RNAseq of Gingival Fibroblasts Exposed to PRF Membrane Lysates and PRF Serum
Abstract
1. Introduction
2. Material and Methods
2.1. Isolation and Treatment of Gingival Fibroblasts
2.2. Preparation of PRF Membrane Lysates and PRF Serum
2.3. Total RNA Isolation, Sequencing and Data Analysis
2.4. Volcano Plot, Venn Diagram and Gene Set Enrichment Analysis
3. Results
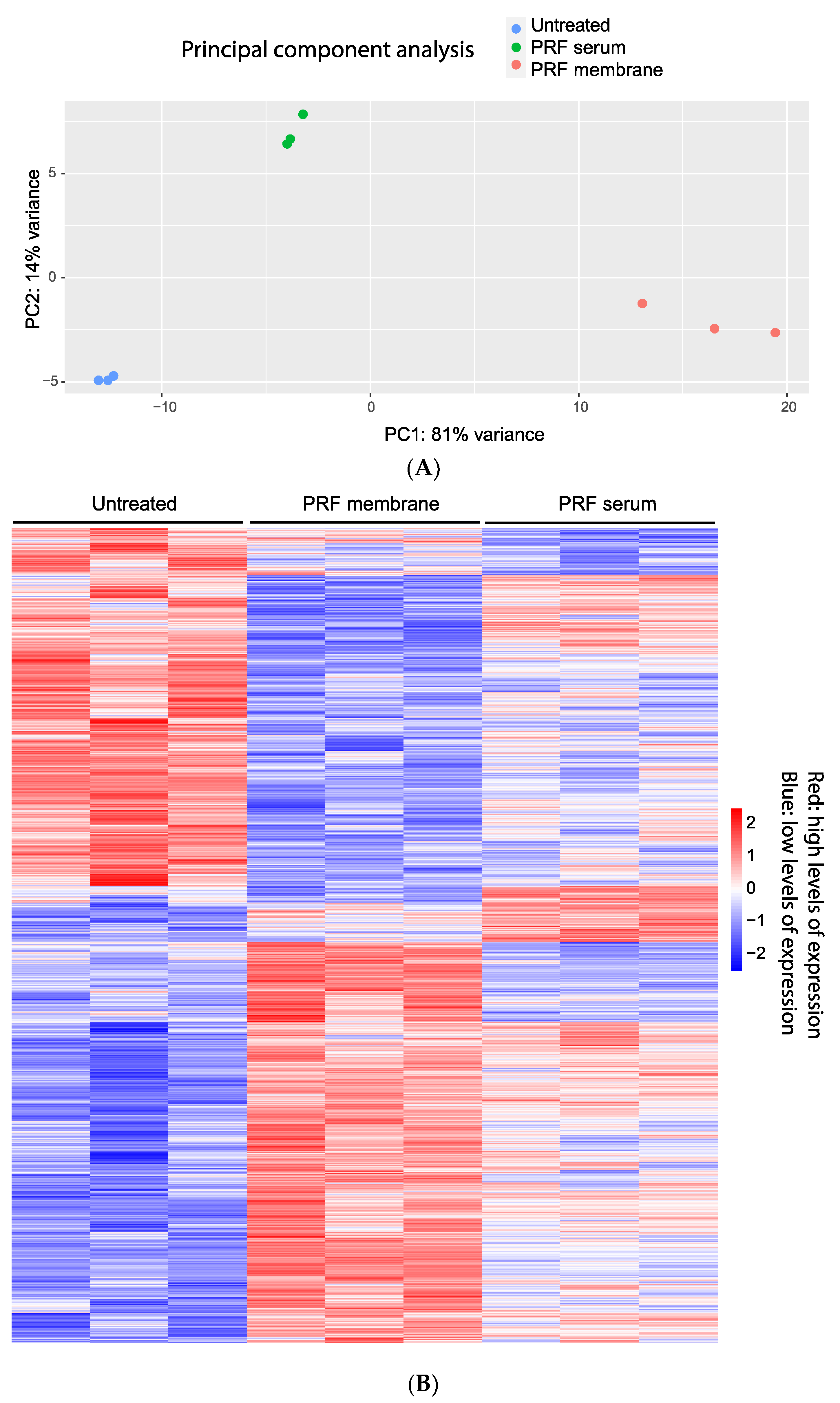
3.1. Principal Component Analysis and Heat Map of Gene Expression Changes by PRF Membrane Lysates and PRF Serum
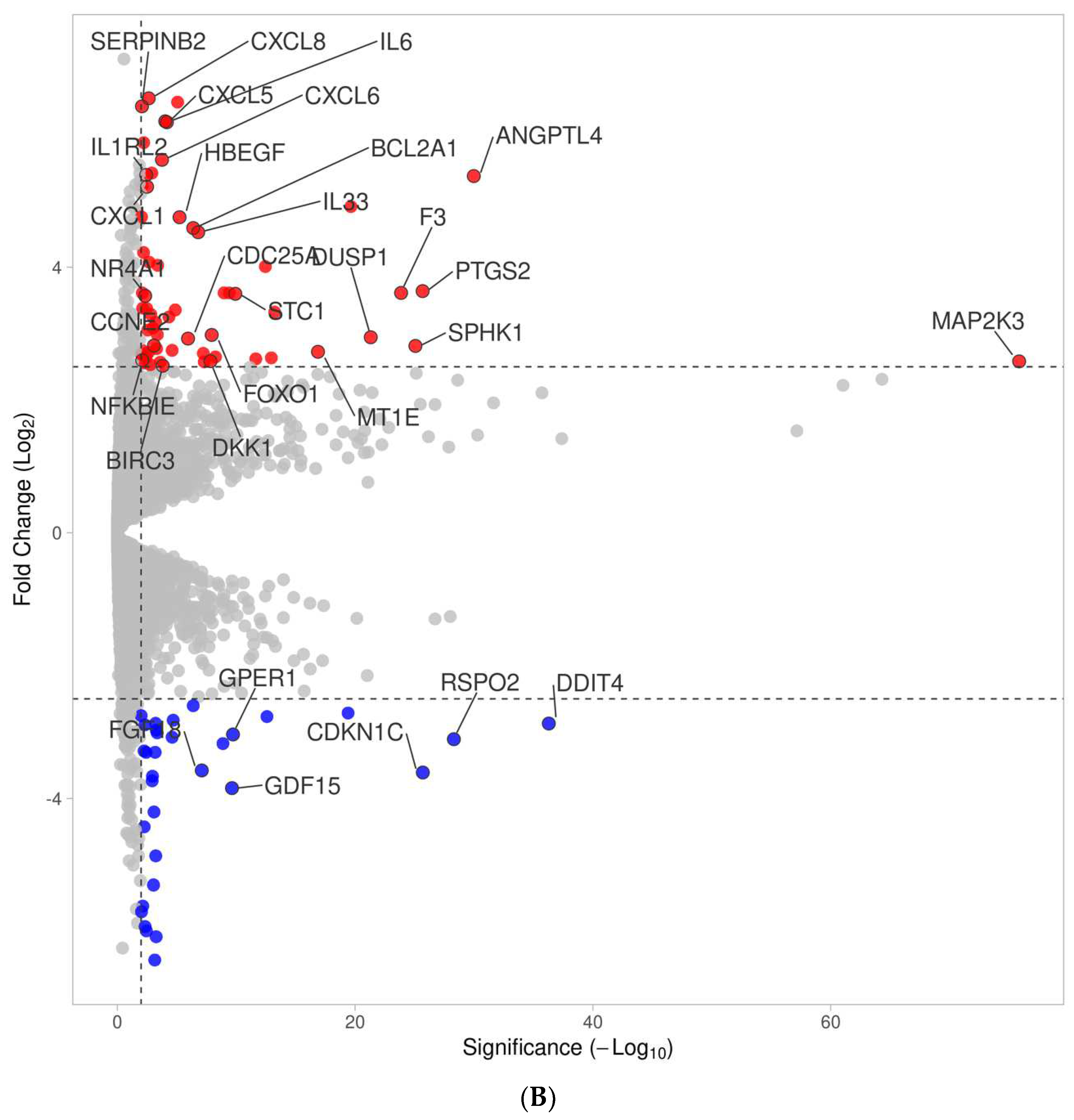
3.2. Volcano Analysis of Gene Expression Changes by PRF Membrane Lysates and PRF Serum
3.3. Venn Analysis of Genes Regulated by PRF Membrane Lysates and PRF Serum
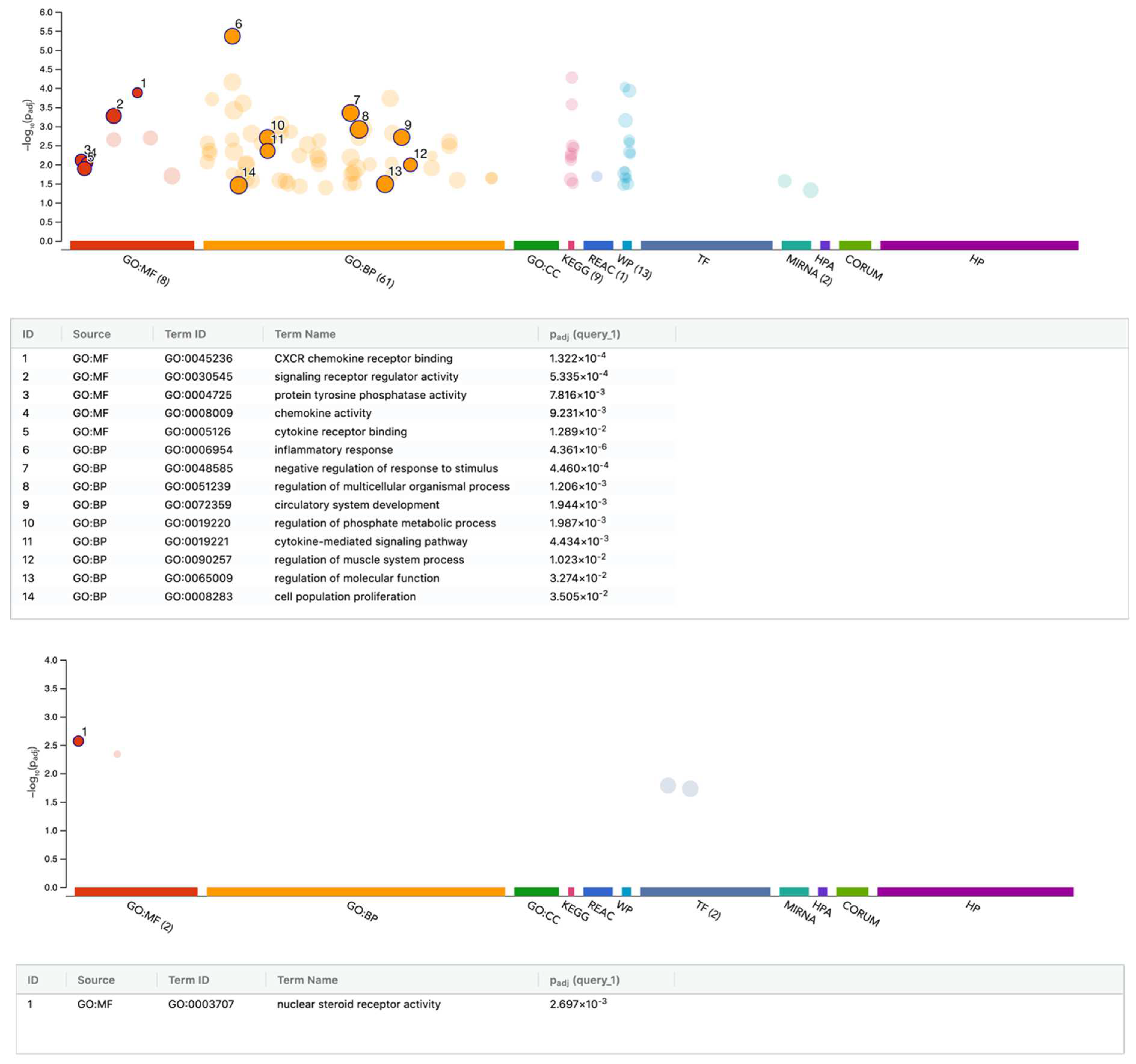
3.4. G:Profiler Analysis of Gene Expression Changes by PRF Membrane Lysates and PRF Serum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quirynen, M.; Siawasch, S.A.M.; Yu, J.; Miron, R.J. Essential principles for blood centrifugation. Periodontol. 2000 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Sculean, A.; Zhang, Y. Optimization of platelet-rich fibrin. Periodontol. 2000 2024, 94, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Blanco, J.; Wang, H.L.; Donos, N.; Temmerman, A.; Castro, A.; Pinto, N. Instructions for the use of L-PRF in different clinical indications. Periodontol. 2000 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Siawasch, S.; Temmerman, A.; Cortellini, S.; Dhondt, R.; Teughels, W.; Castro, A.B. Do autologous platelet concentrates (APCs) have a role in intra-oral bone regeneration? A critical review of clinical guidelines on decision-making process. Periodontol. 2000 2023, 93, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Valentini, P.; Calciolari, E.; Monlezun, S.; Akcali, A.; Donos, N.; Quirynen, M. APCs in sinus floor augmentation. Periodontol. 2000 2024, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Moraschini, V.; Fujioka-Kobayashi, M.; Zhang, Y.; Kawase, T.; Cosgarea, R.; Jepsen, S.; Bishara, M.; Canullo, L.; Shirakata, Y.; et al. Use of platelet-rich fibrin for the treatment of periodontal intrabony defects: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 2461–2478. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Moraschini, V.; Del Fabbro, M.; Piattelli, A.; Fujioka-Kobayashi, M.; Zhang, Y.; Saulacic, N.; Schaller, B.; Kawase, T.; Cosgarea, R.; et al. Use of platelet-rich fibrin for the treatment of gingival recessions: A systematic review and meta-analysis. Clin. Oral Investig. 2020, 24, 2543–2557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wang, X.; Zhao, Y.; Ruan, S.; Cao, H. Effect of leukocyte-platelet fibrin-rich wound reconstruction followed by full-thickness skin grafting in the treatment of diabetic foot Wagner grade 4 ulcer gangrene (toe area). Platelets 2023, 34, 2131752. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.R.; Ubilla, M.; Zamora, Y.; Del Rio, V.; Dohan Ehrenfest, D.M.; Quirynen, M. Leucocyte- and platelet-rich fibrin (L-PRF) as a regenerative medicine strategy for the treatment of refractory leg ulcers: A prospective cohort study. Platelets 2018, 29, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Cecerska-Heryc, E.; Goszka, M.; Serwin, N.; Roszak, M.; Grygorcewicz, B.; Heryc, R.; Dolegowska, B. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022, 64, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Sanchez, F.; Verspecht, T.; Castro, A.B.; Pauwels, M.; Andres, C.R.; Quirynen, M.; Teughels, W. Antimicrobial Mechanisms of Leucocyte- and Platelet Rich Fibrin Exudate Against Planktonic Porphyromonas gingivalis and within Multi-Species Biofilm: A Pilot Study. Front. Cell. Infect. Microbiol. 2021, 11, 722499. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.B.; Herrero, E.R.; Slomka, V.; Pinto, N.; Teughels, W.; Quirynen, M. Antimicrobial capacity of Leucocyte-and Platelet Rich Fibrin against periodontal pathogens. Sci. Rep. 2019, 9, 8188. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, H.; Zhang, Z.; Yan, Z.; Lv, H.; Zhang, Y.; Wu, B. Platelet-rich fibrin exudate promotes the proliferation and osteogenic differentiation of human periodontal ligament cells in vitro. Mol. Med. Rep. 2018, 18, 4477–4485. [Google Scholar] [CrossRef] [PubMed]
- de Witt, S.M.; Verdoold, R.; Cosemans, J.M.; Heemskerk, J.W. Insights into platelet-based control of coagulation. Thromb. Res. 2014, 133 (Suppl. 2), S139–S148. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.B.; Andrade, C.; Li, X.; Pinto, N.; Teughels, W.; Quirynen, M. Impact of g force and timing on the characteristics of platelet-rich fibrin matrices. Sci. Rep. 2021, 11, 6038. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Versura, P.; Buzzi, M.; Primavera, L.; Pellegrini, M.; Campos, E.C. Blood derived eye drops for the treatment of cornea and ocular surface diseases. Transfus. Apher. Sci. 2017, 56, 595–604. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Hodge, C.; Hoque, M.; Petsoglou, C.; Sutton, G. Human Platelets and Derived Products in Treating Ocular Surface Diseases—A Systematic Review. Clin. Ophthalmol. 2020, 14, 3195–3210. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.W. Release of alpha-granule contents during platelet activation. Platelets 2022, 33, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Flaumenhaft, R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. In Fibrous Proteins: Structures and Mechanisms; Subcellular Biochemistry Book Series; Springer: Cham, Switzerland, 2017; Volume 82, pp. 405–456. [Google Scholar] [CrossRef]
- Chang, J.; Blanchard, S.B.; Windsor, L.J.; Gregory, R.L.; Hamada, Y. Levels of growth factors from platelet-rich fibrin from chronic periodontitis versus periodontally healthy subjects: A pilot study. Clin. Oral Investig. 2020, 24, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Koenen, R.R. The prowess of platelets in immunity and inflammation. Thromb. Haemost. 2016, 116, 605–612. [Google Scholar] [CrossRef]
- Anitua, E.; Muruzabal, F.; Pino, A.; Prado, R.; Azkargorta, M.; Elortza, F.; Merayo-Lloves, J. Proteomic Characterization of Plasma Rich in Growth Factors and Undiluted Autologous Serum. Int. J. Mol. Sci. 2021, 22, 12176. [Google Scholar] [CrossRef] [PubMed]
- Perucci, L.O.; Vago, J.P.; Miles, L.A.; Sousa, L.P. Crosstalk between the plasminogen/plasmin system and inflammation resolution. J. Thromb. Haemost. 2023, 21, 2666–2678. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.; Divaris, K.; Bugge, T.H.; Moutsopoulos, N.M. Plasmin-Mediated Fibrinolysis in Periodontitis Pathogenesis. J. Dent. Res. 2023, 102, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Di Summa, F.; Kargarpour, Z.; Nasirzade, J.; Stahli, A.; Mitulovic, G.; Panic-Jankovic, T.; Koller, V.; Kaltenbach, C.; Muller, H.; Panahipour, L.; et al. TGFbeta activity released from platelet-rich fibrin adsorbs to titanium surface and collagen membranes. Sci. Rep. 2020, 10, 10203. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.W.; Greenwell-Wild, T.; Brenchley, L.; Dutzan, N.; Overmiller, A.; Sawaya, A.P.; Webb, S.; Martin, D.; Genomics, N.N.; Computational Biology, C.; et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell 2021, 184, 4090–4104.e15. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Saligan, L.N. Wound Pain and Wound Healing Biomarkers from Wound Exudate: A Scoping Review. J. Wound Ostomy Cont. Nurs. 2020, 47, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K.C.; Nattuthurai, G.S.; Sundararajan, S.K.; Sujith, I.; Joseph, J.; Syedshah, Y.P. Gingival Crevicular Fluid: An Overview. J. Pharm. Bioallied Sci. 2019, 11, S135–S139. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Goedhart, J.; Luijsterburg, M.S. VolcaNoseR is a web app for creating, exploring, labeling and sharing volcano plots. Sci. Rep. 2020, 10, 20560. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Adler, P.; Vilo, J.; Peterson, H. g:Profiler-interoperable web service for functional enrichment analysis and gene identifier mapping (2023 update). Nucleic Acids Res. 2023, 51, W207–W212. [Google Scholar] [CrossRef] [PubMed]
- Kargarpour, Z.; Nasirzade, J.; Panahipour, L.; Miron, R.J.; Gruber, R. Platelet-Rich Fibrin Decreases the Inflammatory Response of Mesenchymal Cells. Int. J. Mol. Sci. 2021, 22, 11333. [Google Scholar] [CrossRef] [PubMed]
- Nasirzade, J.; Kargarpour, Z.; Hasannia, S.; Strauss, F.J.; Gruber, R. Platelet-rich fibrin elicits an anti-inflammatory response in macrophages in vitro. J. Periodontol. 2020, 91, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Imani, A.; Panahipour, L.; Dos Santos Sanches, N.; Wang, L.; Gruber, R. Platelet-Rich Fibrin Increases CXCL8 Expression in Gingival Fibroblasts. Biomedicines 2024, 12, 1326. [Google Scholar] [CrossRef] [PubMed]
- Iyer, V.R.; Eisen, M.B.; Ross, D.T.; Schuler, G.; Moore, T.; Lee, J.C.; Trent, J.M.; Staudt, L.M.; Hudson, J., Jr.; Boguski, M.S.; et al. The transcriptional program in the response of human fibroblasts to serum. Science 1999, 283, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, S.; Tolley, N.D.; Dixon, D.A.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A.; Weyrich, A.S. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J. Cell Biol. 2001, 154, 485–490. [Google Scholar] [CrossRef]
- Di Iorio, A.; Ferrucci, L.; Sparvieri, E.; Cherubini, A.; Volpato, S.; Corsi, A.; Bonafe, M.; Franceschi, C.; Abate, G.; Paganelli, R. Serum IL-1beta levels in health and disease: A population-based study. ‘The InCHIANTI study’. Cytokine 2003, 22, 198–205. [Google Scholar] [CrossRef]
- Santos, A.F.P.; Cervantes, L.C.C.; Panahipour, L.; Souza, F.A.; Gruber, R. Proof-of-Principle Study Suggesting Potential Anti-Inflammatory Activity of Butyrate and Propionate in Periodontal Cells. Int. J. Mol. Sci. 2022, 23, 11006. [Google Scholar] [CrossRef] [PubMed]
- Yucel-Lindberg, T.; Ahola, H.; Nilsson, S.; Carlstedt-Duke, J.; Modeer, T. Interleukin-1 beta induces expression of cyclooxygenase-2 mRNA in human gingival fibroblasts. Inflammation 1995, 19, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylostalo, J.H.; Bazhanov, N.; Kuhlman, J.; Prockop, D.J. Dynamic compaction of human mesenchymal stem/precursor cells into spheres self-activates caspase-dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6, and STC1). Stem Cells 2013, 31, 2443–2456. [Google Scholar] [CrossRef] [PubMed]
- Siamwala, J.H.; Pagano, F.S.; Dubielecka, P.M.; Ivey, M.J.; Guirao-Abad, J.P.; Zhao, A.; Chen, S.; Granston, H.; Jeong, J.Y.; Rounds, S.; et al. IL-1beta-mediated adaptive reprogramming of endogenous human cardiac fibroblasts to cells with immune features during fibrotic remodeling. Commun. Biol. 2023, 6, 1200. [Google Scholar] [CrossRef] [PubMed]
- Alomar, S.Y.; Gentili, A.; Zaibi, M.S.; Kepczynska, M.A.; Trayhurn, P. IL-1beta (interleukin-1beta) stimulates the production and release of multiple cytokines and chemokines by human preadipocytes. Arch. Physiol. Biochem. 2016, 122, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Kargarpour, Z.; Panahipour, L.; Mildner, M.; Miron, R.J.; Gruber, R. Lipids of Platelet-Rich Fibrin Reduce the Inflammatory Response in Mesenchymal Cells and Macrophages. Cells 2023, 12, 634. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.A. Understanding interleukin 11 as a disease gene and therapeutic target. Biochem. J. 2023, 480, 1987–2008. [Google Scholar] [CrossRef] [PubMed]
- Kargarpour, Z.; Nasirzade, J.; Panahipour, L.; Miron, R.J.; Gruber, R. Relative Centrifugal Force (RCF.; G-Force) Affects the Distribution of TGF-beta in PRF Membranes Produced Using Horizontal Centrifugation. Int. J. Mol. Sci. 2020, 21, 7629. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Hamilton, D.W.; Leask, A. ALK5 inhibition blocks TGFss-induced CCN2 expression in gingival fibroblasts. J. Dent. Res. 2010, 89, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Wetzler, M.; Talpaz, M.; Lowe, D.G.; Baiocchi, G.; Gutterman, J.U.; Kurzrock, R. Constitutive expression of leukemia inhibitory factor RNA by human bone marrow stromal cells and modulation by IL-1, TNF-alpha, and TGF-beta. Exp. Hematol. 1991, 19, 347–351. [Google Scholar] [PubMed]
- Usui-Ouchi, A.; Eade, K.; Giles, S.; Ideguchi, Y.; Ouchi, Y.; Aguilar, E.; Wei, G.; Marra, K.V.; Berlow, R.B.; Friedlander, M. Deletion of Tgfbeta signal in activated microglia prolongs hypoxia-induced retinal neovascularization enhancing Igf1 expression and retinal leukostasis. Glia 2022, 70, 1762–1776. [Google Scholar] [CrossRef] [PubMed]
- Kargarpour, Z.; Nasirzade, J.; Panahipour, L.; Mitulovic, G.; Miron, R.J.; Gruber, R. Platelet-Rich Fibrin Increases BMP2 Expression in Oral Fibroblasts via Activation of TGF-beta Signaling. Int. J. Mol. Sci. 2021, 22, 7935. [Google Scholar] [CrossRef]
- Erickson, P.A.; Cleves, P.A.; Ellis, N.A.; Schwalbach, K.T.; Hart, J.C.; Miller, C.T. A 190 base pair, TGF-beta responsive tooth and fin enhancer is required for stickleback Bmp6 expression. Dev. Biol. 2015, 401, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Strand, D.W.; Liang, Y.Y.; Yang, F.; Barron, D.A.; Ressler, S.J.; Schauer, I.G.; Feng, X.H.; Rowley, D.R. TGF-beta induction of FGF-2 expression in stromal cells requires integrated smad3 and MAPK pathways. Am. J. Clin. Exp. Urol. 2014, 2, 239–248. [Google Scholar] [PubMed]
- Stuhlmeier, K.M.; Pollaschek, C. Differential effect of transforming growth factor beta (TGF-beta) on the genes encoding hyaluronan synthases and utilization of the p38 MAPK pathway in TGF-beta-induced hyaluronan synthase 1 activation. J. Biol. Chem. 2004, 279, 8753–8760. [Google Scholar] [CrossRef] [PubMed]
- Oguchi, T.; Ishiguro, N. Differential stimulation of three forms of hyaluronan synthase by TGF-beta, IL-1beta, and TNF-alpha. Connect. Tissue Res. 2004, 45, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Itano, N.; Hata, K.; Ueda, M.; Kimata, K. Differential regulation by IL-1beta and EGF of expression of three different hyaluronan synthases in oral mucosal epithelial cells and fibroblasts and dermal fibroblasts: Quantitative analysis using real-time RT-PCR. J. Investig. Dermatol. 2004, 122, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Xing, J.; Li, X.F.; Yang, Y.L.; Shao, H.; Li, J. Roles of interferon induced protein with tetratricopeptide repeats (IFIT) family in autoimmune disease. Autoimmun. Rev. 2023, 22, 103453. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Lu, W.; Sun, R.; Guo, R.; Cao, X.; Liu, X.; Lyu, C.; Zhao, M. The diagnostic/prognostic roles and biological function of the IFIT family members in acute myeloid leukemia. BMC Med. Genom. 2023, 16, 296. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Kingsley, P.D.; Cho, E.S.; Jiang, R. Osr2, a new mouse gene related to Drosophila odd-skipped, exhibits dynamic expression patterns during craniofacial, limb, and kidney development. Mech. Dev. 2001, 107, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lan, Y.; Chai, Y.; Jiang, R. Antagonistic actions of Msx1 and Osr2 pattern mammalian teeth into a single row. Science 2009, 323, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- Kotsaris, G.; Qazi, T.H.; Bucher, C.H.; Zahid, H.; Pohle-Kronawitter, S.; Ugorets, V.; Jarassier, W.; Borno, S.; Timmermann, B.; Giesecke-Thiel, C.; et al. Odd skipped-related 1 controls the pro-regenerative response of fibro-adipogenic progenitors. npj Regen. Med. 2023, 8, 19. [Google Scholar] [CrossRef]
- Heiland, G.R.; Zwerina, K.; Baum, W.; Kireva, T.; Distler, J.H.; Grisanti, M.; Asuncion, F.; Li, X.; Ominsky, M.; Richards, W.; et al. Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Ann. Rheum. Dis. 2010, 69, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Goes, P.; Dutra, C.; Losser, L.; Hofbauer, L.C.; Rauner, M.; Thiele, S. Loss of Dkk-1 in Osteocytes Mitigates Alveolar Bone Loss in Mice with Periodontitis. Front. Immunol. 2019, 10, 2924. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Adachi, N.; Yoshimoto, Y.; Sasabuchi, A.; Kawashima, N.; Ota, M.S.; Iseki, S. Fibroblast growth factor signalling regulates the development of tooth root. J. Anat. 2024, 244, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, K.; Amirthalingam, S.; Hwang, N.S.; Jayakumar, R. Role of FGF-18 in Bone Regeneration. J. Funct. Biomater. 2023, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Westhrin, M.; Moen, S.H.; Holien, T.; Mylin, A.K.; Heickendorff, L.; Olsen, O.E.; Sundan, A.; Turesson, I.; Gimsing, P.; Waage, A.; et al. Growth differentiation factor 15 (GDF15) promotes osteoclast differentiation and inhibits osteoblast differentiation and high serum GDF15 levels are associated with multiple myeloma bone disease. Haematologica 2015, 100, e511–e514. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Silvestre, R.A.; Diez, J.J. Growth differentiation factor 15 (GDF-15) in endocrinology. Endocrine 2023, 81, 419–431. [Google Scholar] [CrossRef]
- Kargarpour, Z.; Panahipour, L.; Miron, R.J.; Gruber, R. Blood Clots versus PRF: Activating TGF-beta Signaling and Inhibiting Inflammation In Vitro. Int. J. Mol. Sci. 2022, 23, 5897. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imani, A.; Panahipour, L.; Kühtreiber, H.; Mildner, M.; Gruber, R. RNAseq of Gingival Fibroblasts Exposed to PRF Membrane Lysates and PRF Serum. Cells 2024, 13, 1308. https://doi.org/10.3390/cells13151308
Imani A, Panahipour L, Kühtreiber H, Mildner M, Gruber R. RNAseq of Gingival Fibroblasts Exposed to PRF Membrane Lysates and PRF Serum. Cells. 2024; 13(15):1308. https://doi.org/10.3390/cells13151308
Chicago/Turabian StyleImani, Atefe, Layla Panahipour, Hannes Kühtreiber, Michael Mildner, and Reinhard Gruber. 2024. "RNAseq of Gingival Fibroblasts Exposed to PRF Membrane Lysates and PRF Serum" Cells 13, no. 15: 1308. https://doi.org/10.3390/cells13151308
APA StyleImani, A., Panahipour, L., Kühtreiber, H., Mildner, M., & Gruber, R. (2024). RNAseq of Gingival Fibroblasts Exposed to PRF Membrane Lysates and PRF Serum. Cells, 13(15), 1308. https://doi.org/10.3390/cells13151308








