Therapeutic Potential of Olfactory Ensheathing Cells and Adipose-Derived Stem Cells in Osteoarthritis: Insights from Preclinical Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. ADSCs and OECs, Culture and Identification of Their Characteristics
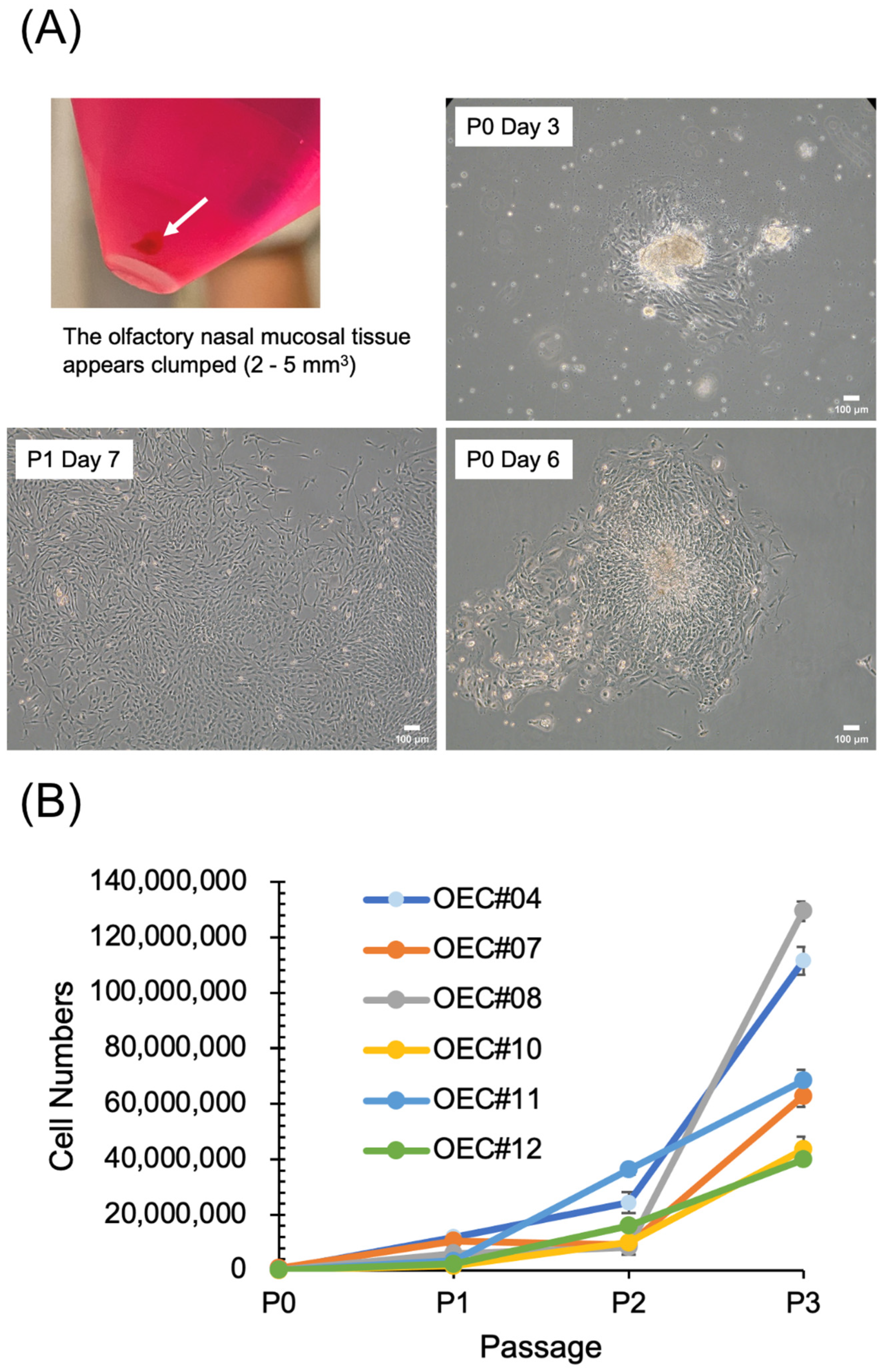
2.2. Cell Proliferation Analysis
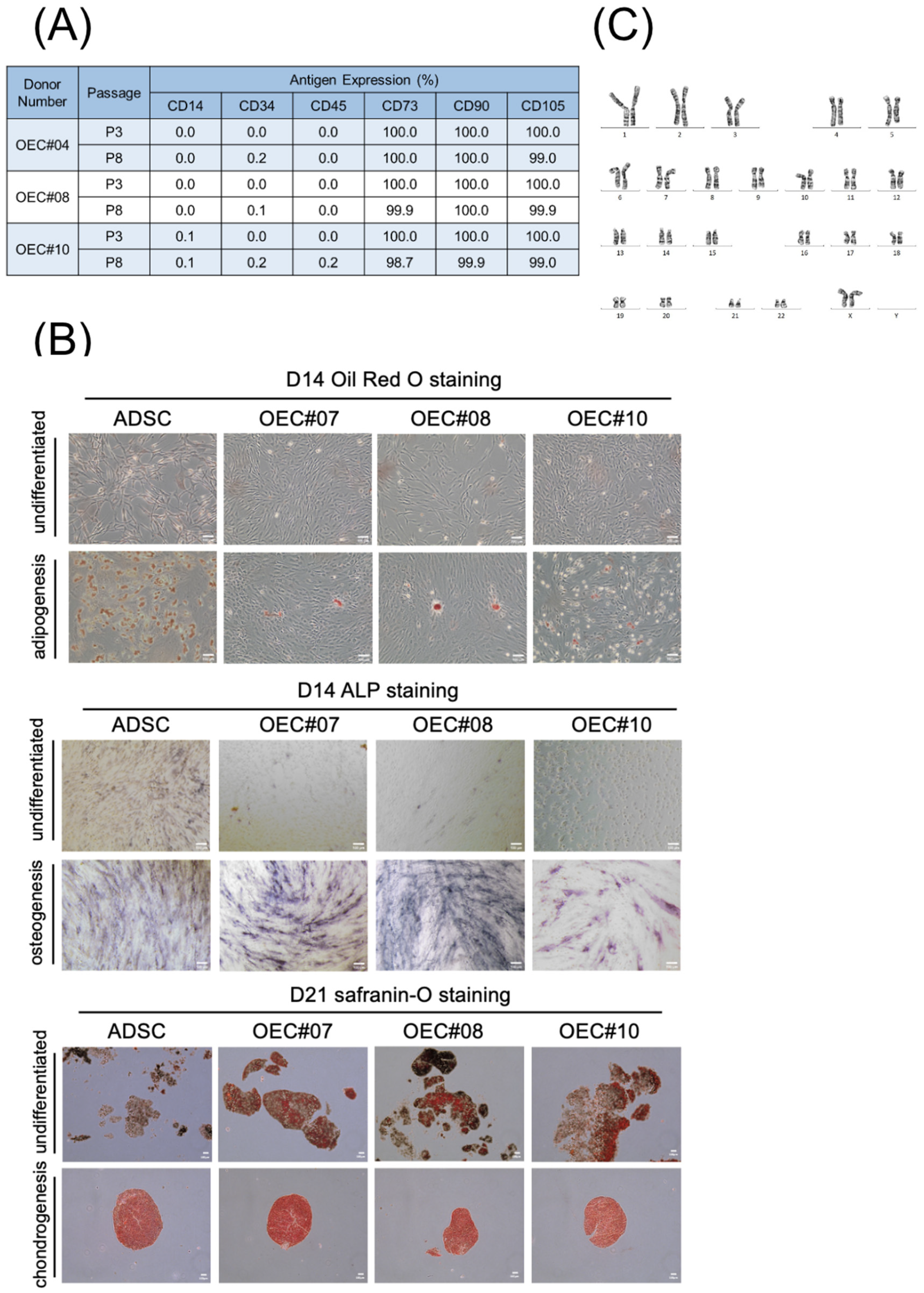
2.3. Flow Cytometry
2.4. Trilineage Differentiation
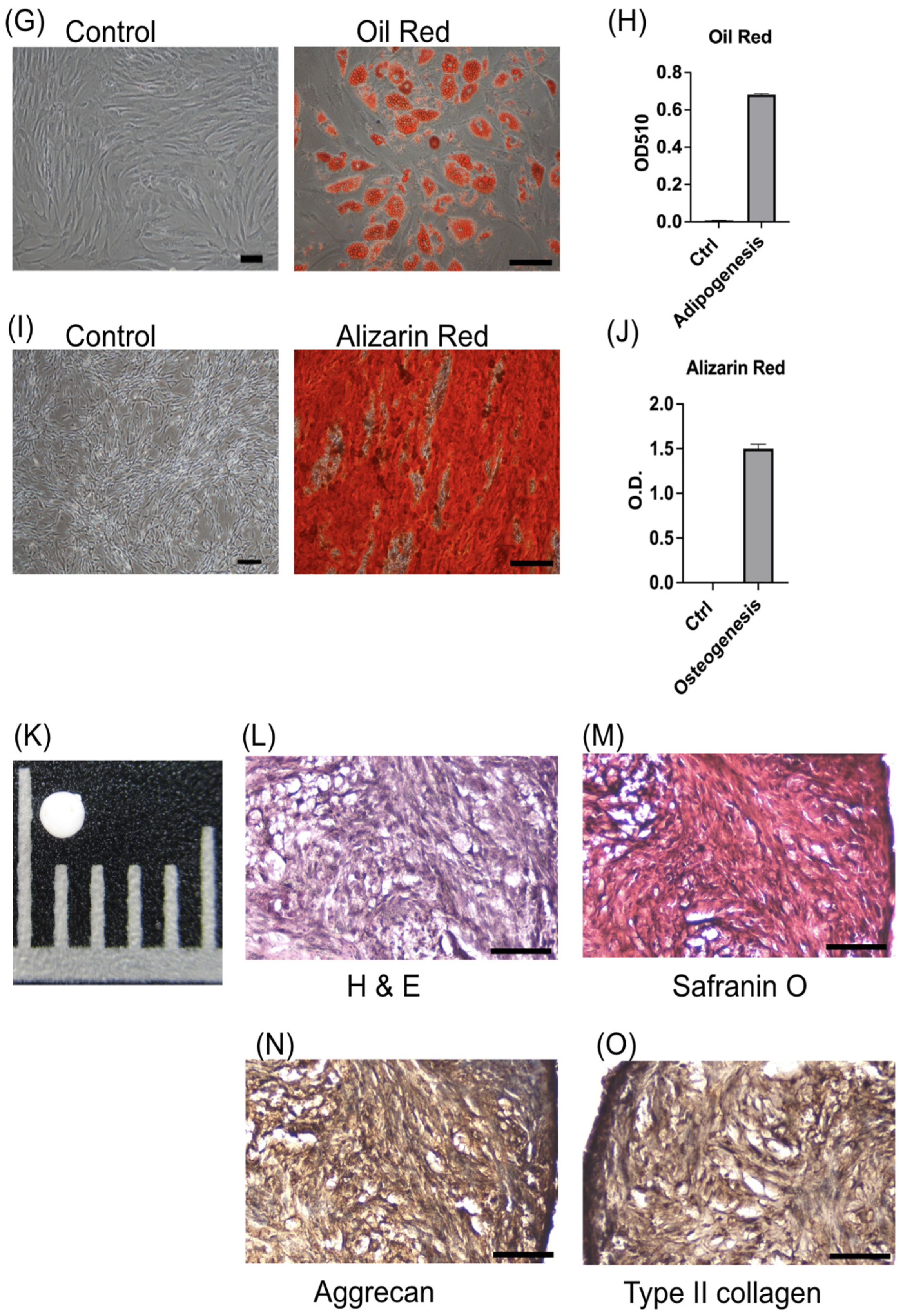
2.4.1. Adipogenesis
2.4.2. Osteogenesis
2.4.3. Chondrogenesis
2.5. Karyotyping
2.6. Quantitative RT-PCR
2.7. Collagenase-Induced Osteoarthritis Model
2.8. Rotarod Test
2.9. Magnetic Resonance Imaging
2.10. Tissue Harvest
2.10.1. Macroscopic Examination
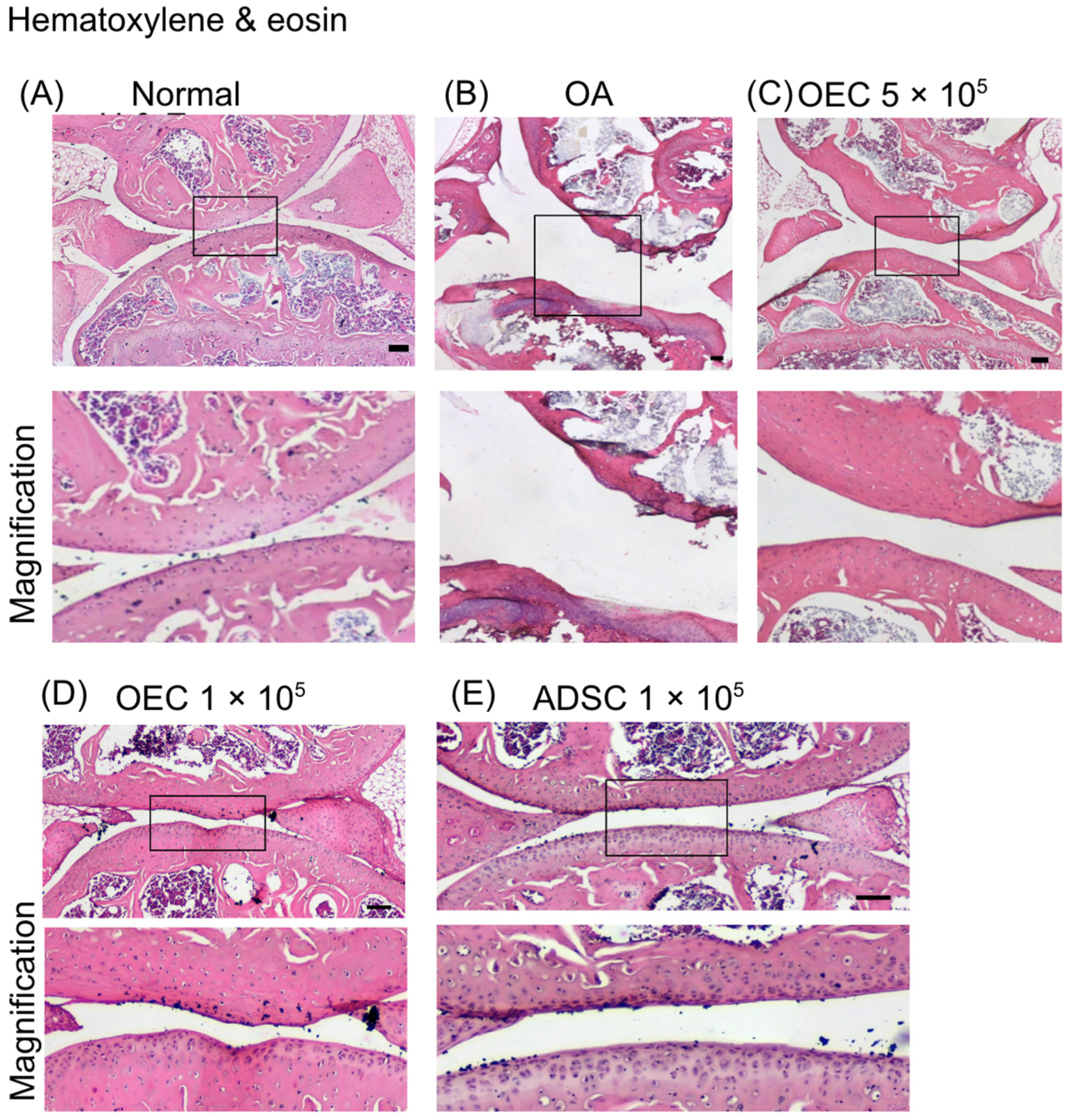
2.10.2. Histological Evaluation
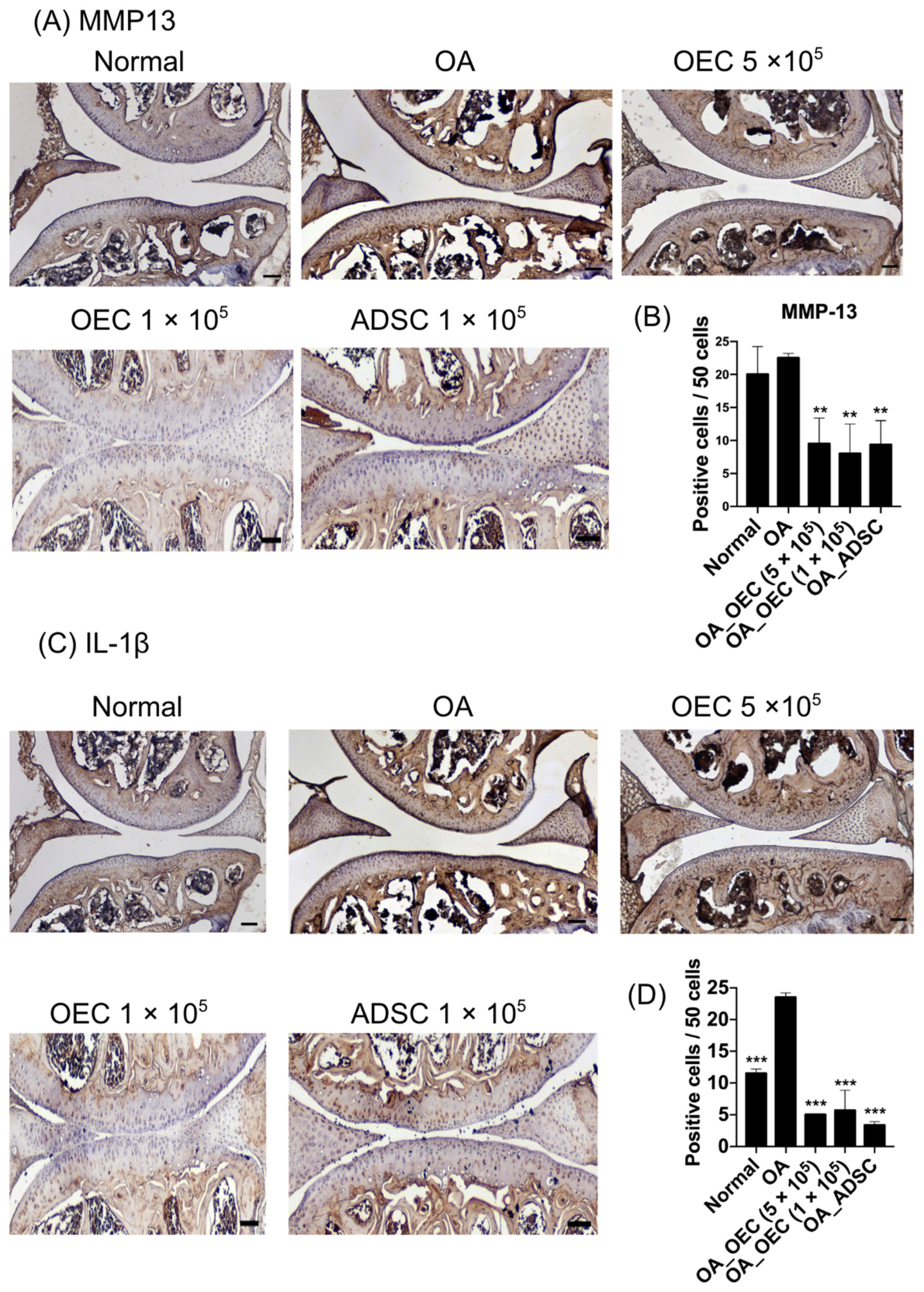
2.11. Immunohistochemistry
2.12. Statistical Analysis
3. Results
3.1. ADSCs Present Typical MSC Characteristics
3.2. OECs Present Typical MSC Characteristics
3.3. Rotarod Behavior after Treatment
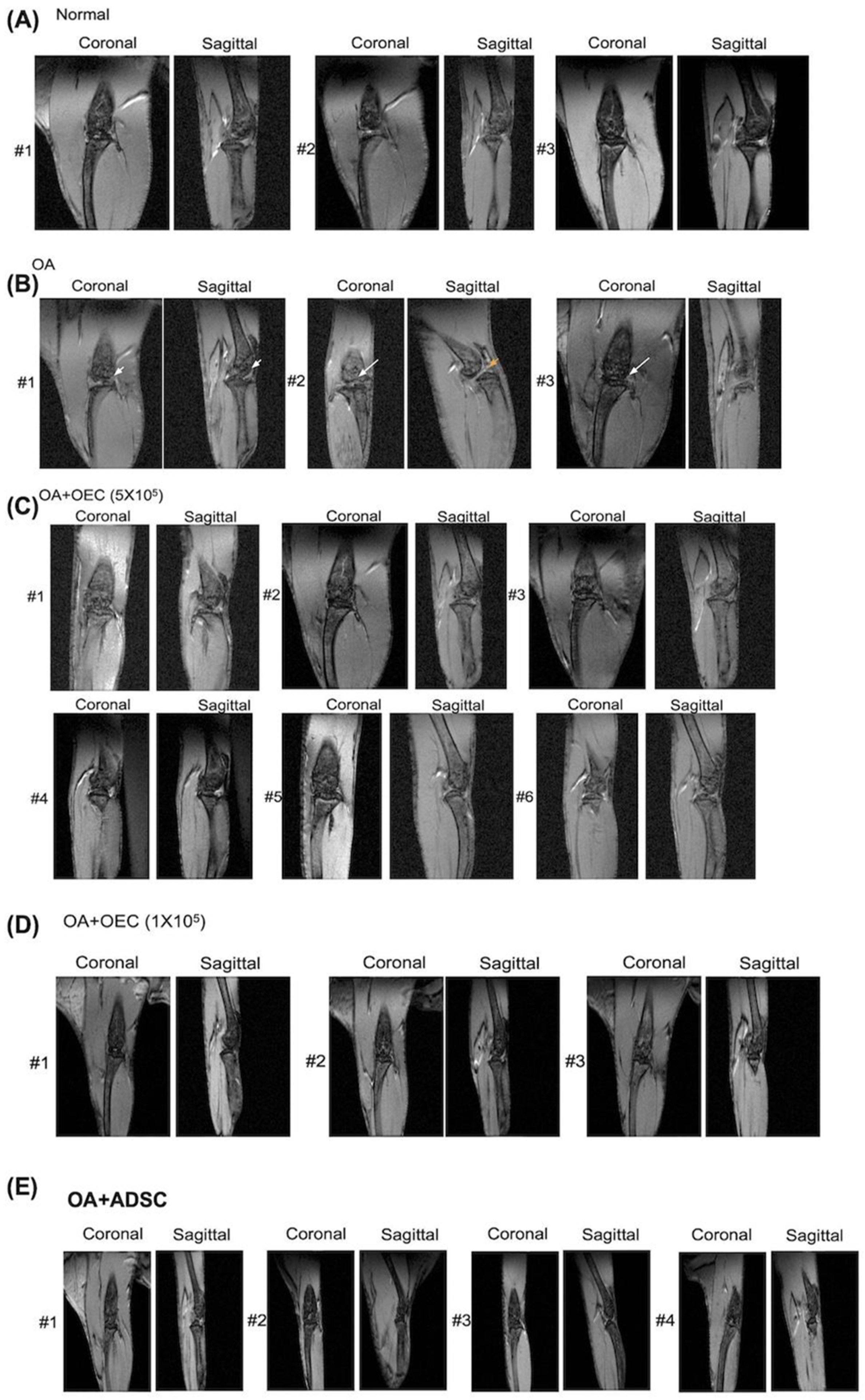
3.4. Magnetic Resonance Image Study
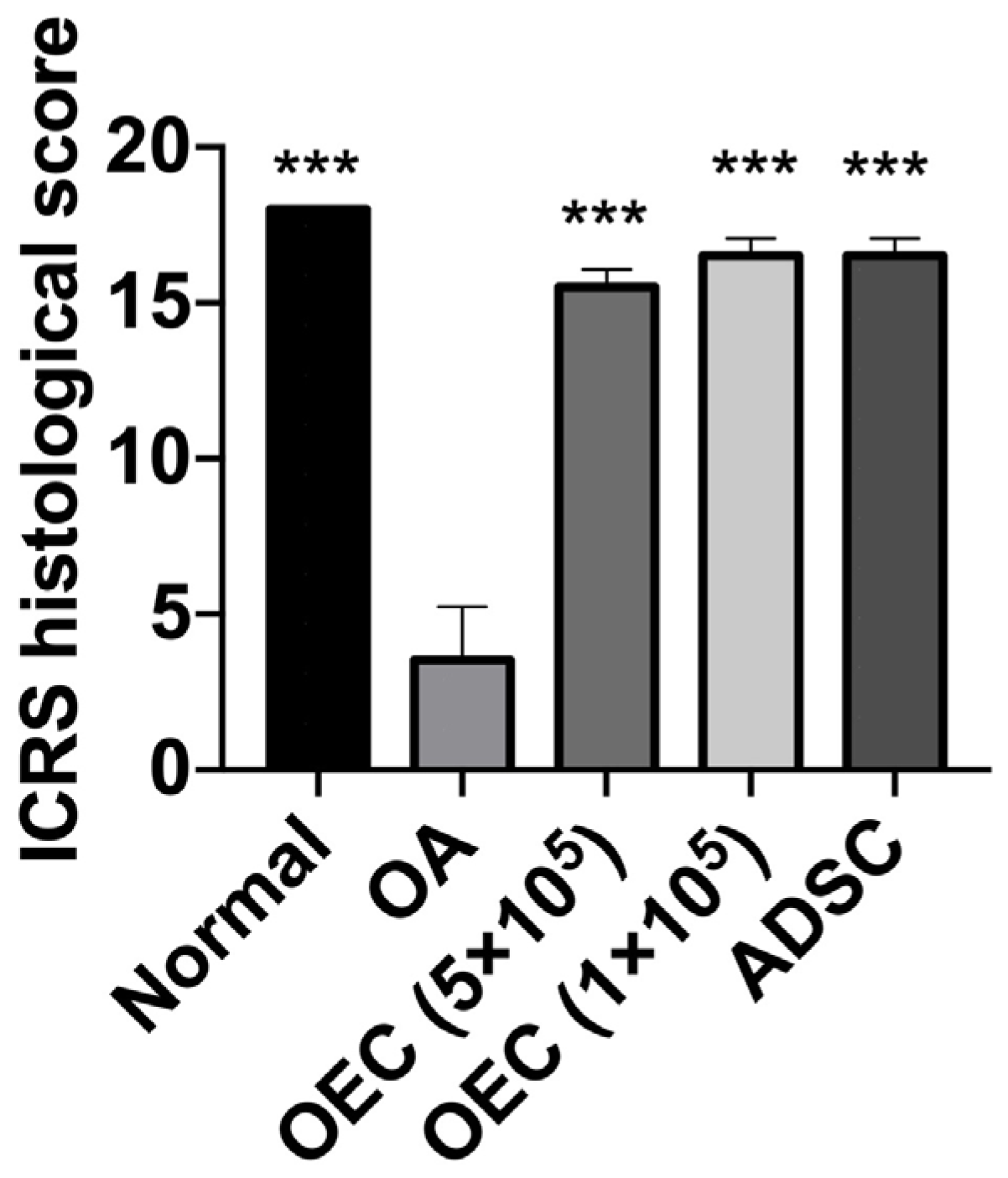
3.5. Histology of Joint Cartilage
3.6. Increased Expression of Type II Collagen and Aggrecan after Transplantation of 5 × 105, 1 × 105 OECs, and 1 × 105 ADSCs
3.7. Decreased Expression of MMP13 and IL-1β in 5 × 105, 1 × 105 OEC, and 1 × 105 ADSC-Treated Cartilages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of Osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- GBD 2021 Osteoarthritis Collaborators. Global, Regional, and National Burden of Osteoarthritis, 1990–2020 and Projections to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings From the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef]
- Hamood, R.; Tirosh, M.; Fallach, N.; Chodick, G.; Eisenberg, E.; Lubovsky, O. Prevalence and Incidence of Osteoarthritis: A Population-Based Retrospective Cohort Study. J. Clin. Med. Res. 2021, 10, 4282. [Google Scholar] [CrossRef]
- Spitaels, D.; Mamouris, P.; Vaes, B.; Smeets, M.; Luyten, F.; Hermens, R.; Vankrunkelsven, P. Epidemiology of Knee Osteoarthritis in General Practice: A Registry-Based Study. BMJ Open 2020, 10, e031734. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Q. Trends and Disparities in Osteoarthritis Prevalence among US Adults, 2005–2018. Sci. Rep. 2021, 11, 21845. [Google Scholar] [CrossRef]
- O’Brien, M.S.; McDougall, J.J. Age and Frailty as Risk Factors for the Development of Osteoarthritis. Mech. Ageing Dev. 2019, 180, 21–28. [Google Scholar] [CrossRef]
- Aubourg, G.; Rice, S.J.; Bruce-Wootton, P.; Loughlin, J. Genetics of Osteoarthritis. Osteoarthr. Cartil. 2022, 30, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Nedunchezhiyan, U.; Varughese, I.; Sun, A.R.; Wu, X.; Crawford, R.; Prasadam, I. Obesity, Inflammation, and Immune System in Osteoarthritis. Front. Immunol. 2022, 13, 907750. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Kean, W.F. Obesity and Knee Osteoarthritis. Inflammopharmacology 2012, 20, 53–58. [Google Scholar] [CrossRef]
- Snoeker, B.; Turkiewicz, A.; Magnusson, K.; Frobell, R.; Yu, D.; Peat, G.; Englund, M. Risk of Knee Osteoarthritis after Different Types of Knee Injuries in Young Adults: A Population-Based Cohort Study. Br. J. Sports Med. 2020, 54, 725–730. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Anderson, D.D.; Brown, T.D.; Tochigi, Y.; Martin, J.A. The Roles of Mechanical Stresses in the Pathogenesis of Osteoarthritis: Implications for Treatment of Joint Injuries. Cartilage 2013, 4, 286–294. [Google Scholar] [CrossRef]
- Clark, K.E.N.; Jawad, A.S.M. Osteoarthritis Management: Does Sex Matter? Trends Urol. Men’s Health 2023, 14, 8–12. [Google Scholar] [CrossRef]
- Wei, G.; Lu, K.; Umar, M.; Zhu, Z.; Lu, W.W.; Speakman, J.R.; Chen, Y.; Tong, L.; Chen, D. Risk of Metabolic Abnormalities in Osteoarthritis: A New Perspective to Understand Its Pathological Mechanisms. Bone Res. 2023, 11, 63. [Google Scholar] [CrossRef]
- Sahinbegovic, E.; Dallos, T.; Aigner, E.; Axmann, R.; Engelbrecht, M.; Schöniger-Hekele, M.; Karonitsch, T.; Farkas, M.; Karger, T.; Willeit, J.; et al. Hereditary Hemochromatosis as a Risk Factor for Joint Replacement Surgery. Am. J. Med. 2010, 123, 659–662. [Google Scholar] [CrossRef]
- Oei, E.H.G.; Runhaar, J. Imaging of Early-Stage Osteoarthritis: The Needs and Challenges for Diagnosis and Classification. Skeletal Radiol. 2023, 52, 2031–2036. [Google Scholar] [CrossRef]
- Karpiński, R. Knee Joint Osteoarthritis Diagnosis Based on Selected Acoustic Signal Discriminants Using Machine Learning. Appl. Comput. Sci. 2022, 18, 71–85. [Google Scholar] [CrossRef]
- Kanamoto, T.; Mae, T.; Yokoyama, T.; Tanaka, H.; Ebina, K.; Nakata, K. Significance and Definition of Early Knee Osteoarthritis. Ann. Jt. 2020, 5, 4. [Google Scholar] [CrossRef]
- Grime, J.; Richardson, J.C.; Ong, B.N. Perceptions of Joint Pain and Feeling Well in Older People Who Reported Being Healthy: A Qualitative Study. Br. J. Gen. Pract. 2010, 60, 597–603. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Tardif, G.; Paiement, P.; Pelletier, J.-P. Common Biochemical and Magnetic Resonance Imaging Biomarkers of Early Knee Osteoarthritis and of Exercise/Training in Athletes: A Narrative Review. Diagnostics 2021, 11, 1488. [Google Scholar] [CrossRef]
- Hayashi, D.; Roemer, F.W.; Link, T.; Li, X.; Kogan, F.; Segal, N.A.; Omoumi, P.; Guermazi, A. Latest Advancements in Imaging Techniques in OA. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221146621. [Google Scholar] [CrossRef]
- Novakofski, K.D.; Pownder, S.L.; Koff, M.F.; Williams, R.M.; Potter, H.G.; Fortier, L.A. High-Resolution Methods for Diagnosing Cartilage Damage In Vivo. Cartilage 2016, 7, 39–51. [Google Scholar] [CrossRef]
- Roemer, F.W.; Guermazi, A.; Demehri, S.; Wirth, W.; Kijowski, R. Imaging in Osteoarthritis. Osteoarthr. Cartil. 2022, 30, 913–934. [Google Scholar] [CrossRef]
- Bernotiene, E.; Bagdonas, E.; Kirdaite, G.; Bernotas, P.; Kalvaityte, U.; Uzieliene, I.; Thudium, C.S.; Hannula, H.; Lorite, G.S.; Dvir-Ginzberg, M.; et al. Emerging Technologies and Platforms for the Immunodetection of Multiple Biochemical Markers in Osteoarthritis Research and Therapy. Front. Med. 2020, 7, 572977. [Google Scholar] [CrossRef]
- Sandhu, A.; Rockel, J.S.; Lively, S.; Kapoor, M. Emerging Molecular Biomarkers in Osteoarthritis Pathology. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X231177116. [Google Scholar] [CrossRef]
- Im, G.-I.; Henrotin, Y. Regenerative Medicine for Early Osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X231194813. [Google Scholar] [CrossRef]
- Krakowski, P.; Karpiński, R.; Maciejewski, R.; Jonak, J.; Jurkiewicz, A. Short-Term Effects of Arthroscopic Microfracturation of Knee Chondral Defects in Osteoarthritis. NATO Adv. Sci. Inst. Ser. E Appl. Sci. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Felson, D.T. Clinical Practice. Osteoarthritis of the Knee. N. Engl. J. Med. 2006, 354, 841–848. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Wu, K.-C.; Ding, D.-C. Chondrogenic Potential of Human Umbilical Cord Mesenchymal Stem Cells Cultured with Exosome-Depleted Fetal Bovine Serum in an Osteoarthritis Mouse Model. Biomedicines 2022, 10, 2773. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Ding, D.-C.; Wu, K.-C. Human Umbilical Mesenchymal Stromal Cells Mixed with Hyaluronan Transplantation Decreased Cartilage Destruction in a Rabbit Osteoarthritis Model. Stem Cells Int. 2021, 2021, 2989054. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, T.; Fan, W. Osteochondral Autograft Transplantation or Autologous Chondrocyte Implantation for Large Cartilage Defects of the Knee: A Meta-Analysis. Cell Tissue Bank. 2016, 17, 59–67. [Google Scholar] [CrossRef]
- Kwon, D.G.; Kim, M.K.; Jeon, Y.S.; Nam, Y.C.; Park, J.S.; Ryu, D.J. State of the Art: The Immunomodulatory Role of MSCs for Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 1618. [Google Scholar] [CrossRef]
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z. Mesenchymal Stem Cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Ding, D.-C.; Chou, H.-L.; Chang, Y.-H.; Hung, W.-T.; Liu, H.-W.; Chu, T.-Y. Characterization of HLA-G and Related Immunosuppressive Effects in Human Umbilical Cord Stroma-Derived Stem Cells. Cell Transplant. 2016, 25, 217–228. [Google Scholar] [CrossRef]
- Murtaza, M.; Mohanty, L.; Ekberg, J.A.K.; St John, J.A. Designing Olfactory Ensheathing Cell Transplantation Therapies: Influence of Cell Microenvironment. Cell Transplant. 2022, 31, 9636897221125685. [Google Scholar] [CrossRef]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal Models of Osteoarthritis: Classification, Update, and Measurement of Outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef]
- Teeple, E.; Jay, G.D.; Elsaid, K.A.; Fleming, B.C. Animal Models of Osteoarthritis: Challenges of Model Selection and Analysis. AAPS J. 2013, 15, 438–446. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of Exosomes Secreted by Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells and Synovial Membrane-Derived Mesenchymal Stem Cells for the Treatment of Osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef]
- Mainil-Varlet, P.; Aigner, T.; Brittberg, M.; Bullough, P.; Hollander, A.; Hunziker, E.; Kandel, R.; Nehrer, S.; Pritzker, K.; Roberts, S.; et al. Histological Assessment of Cartilage Repair: A Report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J. Bone Jt. Surg. Am. 2003, 85 (Suppl. 2), 45–57. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-J.; Choi, K.-U.; Choi, S.-W.; Kim, S.-D.; Kong, S.-K.; Lee, S.; Cho, K.-S. Comparative Analysis of Adipose-Derived Stromal Cells and Their Secretome for Auricular Cartilage Regeneration. Stem Cells Int. 2020, 2020, 8595940. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-H.; Hsu, C.-C.; Hsu, S.-L.; Chou, W.-Y.; Wu, Y.-N.; Kuo, C.-E.A.; Hsu, T.-C.; Shiu, L.-Y.; Jhan, S.-W. Adipose-Derived Mesenchymal Stem Cells-Conditioned Medium Modulates the Expression of Inflammation Induced Bone Morphogenetic Protein-2, -5 and -6 as Well as Compared with Shockwave Therapy on Rat Knee Osteoarthritis. Biomedicines 2021, 9, 1399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Meng, F.; Zhang, Q.; Zou, Z.; Xiao, K.; Zhu, T.; Li, H.; Zhang, W.; Ma, J.; Ma, J. Allogeneic Adipose-Derived Mesenchymal Stem Cells Promote the Expression of Chondrocyte Redifferentiation Markers and Retard the Progression of Knee Osteoarthritis in Rabbits. Am. J. Transl. Res. 2021, 13, 632–645. [Google Scholar] [PubMed]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 8810813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhuang, X.; Chen, Y.; Xia, H. Intravenous Transplantation of Olfactory Bulb Ensheathing Cells for a Spinal Cord Hemisection Injury Rat Model. Cell Transplant. 2019, 28, 1585–1602. [Google Scholar] [CrossRef] [PubMed]
- Denaro, S.; D’Aprile, S.; Alberghina, C.; Pavone, A.M.; Torrisi, F.; Giallongo, S.; Longhitano, L.; Mannino, G.; Lo Furno, D.; Zappalà, A.; et al. Neurotrophic and Immunomodulatory Effects of Olfactory Ensheathing Cells as a Strategy for Neuroprotection and Regeneration. Front. Immunol. 2022, 13, 1098212. [Google Scholar] [CrossRef] [PubMed]
- Minkelyte, K.; Li, D.; Li, Y.; Ibrahim, A. Transplantation of Cryopreserved Olfactory Ensheathing Cells Restores Loss of Functions in an Experimental Model. Cell Transplant. 2023, 32, 9636897231199319. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tan, Z.; Huang, X.; Cheng, X.; Yuan, Y.; Qin, S.; Wang, D.; Hu, X.; Gu, Y.; Qian, W.-J.; et al. Direct Neuronal Reprogramming of Olfactory Ensheathing Cells for CNS Repair. Cell Death Dis. 2019, 10, 646. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Korntner, S.H.; Mullen, A.M.; Zeugolis, D.I. Collagen Type II: From Biosynthesis to Advanced Biomaterials for Cartilage Engineering. Biomater. Biosyst. 2021, 4, 100030. [Google Scholar] [CrossRef]
- Hayes, A.J.; Melrose, J. Aggrecan, the Primary Weight-Bearing Cartilage Proteoglycan, Has Context-Dependent, Cell-Directive Properties in Embryonic Development and Neurogenesis: Aggrecan Glycan Side Chain Modifications Convey Interactive Biodiversity. Biomolecules 2020, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for Articular Cartilage Repair and Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770655. [Google Scholar] [CrossRef] [PubMed]
- Kurenkova, A.D.; Romanova, I.A.; Kibirskiy, P.D.; Timashev, P.; Medvedeva, E.V. Strategies to Convert Cells into Hyaline Cartilage: Magic Spells for Adult Stem Cells. Int. J. Mol. Sci. 2022, 23, 11169. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef] [PubMed]
- Defois, A.; Bon, N.; Charpentier, A.; Georget, M.; Gaigeard, N.; Blanchard, F.; Hamel, A.; Waast, D.; Armengaud, J.; Renoult, O.; et al. Osteoarthritic Chondrocytes Undergo a Glycolysis-Related Metabolic Switch upon Exposure to IL-1b or TNF. Cell Commun. Signal. 2023, 21, 137. [Google Scholar] [CrossRef] [PubMed]
- Alvites, R.; Branquinho, M.; Sousa, A.C.; Lopes, B.; Sousa, P.; Maurício, A.C. Mesenchymal Stem/Stromal Cells and Their Paracrine Activity-Immunomodulation Mechanisms and How to Influence the Therapeutic Potential. Pharmaceutics 2022, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, P.; Rodríguez-Nogales, C.; Jordan, O.; Allémann, E. Combination of Mesenchymal Stem Cells and Bioactive Molecules in Hydrogels for Osteoarthritis Treatment. Eur. J. Pharm. Biopharm. 2022, 172, 41–52. [Google Scholar] [CrossRef]
- Lu, R.; Wang, Y.-G.; Qu, Y.; Wang, S.-X.; Peng, C.; You, H.; Zhu, W.; Chen, A. Dihydrocaffeic Acid Improves IL-1β-Induced Inflammation and Cartilage Degradation via Inhibiting NF-κB and MAPK Signalling Pathways. Bone Jt. Res. 2023, 12, 259–273. [Google Scholar] [CrossRef]




| Gene | Forward (5′->3′) | Reverse (5′->3′) |
|---|---|---|
| PPAR-γ | AGCCTCATGAAGAGCCTTCCA | TCCGGAAGAAACCCTTGCA |
| FABP4 | ATGGGATGGAAAATCAACCA | GTGGAAGTGACGCCTTTCAT |
| ALPL | CCACGTCTTCACATTTGGTG | GCAGTGAAGGGCTTCTTGTC |
| RUNX2 | CGGAATGCCTCTGCTGTTAT | TTCCCGAGGTCCATCTACTG |
| COL2A1 | GGACTTTTCTCCCCTCT CT | GACCCGAAGGTCTTACAGGA |
| ACAN | GAGATGGAGGGTGAGGTC | GAGATGGAGGGTGAGGTC |
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGT GATGGGATTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-H.; Wu, K.-C.; Hsu, C.-J.; Tu, T.-C.; Liu, M.-C.; Chiang, R.Y.-S.; Ding, D.-C. Therapeutic Potential of Olfactory Ensheathing Cells and Adipose-Derived Stem Cells in Osteoarthritis: Insights from Preclinical Studies. Cells 2024, 13, 1250. https://doi.org/10.3390/cells13151250
Chang Y-H, Wu K-C, Hsu C-J, Tu T-C, Liu M-C, Chiang RY-S, Ding D-C. Therapeutic Potential of Olfactory Ensheathing Cells and Adipose-Derived Stem Cells in Osteoarthritis: Insights from Preclinical Studies. Cells. 2024; 13(15):1250. https://doi.org/10.3390/cells13151250
Chicago/Turabian StyleChang, Yu-Hsun, Kun-Chi Wu, Chih-Jung Hsu, Tsui-Chin Tu, Mei-Chun Liu, Raymond Yuh-Shyan Chiang, and Dah-Ching Ding. 2024. "Therapeutic Potential of Olfactory Ensheathing Cells and Adipose-Derived Stem Cells in Osteoarthritis: Insights from Preclinical Studies" Cells 13, no. 15: 1250. https://doi.org/10.3390/cells13151250






