MiRNAs as Regulators of Immune Cells in the Tumor Microenvironment of Ovarian Cancer
Abstract
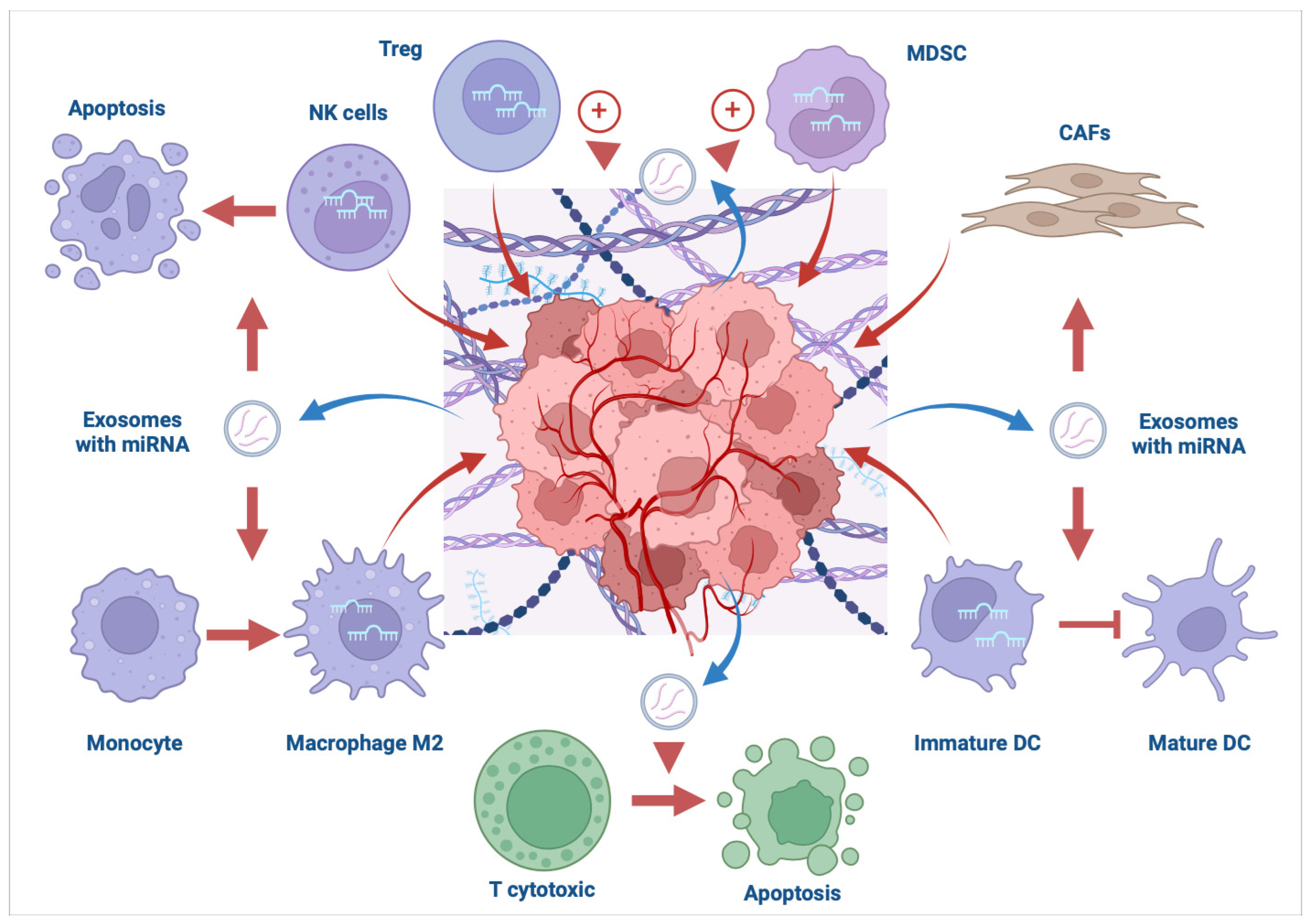
:1. Introduction
2. MiRNAs as Modulators of Immune Response in the TME: General Remarks and Examples from Studies outside the Ovarian Cancer Spectrum
2.1. T Cells
2.2. Macrophages
2.3. Dendritic Cells
2.4. NK Cells
2.5. MDSCs
3. MiRNAs as Modulators of Immune Response in the TME of Ovarian Cancer
| miRNA | Effect | Reference |
|---|---|---|
| Macrophages | ||
| miRNA-125b | Intraperitoneal administration of miRNA-125b in a murine ovarian tumor model caused the repolarization of macrophages to an immune-activating phenotype | [47] |
| miRNA-222-3p | Activation of macrophages to M2 phenotype upon ovarian cancer-derived exosomes through SOCS3/STAT3 pathway | [48] |
| miRNA-223 | miRNA-223 from hypoxic macrophages promoted the drug resistance of ovarian cancer cells via the PTEN-PI3K/AKT pathway | [49] |
| miRNA-200b | KLF6 inhibition decreased the M1 polarization, leading to an immunosuppressive response | [50] |
| miRNA-940 | Exosomal miRNA-940 caused M2 polarization | [51] |
| miRNA-21-3p miRNA-125b-5p miRNA-181d-5p | Exosomes containing the mentioned miRNAs caused M2 polarization | [52] |
| miRNA-181c-5p | SKOV-3 derived exosomes with miRNA-181c-5p caused M2 polarization | [53] |
| miRNA-532-3p | CircATP2B4 served as a competing endogenous RNA of miR-532-3p and induced M2 polarization through miR-532-3p/SREBF1/PI3Ka/AKT axis | [54] |
| miRNA-1246 | Oncogenic exosomal miRNA-1246 promoted tumor progression in the TME via M2-type macrophages | [55] |
| miRNA-221-3p | Exosomes from M2-type macrophages caused EOC proliferation through CDKN1B | [56] |
| miRNA-7 | TWEAK increased miRNA-7 expression in macrophages and its’ exosomes | [59] |
| T cells | ||
| miRNA-29a-3p miRNA-21-5p | Upon transfection of CD4+ T cells with equivalent miRNA mimics STAT3 suppression was observed, which influenced Treg/Th17 cell ratio | [57] |
| miRNA-29a-3p | Exosomal miRNA-29a-3p decreased PD-L1 through the FOXO3-AKT/GSK3β pathway | [58] |
| miRNA-let-7i | Overexpression of miRNA-let-71 caused the increase of T cells presence within the tumor and upregulation of APCs activity | [60] |
| miRNA-142 | Artesunate in murine model upregulated miRNA-142, which suppressed Sirt1 and promoted Th1 differentiation | [64] |
| miRNA-1245 | miRNA-1245 targets MCT1, reduces lactate uptake and impairs immune-suppressive responses of Tregs | [65] |
| miRNA-146a | miRNA-146a targets IL-1 receptor-associated kinase 1 (IRAK1) and tumor necrosis factor receptor-associated factor 6 (TRAF6) leading to NF-κB signaling inhibition; upregulation of miRNA146a leads to CD8+ T cell infiltration | [66] |
| DCs | ||
| miRNA-22 miRNA-503 | miRNA-22 targets YWHAZ and blocks PI3K/Akt and MAPK pathways miRNA-503 downregulates Bcl2 expression Such actions cause reduced survival of DCs | [61] |
| miRNA-155 | miRNA-155 delivery to tumor-associated DCs induced inhibition of tumor progression | [62] |
| NK cells | ||
| miRNA-20a | miRNA-20a targets MICA/B (ligands of the NKG2D receptor), which leads to NK cells’ suppression | [63] |
| MDSCs | ||
| miRNA-211 | miRNA-211 targets C/EBP homologous protein (CHOP); upregulation of miRNA-211 resulted in inhibition of MDCSs’ immunosuppressive actions | [67] |
| miRNA | Effect | Reference |
|---|---|---|
| PD-1/PD-L1 | ||
| miRNA-424(322) | miRNA-424(322) inhibited PD-L1 and CD80 expression | [68] |
| miRNA-576-3p | miRNA-576-3p targets PD-L1 and cyclin D1, leading to an increase in cisplatin sensitivity | [69] |
| miRNA-200c-3p | miRNA-200c-3- reduces PD-L1, c-Myc, and β-catenin, leading to sensitization of ovarian cancer cells to treatment | [70] |
| miRNA-155-5p | Low intake of miRNA-155-5p by macrophages leads to immunosuppression by upregulation of PD-L1 | [71] |
| miRNA-34a-5p | miRNA-34a-5p negatively regulates the expression of PD-L1 | [72] |
| miRNA-92 | LATS2 as a target for miRNA-92; downregulation of LATS2 leads to an increased translocation of YAP1 and upregulation of PD-L1 | [73] |
| miRNA-145 | cisplatin-mediated down-regulation of miRNA-145 is responsible for increased PD-L1 expression via c-Myc in ovarian cancer cells | [75] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cabasag, C.J.; Fagan, P.J.; Ferlay, J.; Vignat, J.; Laversanne, M.; Liu, L.; Van Der Aa, M.A.; Bray, F.; Soerjomataram, I. Ovarian Cancer Today and Tomorrow: A Global Assessment by World Region and Human Development Index Using GLOBOCAN 2020. Int. J. Cancer 2022, 151, 1535–1541. [Google Scholar] [CrossRef]
- Fantone, S.; Piani, F.; Olivieri, F.; Rippo, M.R.; Sirico, A.; Di Simone, N.; Marzioni, D.; Tossetta, G. Role of SLC7A11/xCT in Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 587. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Li, H.; Tong, X.; Zhu, X. Efficacy and Safety of Olaparib in Advanced Ovarian Cancer: A Meta-Analysis. J. Obstet. Gynaecol. 2023, 43, 2151883. [Google Scholar] [CrossRef]
- Truffi, M.; Sorrentino, L.; Corsi, F. Fibroblasts in the Tumor Microenvironment. In Tumor Microenvironment; Birbrair, A., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; Volume 1234, pp. 15–29. ISBN 978-3-030-37183-8. [Google Scholar]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Patil, N.; Allgayer, H.; Leupold, J.H. MicroRNAs in the Tumor Microenvironment. In Tumor Microenvironment; Birbrair, A., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; Volume 1277, pp. 1–31. ISBN 978-3-030-50223-2. [Google Scholar]
- Ambros, V. The Functions of Animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.; Jacks, T. MicroRNAs and Cancer: Short RNAs Go a Long Way. Cell 2009, 136, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, J.; Luo, Y. MicroRNAs in Tumor Immunity: Functional Regulation in Tumor-Associated Macrophages. J. Zhejiang Univ. Sci. B 2020, 21, 12–28. [Google Scholar] [CrossRef]
- Koutsaki, M.; Spandidos, D.A.; Zaravinos, A. Epithelial–Mesenchymal Transition-Associated miRNAs in Ovarian Carcinoma, with Highlight on the miR-200 Family: Prognostic Value and Prospective Role in Ovarian Cancer Therapeutics. Cancer Lett. 2014, 351, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Goradel, N.H.; Mohammadi, N.; Haghi-Aminjan, H.; Farhood, B.; Negahdari, B.; Sahebkar, A. Regulation of Tumor Angiogenesis by microRNAs: State of the Art. J. Cell. Physiol. 2019, 234, 1099–1110. [Google Scholar] [CrossRef]
- Cui, M.; Liu, Y.; Cheng, L.; Li, T.; Deng, Y.; Liu, D. Research Progress on Anti-Ovarian Cancer Mechanism of miRNA Regulating Tumor Microenvironment. Front. Immunol. 2022, 13, 1050917. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Fang, C.; Liu, X.; Liao, Q.; Wu, N.; Wang, J. Immunotherapy and the Ovarian Cancer Microenvironment: Exploring Potential Strategies for Enhanced Treatment Efficacy. Immunology 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kohlhapp, F.J.; Mitra, A.K.; Lengyel, E.; Peter, M.E. MicroRNAs as Mediators and Communicators between Cancer Cells and the Tumor Microenvironment. Oncogene 2015, 34, 5857–5868. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor Microenvironment Complexity and Therapeutic Implications at a Glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Van Der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8+ T Cell States in Human Cancer: Insights from Single-Cell Analysis. Nat. Rev. Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, F.J.; O’Neill, L.A.J. Adding Fuel to Fire: microRNAs as a New Class of Mediators of Inflammation. Ann. Rheum. Dis. 2008, 67, iii50–iii55. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Schambach, F.; DeJong, C.S.; Hammond, S.M.; Reiner, S.L. Micro-RNA-155 Inhibits IFN-γ Signaling in CD4+ T Cells. Eur. J. Immunol. 2010, 40, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Xu, S.; Liu, X.; Zhang, Q.; Xu, X.; Liu, M.; Hua, M.; Li, N.; Yao, H.; Cao, X. The microRNA miR-29 Controls Innate and Adaptive Immune Responses to Intracellular Bacterial Infection by Targeting Interferon-γ. Nat. Immunol. 2011, 12, 861–869. [Google Scholar] [CrossRef]
- Huffaker, T.B.; Hu, R.; Runtsch, M.C.; Bake, E.; Chen, X.; Zhao, J.; Round, J.L.; Baltimore, D.; O’Connell, R.M. Epistasis between MicroRNAs 155 and 146a during T Cell-Mediated Antitumor Immunity. Cell Rep. 2012, 2, 1697–1709. [Google Scholar] [CrossRef]
- Jiang, S.; Li, C.; Olive, V.; Lykken, E.; Feng, F.; Sevilla, J.; Wan, Y.; He, L.; Li, Q.-J. Molecular Dissection of the miR-17-92 Cluster’s Critical Dual Roles in Promoting Th1 Responses and Preventing Inducible Treg Differentiation. Blood 2011, 118, 5487–5497. [Google Scholar] [CrossRef]
- Qin, A.; Wen, Z.; Zhou, Y.; Li, Y.; Li, Y.; Luo, J.; Ren, T.; Xu, L. Micro RNA -126 Regulates the Induction and Function of CD 4+ Foxp3+ Regulatory T Cells through PI 3K/ AKT Pathway. J. Cell. Mol. Med. 2013, 17, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Cai, X.; Chen, X.; Liang, H.; Zhang, Y.; Li, J.; Wang, Z.; Chen, X.; Zhang, W.; Yokoyama, S.; et al. Tumor-Secreted miR-214 Induces Regulatory T Cells: A Major Link between Immune Evasion and Tumor Growth. Cell Res. 2014, 24, 1164–1180. [Google Scholar] [CrossRef] [PubMed]
- Trifari, S.; Pipkin, M.E.; Bandukwala, H.S.; Äijö, T.; Bassein, J.; Chen, R.; Martinez, G.J.; Rao, A. MicroRNA-Directed Program of Cytotoxic CD8+ T-Cell Differentiation. Proc. Natl. Acad. Sci. USA 2013, 110, 18608–18613. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yin, Y.; Li, N.; Zhu, D.; Zhang, J.; Zhang, C.-Y.; Zen, K. Re-Polarization of Tumor-Associated Macrophages to pro-Inflammatory M1 Macrophages by microRNA-155. J. Mol. Cell Biol. 2012, 4, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Z.; Chen, C.; Liu, Y.; Si, Q.; Chuang, T.-H.; Li, N.; Gomez-Cabrero, A.; Reisfeld, R.A.; Xiang, R.; et al. MicroRNA-19a-3p Inhibits Breast Cancer Progression and Metastasis by Inducing Macrophage Polarization through Downregulated Expression of Fra-1 Proto-Oncogene. Oncogene 2014, 33, 3014–3023. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jia, H.; Zhang, H.; Lv, M.; Liu, J.; Zhang, Y.; Huang, T.; Huang, B. TLR4 Signaling Induces the Release of Microparticles by Tumor Cells That Regulate Inflammatory Cytokine IL-6 of Macrophages via microRNA Let-7b. OncoImmunology 2012, 1, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Paone, A.; Calore, F.; Galli, R.; Gaudio, E.; Santhanam, R.; Lovat, F.; Fadda, P.; Mao, C.; Nuovo, G.J.; et al. MicroRNAs Bind to Toll-like Receptors to Induce Prometastatic Inflammatory Response. Proc. Natl. Acad. Sci. USA 2012, 109, E2110–E2116. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vos, K.E.; Abels, E.R.; Zhang, X.; Lai, C.; Carrizosa, E.; Oakley, D.; Prabhakar, S.; Mardini, O.; Crommentuijn, M.H.W.; Skog, J.; et al. Directly Visualized Glioblastoma-Derived Extracellular Vesicles Transfer RNA to Microglia/Macrophages in the Brain. Neuro Oncol. 2016, 18, 58–69. [Google Scholar] [CrossRef]
- Huang, C.; Liu, X.; QunZhou; Xie, J.; Ma, T.; Meng, X.; Li, J. MiR-146a Modulates Macrophage Polarization by Inhibiting Notch1 Pathway in RAW264.7 Macrophages. Int. Immunopharmacol. 2016, 32, 46–54. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Pan, H.; Wang, Y.; Shi, M.; Yu, H.; Wang, C.; Pan, X.; Chen, Z. Exosomes Derived From Macrophages Enhance Aerobic Glycolysis and Chemoresistance in Lung Cancer by Stabilizing C-Myc via the Inhibition of NEDD4L. Front. Cell Dev. Biol. 2021, 8, 620603. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Y.; Mei, S.; Zhang, M.; Xin, J.; Zhang, Y.; Yang, R. MicroRNA-22 Impairs Anti-Tumor Ability of Dendritic Cells by Targeting P38. PLoS ONE 2015, 10, e0121510. [Google Scholar] [CrossRef]
- Dunand-Sauthier, I.; Santiago-Raber, M.-L.; Capponi, L.; Vejnar, C.E.; Schaad, O.; Irla, M.; Seguín-Estévez, Q.; Descombes, P.; Zdobnov, E.M.; Acha-Orbea, H.; et al. Silencing of C-Fos Expression by microRNA-155 Is Critical for Dendritic Cell Maturation and Function. Blood 2011, 117, 4490–4500. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chen, J.; Zhou, L.; Chen, W.; Ding, G.; Cao, L. Pancreatic Cancer Derived Exosomes Regulate the Expression of TLR4 in Dendritic Cells via miR-203. Cell. Immunol. 2014, 292, 65–69. [Google Scholar] [CrossRef]
- Trotta, R.; Chen, L.; Costinean, S.; Josyula, S.; Mundy-Bosse, B.L.; Ciarlariello, D.; Mao, C.; Briercheck, E.L.; McConnell, K.K.; Mishra, A.; et al. Overexpression of miR-155 Causes Expansion, Arrest in Terminal Differentiation and Functional Activation of Mouse Natural Killer Cells. Blood 2013, 121, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Sun, Q.; Yan, J.; Huang, J.; Zhu, S.; Yu, J. Identification of microRNA Transcriptome Involved in Human Natural Killer Cell Activation. Immunol. Lett. 2012, 143, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, M.; Yun, S.; Doh, J.; Greenberg, P.D.; Kim, T.-D.; Choi, I. MicroRNA-150 Regulates the Cytotoxicity of Natural Killers by Targeting Perforin-1. J. Allergy Clin. Immunol. 2014, 134, 195–203.e4. [Google Scholar] [CrossRef]
- Kim, T.-D.; Lee, S.U.; Yun, S.; Sun, H.-N.; Lee, S.H.; Kim, J.W.; Kim, H.M.; Park, S.-K.; Lee, C.W.; Yoon, S.R.; et al. Human microRNA-27a* Targets Prf1 and GzmB Expression to Regulate NK-Cell Cytotoxicity. Blood 2011, 118, 5476–5486. [Google Scholar] [CrossRef] [PubMed]
- Donatelli, S.S.; Zhou, J.-M.; Gilvary, D.L.; Eksioglu, E.A.; Chen, X.; Cress, W.D.; Haura, E.B.; Schabath, M.B.; Coppola, D.; Wei, S.; et al. TGF-β–Inducible microRNA-183 Silences Tumor-Associated Natural Killer Cells. Proc. Natl. Acad. Sci. USA 2014, 111, 4203–4208. [Google Scholar] [CrossRef]
- Berchem, G.; Noman, M.Z.; Bosseler, M.; Paggetti, J.; Baconnais, S.; Le Cam, E.; Nanbakhsh, A.; Moussay, E.; Mami-Chouaib, F.; Janji, B.; et al. Hypoxic Tumor-Derived Microvesicles Negatively Regulate NK Cell Function by a Mechanism Involving TGF-β and miR23a Transfer. OncoImmunology 2016, 5, e1062968. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, L.; Chen, Q.; Song, Y.; Xu, S.; Ma, F.; Wang, X.; Wang, J.; Yu, H.; Cao, X.; et al. MicroRNA-494 Is Required for the Accumulation and Functions of Tumor-Expanded Myeloid-Derived Suppressor Cells via Targeting of PTEN. J. Immunol. 2012, 188, 5500–5510. [Google Scholar] [CrossRef]
- Huang, A.; Zhang, H.; Chen, S.; Xia, F.; Yang, Y.; Dong, F.; Sun, D.; Xiong, S.; Zhang, J. miR-34a Expands Myeloid-Derived Suppressor Cells via Apoptosis Inhibition. Exp. Cell Res. 2014, 326, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, A.; Chen, H.; Yang, Y.; Xia, F.; Jin, L.; Zhang, J. miR-34a Inhibits the Apoptosis of MDSCs by Suppressing the Expression of N-myc. Immunol. Cell Biol. 2016, 94, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Qiu, W.; Liu, Q.; Qian, M.; Wang, S.; Zhang, Z.; Gao, X.; Chen, Z.; Xue, H.; Li, G. Immunosuppressive Effects of Hypoxia-Induced Glioma Exosomes through Myeloid-Derived Suppressor Cells via the miR-10a/Rora and miR-21/Pten Pathways. Oncogene 2018, 37, 4239–4259. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Xin, J.; Liu, Y.; Zhang, Y.; Liang, X.; Su, X.; Yan, H.; Huang, Y.; Yang, R. MicroRNA-200c Promotes Suppressive Potential of Myeloid-Derived Suppressor Cells by Modulating PTEN and FOG2 Expression. PLoS ONE 2015, 10, e0135867. [Google Scholar] [CrossRef] [PubMed]
- Parayath, N.N.; Gandham, S.K.; Leslie, F.; Amiji, M.M. Improved Anti-Tumor Efficacy of Paclitaxel in Combination with MicroRNA-125b-Based Tumor-Associated Macrophage Repolarization in Epithelial Ovarian Cancer. Cancer Lett. 2019, 461, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Wu, Q.; Wu, X.; Zhu, Q.; Wang, X.; Jiang, L.; Chen, X.; Wang, X. Epithelial Ovarian Cancer-Secreted Exosomal miR-222-3p Induces Polarization of Tumor-Associated Macrophages. Oncotarget 2016, 7, 43076–43087. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, H.; Yin, X.; Yang, M.; Wei, H.; Chen, Q.; Feng, F.; Liu, Y.; Xu, W.; Li, Y. Macrophages Derived Exosomes Deliver miR-223 to Epithelial Ovarian Cancer Cells to Elicit a Chemoresistant Phenotype. J. Exp. Clin. Cancer Res. 2019, 38, 81. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; He, X.; Xu, Y.; Zhang, W.; Fu, F. MiR-200b Is Upregulated in Plasma-Derived Exosomes and Functions as an Oncogene by Promoting Macrophage M2 Polarization in Ovarian Cancer. J. Ovarian Res. 2021, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ying, X.; Wang, X.; Wu, X.; Zhu, Q.; Wang, X. Exosomes Derived from Hypoxic Epithelial Ovarian Cancer Deliver microRNA-940 to Induce Macrophage M2 Polarization. Oncol. Rep. 2017, 38, 522–528. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, J.; Li, X.; Wang, X.; Lin, Y.; Wang, X. Exosomes Derived from Hypoxic Epithelial Ovarian Cancer Cells Deliver microRNAs to Macrophages and Elicit a Tumor-Promoted Phenotype. Cancer Lett. 2018, 435, 80–91. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, H.; Xiao, W.; Shao, L.; Zhao, C.; Sun, P. Extracellular Vesicle-packaged miR-181c-5p from Epithelial Ovarian Cancer Cells Promotes M2 Polarization of Tumor-associated Macrophages via the KAT2B/HOXA10 Axis. J. Gene Med. 2022, 24, e3446. [Google Scholar] [CrossRef]
- Wang, F.; Niu, Y.; Chen, K.; Yuan, X.; Qin, Y.; Zheng, F.; Cui, Z.; Lu, W.; Wu, Y.; Xia, D. Extracellular Vesicle–Packaged circATP2B4 Mediates M2 Macrophage Polarization via miR-532-3p/SREBF1 Axis to Promote Epithelial Ovarian Cancer Metastasis. Cancer Immunol. Res. 2023, 11, 199–216. [Google Scholar] [CrossRef]
- Kanlikilicer, P.; Bayraktar, R.; Denizli, M.; Rashed, M.H.; Ivan, C.; Aslan, B.; Mitra, R.; Karagoz, K.; Bayraktar, E.; Zhang, X.; et al. Exosomal miRNA Confers Chemo Resistance via Targeting Cav1/p-Gp/M2-Type Macrophage Axis in Ovarian Cancer. EBioMedicine 2018, 38, 100–112. [Google Scholar] [CrossRef]
- Li, X.; Tang, M. Exosomes Released from M2 Macrophages Transfer miR-221-3p Contributed to EOC Progression through Targeting CDKN1B. Cancer Med. 2020, 9, 5976–5988. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, X.; Wu, X.; Zhang, T.; Zhu, Q.; Wang, X.; Wang, H.; Wang, K.; Lin, Y.; Wang, X. Exosomes Released from Tumor-Associated Macrophages Transfer miRNAs That Induce a Treg/Th17 Cell Imbalance in Epithelial Ovarian Cancer. Cancer Immunol. Res. 2018, 6, 1578–1592. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Ling, W.; Ruan, Z. TAM-Derived Extracellular Vesicles Containing microRNA-29a-3p Explain the Deterioration of Ovarian Cancer. Mol. Ther. Nucleic Acids 2021, 25, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, D.; Wu, A.; Qiu, X.; Di, W.; Huang, L.; Qiu, L. TWEAK-Stimulated Macrophages Inhibit Metastasis of Epithelial Ovarian Cancer via Exosomal Shuttling of microRNA. Cancer Lett. 2017, 393, 60–67. [Google Scholar] [CrossRef]
- Wilkinson, A.N.; Chen, R.; Coleborn, E.; Neilson, T.; Le, K.; Bhavsar, C.; Wang, Y.; Atluri, S.; Irgam, G.; Wong, K.; et al. Let-7i Enhances Anti-Tumour Immunity and Suppresses Ovarian Tumour Growth. Cancer Immunol. Immunother. 2024, 73, 80. [Google Scholar] [CrossRef]
- Min, S.; Liang, X.; Zhang, M.; Zhang, Y.; Mei, S.; Liu, J.; Liu, J.; Su, X.; Cao, S.; Zhong, X.; et al. Multiple Tumor-Associated MicroRNAs Modulate the Survival and Longevity of Dendritic Cells by Targeting YWHAZ and Bcl2 Signaling Pathways. J. Immunol. 2013, 190, 2437–2446. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, J.R.; Baird, J.R.; Tesone, A.J.; Rutkowski, M.R.; Scarlett, U.K.; Camposeco-Jacobs, A.L.; Anadon-Arnillas, J.; Harwood, N.M.; Korc, M.; Fiering, S.N.; et al. Reprogramming Tumor-Associated Dendritic Cells In Vivo Using miRNA Mimetics Triggers Protective Immunity against Ovarian Cancer. Cancer Res. 2012, 72, 1683–1693. [Google Scholar] [CrossRef]
- Xie, J.; Liu, M.; Li, Y.; Nie, Y.; Mi, Q.; Zhao, S. Ovarian Tumor-Associated microRNA-20a Decreases Natural Killer Cell Cytotoxicity by Downregulating MICA/B Expression. Cell Mol. Immunol. 2014, 11, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Zhang, G.; Gao, Y. Artesunate Promotes Th1 Differentiation from CD4+ T Cells to Enhance Cell Apoptosis in Ovarian Cancer via miR-142. Braz J. Med. Biol. Res. 2019, 52, e7992. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Lin, Y.-Q.; Ye, H.-Y.; Lin, W.-M. miR-124 Delivered by BM-MSCs-Derived Exosomes Targets MCT1 of Tumor-Infiltrating Treg Cells and Improves Ovarian Cancer Immunotherapy. Neoplasma 2024, 70, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Coleborn, E.; Bhavsar, C.; Wang, Y.; Alim, L.; Wilkinson, A.N.; Tran, M.A.; Irgam, G.; Atluri, S.; Wong, K.; et al. miR-146a inhibits ovarian tumor growth in vivo via targeting immunosuppressive neutrophils and enhancing CD8+ T cell infiltration. Mol. Ther. Oncolytics. 2023, 31, 100725. [Google Scholar] [CrossRef]
- Zheng, L.E.; Huang, M.; Ye, Y.; Sun, P. MicroRNA-211 Regulates Proliferation, Expansion, and Immune Inhibitory Function of Myeloid-Derived Suppressor Cells via Mediation of CHOP Expression. Immunol. Investig. 2023, 52, 616–634. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tao, Z.; Hai, B.; Liang, H.; Shi, Y.; Wang, T.; Song, W.; Chen, Y.; OuYang, J.; Chen, J.; et al. miR-424(322) Reverses Chemoresistance via T-Cell Immune Response Activation by Blocking the PD-L1 Immune Checkpoint. Nat. Commun. 2016, 7, 11406. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Zheng, W.; Tang, Q.; Liu, J.; Wang, S.; Xin, C. miR-576-3p Overexpression Enhances Cisplatin Sensitivity of Ovarian Cancer Cells by Dysregulating PD-L1 and Cyclin D1. Mol. Med. Rep. 2020, 23, 81. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Messina, E.; Sanavia, T.; Mundo, L.; Farinella, F.; Lazzi, S.; Megiorni, F.; Ceccarelli, S.; Pontecorvi, P.; Marampon, F.; et al. MiR-200c-3p Contrasts PD-L1 Induction by Combinatorial Therapies and Slows Proliferation of Epithelial Ovarian Cancer through Downregulation of β-Catenin and c-Myc. Cells 2021, 10, 519. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, S.; Mu, W.; Barry, J.; Han, A.; Carpenter, R.L.; Jiang, B.-H.; Peiper, S.C.; Mahoney, M.G.; Aplin, A.E.; et al. Reactive Oxygen Species Reprogram Macrophages to Suppress Antitumor Immune Response through the Exosomal miR-155-5p/PD-L1 Pathway. J. Exp. Clin. Cancer Res. 2022, 41, 41. [Google Scholar] [CrossRef]
- Zuo, Y.; Zheng, W.; Liu, J.; Tang, Q.; Wang, S.S.; Yang, X.S. MiR-34a-5p/PD-L1 Axis Regulates Cisplatin Chemoresistance of Ovarian Cancer Cells. Neoplasma 2020, 67, 93–101. [Google Scholar] [CrossRef]
- Feng, S.; Sun, H.; Zhu, W. MiR-92 Overexpression Suppresses Immune Cell Function in Ovarian Cancer via LATS2/YAP1/PD-L1 Pathway. Clin. Transl. Oncol. 2021, 23, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, Y. Extracellular Vesicles Derived from M2-Polarized Tumor-Associated Macrophages Promote Immune Escape in Ovarian Cancer through NEAT1/miR-101-3p/ZEB1/PD-L1 Axis. Cancer Immunol. Immunother. 2023, 72, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Q.; Zhang, Y.; Wang, Z.; Ding, J.; Song, Y.; Zhao, W. Cisplatin-Mediated down-Regulation of miR-145 Contributes to up-Regulation of PD-L1 via the c-Myc Transcription Factor in Cisplatin-Resistant Ovarian Carcinoma Cells. Clin. Exp. Immunol. 2020, 200, 45–52. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilczyński, M.; Wilczyński, J.; Nowak, M. MiRNAs as Regulators of Immune Cells in the Tumor Microenvironment of Ovarian Cancer. Cells 2024, 13, 1343. https://doi.org/10.3390/cells13161343
Wilczyński M, Wilczyński J, Nowak M. MiRNAs as Regulators of Immune Cells in the Tumor Microenvironment of Ovarian Cancer. Cells. 2024; 13(16):1343. https://doi.org/10.3390/cells13161343
Chicago/Turabian StyleWilczyński, Miłosz, Jacek Wilczyński, and Marek Nowak. 2024. "MiRNAs as Regulators of Immune Cells in the Tumor Microenvironment of Ovarian Cancer" Cells 13, no. 16: 1343. https://doi.org/10.3390/cells13161343




