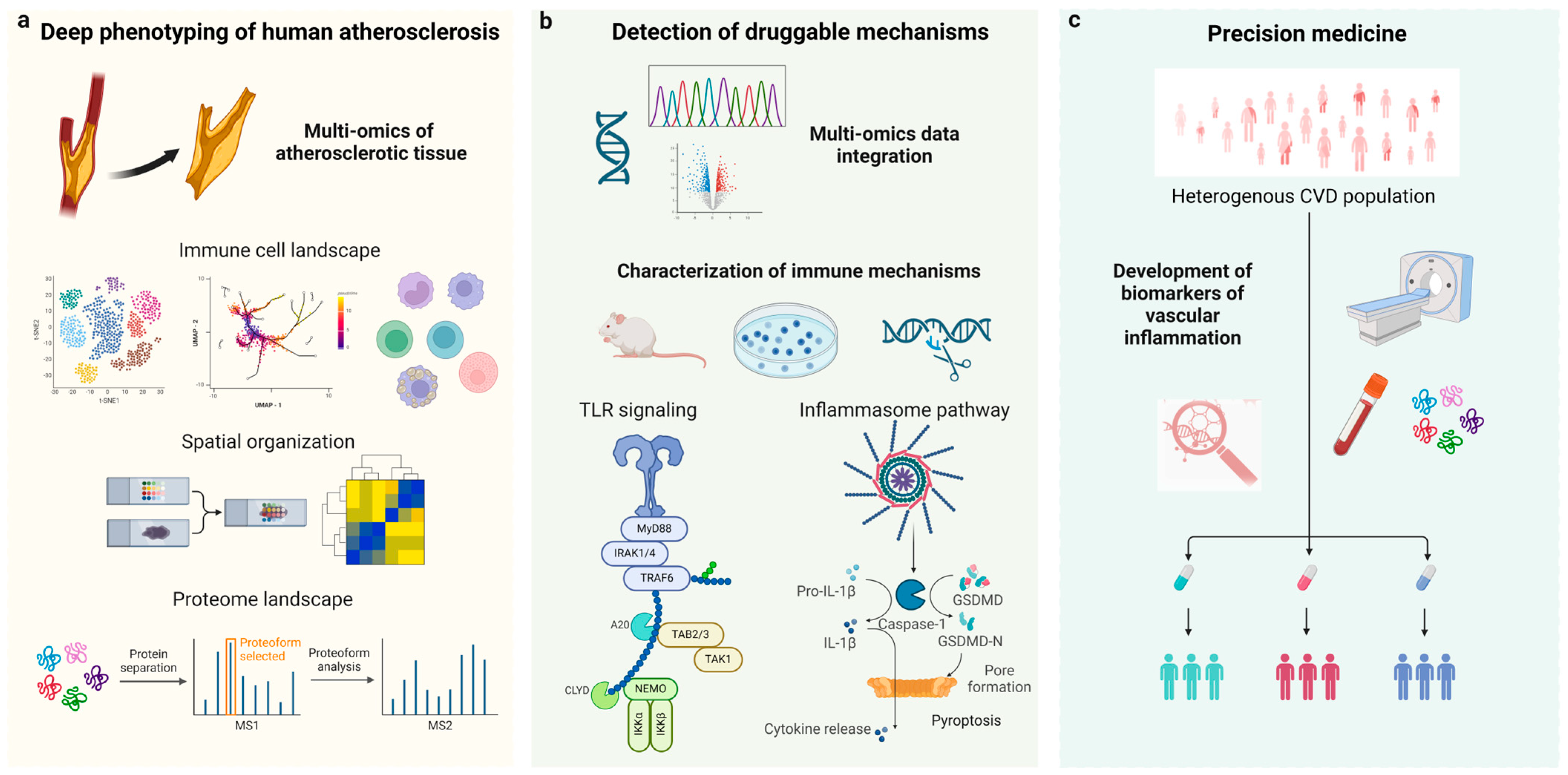
Translating Anti-Inflammatory Strategies for Atherosclerosis: Deep Phenotyping, Next-Generation Drug Targets, and Precision Medicine
Funding
Conflicts of Interest
List of Contributions
- Blagov, A.V.; Markin, A.M.; Bogatyreva, A.I.; Tolstik, T.V.; Sukhorukov, V.N.; Orekhov, A.N. The Role of Macrophages in the Pathogenesis of Atherosclerosis. Cells 2023, 12, 522. https://doi.org/10.3390/cells12040522.
- Hinkley, H.; Counts, D.A.; VonCanon, E.; Lacy, M. T Cells in Atherosclerosis: Key Players in the Pathogenesis of Vascular Disease. Cells 2023, 12, 2152. https://doi.org/10.3390/cells12172152.
- Ebert, S.; Zang, L.; Ismail, N.; Otabil, M.; Frohlich, A.; Egea, V.; Acs, S.; Hoeberg, M.; Berres, M.L.; Weber, C.; et al. Tissue Inhibitor of Metalloproteinases-1 Interacts with CD74 to Promote AKT Signaling, Monocyte Recruitment Responses, and Vascular Smooth Muscle Cell Proliferation. Cells 2023, 12, 1899. https://doi.org/10.3390/cells12141899.
- Aronova, A.; Tosato, F.; Naser, N.; Asare, Y. Innate Immune Pathways in Atherosclerosis From Signaling to Long-Term Epigenetic Reprogramming. Cells 2023, 12, 2359. https://doi.org/10.3390/cells12192359.
- Mohanta, S.K.; Sun, T.; Lu, S.; Wang, Z.; Zhang, X.; Yin, C.; Weber, C.; Habenicht, A.J.R. The Impact of the Nervous System on Arteries and the Heart: The Neuroimmune Cardiovascular Circuit Hypothesis. Cells 2023, 12, 2485. https://doi.org/10.3390/cells12202485.
- McCabe, J.J.; Evans, N.R.; Gorey, S.; Bhakta, S.; Rudd, J.H.F.; Kelly, P.J. Imaging Carotid Plaque Inflammation Using Positron Emission Tomography: Emerging Role in Clinical Stroke Care, Research Applications, and Future Directions. Cells 2023, 12, 2073. https://doi.org/10.3390/cells12162073.
References
- Björkegren, J.L.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Gibbs, B.B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, 8. [Google Scholar] [CrossRef] [PubMed]
- Aqel, N.M.; Ball, R.Y.; Waldmann, H.; Mitchinson, M.J. Identification of macrophages and smooth muscle cells in human ath-erosclerosis using monoclonal antibodies. J. Pathol. 1985, 146, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Trogan, E.; Ginsberg, M.; Grigaux, C.; Tian, J.; Miyata, M. Decreased atherosclerosis in mice deficient in both mac-rophage colony-stimulating factor (op) and apolipoprotein E. Proc. Natl. Acad. Sci. USA 1995, 92, 8264–8268. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 1997, 336, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; Salem, H.K.; Fu, X.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.F.; Libby, P. Colchicine’s Role in Cardiovascular Disease Management. Arter. Thromb. Vasc. Biol. 2024, 44, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.M.; Giannarelli, C. Immune cell profiling in atherosclerosis: Role in research and precision medicine. Nat. Rev. Cardiol. 2022, 19, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Bernhagen, J.; Heitman, L.H.; Weber, C.; Dichgans, M. Targeting the CCL2–CCR2 axis for atheroprotection. Eur. Hear. J. 2022, 43, 1799–1808. [Google Scholar] [CrossRef]
- Gill, D.; Georgakis, M.K.; Walker, V.M.; Schmidt, A.F.; Gkatzionis, A.; Freitag, D.F.; Finan, C.; Hingorani, A.D.; Howson, J.M.M.; Burgess, S.; et al. Mendelian randomization for studying the effects of perturbing drug targets. Wellcome Open Res. 2021, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Prapiadou, S.; Živković, L.; Thorand, B.; George, M.J.; van der Laan, S.W.; Malik, R.; Herder, C.; Koenig, W.; Ueland, T.; Kleveland, O.; et al. Proteogenomic Data Integration Reveals CXCL10 as a Potentially Downstream Causal Mediator for IL-6 Signaling on Atherosclerosis. Circulation 2024, 149, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Angelopoulos, A.; Papanikolaou, P.; Simantiris, S.; Oikonomou, E.K.; Vamvakaris, K.; Koumpoura, A.; Farmaki, M.; Trivellam, M.; Vlachopoulos, C.; et al. Biomarkers of Vascular Inflammation for Cardiovascular Risk Prognostication: A Meta-Analysis. JACC Cardiovasc. Imaging 2022, 15, 460–471. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asare, Y.; Georgakis, M.K. Translating Anti-Inflammatory Strategies for Atherosclerosis: Deep Phenotyping, Next-Generation Drug Targets, and Precision Medicine. Cells 2024, 13, 1306. https://doi.org/10.3390/cells13151306
Asare Y, Georgakis MK. Translating Anti-Inflammatory Strategies for Atherosclerosis: Deep Phenotyping, Next-Generation Drug Targets, and Precision Medicine. Cells. 2024; 13(15):1306. https://doi.org/10.3390/cells13151306
Chicago/Turabian StyleAsare, Yaw, and Marios K. Georgakis. 2024. "Translating Anti-Inflammatory Strategies for Atherosclerosis: Deep Phenotyping, Next-Generation Drug Targets, and Precision Medicine" Cells 13, no. 15: 1306. https://doi.org/10.3390/cells13151306




