Co-Targeting of DTYMK and PARP1 as a Potential Therapeutic Approach in Uveal Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical and Histologic Information
2.2. RNA Sequencing Analyses
2.3. Immunohistochemistry
2.4. Statistical Analyses
2.5. Cell Culture and Reagents
2.6. Treatment of Cells with Inhibitors
2.7. Viability Evaluation and Synergy Assessment
2.8. Cell Proliferation Analysis
2.9. Western Blot Analysis
2.10. Single-Cell Migration Assay
2.11. Immunofluorescence Techniques and Microscopy
3. Results
3.1. Selection of DTYMK and PARP1 as Potential Therapeutic Targets Using In Silico Analyses
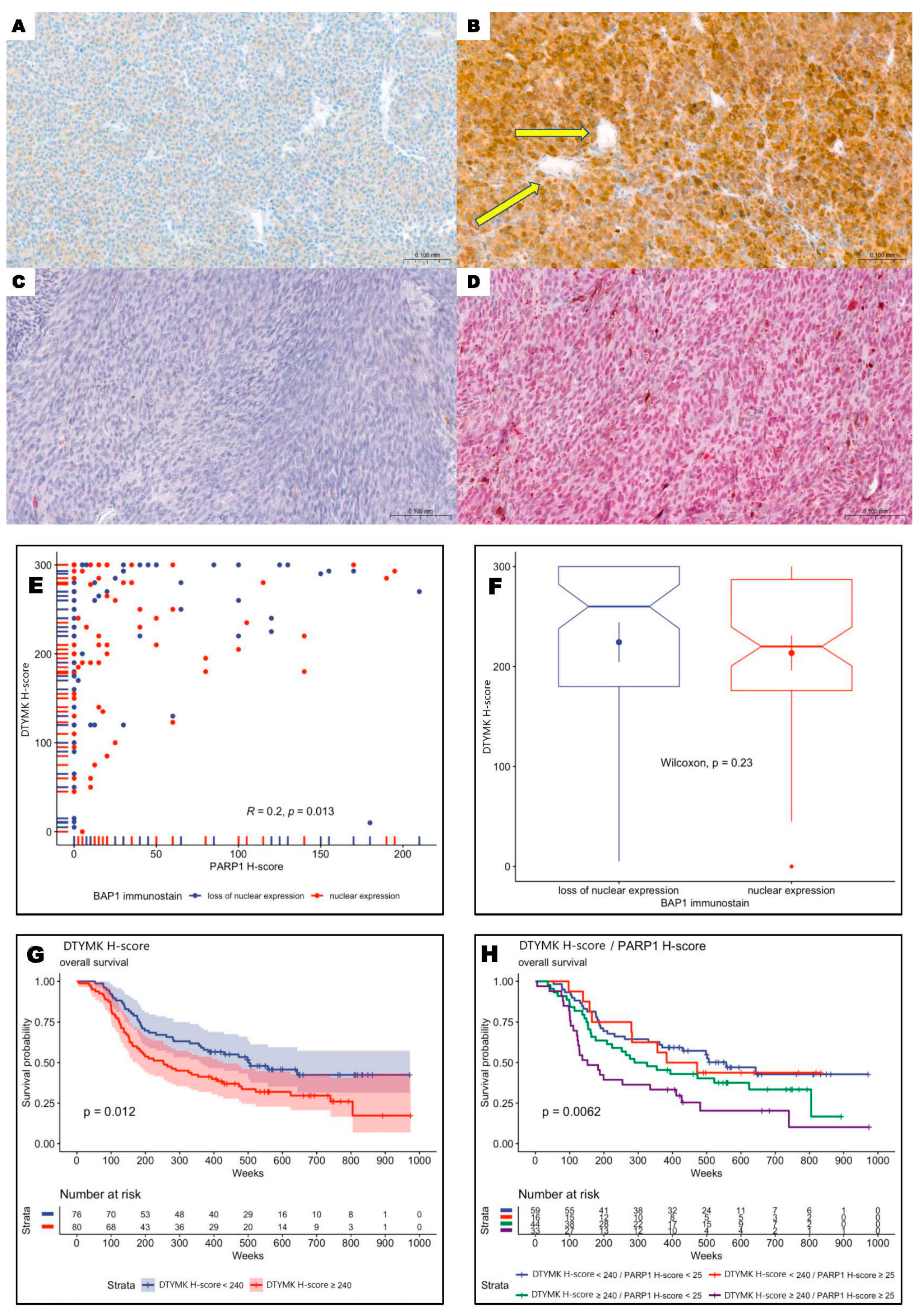
3.2. DTYMK and PARP1 Expression in Uveal Melanoma Patients
3.3. Overexpression of DTYMK and PARP1 Protein Levels Correlated with Worst Long-Term Prognosis
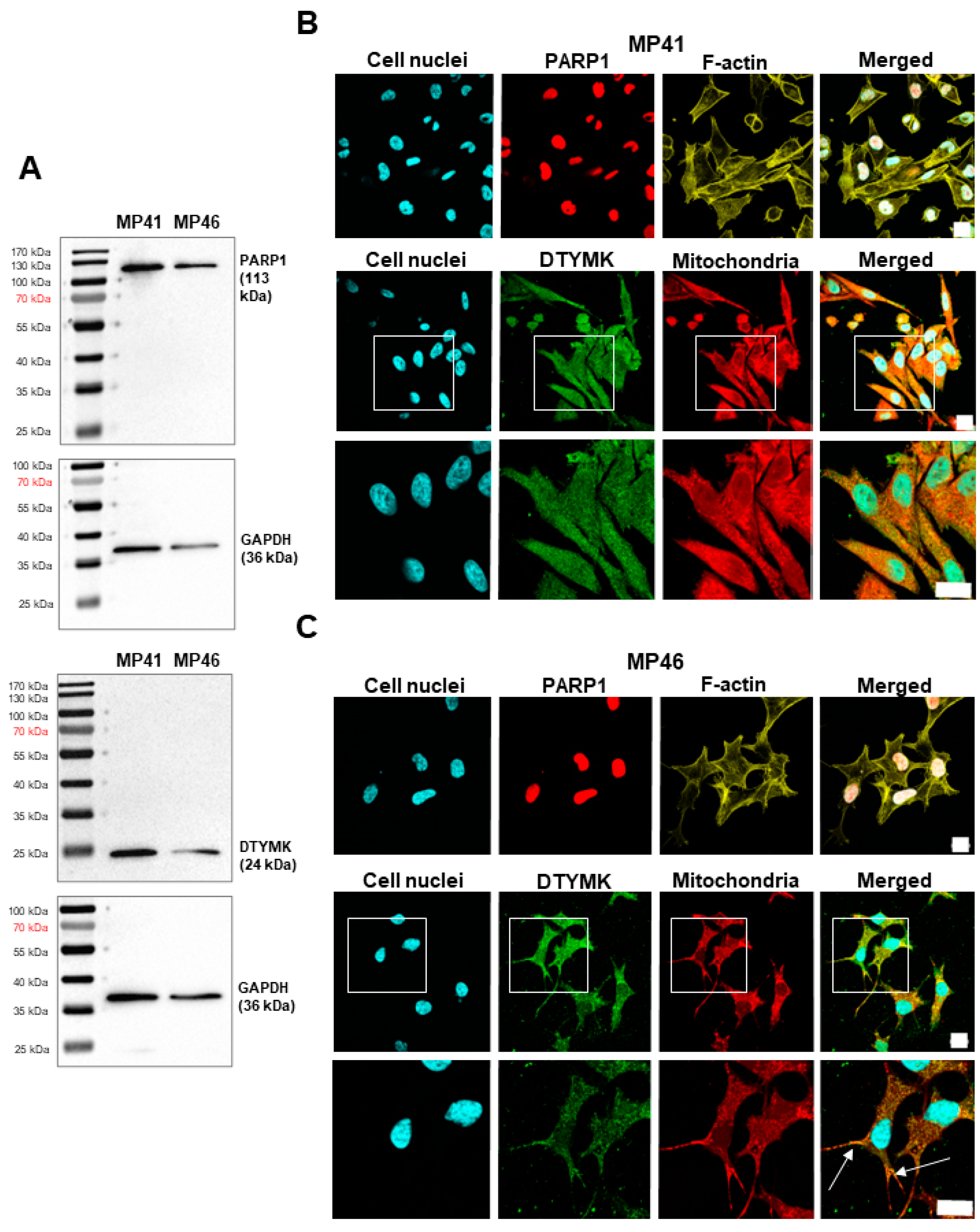
3.4. Validation of the Presence of DTYMK and PARP1 in Studied Cell Lines
3.5. Determination of the Cellular Localization of DTYMK and PARP1
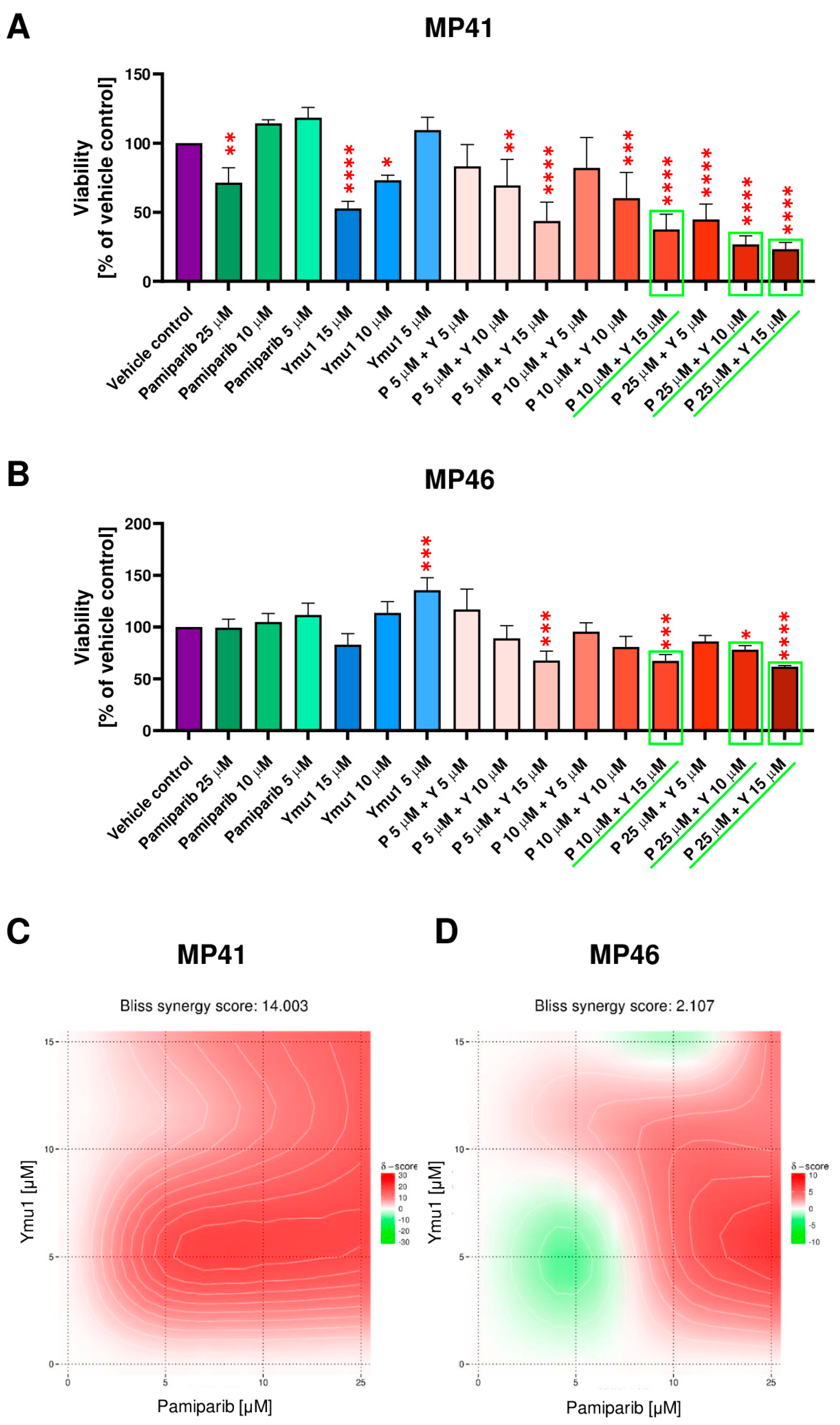
3.6. The Cytotoxic Effect of DTYMK and PARP1 Inhibitors on the Uveal Melanoma Cells
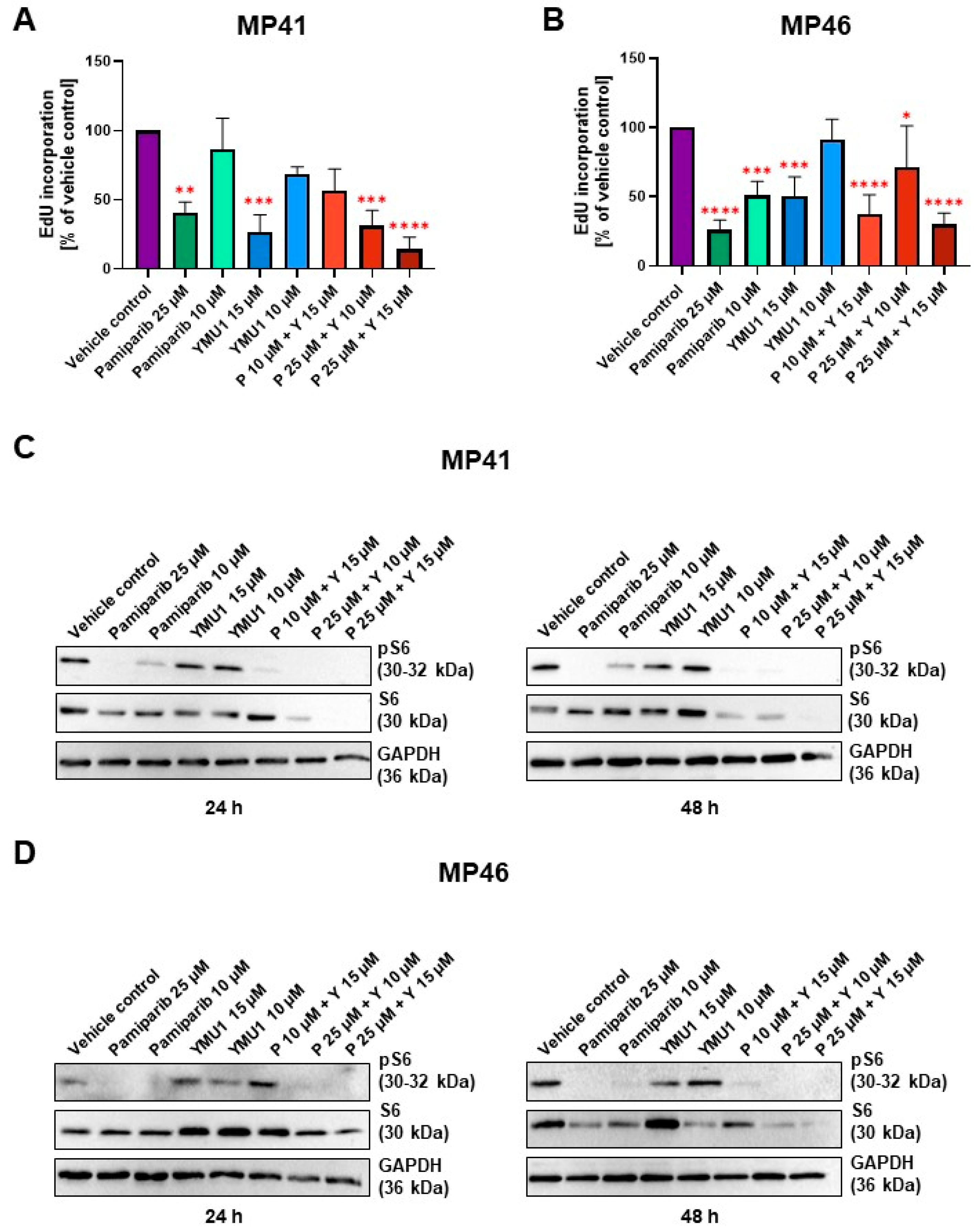
3.7. Effect of Pamiparib and Ymu1 and Their Combinations on EdU Incorporation
3.8. The Influence of DTYMK and PARP1 Inhibitors on the mTOR Signaling Pathway
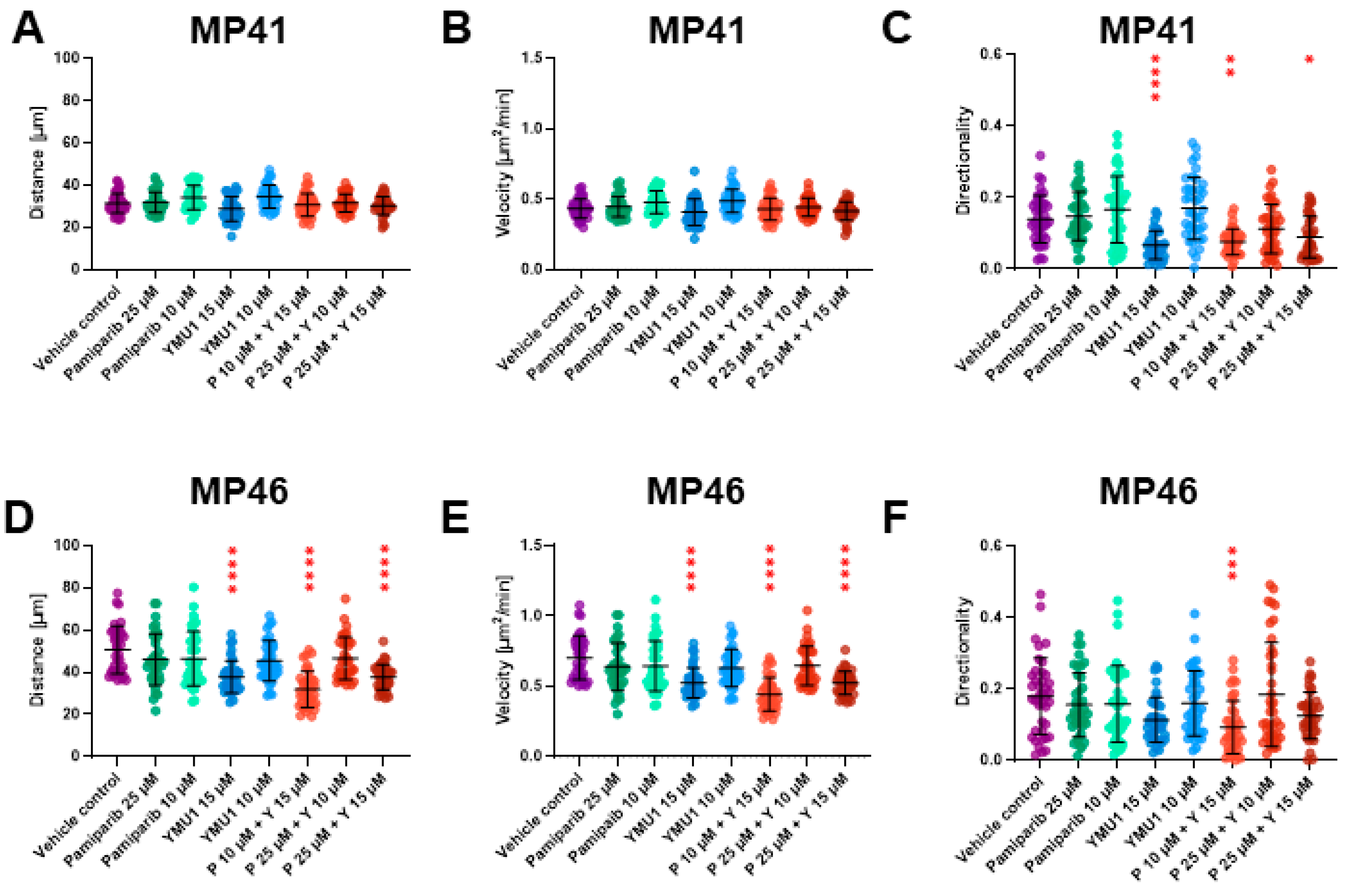
3.9. The Effect of Drug Treatment on Cells’ Motility Abilities and Their Morphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smit, K.N.; Jager, M.J.; de Klein, A.; Kiliç, E. Uveal melanoma: Towards a molecular understanding. Prog. Retin. Eye Res. 2020, 75, 100800. [Google Scholar] [CrossRef] [PubMed]
- Eye Cancer Survival Rates. Ocular Melanoma Survival Rates. American Cancer Society. Available online: https://www.cancer.org/cancer/types/eye-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 4 April 2024).
- Schank, T.E.; Hassel, J.C. Immunotherapies for the Treatment of Uveal Melanoma—History and Future. Cancers 2019, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Luo, W.; Huang, S.; Zhao, W.; Chen, H.; Ma, Y.; Ye, M.; Nie, Y.; Zhang, Y.; Huang, C.; et al. DTYMK Expression Predicts Prognosis and Chemotherapeutic Response and Correlates with Immune Infiltration in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Marks, K.; Cowley, G.S.; Carretero, J.; Liu, Q.; Nieland, T.J.; Xu, C.; Cohoon, T.J.; Gao, P.; Zhang, Y.; et al. Metabolic and functional genomic studies identify deoxythymidylate kinase as a target in LKB1-mutant lung cancer. Cancer Discov. 2013, 3, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yuan, Y.; Zhou, F.; Huang, X.; Pu, J.; Niu, X.; Jiang, X.; Ding, X. Comprehensive analysis of DTYMK for estimating the immune microenvironment, diagnosis, prognosis effect in patients with lung adenocarcinoma. Aging 2022, 14, 7866–7876. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Liu, Y.; Gong, T.; Pan, Q.; Xiang, T.; Zhao, J.; Tang, Y.; Chen, H.; Han, Y.; Song, M.; et al. Inhibition of DTYMK significantly restrains the growth of HCC and increases sensitivity to oxaliplatin. Cell Death Dis. 2021, 12, 1093. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Yang, H.; Zhou, S.; Huang, F.; Wei, Z.; Peng, Z. Deoxythymidylate kinase (DTYMK) participates in cell cycle arrest to promote pancreatic adenocarcinoma progression regulated by miR-491-5p through TP53 and is associated with tumor immune infiltration. J. Gastrointest. Oncol. 2023, 14, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Zheng, J.-Y.; Huang, L.-L.; Wang, Y.-H.; Yao, D.-F.; Dai, H.-B. Human PARP1 substrates and regulators of its catalytic activity: An updated overview. Front. Pharmacol. 2023, 14, 1137151. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, J.O.; Van Houten, B.; Durrant, J.D. PARP1: Structural insights and pharmacological targets for inhibition. DNA Repair 2021, 103, 103125. [Google Scholar] [CrossRef]
- Kupczyk, P.; Simiczyjew, A.; Marczuk, J.; Dratkiewicz, E.; Beberok, A.; Rok, J.; Pieniazek, M.; Biecek, P.; Nevozhay, D.; Slowikowski, B.; et al. PARP1 as a Marker of an Aggressive Clinical Phenotype in Cutaneous Melanoma-A Clinical and an In Vitro Study. Cells 2021, 10, 286. [Google Scholar] [CrossRef]
- Peng, X.; Pan, W.; Jiang, F.; Chen, W.; Qi, Z.; Peng, W.; Chen, J. Selective PARP1 inhibitors, PARP1-based dual-target inhibitors, PROTAC PARP1 degraders, and prodrugs of PARP1 inhibitors for cancer therapy. Pharmacol. Res. 2022, 186, 106529. [Google Scholar] [CrossRef] [PubMed]
- Donizy, P.; Krzyzinski, M.; Markiewicz, A.; Karpinski, P.; Kotowski, K.; Kowalik, A.; Orlowska-Heitzman, J.; Romanowska-Dixon, B.; Biecek, P.; Hoang, M.P. Machine learning models demonstrate that clinicopathologic variables are comparable to gene expression prognostic signature in predicting survival in uveal melanoma. Eur. J. Cancer 2022, 174, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Grossman, R.L.; Heath, A.P.; Ferretti, V.; Varmus, H.E.; Lowy, D.R.; Kibbe, W.A.; Staudt, L.M. Toward a Shared Vision for Cancer Genomic Data. N. Engl. J. Med. 2016, 375, 1109–1112. [Google Scholar] [CrossRef]
- Robertson, A.G.; Shih, J.; Yau, C.; Gibb, E.A.; Oba, J.; Mungall, K.L.; Hess, J.M.; Uzunangelov, V.; Walter, V.; Danilova, L.; et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell 2017, 32, 204–220.e15. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.A.; Rodriguez, D.A.; Kurtenbach, S.; Kuznetsov, J.N.; Sanchez, M.I.; Decatur, C.L.; Snyder, H.; Feun, L.G.; Livingstone, A.S.; Harbour, J.W. Single-cell analysis reveals new evolutionary complexity in uveal melanoma. Nat. Commun. 2020, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.; Hoffman, P.; Smibert, P.; Papalexi, E.; Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018, 36, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Donizy, P.; Spytek, M.; Krzyziński, M.; Kotowski, K.; Markiewicz, A.; Romanowska-Dixon, B.; Biecek, P.; Hoang, M.P. Ki67 is a better marker than PRAME in risk stratification of BAP1-positive and BAP1-loss uveal melanomas. Br. J. Ophthalmol. 2023, 108, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Yu, A.; Kim, S.; Kang, J.; Hong, S.-M. Robust Likelihood-Based Survival Modeling with Microarray Data. J. Stat. Softw. 2009, 29, 1–16. [Google Scholar] [CrossRef]
- Vazquez, F.; Boehm, J.S. The Cancer Dependency Map enables drug mechanism-of-action investigations. Mol. Syst. Biol. 2020, 16, e9757. [Google Scholar] [CrossRef]
- Lausen, B.; Hothorn, T.; Bretz, F.; Schumacher, M. Assessment of Optimal Selected Prognostic Factors. Biom. J. 2004, 46, 364–374. [Google Scholar] [CrossRef]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 9 April 2024).
- Therneau, T. A Package for Survival Analysis in R; The R Project: Vienna, Austria, 2024. [Google Scholar]
- Kassambara, A.; Kosinski, M.; Biecek, P. Drawing Survival Curves Using “ggplot2” [R Package Survminer Version 0.4.8]; The R Project: Vienna, Austria, 2020. [Google Scholar]
- Contal, C.; O’Quigley, J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput. Stat. Data Anal. 1999, 30, 253–270. [Google Scholar] [CrossRef]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 3.0: An interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res. 2022, 50, W739–W743. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Masuzzo, P.; Huyck, L.; Simiczyjew, A.; Ampe, C.; Martens, L.; Van Troys, M. An end-to-end software solution for the analysis of high-throughput single-cell migration data. Sci. Rep. 2017, 7, 42383. [Google Scholar] [CrossRef] [PubMed]
- Mazurkiewicz, E.; Makowiecka, A.; Mrówczyńska, E.; Kopernyk, I.; Nowak, D.; Mazur, A.J. Gelsolin contributes to the motility of a375 melanoma cells and this activity is mediated by the fibrous extracellular matrix protein profile. Cells 2021, 10, 1848. [Google Scholar] [CrossRef] [PubMed]
- Amirouchene-Angelozzi, N.; Nemati, F.; Gentien, D.; Nicolas, A.; Dumont, A.; Carita, G.; Camonis, J.; Desjardins, L.; Cassoux, N.; Piperno-Neumann, S.; et al. Establishment of novel cell lines recapitulating the genetic landscape of uveal melanoma and preclinical validation of mTOR as a therapeutic target. Mol. Oncol. 2014, 8, 1508–1520. [Google Scholar] [CrossRef]
- Frisk, J.H.; Örn, S.; Pejler, G.; Eriksson, S.; Wang, L. Differential expression of enzymes in thymidylate biosynthesis in zebrafish at different developmental stages: Implications for dtymk mutation-caused neurodegenerative disorders. BMC Neurosci. 2022, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Lin, D.W.; Chang, Z.F. Identification of a putative human mitochondrial thymidine monophosphate kinase associated with monocytic/macrophage terminal differentiation. Genes Cells 2008, 13, 679–689. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2019, 20, 74–88. [Google Scholar] [CrossRef]
- Magnuson, B.; Ekim, B.; Fingar, D.C. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 2012, 441, 1–21. [Google Scholar] [CrossRef]
- Wu, A.; Mazurkiewicz, E.; Donizy, P.; Kotowski, K.; Pieniazek, M.; Mazur, A.J.; Czogalla, A.; Trombik, T. ABCA1 transporter promotes the motility of human melanoma cells by modulating their plasma membrane organization. Biol. Res. 2023, 56, 32. [Google Scholar] [CrossRef]
- Heydari, N.; Mahdizadeh, M.; Jafari, S.M. The evolving landscape of involvement of DTYMK enzymes in cancer. Med. Oncol. 2023, 40, 213. [Google Scholar] [CrossRef] [PubMed]
- Teyssonneau, D.; Margot, H.; Cabart, M.; Anonnay, M.; Sargos, P.; Vuong, N.-S.; Soubeyran, I.; Sevenet, N.; Roubaud, G. Prostate cancer and PARP inhibitors: Progress and challenges. J. Hematol. Oncol. 2021, 14, 51. [Google Scholar] [CrossRef]
- Bhamidipati, D.; Haro-Silerio, J.I.; Yap, T.A.; Ngoi, N. PARP inhibitors: Enhancing efficacy through rational combinations. Br. J. Cancer 2023, 129, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Chen, S.; Sun, R.; Ashby, C.R., Jr.; Wei, L.; Huang, Z.; Chen, Z.-S. Darovasertib, a novel treatment for metastatic uveal melanoma. Front. Pharmacol. 2023, 14, 1232787. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xie, R.; Zhang, C.; Lu, G.; Kong, H. Pan-cancer analysis of prognostic and immunological role of DTYMK in human tumors. Front. Genet. 2022, 13, 989460. [Google Scholar] [CrossRef]
- Zhou, T.; Qin, R.; Shi, S.; Zhang, H.; Niu, C.; Ju, G.; Miao, S. DTYMK promote hepatocellular carcinoma proliferation by regulating cell cycle. Cell Cycle 2021, 20, 1681–1691. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Liu, Y.; Yang, J.; Zuo, X.; Dong, M.; Zhang, Z.; Shi, Y.; Deng, X.; Lv, Y. Comprehensive analysis of DTYMK in pan-cancer and verification in lung adenocarcinoma. Biosci. Rep. 2022, 42, 20221170. [Google Scholar] [CrossRef]
- Kim, S.; Lee, E.-W.; Oh, D.-B.; Seo, J. BAP1 controls mesenchymal stem cell migration by inhibiting the ERK signaling pathway. BMB Rep. 2024, 57, 250–255. [Google Scholar] [CrossRef]
- Zhang, X.; Cong, X.; Jin, X.; Liu, Y.; Zhang, T.; Fan, X.; Shi, X.; Zhang, X.; Wang, X.; Yang, Y.G.; et al. Deficiency of BAP1 inhibits neuroblastoma tumorigenesis through destabilization of MYCN. Cell Death Dis. 2023, 14, 504. [Google Scholar] [CrossRef] [PubMed]
- Gajdzis, M.; Theocharis, S.; Klijanienko, J.; Cassoux, N.; Gardrat, S.; Donizy, P.; Kaczmarek, R.; Gajdzis, P. The Prognostic Values of PARP-1 Expression in Uveal Melanoma. Cells 2021, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Doherty, R.E.; Bryant, H.E.; Valluru, M.K.; Rennie, I.G.; Sisley, K. Increased Non-Homologous End Joining Makes DNA-PK a Promising Target for Therapeutic Intervention in Uveal Melanoma. Cancers 2019, 11, 1278. [Google Scholar] [CrossRef] [PubMed]


| MP41 Cell Line (BAP-Positive, c.626al>A/T Mutation in the GNA11 Gene) | MP46 Cell Line (BAP-Negative, c.626 A>T Mutation in the GNAQ Gene) | |||||
|---|---|---|---|---|---|---|
| Pamiparib | YMU1 | Combinations of Inhibitors | Pamiparib | YMU1 | Combinations of Inhibitors | |
| XTT assay | IC50 = 29.07 μM | IC50 = 7.49 μM | High cytotoxicity | No cytotoxicity | IC50 = 19.87 μM | Middle level cytotoxicity |
| Incoropration of EdU | Reduction by approx. 60% (P 25 μM) | Reduction by approx. 70% (Y 15 μM) | Reduction by approx. 60–80% (P 25 μM + Y 10 μM P 25 μM + Y 15 μM) | Reduction by approx. 50–70% (P 10 μM, P 25 μM) | Reduction by approx. 50% (Y 15 μM) | Reduction by approx. 70% (P 10 μM + Y 15 μM P 25 μM + Y 15 μM) |
| Western Blot analysis | Lack of phosphorylated S6 | S6 is phosphorylated | Lack of phosphorylated S6 | Lack of phosphorylated S6 | S6 is phosphorylated | Lack of phosphorylated S6 |
| Morphology | No changes | Cells are rounded (Y 10 μM, Y 15 μM) | Cells are rounded | No changes | Cells are rounded (Y 15 μM) | Cells are rounded (P 10 μM + Y 15 Μm P 25 μM + Y 15 μM) |
| Single-cell migration assay | No changes | Changed directionality (Y 15 μM) | Changed directionality (P 10 μM + Y 15 μM) | No changes | The covered distance is shortened, and the velocity is reduced (Y 15 μM) | The covered distance is shortened, and the velocity is reduced (P10 μM + Y 15 μM P 25 μM + Y 15 μM) Changed directionality (P 10 μM + Y 15 μM) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oziębło, S.; Mizera, J.; Górska, A.; Krzyziński, M.; Karpiński, P.; Markiewicz, A.; Sąsiadek, M.M.; Romanowska-Dixon, B.; Biecek, P.; Hoang, M.P.; et al. Co-Targeting of DTYMK and PARP1 as a Potential Therapeutic Approach in Uveal Melanoma. Cells 2024, 13, 1348. https://doi.org/10.3390/cells13161348
Oziębło S, Mizera J, Górska A, Krzyziński M, Karpiński P, Markiewicz A, Sąsiadek MM, Romanowska-Dixon B, Biecek P, Hoang MP, et al. Co-Targeting of DTYMK and PARP1 as a Potential Therapeutic Approach in Uveal Melanoma. Cells. 2024; 13(16):1348. https://doi.org/10.3390/cells13161348
Chicago/Turabian StyleOziębło, Sylwia, Jakub Mizera, Agata Górska, Mateusz Krzyziński, Paweł Karpiński, Anna Markiewicz, Maria Małgorzata Sąsiadek, Bożena Romanowska-Dixon, Przemysław Biecek, Mai P. Hoang, and et al. 2024. "Co-Targeting of DTYMK and PARP1 as a Potential Therapeutic Approach in Uveal Melanoma" Cells 13, no. 16: 1348. https://doi.org/10.3390/cells13161348
APA StyleOziębło, S., Mizera, J., Górska, A., Krzyziński, M., Karpiński, P., Markiewicz, A., Sąsiadek, M. M., Romanowska-Dixon, B., Biecek, P., Hoang, M. P., Mazur, A. J., & Donizy, P. (2024). Co-Targeting of DTYMK and PARP1 as a Potential Therapeutic Approach in Uveal Melanoma. Cells, 13(16), 1348. https://doi.org/10.3390/cells13161348









