derived Mesenchymal Stem/Stromal Cells from Early OA Patients Show Comparable In Vitro Properties to Those of Non-OA Patients. Cells, 13(15), 1238. https://doi.org/10.3390/cells13151238









