Wnt5a Promotes Axon Elongation in Coordination with the Wnt–Planar Cell Polarity Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Cortical Neuron Isolation, Culturing, and Transfection
2.3. Mammalian Cell Culture
2.4. Preparation of the Conditioned Media
2.5. Isolation of the sEVs Using Differential Centrifugation
2.6. Isolation of the sEVs Using Density Gradient Purification
2.7. Nanoparticle Tracking Analysis
2.8. Electron Microscopy
2.9. Immunofluorescence Microscopy
2.10. Immunoblotting
2.11. RT and qPCR
2.12. Quantification
2.13. Statistical Analyses
3. Results
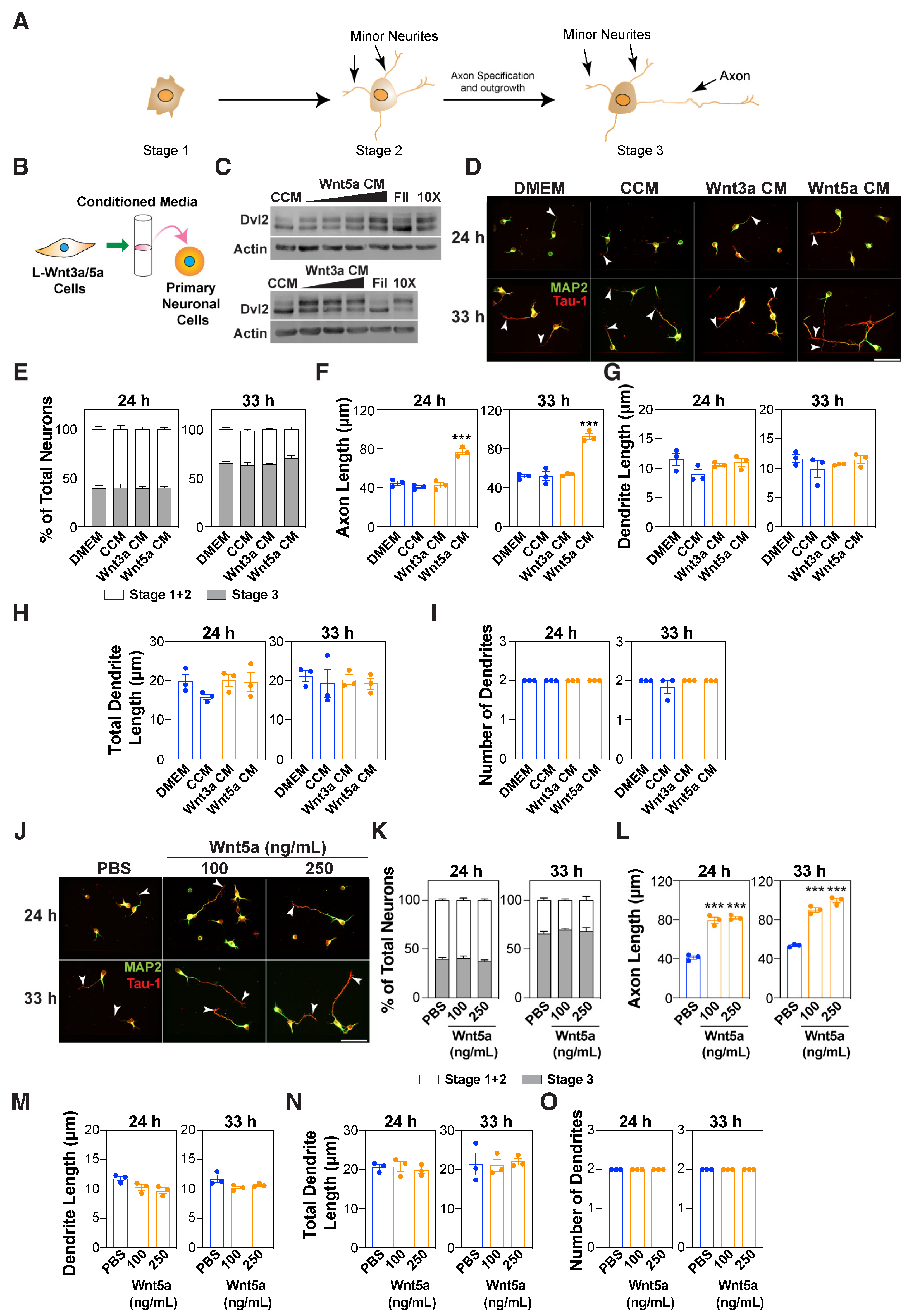
3.1. Wnt5a Promotes Axon Outgrowth
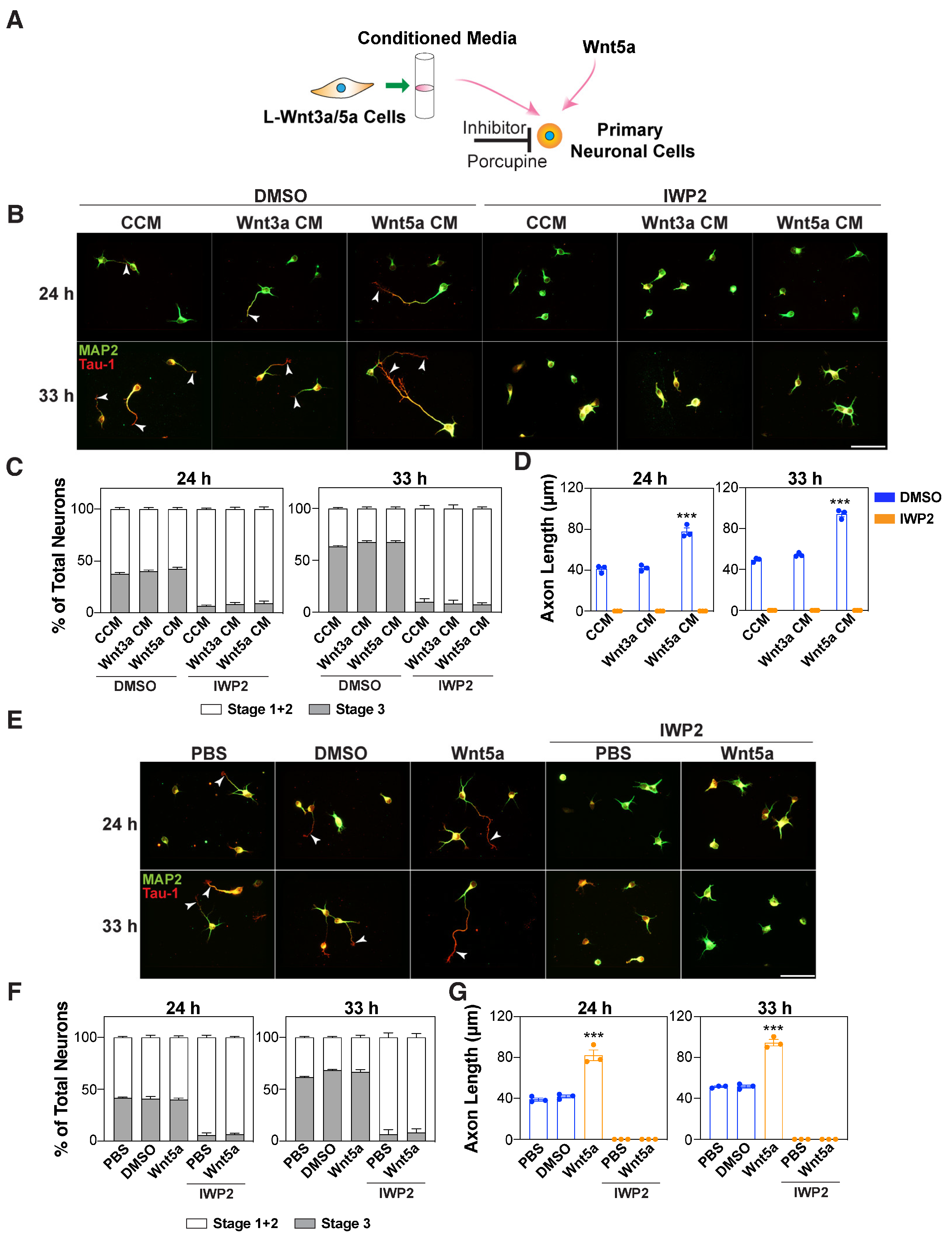
3.2. Autocrine Wnts are Required for Wnt5a-Induced Axon Outgrowth
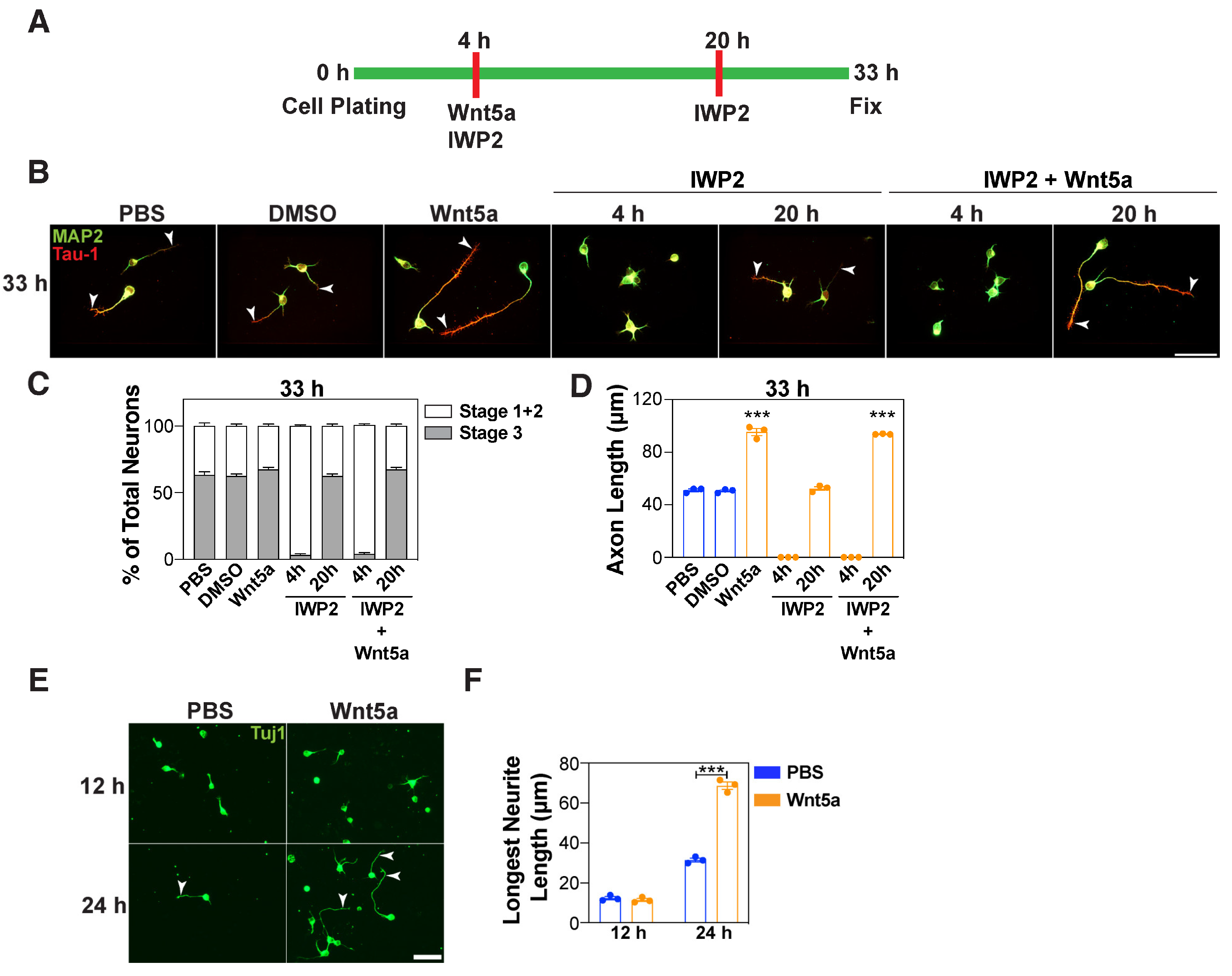
3.3. Wnt5a Promotes Axon Elongation in a Distinct Timeframe
3.4. Wnt5a-Induced Outgrowth of the Prospective Axon Requires Coordination with PCP Components
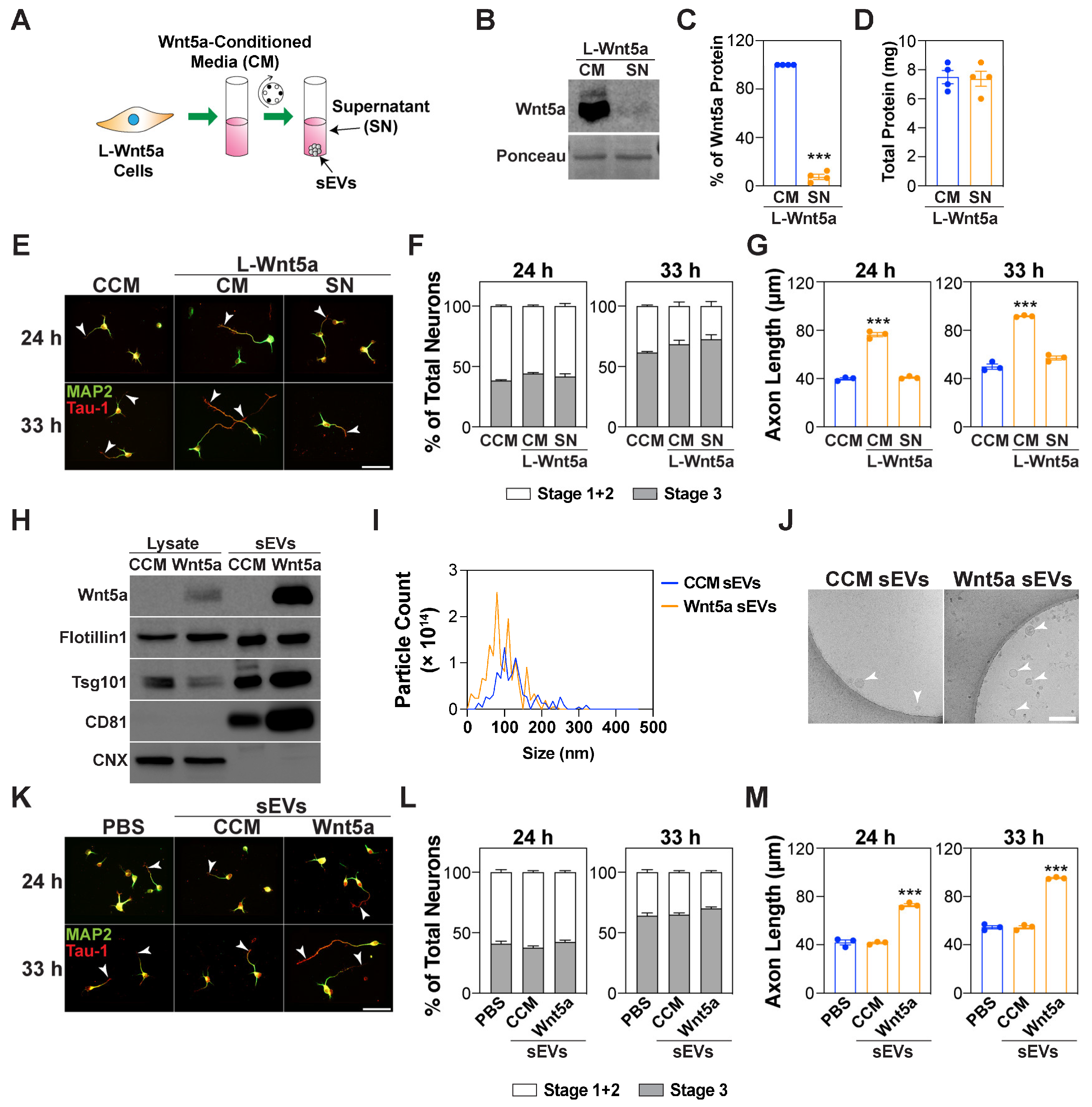
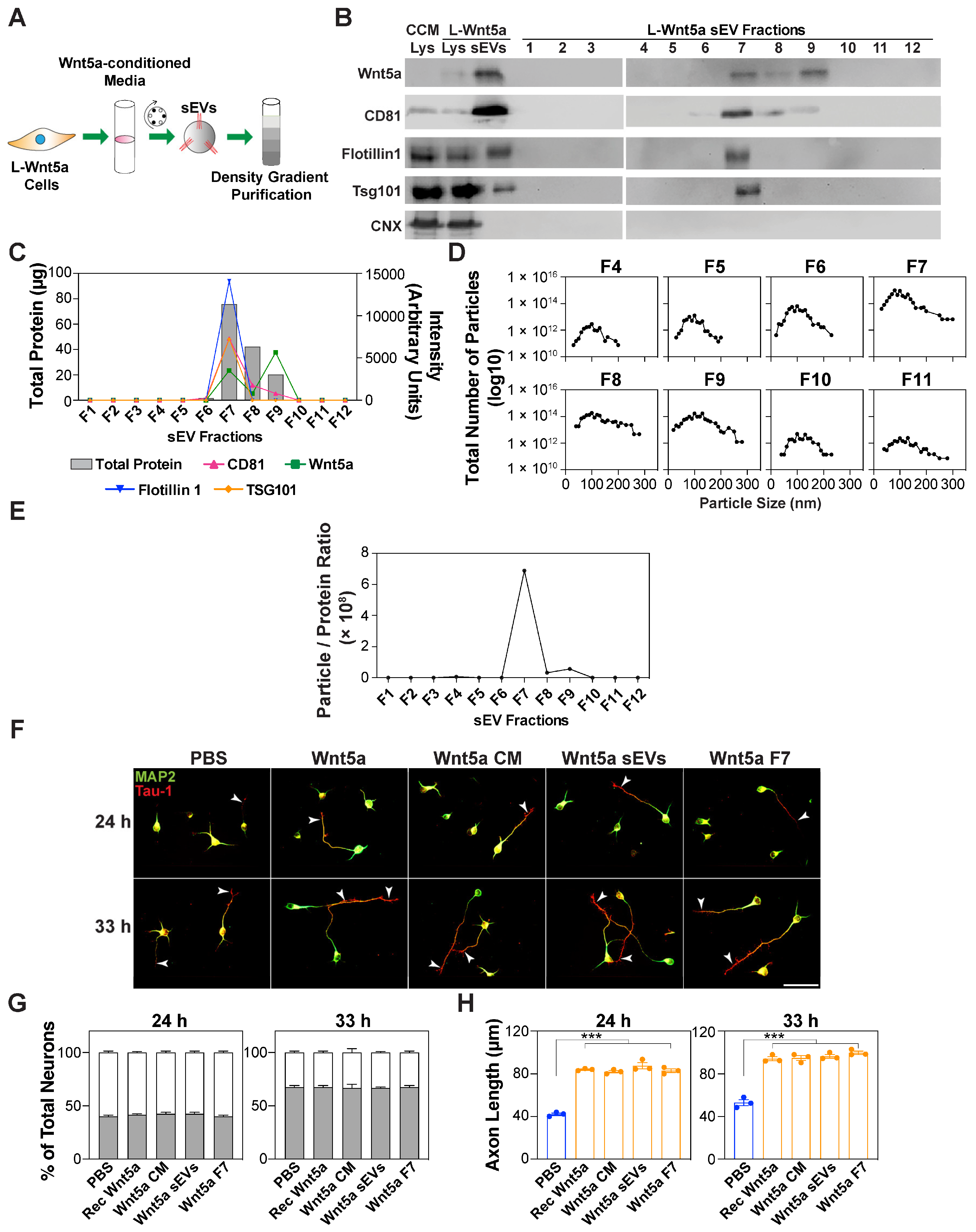
3.5. Wnt5a-Containing sEVs Promote Axon Elongation
4. Discussion
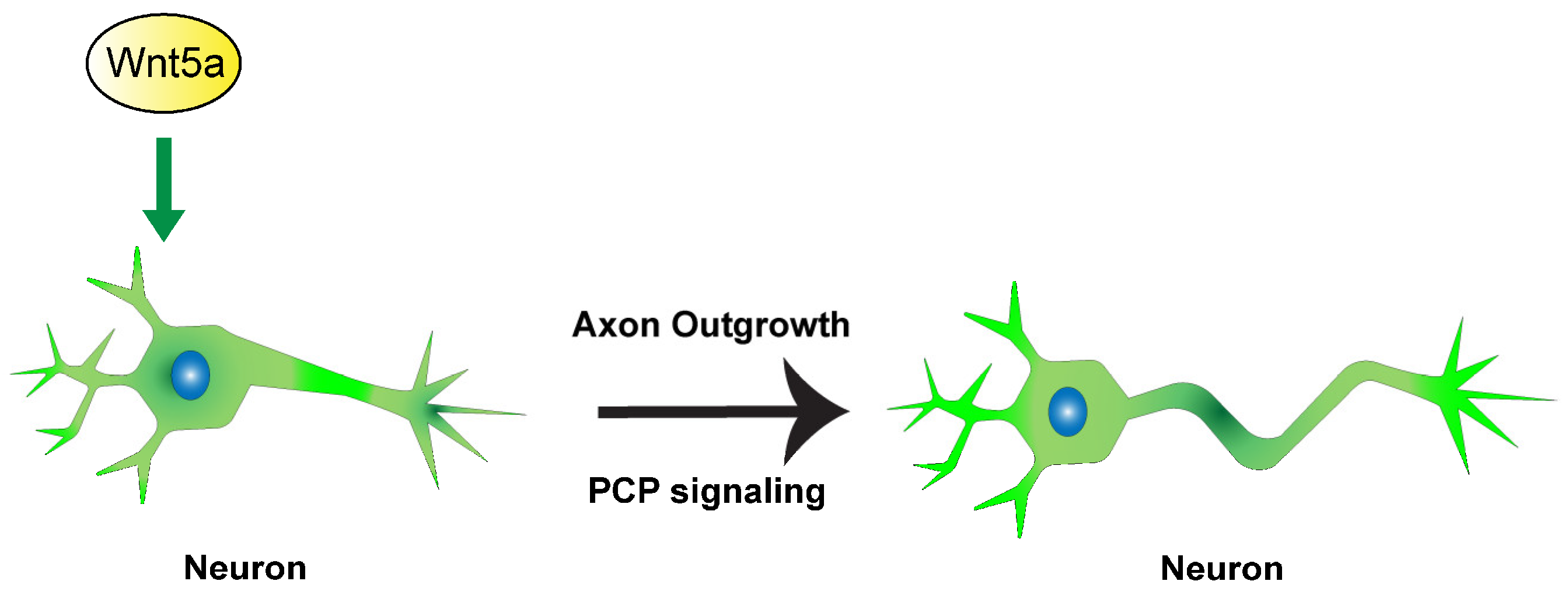
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arimura, N.; Kaibuchi, K. Neuronal polarity: From extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 2007, 8, 194–205. [Google Scholar] [CrossRef]
- Takano, T.; Xu, C.; Funahashi, Y.; Namba, T.; Kaibuchi, K. Neuronal polarization. Development 2015, 142, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Funahashi, Y.; Kaibuchi, K. Neuronal Polarity: Positive and Negative Feedback Signals. Front. Cell Dev. Biol. 2019, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Dotti, C.G.; Sullivan, C.A.; Banker, G.A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988, 8, 1454–1468. [Google Scholar] [CrossRef] [PubMed]
- van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.D.; Nusse, R. Wnt signaling: Multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 2006, 281, 22429–22433. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.E.; Nourse, C.C.; Cooper, H.M. The tangled web of non-canonical Wnt signalling in neural migration. Neurosignals 2012, 20, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Salinas, P.C. Wnt signaling in the vertebrate central nervous system: From axon guidance to synaptic function. Cold Spring Harb. Perspect. Biol. 2012, 4, a008003. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.; Salinas, P.C. Wnt-Frizzled Signaling Regulates Activity-Mediated Synapse Formation. Front. Mol. Neurosci. 2021, 14, 683035. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Yang, G.Y.; Wang, Q.J.; Qian, L.; Chen, Y.M.; Chen, F.; Tao, Y.; Hu, H.S.; Wang, T.; et al. Dishevelled promotes axon differentiation by regulating atypical protein kinase C. Nat. Cell. Biol. 2007, 9, 743–754. [Google Scholar] [CrossRef]
- Shafer, B.; Onishi, K.; Lo, C.; Colakoglu, G.; Zou, Y. Vangl2 promotes Wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance. Dev. Cell 2011, 20, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Horigane, S.; Ageta-Ishihara, N.; Kamijo, S.; Fujii, H.; Okamura, M.; Kinoshita, M.; Takemoto-Kimura, S.; Bito, H. Facilitation of axon outgrowth via a Wnt5a-CaMKK-CaMKIalpha pathway during neuronal polarization. Mol. Brain 2016, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fothergill, T.; Hutchins, B.I.; Dent, E.W.; Kalil, K. Wnt5a evokes cortical axon outgrowth and repulsive guidance by tau mediated reorganization of dynamic microtubules. Dev. Neurobiol. 2014, 74, 797–817. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hutchins, B.I.; Kalil, K. Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. J. Neurosci. 2009, 29, 5873–5883. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, B.I.; Li, L.; Kalil, K. Wnt/calcium signaling mediates axon growth and guidance in the developing corpus callosum. Dev. Neurobiol. 2011, 71, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.R.; Prakash, N.; Cajanek, L.; Minina, E.; Bryja, V.; Bryjova, L.; Yamaguchi, T.P.; Hall, A.C.; Wurst, W.; Arenas, E. Wnt5a regulates ventral midbrain morphogenesis and the development of A9–A10 dopaminergic cells in vivo. PLoS ONE 2008, 3, e3517. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, S.B.; Guerrero, F.G.; Herrera-Soto, A.; Jensen-Flores, J.; Bustamante, D.B.; Onate-Ponce, A.; Henny, P.; Varas-Godoy, M.; Inestrosa, N.C.; Varela-Nallar, L. Wnt5a promotes differentiation and development of adult-born neurons in the hippocampus by noncanonical Wnt signaling. Stem Cells 2020, 38, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, V.T.; Ramos-Fernandez, E.; Henriquez, J.P.; Lorenzo, A.; Inestrosa, N.C. Wnt-5a/Frizzled9 Receptor Signaling through the Galphao-Gbetagamma Complex Regulates Dendritic Spine Formation. J. Biol. Chem. 2016, 291, 19092–19107. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.J.; Miao, W.Y.; He, S.J.; Wan, Z.F.; Luo, Z.G.; Yu, X. A novel Wnt5a-Frizzled4 signaling pathway mediates activity-independent dendrite morphogenesis via the distal PDZ motif of Frizzled 4. Dev. Neurobiol. 2015, 75, 805–822. [Google Scholar] [CrossRef]
- Green, J.; Nusse, R.; van Amerongen, R. The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb. Perspect. Biol. 2014, 6, a009175. [Google Scholar] [CrossRef]
- Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, J.; Lu, C.C.; Wang, Z.B.; Lyuksyutova, A.I.; Song, X.J.; Zou, Y. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat. Neurosci. 2005, 8, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Keeble, T.R.; Halford, M.M.; Seaman, C.; Kee, N.; Macheda, M.; Anderson, R.B.; Stacker, S.A.; Cooper, H.M. The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J. Neurosci. 2006, 26, 5840–5848. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.E.; Richards, L.J.; Stacker, S.A.; Cooper, H.M. Wnt5a induces Ryk-dependent and -independent effects on callosal axon and dendrite growth. Growth Factors 2014, 32, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Beckett, K.; Monier, S.; Palmer, L.; Alexandre, C.; Green, H.; Bonneil, E.; Raposo, G.; Thibault, P.; Le Borgne, R.; Vincent, J.P. Drosophila S2 cells secrete wingless on exosome-like vesicles but the wingless gradient forms independently of exosomes. Traffic 2013, 14, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.C.; Chaudhary, V.; Bartscherer, K.; Boutros, M. Active Wnt proteins are secreted on exosomes. Nat. Cell Biol. 2012, 14, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Christova, T.; Ho, S.K.; Liu, Y.; Gill, M.; Attisano, L. LTK and ALK promote neuronal polarity and cortical migration by inhibiting IGF1R activity. EMBO Rep. 2023, 24, e56937. [Google Scholar] [CrossRef] [PubMed]
- Labbe, E.; Lock, L.; Letamendia, A.; Gorska, A.E.; Gryfe, R.; Gallinger, S.; Moses, H.L.; Attisano, L. Transcriptional cooperation between the transforming growth factor-beta and Wnt pathways in mammary and intestinal tumorigenesis. Cancer Res. 2007, 67, 75–84. [Google Scholar] [CrossRef]
- Jackson, M.R.; Cohen-Doyle, M.F.; Peterson, P.A.; Williams, D.B. Regulation of MHC class I transport by the molecular chaperone, calnexin (p88, IP90). Science 1994, 263, 384–387. [Google Scholar] [CrossRef]
- Bao, R.; Christova, T.; Song, S.; Angers, S.; Yan, X.; Attisano, L. Inhibition of tankyrases induces Axin stabilization and blocks Wnt signalling in breast cancer cells. PLoS ONE 2012, 7, e48670. [Google Scholar] [CrossRef]
- Dupraz, S.; Hilton, B.J.; Husch, A.; Santos, T.E.; Coles, C.H.; Stern, S.; Brakebusch, C.; Bradke, F. RhoA Controls Axon Extension Independent of Specification in the Developing Brain. Curr. Biol. 2019, 29, 3874–3886.e3879. [Google Scholar] [CrossRef] [PubMed]
- Salinas, P.C.; Zou, Y. Wnt signaling in neural circuit assembly. Annu. Rev. Neurosci. 2008, 31, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Willert, K.; Nusse, R. Wnt proteins. Cold Spring Harb. Perspect. Biol. 2012, 4, a007864. [Google Scholar] [CrossRef] [PubMed]
- Banziger, C.; Soldini, D.; Schutt, C.; Zipperlen, P.; Hausmann, G.; Basler, K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 2006, 125, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Mikels, A.J.; Nusse, R. Wnts as ligands: Processing, secretion and reception. Oncogene 2006, 25, 7461–7468. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dodge, M.E.; Tang, W.; Lu, J.; Ma, Z.; Fan, C.W.; Wei, S.; Hao, W.; Kilgore, J.; Williams, N.S.; et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 2009, 5, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Fenstermaker, A.G.; Prasad, A.A.; Bechara, A.; Adolfs, Y.; Tissir, F.; Goffinet, A.; Zou, Y.; Pasterkamp, R.J. Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J. Neurosci. 2010, 30, 16053–16064. [Google Scholar] [CrossRef]
- Ban, Y.; Yu, T.; Feng, B.; Lorenz, C.; Wang, X.; Baker, C.; Zou, Y. Prickle promotes the formation and maintenance of glutamatergic synapses by stabilizing the intercellular planar cell polarity complex. Sci. Adv. 2021, 7, eabh2974. [Google Scholar] [CrossRef]
- Dorrego-Rivas, A.; Ezan, J.; Moreau, M.M.; Poirault-Chassac, S.; Aubailly, N.; De Neve, J.; Blanchard, C.; Castets, F.; Freal, A.; Battefeld, A.; et al. The core PCP protein Prickle2 regulates axon number and AIS maturation by binding to AnkG and modulating microtubule bundling. Sci. Adv. 2022, 8, eabo6333. [Google Scholar] [CrossRef]
- Lu, W.; Yamamoto, V.; Ortega, B.; Baltimore, D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 2004, 119, 97–108. [Google Scholar] [CrossRef]
- Stanganello, E.; Zahavi, E.E.; Burute, M.; Smits, J.; Jordens, I.; Maurice, M.M.; Kapitein, L.C.; Hoogenraad, C.C. Wnt Signaling Directs Neuronal Polarity and Axonal Growth. iScience 2019, 13, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Dickins, E.M.; Salinas, P.C. Wnts in action: From synapse formation to synaptic maintenance. Front. Cell. Neurosci. 2013, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- McLeod, F.; Salinas, P.C. Wnt proteins as modulators of synaptic plasticity. Curr. Opin. Neurobiol. 2018, 53, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Wallingford, J.B. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 2012, 28, 627–653. [Google Scholar] [CrossRef] [PubMed]
- Dos-Santos Carvalho, S.; Moreau, M.M.; Hien, Y.E.; Garcia, M.; Aubailly, N.; Henderson, D.J.; Studer, V.; Sans, N.; Thoumine, O.; Montcouquiol, M. Vangl2 acts at the interface between actin and N-cadherin to modulate mammalian neuronal outgrowth. Elife 2020, 9, e51822. [Google Scholar] [CrossRef]
- Hua, Z.L.; Jeon, S.; Caterina, M.J.; Nathans, J. Frizzled3 is required for the development of multiple axon tracts in the mouse central nervous system. Proc. Natl. Acad. Sci. USA 2014, 111, E3005–E3014. [Google Scholar] [CrossRef]
- Mikels, A.J.; Nusse, R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 2006, 4, e115. [Google Scholar] [CrossRef] [PubMed]
- Voloshanenko, O.; Gmach, P.; Winter, J.; Kranz, D.; Boutros, M. Mapping of Wnt-Frizzled interactions by multiplex CRISPR targeting of receptor gene families. FASEB J. 2017, 31, 4832–4844. [Google Scholar] [CrossRef]
- Gross, J.C. Extracellular WNTs: Trafficking, Exosomes, and Ligand-Receptor Interaction. Handb. Exp. Pharmacol. 2021, 269, 29–43. [Google Scholar]
- Menck, K.; Sonmezer, C.; Worst, T.S.; Schulz, M.; Dihazi, G.H.; Streit, F.; Erdmann, G.; Kling, S.; Boutros, M.; Binder, C.; et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J. Extracell. Vesicles 2017, 6, 1378056. [Google Scholar] [CrossRef]
- Tuysuz, N.; van Bloois, L.; van den Brink, S.; Begthel, H.; Verstegen, M.M.; Cruz, L.J.; Hui, L.; van der Laan, L.J.; de Jonge, J.; Vries, R.; et al. Lipid-mediated Wnt protein stabilization enables serum-free culture of human organ stem cells. Nat. Commun. 2017, 8, 14578. [Google Scholar] [CrossRef] [PubMed]
- Dhamdhere, G.R.; Fang, M.Y.; Jiang, J.; Lee, K.; Cheng, D.; Olveda, R.C.; Liu, B.; Mulligan, K.A.; Carlson, J.C.; Ransom, R.C.; et al. Drugging a stem cell compartment using Wnt3a protein as a therapeutic. PLoS ONE 2014, 9, e83650. [Google Scholar] [CrossRef] [PubMed]


| Gene | Dharmacon Catalog # |
|---|---|
| Prickle1 | MQ-042729-01-0002 |
| Prickle2 | MQ-056882-02-0002 |
| Vangl1 | MQ-057276-01-0002 |
| Vangl2 | MQ-059396-01-0002 |
| Porcupine | MQ-049203-01-0002 |
| Wntless | MQ-060922-01-0002 |
| Antibodies | Source | Identifier |
|---|---|---|
| Mouse anti- MAP2 (Clone AP20, 1:500) | Millipore (Oakville, ON, Canada) | MAB3418 |
| Mouse anti-Tau-1 (Clone PC1C6, 1:300) | Millipore | MAB3420 |
| Mouse anti-Tubulin β 3 (Clone Tuj1, 1:3000) | Biolegend (San Diego, CA, USA) | 801202 |
| Mouse anti-TSG101 (1:1500) | GeneTex (Irvine, CA, USA) | GTX70255 |
| Mouse anti-Flotillin-1 (1:1500) | BD Transduction (Franklin Lakes, NJ, USA) | 618021 |
| Mouse anti-CD81 (B-11, 1:1500) | Santa Cruz (Dallas, TX, USA) | sc-166029 |
| Goat anti-Wnt5a (1:1000) | R&D Systems | AF645 |
| Rabbit anti-Actin (1:1000) | Sigma-Aldrich | A2066 |
| Rabbit anti-Dvl2 (H-75, 1:1000) | Santa Cruz | sc-13974 |
| Rabbit anti-Calnexin (1:2000) | [29] | N/A |
| Goat anti-Mouse Alexa Fluor® 488 (1:1000) | Invitrogen | A11029 |
| Goat anti-Mouse Alexa FluorTM 488 (1:500) | Thermo Fisher Scientific | A21121 |
| Goat anti-Mouse Alexa FluorTM 568 (1:500) | Thermo Fisher Scientific | A21134 |
| Goat anti-Mouse Alexa Fluor® 594 (1:1000) | Invitrogen | A11032 |
| Donkey anti-Goat IgG-HRP (1:5000) | Jackson ImmunoResearch (West Grove, PA, USA) | 705-035-147 |
| Donkey anti-Mouse IgG-HRP (1:5000) | Jackson ImmunoResearch | 715-035-150 |
| Goat anti-Rabbit IgG-HRP (1:5000) | Jackson ImmunoResearch | 711-035-152 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Prickle1 | TCCCGAAACAAGGTCAGATTTA | TCTCTGGATCTGGCTGACT |
| Prickle2 | CACTGCTTTGAGTCCCTGTATG | TCTGTAGCATGCCAGTGTTG |
| Vangl1 | GCTGGCCTGAAAGTCTACAA | CGTGTTCGGCCTCTTCATAATA |
| Vangl2 | ACTCGGGCTATTCCTACAAGT | TGATTTATCTCCACGACTCCCAT |
| Wntless | TGGGAAGCAGTCTAGCCTCC | GCAGCACAAGCCAAGGTGATA |
| Porcupine | GAGAAGGACCACCTGGAATG | ATAAGACATGGGCAGGTTCC |
| Gapdh | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.; Attisano, L. Wnt5a Promotes Axon Elongation in Coordination with the Wnt–Planar Cell Polarity Pathway. Cells 2024, 13, 1268. https://doi.org/10.3390/cells13151268
Ahmad S, Attisano L. Wnt5a Promotes Axon Elongation in Coordination with the Wnt–Planar Cell Polarity Pathway. Cells. 2024; 13(15):1268. https://doi.org/10.3390/cells13151268
Chicago/Turabian StyleAhmad, Samar, and Liliana Attisano. 2024. "Wnt5a Promotes Axon Elongation in Coordination with the Wnt–Planar Cell Polarity Pathway" Cells 13, no. 15: 1268. https://doi.org/10.3390/cells13151268





