Abstract
Chronic Obstructive Pulmonary Disease (COPD) is the third leading cause of global mortality. Despite clinical predictors (age, severity, comorbidities, etc.) being established, proteomics offers comprehensive biological profiling to obtain deeper insights into COPD pathophysiology and survival prognoses. This pilot study aimed to identify proteomic footprints that could be potentially useful in predicting mortality in stable COPD patients. Plasma samples from 40 patients were subjected to both blind (liquid chromatography–mass spectrometry) and hypothesis-driven (multiplex immunoassays) proteomic analyses supported by artificial intelligence (AI) before a 4-year clinical follow-up. Among the 34 patients whose survival status was confirmed (mean age 69 ± 9 years, 29.5% women, FEV1 42 ± 15.3% ref.), 32% were dead in the fourth year. The analysis identified 363 proteins/peptides, with 31 showing significant differences between the survivors and non-survivors. These proteins predominantly belonged to different aspects of the immune response (12 proteins), hemostasis (9), and proinflammatory cytokines (5). The predictive modeling achieved excellent accuracy for mortality (90%) but a weaker performance for days of survival (Q2 0.18), improving mildly with AI-mediated blind selection of proteins (accuracy of 95%, Q2 of 0.52). Further stratification by protein groups highlighted the predictive value for mortality of either hemostasis or pro-inflammatory markers alone (accuracies of 95 and 89%, respectively). Therefore, stable COPD patients’ proteomic footprints can effectively forecast 4-year mortality, emphasizing the role of inflammatory, immune, and cardiovascular events. Future applications may enhance the prognostic precision and guide preventive interventions.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a very prevalent disorder, and is the third leading cause of death worldwide [1], which also negatively impacts not only health systems (costs, overload) but socio-economic outcomes in general [2]. Therefore, it is important to establish good predictors for the more relevant events occurring in patients’ lives: exacerbations, hospitalizations, disability, and mortality [3]. The rate of the latter depends on the characteristics of the patients but also on the specific health system, oscillating from 25 to 40% at 4–5 years in individuals with a stable clinical condition to around 50% at 2 years in hospitalized patients [4,5,6,7,8,9]. Most of the long-term deaths are attributable to respiratory causes, with lower percentages attributed to cardiovascular/cerebrovascular events or cancer [4,10]. Many different clinical predictors of mortality, including age, obesity, dyspnea, hypoxemia, anemia, exercise capacity, disease severity, nutritional status, comorbidities, and multidimensional indices such as BODE, have already been described [3,4,11,12,13,14,15,16,17,18,19]. There have also been some attempts to obtain blood markers that can predict patients’ death, for example, neutrophil, eosinophil, or lymphocyte counts, and the levels of proatrial natriuretic peptide (MRproANP), copeptin, endotrophin, von Willebrand factor (VWF), and D-dimer, among others [20,21,22,23,24]. Many of these studies were, however, retrospective. Moreover, it is worth noting that a trend toward better prognoses (disease progression, exacerbations, and mortality) has been observed in the last few years. This phenomenon may be due to recent changes in treatment [3,11,19,25,26,27] and involves the need for new studies on the current predictors of mortality.
We hypothesized that the plasma proteomic profile of stable COPD patients, which is a surrogate reflecting their pathophysiological abnormalities, could be useful in determining their survival prognosis. Therefore, the objective of this pilot study was to identify proteomic markers and specific multiprotein signatures that could be useful in predicting long-term mortality, identifying the related pathways and contributing to our knowledge on the mechanisms linked to a poor prognosis for COPD patients.
2. Materials and Methods
2.1. Study Design and Ethics
This prospective study was performed with patients from the BIOMEPOC cohort (269 patients and 83 healthy subjects), whose details have been published elsewhere [28]. The investigation was designed and conducted according to the Helsinki Declaration and all procedures were approved by our institutional ethics committee (ref. 2014/5895/I). Moreover, all participants signed the corresponding written informed consent form.
2.2. Study Population
For the current pilot analysis, a subset from the BIOMEPOC cohort comprising randomly chosen stable COPD patients was included and followed for 4 years. All patients were Caucasians from the European Mediterranean area. The diagnosis of COPD was based on smoking history and a post-bronchodilator spirometry ratio (FEV1/FVC) < 0.7 [2,28], whilst clinical stability was defined as the absence of any significant clinical changes including acute exacerbations in the three months preceding the beginning of the study. The exclusion criteria included all other respiratory or chronic inflammatory conditions. Clinical data were recorded at the beginning of the study.
2.3. Biological Sample Obtention
Blood samples were collected by venipuncture at the onset of the study, which were immediately placed in K3-EDTA tubes for plasma analyses. These tubes were centrifuged at 1500 r.p.m. at 4° C for 15 min, and the supernatants were moved to new tubes and stored at −80 °C until the proteomic analyses were performed. The latter were performed through two different but complementary techniques.
2.4. Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS)
Details of the sample preparation, instrument parameters, and protein identification process have been published elsewhere [29,30]. Briefly, the proteins were cleaved with trypsin (1:100 w:w, 8 h) (Promega, Madison, WI, USA) and endoproteinase LysC (1:100 w:w, overnight) (Merck, Wako, Neuss, Germany). The reactions were carried out at 37 °C and the peptides were desalted in C18 Hypersep columns (Thermo Fisher Scientific, Waltham, MA, USA). The peptides were analyzed by LC–MS/MS using an LTQ-Orbitrap Fusion Lumos mass spectrometer linked to an EASY nLC 1000 nanocapillary and microflow system (Thermo Fisher Scientific). Digested samples from each patient were tested in duplicate, and label-free quantification (LFQ) and database inquiries were performed using the MaxQuant LFQ software (version 1.6.1.0, Max Planck Institute for Biochemistry, Martinsried, Germany), the Andromeda algorithm, and the H. sapiens database (SwissProt, Geneva, Switzerland 2018). Protein amounts were approximated using MaxQuant LFQ values.
2.5. Immune-Based Multiplexing
The results obtained with the previous blind method were expanded by using a complementary hypothesis-driven approach focused on soluble inflammatory markers, including cytokines, chemokines, growth factors, and acute-phase proteins. This was achieved using 3 multiplex bead-based immunoassays and a Bio-Plex 200 array system (Bio-Rad, Hercules, CA, USA). Sixty-three distinct plasma proteins were assessed with this procedure following the manufacturer’s recommendations. The validation of randomly chosen proteins from the immunoassay results was conducted using ELISA in the standard way. The details have been described previously [29].
2.6. Data Analysis
2.6.1. Calculation of the Sample Size
The minimum sample size to be included in the present pilot study was based on previously published studies and calculated using the GRANMO software (version 8.0) [29,30,31], assuming a loss of 10% of patients in the follow-up period.
2.6.2. Descriptive Statistics and Comparisons between Groups
Clinical variables were tested for normal distribution using the Kolmogorov–Smirnov test. Since all of them showed a normal distribution, their results are presented as mean ± SD, and comparisons between the survivor and non-survivor groups were performed using the unpaired t-test.
Regarding proteomic data, the results from both techniques were merged into a single dataset and protein values were log2 transformed to diminish the skewness of their distributions. These data are presented as mean ± SD or median and quartiles depending on the presence or absence of normality in their distributions. Proteomic data comparisons were analyzed using complementary quantitative and qualitative approaches (see the flow chart diagram in Figure S1).
A “95% minimum imputation” of missing values was performed [30,32] and quantitative comparisons between the survivor and non-survivor groups were made for the proteins detected in at least 80% of patients from each group. Independent sample t- or Mann–Whitney tests were employed for comparisons between groups, as appropriate. The protein log-base-2-fold change (log2FC) value was determined by subtracting the average log2 values of the proteins from each comparison of the survivor and non-survivor groups. The remaining proteins were included in a qualitative analysis to identify the ones that were consistently present in all/almost all of the patients from one of the two groups but were absent in the other (P-A Proteins). This analysis was performed using the Barnard test. The proteins showing differences between the two groups in any of the statistical approaches (identified as differentially abundant proteins, DAPs) were used for the network analysis.
The SPSS v.28 software (SPSS Inc.; Chicago, IL, USA) was used for the descriptive analysis of the clinical data, whereas Python 3.8.3, in a Jupyter notebook v6.0.3 associated with an Anaconda environment v4.9.1 (NumPy v1.18.5, Pandas v1.0.5, SciPy v1.5.0, and Statsmodels v0.11.1 Python modules) was used for the proteomic data.
2.7. Functional Classification of Proteins and Network Analysis
The proteins were categorized using Reactome v.88 and UniProtKB v.2024_03 into 7 categories (lipid metabolism-related, hemostasis, inflammatory mediators, complement system, adaptive immunity, other immune-related proteins, and “orphans”). The resultant classification was manually refined in certain cases. Detailed descriptions are available in the Supplementary Data.
To further assess the biological relevance of DAPs, protein–protein interactions (PPIs) were explored through the STRING digital platform (STRING Consortium, Kearney, NE, USA), using a significance score of ≥0.4. Interactions were visualized by generating network representations through Cytoscape (Cytoscape Consortium, San Diego, CA, USA). The proteins that are not included in the STRING database were manually added. Further details can be found in a previous article from our group [29].
2.8. Generation of Predictive Models
Predictive models of both mortality (categorical) and days of survival (continuous) were developed using AI–machine learning techniques: Random Forest (RF), Partial Least Squares Regression (PLSR), and K-Nearest Neighbors (KNN). These models allow for a deep understanding of the characteristics of the predictions and their level of reliability. Two different approaches were chosen to generate the models, which were assisted by AI. First, only quantitative and qualitative DAPs were included to build the models, whereas in the second step, AI was allowed to freely choose the proteins that would build the best predictive model. For this second step, although the set was constituted by the qualitative data of all proteins, only the quantitative data from the proteins with 100% coverage in all patients were included. This was chosen to counterbalance the potential limitations of individual imputations, improving the consistency of our model [30].
The specialized software named Flame, an open and free tool developed in our center for predictive modeling (version 1.2.2; https://phi.upf.edu/phi/index.php/software/flame/) [33], was used for the analysis due to its ease of use as well as its robust and replicable results.
Initial fitting. The data were preprocessed using standard scaling. The All-KNN method was used as a sub/oversampling strategy to correct class imbalances (death and survival) for continuous modeling (days of survival), and the Instance Hardness Threshold was used for categorical modeling (mortality at 4 years). In all cases, the Random Forest (RF) method was used in a conformal variant with the confidence level set at 0.8, coupled with K-best feature selection using the “auto” feature number selection criterion. Two hundred trees were used in the RF model, and their class weights were considered balanced because of the pre-processing procedure. RF-max features were calculated through the square array of the previously selected features. A minimum of 2 samples were required for RF-split. An internal method with out-of-the-bag samples was used to estimate the generalization accuracy. A random seed of 46 was used for reproducibility. A conformal analysis was conducted using Aggregated Conformal Predictors (ACPs), with model normalization achieved using a KNN method that included the 15 nearest neighbors. These ACPs aggregated conformal predictions derived from 10 models, and a bootstrap sampler strategy was employed to select calibration sets. Median aggregation was employed to combine the resulting p-values.
Internal validation. The obtained models were validated using a 5-fold cross-validation approach, with a specific permutation importance approach being developed to discern the significance of each molecule within each one of the models [33].
Model evaluation. Based on coincidences or discrepancies between the predicted and the real survival situation, a confusional matrix was generated with the latter, and sensibility (SE), specificity (SP), Predictive Positive Value (PPV), Predictive Negative Value (PNV), Overall Detection Accuracy (Acc), and the Matthews correlation coefficient (MCC, commonly known as the phi coefficient, φ or rφ) were calculated. Both our own death rate and the mortality data provided by a large study (close to 9000 patients) from the COPD Cohorts Collaborative International Assessment (3CIA) (Appendix B) [34] were used to calculate PNV, PPV, and Acc.
3. Results
3.1. General Characteristics of the Patients
The survival outcome at the end of the 4-year follow-up was impossible to confirm in six COPD patients. The remaining 34 were mainly in the seventh and eighth decades of life (mean age: 69 ± 9 years old), 29.5% were women, and all were current or former smokers. The severity of the disease was either mild–moderate (26.5%) or severe–very severe (73.5%), with several exacerbations in the previous year, mostly ranging in number from 0 to 3. The prescribed treatments included long-acting bronchodilators (95%), inhaled or oral steroids (41% and 3%, respectively), regularly scheduled antibiotics (8%), platelet antiaggregants (26%), anticoagulants (11%), and home oxygen therapy (6%). The main comorbidities were cardiovascular disorders (57.8%, including ischemic heart disease, 12.5%; cerebrovascular disease, 7.9%; and peripheral vascular disease, 4.8%), sleep apnea syndrome (25.4%), and diabetes (14.3%), which resulted in a Charlson index of 3.6 ± 1.6. The mortality rates were 6, 18, 24, and 32% at the first, second, third, and fourth year of follow-up, respectively. The main causes of death were respiratory and cardiovascular events (35% and 27%, respectively). Table 1 shows the main clinical differences between 4-year survivors and non-survivors. As expected, the proportion of frequent exacerbators who died in the follow-up period was much higher than that of non-frequent exacerbators (50% vs. 20%, respectively).

Table 1.
Clinical characteristics of the study population.
3.2. Proteomic Profile
From a total of 363 proteins/peptides identified through either LC-MS or multiplexing (listed in Table S1 in the Supplementary Tables), the data for 208 were at least 80% valid in both groups and were included in the quantitative comparisons between the survivors and non-survivors (Figure S1 in Supplementary Materials). Fifteen of them showed significant differences, and their categorization revealed that they were mostly associated with four different biological processes: hemostasis (n = 5), proinflammatory processes (cytokines and chemokines, n = 3), the complement system (n = 3), and other immunological pathways (n = 2, with one of them being an immunoglobulin fraction). The two proteins that were not grouped into any of the categories were called “orphans” (Table 2, Tables S2 and S3)

Table 2.
Quantitative differentially abundant proteins (DAPs).
Moreover, sixteen more proteins/peptides with less than 80% valid values were significantly more present in one of the groups, and are or are involved in hemostasis (n = 4), proinflammatory mediators (n = 2), the complement system (n = 1), other immunological pathways (n = 6, with three of them being immunoglobulin fractions), and “orphan” proteins (n = 3) (Table 3, Tables S2 and S3).

Table 3.
Qualitative differentially abundant proteins (DAPs).
Globally, in the joint analysis from both statistical approaches, 31 proteins showed statistically significant differences. A protein–protein interaction network was elaborated based on the STRING database and our functional classification groups (Figure 1).
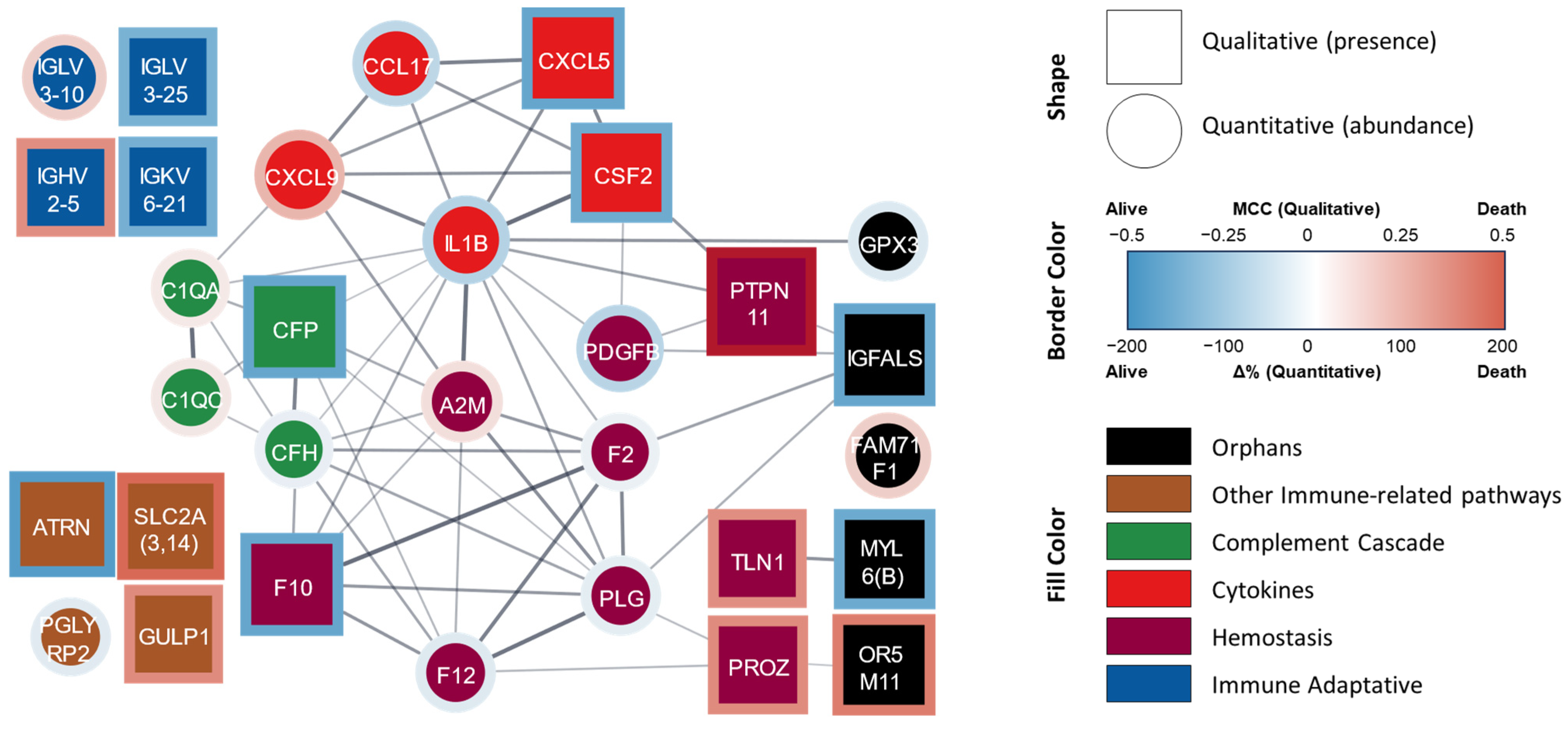
Figure 1.
Protein–protein interaction network of proteins significantly related to mortality at four years. STRING-generated network where each node lists the gene symbol or the Ig fraction of the identified proteins. Square nodes represent DAPs identified by the qualitative analysis whereas circle nodes represent the DAPs identified by quantitative analysis. The border color represents the stats value. The fill color represents the assigned functional group: ‘hemostasis’ (purple), ‘cytokine’ (red), ‘complement cascade’ (green), ‘immune adaptive’ (blue), ‘other immune-related pathways’ (brown), and ‘orphan’ (black). A0A075B6K4 (IGLV3-10), A0A0C4DH24 (IGKV6-21), P01717 (IGLV3-25), and P01817 (IGHV2-5) were manually added since they are not included in the STRING database. Abbreviations: Δ%, percent change; MCC, Matthews correlation coefficient (also called the phi coefficient, φ or rφ).
In this joint analysis, the hemostasis group comprised nine proteins. The abundances of coagulation factors II (F2, prothrombin), X (F10, thrombokinase), and XII (F12), the subunit B of platelet-derived growth factor (PDGFB), and plasminogen (PLG, including PLGLA and PLGB1) were lower or mostly absent in the non-survivor patients when compared with the survivors. In contrast, the abundances of the prothrombotic factors talin-1 (TLN1) and protein Z (PROZ), as well as the antihemostatics α-2 macroglobulin (A2M) and tyrosine-protein phosphatase non-receptor type 11 (PTPN11), were higher or mostly present in the patients who did not survive.
The group of inflammatory mediators was composed of five proteins/peptides. The abundances of IL-1β and CCL17 were lower or absent and that of CXCL9 was higher in the patients who died; CXCL5 and CSF2 (also known as GM-CSF, granulocyte–macrophage colony-stimulating factor) were mostly absent in this group.
The immune-related pathway group contained a total of 12 proteins, which can be further subdivided into three different subgroups: those related to the complement system (innate immunity), the adaptive immune response, and ‘other immune pathways’. Four proteins were associated with the complement system: the abundances of subunits A and C of the subcomponent C1q (C1QA and C1QC, respectively) were higher in the patients who were deceased at the 4-year follow-up, whereas the abundances of factor H (CFH) and its inhibitor properdin (CFP) were lower or mostly absent. The adaptive immunity subgroup comprised immunoglobulin light chain λ variable 3-25 (IGLV3-25) and light chain Ϗ variable 6-21 (IGKV6-21), which were mostly absent in patients who died, as well as immunoglobulin light chain λ variable 3-10 (IGLV3-10) and heavy chain variable 1-5 (IGHV2-5), whose abundances were higher in this group. The proteins included in the ‘other immunological pathways’ group were peptidoglycan recognition protein 2 (PGLYRP2, an enzyme that hydrolyzes a component of bacterial cell walls) and attractin (ATRN, also involved in the inflammatory response), whose abundances were lower or mostly absent in non-survivors, whereas solute carriers from family 2 (SLC2A, including SLC2A3 and SLC2A14 members) and the engulfment protein GULP adaptor 1 (GULP1) were mainly present in non-survivors at 4 years.
Finally, the five differentially abundant proteins that were not included in the previous groups (“orphan” proteins) are the antioxidant glutathione peroxidase 3 (GPX3), myosin light chain 6 (including MYL6 and MYL6B, which are motor proteins expressed in different tissues), and an insulin-like growth factor-binding protein (IGFALS, a facilitator of the actions of insulin-like growth factor); all of their abundances were lower or absent in the non-survivor patients. Meanwhile, the abundances of an olfactory receptor from family 5, subfamily M (member 11 (OR5M11), which is involved in the transduction of odorant signals) and FAM71F1 (regulates acrosome formation) were higher in this subpopulation.
The details of the results for patients taking either platelet antiaggregants or anticoagulants were excluded from the analysis but can be found in the Supplementary Materials. Briefly, some of the DAPs in the hemostasis group remained significant while the others did not reach statistical significance.
3.3. Prediction of Death and Days of Survival (Table 4 and Table 5)
3.3.1. Conventional Approach
In a first step, two models were developed to predict mortality: one is categorical, based on death/survival at the 4th year, and the other is ‘continuous’, based on days of survival. For this purpose, the 31 DAPs identified in the previous phases of the analysis were initially used.
The categorical model gave excellent results in both the initial fitting (accuracy of 100%) and the subsequent internal validation, based on either the reported literature mortality (47%, Appendix B) or deaths in our cohort (32%) (accuracies of 90 and 93%, respectively; Table 4a).

Table 4.
Summary of the results for categorical models (mortality).
Table 4.
Summary of the results for categorical models (mortality).
| Fitting | Prediction (Internal Validation) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model Name | Prot | Se/Sp/Acc/MCC | Cov | Se | Sp | MCC | Cov | PPV (Rep|Our) | NPV (Rep|Our) | Acc (Rep|Our) |
| 31 | 1.00 | 1.00 | 0.78 | 1.00 | 0.79 | 0.77 | 1.00|1.00 | 0.84|0.91 | 0.90|0.93 |
| 10 | 1.00 | 1.00 | 0.89 | 1.00 | 0.89 | 0.82 | 1.00|1.00 | 0.91|0.95 | 0.95|0.96 |
| 10 | 1.00 | 1.00 | 1.00 | 0.90 | 0.88 | 0.73 | 0.90|0.82 | 1.00|1.00 | 0.95|0.93 |
| 10 | 1.00 | 0.68 | 0.80 | 1.00 | 0.80 | 0.53 | 0.82|0.70 | 1.00|1.00 | 0.89|0.86 |
Abbreviations: Prot, proteins; Se, sensitivity; Sp, specificity; MCC, Matthews correlation coefficient; rep, based on reported mortality rate (47%, Appendix B); ours, based in our mortality rate (32%); Cov, coverage; PPV, positive predicted value; NPV, negative predicted value; Acc, accuracy.

Table 5.
Summary of results for continuous models (days of survival).
Table 5.
Summary of results for continuous models (days of survival).
| Fitting | Prediction | ||||
|---|---|---|---|---|---|
| Model Name | Proteins | R2 | Conformal Accuracy | Q2 | Conformal Accuracy |
| 31 | 0.64 | 1.00 | 0.18 | 0.95 |
| 10 | 0.81 | 1.00 | 0.52 | 0.95 |
| 10 | 0.64 | 1.00 | 0.25 | 0.91 |
| 10 | 0.71 | 1.00 | 0.36 | 0.95 |
The continuous model showed an initial R2 of 0.64 (usually considered as “acceptable” [35]) between the model prediction and the actual days of survival. However, the R2 dropped to just a Q2 of 0.18 (considered “weak”) in the internal validation (Table 5a).
3.3.2. AI Free Choice of Proteins
In the second step to improve the results of our initial prediction, we allowed the AI modeling to freely chose the 10 proteins that best contributed to generating the most accurate prediction model.
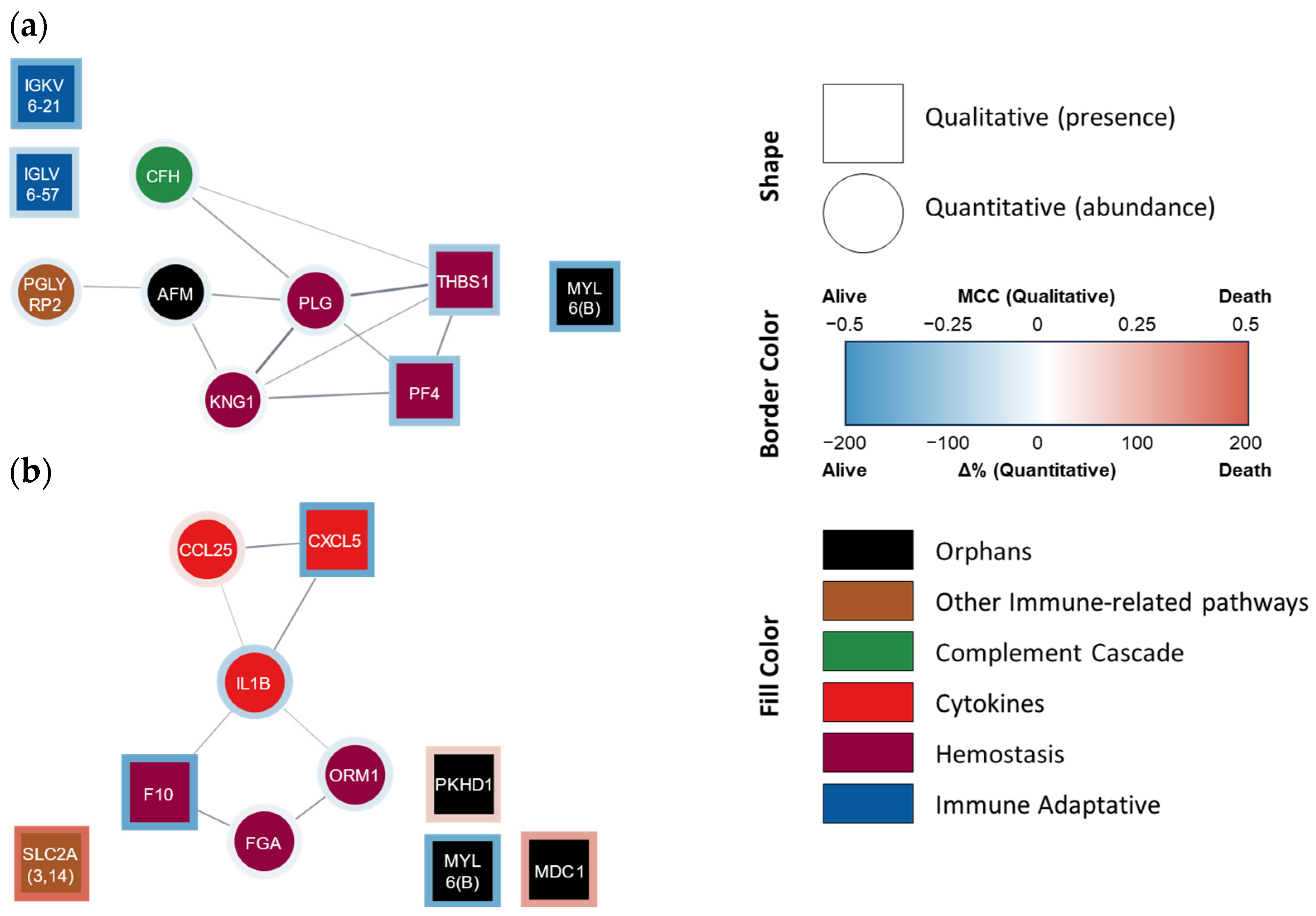
Indeed, the categorical model (i.e., death vs. survival at 4 years, Table 4b) obtained was able to better predict mortality at 4 years, not only in the fitting but also in the internal validation steps, using either literature data or our own mortality data (accuracies of 100, 95, and 93%, respectively). The ten proteins used in this model belonged to the following functional groups: hemostasis [n = 4; kininogen (KNG1), PLG, platelet factor 4 (PF4), and thrombospondin 1 (TYHBS1)], innate and adaptive immunity pathways (n = 4; CFH from the complement system, as well as IGKV6-21, IGLV6-57, and PGLYRP2), and ‘orphan’ proteins (n = 2, including MYL6 and a nutrition-related protein known as afamin) (Figure 2a).
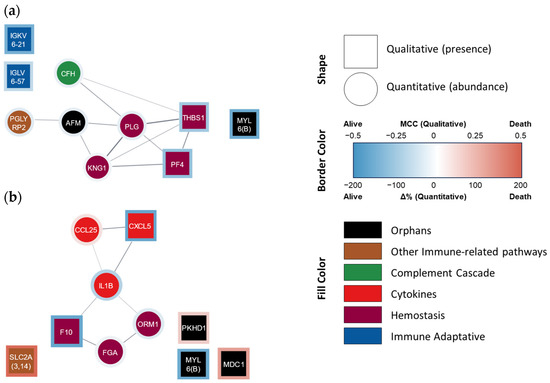
Figure 2.
Protein–protein interaction network of proteins that AI selected for (a) categorical and (b) continuous mortality models. STRING-generated network where each node lists the gene name/Ig fraction of the AI-selected proteins. Square nodes represent qualitative variables, while circle nodes represent quantitative variables. The border color represents the presence/abundance at 4 years in non-survivor (red) and survivor (blue) patients. The fill color represents the assigned functional group: ‘hemostasis’ (purple), ‘cytokine’ (red), ‘complement cascade’ (green), ‘adaptive immunity’ (blue), ‘other immune-related pathways’ (brown), and ‘orphan’ (black). Ig fractions A0A0C4DH24 (IGKV6-21) and P01721 (IGLV6-57) were manually added since they are not included in the STRING database.
The continuous model (i.e., days of survival) initially disclosed an R2 of 0.81 (strong) between the predicted and actual days of survival, decreasing to 0.52 (‘acceptable’) in the validation step (Table 5b). The 10 proteins/peptides present in this latter model belonged to the proinflammatory mediator (n = 3; IL-1β, CXCL5, and CLL25), hemostasis [n = 3; F10, fibrinogen (FG), and α-1-acid glycoprotein 1 (ORM1, which interacts with PAI-1 favoring coagulation, although it can also be considered as part of the inflammatory response)], immunological pathway (n = 1; SLC2A), and ‘orphan’ [n = 3, including the component of structural muscle proteins MYL6, as well as fibrocystin (PKHD1) and mediator of DNA damage checkpoint protein 1 (MDC1)] groups (Figure 2b).
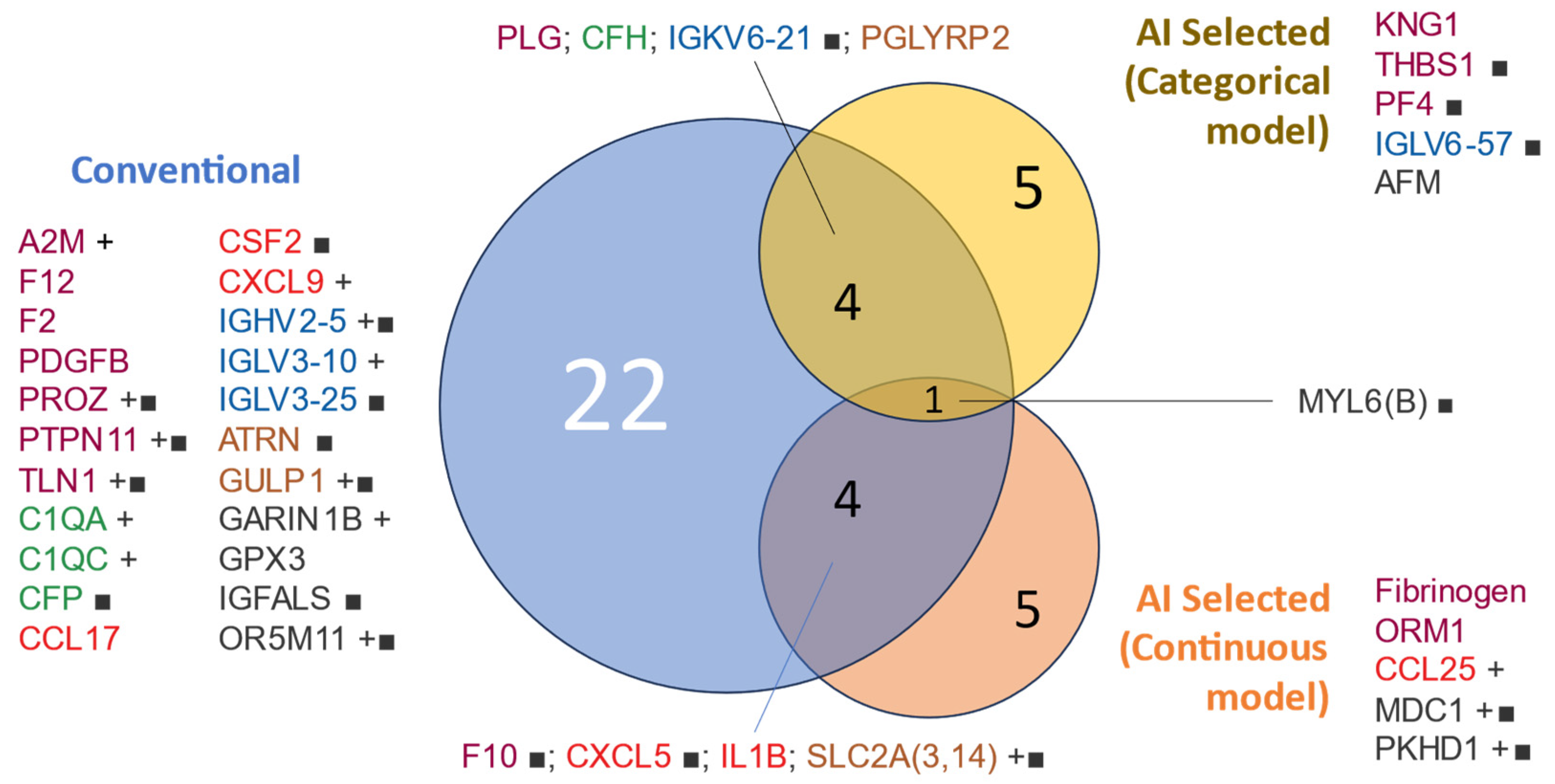
Figure 3 shows a visualization of the interactions between the proteins identified as predictive in the initial and more conventional analysis and those freely chosen by the AI for both categorical and continuous predictions (death and days of survival, respectively).
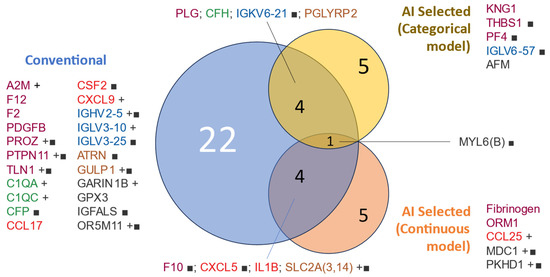
Figure 3.
Venn diagram of protein variables used for modeling by selection method. Proteins included in the mortality modeling were selected based on a conventional univariable analysis (t-test/Bernard test) or AI selection. The text color represents the assigned functional group: ‘hemostasis’ (purple), ‘cytokine’ (red), ‘complement cascade’ (green), ‘adaptive immunity’ (blue), ‘other immune-related pathways’ (brown), and ‘orphan’ (black). Protein lists are sorted by group and name. ■: qualitative variable (present/absent), +: higher abundance in non-survivors.
When the models were built with only one of the five biological groups chosen for the analysis, those that included hemostasis or proinflammatory mediators produced the best results in both analyses, in close accordance with their relative importance in the models (Figure S2).
Indeed, the hemostasis group reached 100% accuracy in the fitting step, as well as accuracies of 95 and 93% in the validations (calculated using, as always, the reported and our own mortality rates, respectively) in the categorical prediction (Table 4b). However, the 0.61 R2 value obtained in the fitting of the continuous analysis fell to a Q2 of 0.25 in the validation step (Table 5b).
Similarly, the group of proinflammatory mediators (cytokines) produced excellent results in the categorical prediction (accuracies of 100% for fitting, and 89 and 86% in both validations) (Table 4b) and the fitting of the continuous one (R2 of 0.71, Table 5b). However, the validation of the latter decreased to a ‘weak’ classification (Q2 of 0.36, Table 5b). Aggregations of the same five biological groups into sets of two did not improve these results.
Details on the models obtained for patients taking antihemostatic drugs were omitted but can be found in the Supplementary Materials. In summary, when the patients taking platelet antiaggregants were excluded, the models did not vary substantially. Moreover, when the patients under anticoagulant therapy were discarded, the AI chose six instead of four proteins from the hemostasis group, improving the accuracy of the model for categorical prediction. However, the results for the continuous prediction were modest.
4. Discussion
To our knowledge, this is the first study to analyze the predictive ability of blood proteomics on long-term mortality (4 years) in stable COPD patients through the combination of two complementary conceptual approaches (non-hypothesis-biased and hypothesis-driven analyses). Although this was a pilot study with a relatively low number of patients, our results indicate that the plasma proteomic profile can be used for death prognosis purposes. The markers obtained during clinical stability and linked to proinflammatory status, as well as to hemostasis and different components of the immune response, appear to be the main determinants of the long-term death rate.
4.1. Previous Studies
The mortality rate of the present pilot study is similar to that reported in most of the previous studies carried out in stable COPD patients from different developed countries [4,7,10,36,37,38]. Previous investigations on factors associated with death in COPD patients have mainly assessed clinical variables, identifying predictors such as comorbidities, frailty, functional capacity, physical activity, nutritional status, dynamic hyperinflation, and exertional desaturation, among others [39,40,41,42,43,44,45]. Interestingly, however, and in close accordance with our proteomic results, respiratory events of infectious origin and cardiovascular disorders, together with cancer, clearly stand out among the comorbidities closely associated with COPD mortality [46,47,48,49,50,51,52]. Fewer studies have focused on prognostic blood markers, and normally, they have been restricted to those obtained in routine tests. This is the case for percentages of neutrophils and lymphocytes, and fibrinogen, hemoglobin, D-dimer, and oxyhemoglobin concentrations [23,53,54,55,56,57]. Other authors have investigated specific markers that are not currently tested in COPD patients, such as IL-6, cholinesterase, copeptin, MRproANP, p-calprotectin, and soluble suppression of tumorigenicity 2 (sST2) [20,58,59,60,61]. However, it seems increasingly clear that only aggregations of various protein biomarkers can identify a signature with a clear predictive value for COPD outcomes. This is the case, for instance, for the combination of CC16, SRAGE, fibrinogen, CRP, and SP-D proposed by Zemans et al. to predict patient mortality [62], or the one introduced by Agustí et al. based on white blood cell (WBC) counts and C-reactive protein (CRP), IL-6, IL-8, fibrinogen, and TNF-α levels [63]. Nevertheless, all these were target-driven studies, addressing specific molecules that were already considered potential candidates for being predictive of negative outcomes. Only a few studies have attempted a wider biological approach to clinical COPD outcomes using large-scale techniques [64,65]. Unfortunately, most of them did not address mortality. This is the case for Zhang et al., who used a combination of metabolomics and proteomics to identify severity biomarkers for the disease [66]. These authors found that different proteins associated with hemostasis and/or endothelial function were dysregulated in COPD, although only cadherin (5CDH5) was a good marker of an advanced disease. From those that investigated markers of future death in stable COPD patients, Gregory et al. clearly stand out. These authors performed a cluster analysis using transcriptomics and proteomics in a large cohort of COPD patients (COPD Gene) [67], obtaining three groups of predictive proteins: those linked to innate immunity, mitochondrial function and cytoskeleton rearrangements-fatty acid metabolism.
Certainly, some complementary studies have also investigated predictors of mortality during acute COPD exacerbations. However, this is a totally different subject and is more focused on short- to medium-term prognoses in already unstable patients. As it is also the case in stable patients, the clinical findings have been prominently used to establish a survival prognosis [68,69], but some blood biomarkers, such as troponin levels, the eosinophil-to-platelet ratio, the triglyceride–glucose index (TyG), and CRP levels, among others, seem to be useful for this purpose [68,69,70,71].
4.2. Interpretation of Novel Findings
4.2.1. Differentially Abundant Proteins
The present investigation revealed that immune-related proteins, including those belonging to the complement system, and proteins associated with hemostasis are the main groups of markers that were differentially abundant between survivors and non-survivors. This was a prominent feature in the results from both the more conventional and the free-choice AI analyses.
Regarding immune-related pathways, we considered three different components; one of these is proteins linked to adaptive immunity, such as immunoglobulins, but our results initially seemed to be partially contradictory. There was an increase or a differential presence of some immunoglobulin fractions (IGLV3-10 and IGHV2-5) in the patients who later died, but other fractions were absent (IGLV3-25 and IGKV6-21). Immunoglobulins are key elements of the humoral response, allowing for the control of infections through opsonization, complement cascade activation (see the following paragraph), and cytotoxicity. A possible interpretation of the present findings may be that the activation of the adaptive immune response in apparently stable patients occurred, but it was partially compromised. This would be consistent with the observations of our and other previous studies in patients with frequent exacerbations [29,30,72], a specific COPD phenotype that (as also happened in the present study) consistently shows an increased mortality rate [73].
Moreover, other immune mechanisms may also have been defective in patients who would die later, as suggested by the absence of ATRN, a known modulator of the immune and inflammatory response, or the lower level of PGLYRP2, which is involved in the destruction of bacterial walls. On the contrary, other immune-related proteins seem to have been activated in those patients who subsequently died. This would have been the case for the presence of either SLC2A, which is involved with lymphocyte signaling and the subsequent activation of the response against infections and tumors [74], or GULP, implicated in the phagocytic elimination of cells and microorganisms that are already inactive, in this COPD group.
The complement system, in turn, showed signs of activation in the first steps of the classical pathway (moderate increases in C1q A and C chains), but a probable restriction of the common final steps of the system, which are especially critical for the alternative pathway, through the inhibition of its C3 self-amplification loop (i.e., the decrease in CFH and the apparent absence of CFP). The complement cascade is an essential part of the defense system since it directly induces bacterial lysis and opsonization, and contributes to improving the action of antibodies and clearing immune complexes [75]. This system is made up of three different pathways that converge in their final steps. In the main one (‘classical’), the complement component C1 can bind to immune complexes or react with different polyanions, CRP, or even components of bacteria walls. The so-called lectin or MB-lectin path has some similarities with the main pathway, but here, the activation of the system occurs when mannose-binding lectin (MBL) recognizes foreign carbohydrates present in bacterial walls, which is totally independent of antibodies. It is worth noting that MBL deficiency has been associated with the pathogenesis of COPD and high serum MBL levels have been associated with increased survival in stable patients [76]. The third path, usually known as the ‘alternative pathway’, is only slightly active in normal conditions but initiated when components of bacterial walls, such as bacterial lipopolysaccharide (LPS), or even immunoglobulins induce a moderate split of C3. However, these three distinct paths converge when the action of a specific convertase splits C3 into C3a and C3b, leading to the generation of the membrane attack complex (MAC, composed of C5b, C6, C7, C8, and C9), which disrupts the membrane of pathogens, leading to their death. Other authors have also shown that some components of the complement system can be altered in COPD patients [66,67], but the present report contributes to clarify some of these abnormalities and their association with mortality. As previously mentioned, our results suggest that although patients who will die in the next few years have a certain activation of the initial steps of the complement system (which would involve the presence of a relatively permanent harmful noxae), and the final response is impaired, even in periods of clinical stability (a factor that may be a determinant in their future defense capacity against infections) [76]. Indeed, this is in close agreement with our previous observations in either stable or exacerbated patients [29,30]. Although other authors have reported that C9 was one of the main proteins associated with an increased risk of death [67], no differences were found for this component of the complement system between our two groups of patients.
Hemostasis was one of the two groups will more proteins associated with death in our COPD patients. This is in close agreement with some previous clinical studies that evidenced a high prevalence of cardiovascular comorbidities in COPD [49,50,51,52,77]. Moreover, factors involved in thrombogenicity that contribute to severe cardiovascular events, such as increases in coagulation factors, platelet activation, and even endothelial dysfunction, have been described in both stable and exacerbated COPD patients [78]. Other studies have specifically highlighted the relevance of thrombotic events in their survival prognosis [49,50,51,52]. Interestingly, however, the hemostasis-linked proteins/peptides identified in the present study seem to indicate an ambivalent situation in deceased patients. On the one hand, there are elements whose presence or increased levels would either favor the generation of thrombi (TLN1 and PROZ) or hinder their resolution (decreases in PLG). It appears, however, that the levels of other proteins would have potential antithrombotic effects (i.e., the increase in A2M and the presence of PTPN11, as well as decreases or an absence of F2, F10, F12, and PDGFB in models from the conventional analysis). Certainly, an alternative explanation for the results of the latter proteins would be that they have been extensively used in the generation of subclinical thrombi [79]. Moreover, other authors have related low levels of some factors related to hemostasis with difficulties in the repair of previously injured airways and lung tissues in COPD patients [80]. Other researchers have also observed dysregulation in these and other prothrombotic factors (such as F11a and tissue factor, TF) in stable COPD patients, suggesting that this may contribute to an elevated risk of cardiovascular complications [66,81,82]. Moreover, Sand et al. and Manon-Jensen et al. reported that high levels of markers of fibrin clot resolution are indicative of a poor survival prognosis in COPD patients [79,83]. Similarly, Ronnow et al. and Langholm et al. described an increase in mortality in patients with high levels of Von Willebrand factor epitopes [24,84].
Regarding inflammatory mediators, the absence or reduced presence of various cytokines and chemokines in the group of patients who died before the 4-year follow-up may have contributed to impaired modulation of the inflammatory response. For example, reduced levels of CCL17 and CXCL5 may have resulted in a decreased leukocyte chemotaxis, and reduced levels of IL-1β and CSF2 may account for the problems in the differentiation and proliferation of various types of leukocytes [85,86]. In contrast, the CXCL9 level was higher in this group than in the survivors. This Th1-type monokine, which is released by neutrophils and induced by interferon-γ, has been described as being elevated in the airways of COPD patients [87], and its blood levels have been reported as being predictive of hospital readmissions [88]. Moreover, multiple studies have shown mild-to-moderate increases of inflammatory mediators in the blood of stable COPD patients, reflecting a low grade of chronic systemic inflammation [89,90,91,92]. Although some previous work described isolated or groups of inflammatory mediators as associated with mortality (as is the case for CRP) [93,94], and others have used microarrays with the same objective [3,95], this is the first article that provides a broader profile of the cytokines and chemokines associated with death in a long-term follow up. Moreover, our set of proinflammatory mediators was one the best protein groups that, alone, was able to predict mortality in COPD patients. This finding is in close agreement with the results published by Agustí et al. in the ECLIPSE cohort [63] and adds new predictive proinflammatory biomarkers.
Finally, the ‘orphan’ proteins/peptides with differential presence between the survivors and non-survivors included an antioxidant (GPX3), an insulin-like growth factor 1 (IGF) facilitator (IGFALS), and a fraction of muscle structural proteins (MYL6), among others. Although purely speculative, the lower level of GPX3 may have contributed to an environment of oxidative stress in patients, while the absence of IGFALS could have resulted in decreased signaling for cell proliferation (including in leukocytes) and, along with the absence of MLY6, may have had an impact on muscle growth. It is well known that a significant number of patients with COPD present a reduction in their muscle mass [96], and this constitutes a factor for a poorer survival prognosis [97]. Although there were no differences in BMI between the survivors and non-survivors in our study, this plasma finding may suggest the presence of a mechanism that does not show a clear clinical expression. Unfortunately, we did not evaluate the body composition at the time of blood extraction or obtain a BMI value closer to the patients’ death.
4.2.2. Prediction of Death and Days of Survival
The models generated by both the more traditional analysis based on DAPs and through the AI free choice of proteins were enormously efficient in predicting deaths at 4 years, although they were much less accurate in predicting the days of survival for each COPD patient. The prediction of death at 4 years using the more conventional statistical analysis was excellent when all the differential proteins meeting the initial study criteria (presence in 80% of patients or being present in only one of the two groups) were analyzed, obtaining the maximum specificity, although the sensitivity was only moderate [98]. However, this latter parameter improved substantially through the use of the AI free choice option.
Moreover, when separately analyzing the four mechanistic groups of proteins separately, it becomes evident that those constituted by inflammatory or hemostasis mediators could generate reasonable approximations for the occurrence of death.
The type of analysis presented here could eventually provide a new tool for predicting mortality or even clinically unexpected deaths [99,100] based only on the pathophysiological mechanisms that may be involved in the prognosis. In this regard, this tool could help us to identify the patients that require special management. Moreover, our results may help in developing future predictive blood tests.
4.3. Strengths and Potential Limitations
The complementary approach provided by both the hypothesis-driven and blinded techniques (immune-based assays and mass spectrometry, respectively) stands out as the most powerful point of the present study. Indeed, the immune-based assay approach allowed for the analysis of protein markers known to be associated with pulmonary disease and with a possible impact on its mortality, while the mass spectrometry approach allowed for a very broad screening of unbiased biomarkers, which are less frequently analyzed in other searches. Furthermore, our research group directly obtained and verified all the clinical data and carried out all the different phases of the proteomic study, a characteristic that is very uncommon in most of the previous omic studies on blood biomarkers. Furthermore, our biological tool could be combined with clinical data in the future to increase its predictive power.
Another interesting point is that, in contrast to many previous studies, our sample consisted of around 30% female COPD patients. It is worth noting that a significant increase in the prevalence of COPD in women has been observed worldwide in recent years, and some authors have related this to the changes in global mortality attributed to the disease [101,102]. Our study is therefore more representative of the present COPD population than some of the previous ones [4,19]. This representativity of the results also account for comorbidities, since around 80–90% of COPD patients are associated with at least one additional chronic condition [102].
However, we should recognize that our population sample is relatively small, which is intrinsic to pilot studies but can be considered a potential limitation. Nevertheless, although this restriction may have reduced the power of our analysis, many interesting associations between protein markers and mortality were evidenced. On the other hand, our sample is similar to that of other previous reports using omics to explore biomarkers in COPD [64,66,103,104,105]. We are currently developing a validation approach using multiomics in a much larger sample of patients and with a longer prospective follow-up [106], which might also facilitate the investigation of the impact of co-morbidities and other clinical factors that were not part of the objectives of the present pilot study. Moreover, most of the previous studies on mortality with a higher number of participants were retrospective and/or based solely on clinical variables. In addition to these common clinical or biological variables, our study also identified a wide number of proteins/peptides present in the blood of stable COPD patients who have been prospectively followed for at least 4 years.
It could also be considered that the inclusion of patients on platelet antiaggregant or anticoagulant treatment interferes with the results. However, this situation corresponds to the real world, where a significant percentage of COPD patients shows vascular comorbidities (cardiovascular or neurovascular comorbidities, or involvement of peripheral vessels). Furthermore, our results remain essentially similar regarding the influence of each protein group if such patients are excluded.
Our findings may be subject to variability when new medications or treatment recommendations are introduced. Therefore, ongoing refinement and updates of this biological approximation will be required to achieve real and applicable personalized medicine.
Finally, we should also admit that some of the differentially abundant proteins identified in the present study may have been allocated into more than one biological group. However, we tried to include them in the one that seemed most appropriate for the objectives of the study.
5. Conclusions
A proteomic blood signature found in stable COPD patients can help in establishing their long-term (4-year) survival prognosis. The most important elements for establishing this prognosis are proteins/peptides linked to the hemostatic and inflammatory statuses during the stable phase, as well as different elements of the immune response. Furthermore, the present results may help us to better understand the biological mechanisms involved in future deaths in long-term patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells13161351/s1, Figure S1: Flow chart with information regarding proteins, quantitative and qualitative approaches, and main results; Figure S2: Comparison of importance of (A) proteins and (B) functional groups in the models. Supplementary Data also include “Main results when patients taking antihemostatic drugs were excluded”, “UNIPROT codes used for protein functional classification” and “Semi-code for functional classification”. EXCEL file (version 2406, 2024; Microsoft, Redmont, WA, USA) includes, Table S1: Identified proteins (information based on UNIPROT) [+printable version]; Table S2: Identified and significantly different proteins between survivors and non-survivors, identified through the Barnard test (qualitative) and t-test (quantitative); and Table S3: Extended data for proteins selected by conventional and AI free selection for mortality models.
Author Contributions
Conceptualization, C.J.E.-R., C.C., S.P.-G. and J.G.; Methodology, C.J.E.-R., E.B. and J.G.; Software, C.J.E.-R.; Validation, C.J.E.-R. and C.C.; Formal analysis, C.J.E.-R., C.C. and J.G.; Data curation, C.J.E.-R., C.C. and S.P.-G.; Writing—original draft preparation, C.J.E.-R. and J.G.; Writing—review and editing, all authors; Supervision, J.G.; Project administration, C.J.E.-R. and J.G.; Funding acquisition, C.J.E.-R., E.B. and J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-funded by the Spanish Ministerio de Economía y Competitividad and the European Union (project SAF2014-54371); the Instituto de Salud Carlos III (ISCIII) and European Union (projects PI21/00785 and M-BAE BA22/00009); SEPAR Grants (2015, 2016 and 2019); an FUCAP Grant (2014); an SOCAP Grant (2020); and Menarini Spain (unrestricted Grant 2015-19). César J. Enríquez-Rodríguez is a recipient of a Predoctoral Grant PFIS (ref. FI22/00003) and a Mobility of Research Personnel Grant (M-AES MV23/00012) from ISCIII and co-funded by the European Union.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Clinical Research Ethics Committee (CEIC) of IMIM (ref. 2014/5895/I, 22 December 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We would like to thank all patients for their generous contribution to the study, as well as all research members of the BIOMEPOC and EARLY COPD groups; Manuel Pastor from the PharmacoInformatics Group, MELIS Dpt., Universitat Pompeu Fabra, and Hospital del Mar Research Institute (Barcelona) for his contribution with Flame; Jonathan McFarland for his editing help; and the Proteomics Unit of the Pompeu Fabra University and the Centre for Genomic Regulation (both in Barcelona) for their help and kindness.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
Members of the BIOMEPOC group (in alphabetical order): Mireia Admetlló, Alvar Agustí, Carlos Alvarez-Martínez, Esther Barreiro, Oswaldo Antonio Caguana, Carme Casadevall, Ferran Casals, Robert Castelo, Ady Castro-Acosta, Rocío Córdova, Borja G Cosío, Rosa Faner, Laura I Furlong, Marian García, José G. González-García, Carmen Hernández-Carcereny, José Luis López-Campos, Eduardo Márquez, Eduard Monsó, Concepción Montón, Miren Josune Ormaza, Alexandre Palou, Sergi Pascual, Germán Peces-Barba, Pau Puigdevall, Diego A. Rodríguez-Chiaradia, Ferran Sanz, Luis Seijo, Montserrat Torà, Yolanda Torralba, and Carles Vilaplana.
Appendix B
The mortality ratio based on the literature was estimated according to the data provided by a large study carried out in the COPD Cohorts Collaborative International Assessment (3CIA). This study provides a survival ratio for 1 to 5 years of follow-up for each 2019 GOLD group [34].
The calculation applied followed the next formula (based on weighted averages):
So, applying our 2019 GOLD proportion and the ratio of the study [34], it was
References
- WHO. COPD Factsheet. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 19 July 2024).
- Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 Report). 2023. Available online: www.goldcopd.org (accessed on 19 July 2024).
- Celli, B.R.; Fabbri, L.M.; Aaron, S.D.; Agusti, A.; Brook, R.; Criner, G.J.; Franssen, F.M.E.; Humbert, M.; Hurst, J.R.; O’donnell, D.; et al. An Updated Definition and Severity Classification of Chronic Obstructive Pulmonary Disease Exacerbations: The Rome Proposal. Am. J. Respir. Crit. Care Med. 2021, 204, 1251–1258. [Google Scholar] [CrossRef]
- Esteban, C.; Quintana, J.M.; Aburto, M.; Moraza, J.; Egurrola, M.; España, P.P.; Pérez-Izquierdo, J.; Capelastegui, A. Predictors of mortality in patients with stable COPD. J. Gen. Intern. Med. 2008, 23, 1829–1834. [Google Scholar] [CrossRef]
- Nishimura, K.; Izumi, T.; Tsukino, M.; Oga, T. Dyspnea Is a Better Predictor of 5-Year Survival Than Airway Obstruction in Patients with COPD. Chest 2002, 121, 1434–1440. [Google Scholar] [CrossRef]
- Oga, T.; Nishimura, K.; Tsukino, M.; Sato, S.; Hajiro, T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: Role of exercise capacity and health status. Am. J. Respir. Crit. Care Med. 2003, 167, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Salvany, A.; Lamarca, R.; Ferrer, M.; Garcia-Aymerich, J.; Alonso, J.; Félez, M.; Khalaf, A.; Marrades, R.M.; Monsó, E.; Serra-Batlles, J.; et al. Health-related Quality of Life and Mortality in Male Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2002, 166, 680–685. [Google Scholar] [CrossRef]
- Almagro, P.; Calbo, E.; de Echaguïen, A.O.; Barreiro, B.; Quintana, S.; Heredia, J.L.; Garau, J. Mortality After Hospitalization for COPD. Chest 2002, 121, 1441–1448. [Google Scholar] [CrossRef]
- Connors, A.F., Jr.; Dawson, N.V.; Thomas, C.; Harrell, F.E., Jr.; Desbiens, N.; Fulkerson, W.J.; Kussin, P.; Bellamy, P.; Goldman, L.; Knaus, W.A. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am. J. Respir. Crit. Care Med. 1996, 154, 959–967. [Google Scholar] [CrossRef]
- Solanes-Garcia, I.; Casan, P. Causes of death and prediction of mortality in COPD. Arch Bronconeumol. 2010, 46, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Lüthi-Corridori, G.; Boesing, M.; Roth, A.; Giezendanner, S.; Leuppi-Taegtmeyer, A.B.; Schuetz, P.; Leuppi, J.D. Predictors of Length of Stay, Rehospitalization and Mortality in Community-Acquired Pneumonia Patients: A Retrospective Cohort Study. J. Clin. Med. 2023, 12, 5601. [Google Scholar] [CrossRef]
- Hartl, S.; Lopez-Campos, J.L.; Pozo-Rodriguez, F.; Castro-Acosta, A.; Studnicka, M.; Kaiser, B.; Roberts, C.M. Risk of death and re-admission of hospital-admitted COPD exacerbations: European COPD Audit. Eur. Respir. J. 2016, 47, 113–121. [Google Scholar] [CrossRef]
- Han, M.-Z.; Hsiue, T.-R.; Tsai, S.-H.; Huang, T.-H.; Liao, X.-M.; Chen, C.-Z. Validation of the GOLD 2017 and new 16 subgroups (1A–4D) classifications in predicting exacerbation and mortality in COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3425–3433. [Google Scholar] [CrossRef] [PubMed]
- de Torres, J.P.; Casanova, C.; Marín, J.M.; Pinto-Plata, V.; Divo, M.; Zulueta, J.J.; Berto, J.; Zagaceta, J.; Sanchez-Salcedo, P.; Cabrera, C.; et al. Prognostic evaluation of COPD patients: GOLD 2011 versus BODE and the COPD comorbidity index COTE. Thorax 2014, 69, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.W.V.; MacDonald, T.M.; Chalmers, J.D.; Schembri, S. The effect of changes to GOLD severity stage on long term morbidity and mortality in COPD. Respir. Res. 2018, 19, 249. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Wrobel, J.P. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 871–888. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G.; et al. Comorbidities and Risk of Mortality in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef]
- Negewo, N.A.; Gibson, P.G.; McDonald, V.M. COPD and its comorbidities: Impact, measurement and mechanisms. Respirology 2015, 20, 1160–1171. [Google Scholar] [CrossRef]
- Sato, S.; Oga, T.; Muro, S.; Tanimura, K.; Tanabe, N.; Nishimura, K.; Hirai, T. Changes in mortality among patients with chronic obstructive pulmonary disease from the 1990s to the 2000s: A pooled analysis of two prospective cohort studies. BMJ Open 2023, 13, e065896. [Google Scholar] [CrossRef]
- Fähndrich, S.; Herr, C.; Teuteberg, S.; Söhler, S.; Soriano, D.; Classen, J.; Adams, J.; Weinhold, V.; Waschki, B.; Zeller, T.; et al. Midregional proatrial naturetic peptide (MRproANP) and copeptin (COPAVP) as predictors of all-cause mortality in recently diagnosed mild to moderate COPD—Results from COSYCONET. Respir. Res. 2024, 25, 56. [Google Scholar] [CrossRef]
- Hu, H.S.; Wang, Z.; Jian, L.Y.; Zhao, L.M.; Liu, X.D. Optimizing inhaled corticosteroid use in patients with chronic obstructive pul-monary disease: Assessing blood eosinophils, neutrophil-to-lymphocyte ratio, and mortality outcomes in US adults. Front Immunol. 2023, 14, 1230766. [Google Scholar] [CrossRef]
- Echevarria, C.; Steer, J.; Prasad, A.; Quint, J.K.; Bourke, S.C. Admission blood eosinophil count, inpatient death and death at 1 year in exacerbating patients with COPD. Thorax 2023, 78, 1090–1096. [Google Scholar] [CrossRef]
- Husebø, G.R.; Gabazza, E.C.; D’Alessandro Gabazza, C.; Yasuma, T.; Toda, M.; Aanerud, M.; Nielsen, R.; Bakke, P.S.; Eagan, T.M.L. Coag-ulation markers as predictors for clinical events in COPD. Respirology 2021, 26, 342–351. [Google Scholar] [CrossRef]
- Rønnow, S.R.; Langholm, L.L.; Karsdal, M.A.; Manon-Jensen, T.; Tal-Singer, R.; Miller, B.E.; Vestbo, J.; Leeming, D.J.; Sand, J.M.B. Endo-trophin, an extracellular hormone, in combination with neoepitope markers of von Willebrand factor improves prediction of mortality in the ECLIPSE COPD cohort. Respir. Res. 2020, 21, 202. [Google Scholar] [CrossRef]
- Rabe, K.F.; Martinez, F.J.; Ferguson, G.T.; Wang, C.; Singh, D.; Wedzicha, J.A.; Trivedi, R.; Rose, E.S.; Ballal, S.; McLaren, J.; et al. Triple Inhaled Therapy at Two Glucocorticoid Doses in Moderate-to-Very-Severe COPD. N. Engl. J. Med. 2020, 383, 35–48. [Google Scholar] [CrossRef]
- Calverley, P.M.; Anzueto, A.R.; Carter, K.; Grönke, L.; Hallmann, C.; Jenkins, C.; Wedzicha, J.; Rabe, K.F. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): A double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir. Med. 2018, 6, 337–344. [Google Scholar] [CrossRef]
- Lipson, D.A.; Crim, C.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Jones, C.E.; Kilbride, S.; Lange, P.; et al. Reduction in All-Cause Mortality with Fluticasone Furoate/Umeclidinium/Vilanterol in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2020, 201, 1508–1516. [Google Scholar] [CrossRef]
- Gea, J.; Pascual, S.; Castro-Acosta, A.; Hernández-Carcereny, C.; Castelo, R.; Márquez-Martín, E.; Montón, C.; Palou, A.; Faner, R.; Furlong, L.I.; et al. The BIOMEPOC Project: Personalized Biomarkers and Clinical Profiles in Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2019, 55, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Enríquez-Rodríguez, C.J.; Casadevall, C.; Faner, R.; Castro-Costa, A.; Pascual-Guàrdia, S.; Seijó, L.; López-Campos, J.L.; Peces-Barba, G.; Monsó, E.; Barreiro, E.; et al. COPD: Systemic proteomic profiles in frequent and infrequent exacerbators. ERJ Open Res. 2024, 10. [Google Scholar] [CrossRef]
- Enríquez-Rodríguez, C.J.; Pascual-Guardia, S.; Casadevall, C.; Caguana-Vélez, O.A.; Rodríguez-Chiaradia, D.; Barreiro, E.; Gea, J. Proteomic Blood Profiles Obtained by Totally Blind Biological Clustering in Stable and Exacerbated COPD Patients. Cells 2024, 13, 866. [Google Scholar] [CrossRef]
- Arostegui, I.; Legarreta, M.J.; Barrio, I.; Esteban, C.; Garcia-Gutierrez, S.; Aguirre, U.; Quintana, J.M. IRYSS-COPD Group A Computer Application to Predict Adverse Events in the Short-Term Evolution of Patients with Exacerbation of Chronic Obstructive Pulmonary Disease. JMIR Med. Inform. 2019, 7, e10773. [Google Scholar] [CrossRef]
- Austin, P.C.; White, I.R.; Lee, D.S.; van Buuren, S. Missing Data in Clinical Research: A Tutorial on Multiple Imputation. Can. J. Cardiol. 2021, 37, 1322–1331. [Google Scholar] [CrossRef]
- Pastor, M.; Gomez-Tamayo, J.C.; Sanz, F. Flame: An open-source framework for model development, hosting, and usage in production envoiironments. J. Cheminform. 2021, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Castillo, E.G.; Pérez, T.A.; Ancochea, J.; Sanz, M.T.P.; Almagro, P.; Martínez-Camblor, P.; Miravitlles, M.; Rodríguez-Carballeira, M.; Navarro, A.; Lamprecht, B.; et al. Mortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2015 and GOLD 2019 staging: A pooled analysis of individual patient data. ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef]
- Chicco, D.; Warrens, M.J.; Jurman, G. The coefficient of determination R-squared is more informative than SMAPE, MAE, MAPE, MSE and RMSE in regression analysis evaluation. PeerJ Comput. Sci. 2021, 7, e623. [Google Scholar] [CrossRef]
- Kiddle, S.J.; Whittaker, H.R.; Seaman, S.R.; Quint, J.K. Prediction of five-year mortality after COPD diagnosis using primary care records. PLoS ONE 2020, 15, e0236011. [Google Scholar] [CrossRef]
- Gedebjerg, A.; Szépligeti, S.K.; Wackerhausen, L.-M.H.; Horváth-Puhó, E.; Dahl, R.; Hansen, J.G.; Sørensen, H.T.; Nørgaard, M.; Lange, P.; Thomsen, R.W. Prediction of mortality in patients with chronic obstructive pulmonary disease with the new Global Initiative for Chronic Obstructive Lung Disease 2017 classification: A cohort study. Lancet Respir. Med. 2018, 6, 204–212. [Google Scholar] [CrossRef]
- van Hirtum, P.V.; Sprooten, R.T.M.; van Noord, J.A.; van Vliet, M.; de Kruif, M.D. Long term survival after admission for COPD exacerbation: A comparison with the general population. Respir. Med. 2018, 137, 77–82. [Google Scholar] [CrossRef]
- Lorenzana, I.; Galera, R.; Casitas, R.; Martínez-Cerón, E.; Castillo, M.A.; Alfaro, E.; Cubillos-Zapata, C.; García-Río, F. Dynamic hy-perinflation is a risk factor for mortality and severe exacerbations in COPD patients. Respir. Med. 2024, 225, 107597. [Google Scholar] [CrossRef]
- Nishimura, K.; Kusunose, M.; Shibayama, A.; Nakayasu, K. Is Frailty a Mortality Predictor in Subjects with Chronic Obstructive Pulmonary Disease? Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 2955–2960. [Google Scholar] [CrossRef]
- Nishimura, K.; Kusunose, M.; Sanda, R.; Mori, M.; Shibayama, A.; Nakayasu, K. Comparison of Predictive Properties between Tools of Patient-Reported Outcomes: Risk Prediction for Three Future Events in Subjects with COPD. Diagnostics 2023, 13, 2269. [Google Scholar] [CrossRef]
- Medina-Mirapeix, F.; Valera-Novella, E.; Morera-Balaguer, J.; Bernabeu-Mora, R. Prognostic value of the five-repetition sit-to-stand test for mortality in people with chronic obstructive pulmonary disease. Ann. Phys. Rehabil. Med. 2022, 65, 101598. [Google Scholar] [CrossRef]
- Liu, S.-F.; Chin, C.-H.; Tseng, C.-W.; Chen, Y.-C.; Kuo, H.-C. Exertional Desaturation Has Higher Mortality Than Non-Desaturation in COPD. Medicina 2021, 57, 1110. [Google Scholar] [CrossRef]
- Waschki, B.; Kirsten, A.; Holz, O.; Müller, K.C.; Meyer, T.; Watz, H.; Magnussen, H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest 2011, 140, 331–342. [Google Scholar] [CrossRef]
- Deng, M.; Lu, Y.; Zhang, Q.; Bian, Y.; Zhou, X.; Hou, G. Global prevalence of malnutrition in patients with chronic obstructive pulmonary disease: Systemic review and meta-analysis. Clin. Nutr. 2023, 42, 848–858. [Google Scholar] [CrossRef]
- van Gestel, Y.R.; Hoeks, S.E.; Sin, D.D.; Hüzeir, V.; Stam, H.; Mertens, F.W.; van Domburg, R.T.; Bax, J.J.; Poldermans, D. COPD and cancer mortality: The influence of statins. Thorax 2009, 64, 963–967. [Google Scholar] [CrossRef][Green Version]
- Wasswa-Kintu, S.; Gan, W.Q.; Man, S.F.P.; Pare, P.D.; Sin, D.D. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: A systematic review and meta-analysis. Thorax 2005, 60, 570–575. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Zheng, X.; Peng, J.; Chen, Y.; Yu, K.; Yang, Y.; Wang, X.; Yang, X.; Qian, J.; et al. Deaths from COPD in patients with cancer: A population-based study. Aging 2021, 13, 12641–12659. [Google Scholar] [CrossRef]
- Lüthi-Corridori, G.; Boesing, M.; Ottensarendt, N.; Leuppi-Taegtmeyer, A.B.; Schuetz, P.; Leuppi, J.D. Predictors of Length of Stay, Mortality and Rehospitalization in COPD Patients: A Retrospective Cohort Study. J. Clin. Med. 2023, 12, 5322. [Google Scholar] [CrossRef]
- Abdullah, A.S.; Eigbire, G.; Ali, M.; Awadalla, M.; Wahab, A.; Ibrahim, H.; Salama, A.; Alweis, R. Relationship of Atrial Fibrillation to Outcomes in Patients Hospitalized for Chronic Obstructive Pulmonary Disease Exacerbation. J. Atr. Fibrillation 2019, 12, 2117. [Google Scholar] [CrossRef]
- Liao, K.-M.; Chen, C.-Y. Incidence and risk factors of atrial fibrillation in Asian COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2523–2530. [Google Scholar] [CrossRef]
- Warming, P.E.; Garcia, R.; Hansen, C.J.; Simons, S.O.; Torp-Pedersen, C.; Linz, D.; Tfelt-Hansen, J. Atrial fibrillation and chronic ob-structive pulmonary disease: Diagnostic sequence and mortality risk. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 128–134. [Google Scholar] [CrossRef]
- Trudzinski, F.C.; Jörres, R.A.; Alter, P.; Kahnert, K.; Waschki, B.; Herr, C.; Kellerer, C.; Omlor, A.; Vogelmeier, C.F.; Fähndrich, S.; et al. Associations of oxygenated hemoglobin with disease burden and prognosis in stable COPD: Results from COSYCONET. Sci. Rep. 2020, 10, 10544. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, E.C.; Özgül, M.A.; Tutar, N.; Ömür, I.; Uysal, A.; Altın, S. Red Blood Cell Distribution and Survival in Patients with Chronic Obstructive Pulmonary Disease. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10, 416–424. [Google Scholar] [CrossRef]
- Valvi, D.; Mannino, D.M.; Müllerova, H.; Tal-Singer, R. Fibrinogen, chronic obstructive pulmonary disease (COPD) and outcomes in two United States cohorts. Int. J. Chronic Obstr. Pulm. Dis. 2012, 7, 173–182. [Google Scholar] [PubMed]
- Oh, Y.-M.; Park, J.H.; Kim, E.-K.; Hwang, S.C.; Kim, H.J.; Kang, D.R.; Yoo, K.H.; Lee, J.-H.; Kim, T.-H.; Lim, S.Y.; et al. Anemia as a clinical marker of stable chronic obstructive pulmonary disease in the Korean obstructive lung disease cohort. J. Thorac. Dis. 2017, 9, 5008–5016. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lim, B.; Kyung, S.Y.; Park, J.-W.; Jeong, S.H. Comorbidity and Inflammatory Markers May Contribute to Predict Mortality of High-Risk Patients with Chronic Obstructive Pulmonary Disease Exacerbation. J. Clin. Med. Res. 2016, 8, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zha, L.; Feng, G.; An, Q.; Shi, F.; Xu, J.; Xu, Q.; Xia, H.; Zhang, M.; Li, L. Prognostic Value of Serum Cholinesterase Levels for In-Hospital Mortality among Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. COPD J. Chronic Obstr. Pulm. Dis. 2023, 20, 178–185. [Google Scholar] [CrossRef]
- Urban, M.H.; Stojkovic, S.; Demyanets, S.; Hengstenberg, C.; Valipour, A.; Wojta, J.; Burghuber, O.C. Soluble ST2 and All-Cause Mortality in Patients with Chronic Obstructive Pulmonary Disease—A 10-Year Cohort Study. J. Clin. Med. 2021, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Holmgaard, D.B.; Mygind, L.H.; Titlestad, I.; Madsen, H.; Pedersen, S.S.; Mortensen, O.H.; Pedersen, C. Calprotectin—A Marker of Mortality in COPD? Results from a Prospective Cohort Study. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Tanni, S.E.; Caram, L.M.; Corrêa, C.; Corrêa, C.R.; Godoy, I. Three-year follow-up of Interleukin 6 and C-reactive protein in chronic obstructive pulmonary disease. Respir. Res. 2013, 14, 24. [Google Scholar] [CrossRef]
- Zemans, R.L.; Jacobson, S.; Keene, J.; Kechris, K.; Miller, B.E.; Tal-Singer, R.; Bowler, R.P. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir. Res. 2017, 18, 117. [Google Scholar] [CrossRef]
- Agustí, A.; Edwards, L.D.; Rennard, S.I.; MacNee, W.; Tal-Singer, R.; Miller, B.E.; Vestbo, J.; Lomas, D.A.; Calverley, P.M.A.; Wouters, E.; et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Persistent Systemic Inflammation is Associated with Poor Clinical Outcomes in COPD: A Novel Phenotype. PLoS ONE 2012, 7, e37483. [Google Scholar] [CrossRef]
- Wu, S.; Huang, K.; Chang, C.; Chu, X.; Zhang, K.; Li, B.; Yang, T. Serum Proteomic Profiling in Patients with Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 1623–1635. [Google Scholar] [CrossRef]
- Ubhi, B.K.; Cheng, K.K.; Dong, J.; Janowitz, T.; Jodrell, D.; Tal-Singer, R.; MacNee, W.; Lomas, D.A.; Riley, J.H.; Griffin, J.L.; et al. Targeted metabolomics identifies perturbations in amino acid metabolism that sub-classify patients with COPD. Mol. Biosyst. 2012, 8, 3125–3133. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Li, Y.; Liu, F.; Chen, L.; He, S.; Lin, F.; Wei, X.; Fang, Y.; Li, Q.; et al. Proteomics and metabolomics profiling reveal panels of circulating diagnostic biomarkers and molecular subtypes in stable COPD. Respir. Res. 2023, 24, 73. [Google Scholar] [CrossRef]
- Gregory, A.; Xu, Z.; Pratte, K.; Lee, S.; Liu, C.; Chase, R.; Yun, J.; Saferali, A.; Hersh, C.P.; Bowler, R.; et al. Clustering-based COPD subtypes have distinct longitudinal outcomes and multi-omics biomarkers. BMJ Open Respir. Res. 2022, 9, e001182. [Google Scholar] [CrossRef]
- Gandhi, R.; Kalsariya, V.; Katara, R.; Murugan, Y. Sarcopenia, Eosinophil-to-Platelet Ratio, and C-reactive Protein as Predictors of Adverse Events in Patients with Acute Exacerbations of Chronic Obstructive Pulmonary Disease: A Prospective Observational Study. Cureus 2024, 16, e56651. [Google Scholar] [CrossRef]
- Zhou, R.; Pan, D. Association between admission heart rate and in-hospital mortality in patients with acute exacerbation of chronic obstructive pulmonary disease and respiratory failure: A retrospective cohort study. BMC Pulm. Med. 2024, 24, 111. [Google Scholar] [CrossRef]
- Bhat, R.; Kamath, S.; Jain, A.; Acharya, V.; Antony, T.; Holla, R.; Jha, A. RV in COPD—The complicated matters of the heart—Correlation of ECHO and biomarker with COPD severity and outcome. Lung India 2024, 41, 192–199. [Google Scholar] [CrossRef]
- Zhou, W.-Q.; Song, X.; Dong, W.-H.; Chen, Z. Independent effect of the triglyceride-glucose index on all-cause mortality in critically ill patients with chronic obstructive pulmonary disease and asthma: A retrospective cohort study. Chronic Respir. Dis. 2024, 21. [Google Scholar] [CrossRef]
- Holm, A.M.; Andreassen, S.L.; Christensen, V.L.; Kongerud, J.; Almås, Ø.; Auråen, H.; Henriksen, A.H.; Aaberge, I.S.; Klingenberg, O.; Rustøen, T. Hypogammaglobulinemia and Risk of Exacerbation and Mortality in Patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 799–807. [Google Scholar] [CrossRef]
- Fortis, S.; Wan, E.S.; Kunisaki, K.; Eyck, P.T.; Ballas, Z.K.; Bowler, R.P.; Crapo, J.D.; Hokanson, J.E.; Wendt, C.; Silverman, E.K.; et al. Increased mortality associated with frequent exacerbations in COPD patients with mild-to-moderate lung function impairment, and smokers with normal spirometry. Respir. Med. X 2021, 3, 100025. [Google Scholar] [CrossRef]
- Song, W.; Li, D.; Tao, L.; Luo, Q.; Chen, L. Solute carrier transporters: The metabolic gatekeepers of immune cells. Acta Pharm. Sin. B 2020, 10, 61–78. [Google Scholar] [CrossRef]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I—Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Mandal, J.; Malla, B.; Steffensen, R.; Costa, L.; Egli, A.; Trendelenburg, M.; Blasi, F.; Kostikas, K.; Welte, T.; Torres, A.; et al. Man-nose-binding lectin protein and its association to clinical outcomes in COPD: A longitudinal study. Respir. Res. 2015, 16, 150. [Google Scholar] [CrossRef]
- Santos, N.C.D.; Miravitlles, M.; Camelier, A.A.; Almeida, V.D.C.; Maciel, R.R.B.T.; Camelier, F.W.R. Prevalence and Impact of Comor-bidities in Individuals with Chronic Obstructive Pulmonary Disease: A Systematic Review. Tuberc. Respir. Dis. 2022, 85, 205–220. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.; Chronis, C.; Papapetrou, E.; Tatsioni, A.; Gartzonika, K.; Tsaousi, C.; Gogali, A.; Katsanos, C.; Vaggeli, A.; Tselepi, C.; et al. Prothrombotic state in patients with stable COPD: An observational study. ERJ Open Res. 2021, 7. [Google Scholar] [CrossRef]
- Manon-Jensen, T.; Langholm, L.L.; Rønnow, S.R.; Karsdal, M.A.; Tal-Singer, R.; Vestbo, J.; Leeming, D.J.; Miller, B.E.; Bülow Sand, J.M. End-product of fibrinogen is elevated in emphysematous chronic obstructive pulmonary disease and is predictive of mortality in the ECLIPSE cohort. Respir. Med. 2019, 160, 105814. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Li, C.; Ma, C.; Ge, W. Proteome Profiling of Lung Tissues in Chronic Obstructive Pulmonary Disease (COPD): Platelet and Macrophage Dysfunction Contribute to the Pathogenesis of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 973–980. [Google Scholar] [CrossRef]
- Jankowski, M.; Undas, A.; Kaczmarek, P.; Butenas, S. Activated factor XI and tissue factor in chronic obstructive pulmonary disease: Links with inflammation and thrombin generation. Thromb. Res. 2011, 127, 242–246. [Google Scholar] [CrossRef]
- Mannino, D.M.; Tal-Singer, R.; Lomas, D.A.; Vestbo, J.; Barr, G.; Tetzlaff, K.; Lowings, M.; Rennard, S.I.; Snyder, J.; Goldman, M.; et al. Plasma Fibrinogen as a Biomarker for Mortality and Hospitalized Exacerbations in People with COPD. Chronic Obstr. Pulm. Dis. 2015, 2, 23–34. [Google Scholar] [CrossRef]
- Sand, J.M.B.; Rønnow, S.R.; Langholm, L.L.; Karsdal, M.A.; Manon-Jensen, T.; Tal-Singer, R.; Miller, B.E.; Vestbo, J.; Leeming, D.J. Combining biomarkers of clot resolution and alveolar basement membrane destruction predicts mortality in the ECLIPSE COPD cohort. Respir. Med. 2020, 173, 106185. [Google Scholar] [CrossRef]
- Langholm, L.L.; Rønnow, S.R.; Sand, J.M.B.; Leeming, D.J.; Tal-Singer, R.; Miller, B.E.; Vestbo, J.; Karsdal, M.A.; Manon-Jensen, T. Increased von Willebrand Factor Processing in COPD, Reflecting Lung Epithelium Damage, Is Associated with Emphysema, Exacer-bations and Elevated Mortality Risk. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 543–552. [Google Scholar] [CrossRef]
- Foti, M.; Locati, M. (Eds.) Cytokine Effector Functions in Tissues; Academic Press: Cambridge, MA, USA, 2017; ISBN 978-0-12-804214-4. [Google Scholar]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef]
- Costa, C.; Rufino, R.; Traves, S.L.; e Silva, J.R.L.; Barnes, P.J.; Donnelly, L.E. CXCR3 and CCR5 Chemokines in Induced Sputum from Patients with COPD. Chest 2008, 133, 26–33. [Google Scholar] [CrossRef]
- Peng, J.; Yu, Q.; Fan, S.; Chen, X.; Tang, R.; Wang, D.; Qi, D. High Blood Eosinophil and YKL-40 Levels, as Well as Low CXCL9 Levels, are Associated with Increased Readmission in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 795–806. [Google Scholar] [CrossRef]
- Kubysheva, N.I.; Postnikova, L.B.; Soodaeva, S.K.; Novikov, D.V.; Eliseeva, T.I.; Novikov, V.V.; Karaulov, A.V. Comparative Study of the Levels of IL-1β, IL-4, IL-8, TNFα, and IFNγ in Stable Course and Exacerbation of Chronic Obstructive Pulmonary Disease of Varying Severity. Bull. Exp. Biol. Med. 2022, 173, 745–748. [Google Scholar] [CrossRef]
- Di Francia, M.; Barbier, D.; Mege, J.L.; Orehek, J. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pul-monary disease. Am. J. Respir. Crit. Care Med. 1994, 150, 1453–1455. [Google Scholar] [CrossRef]
- Schols, A.M.; Buurman, W.A.; Van den Brekel, A.S.; Dentener, M.A.; Wouters, E.F. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax 1996, 51, 819–824. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Herting, J.R.; Pike, K.C.; Gharib, S.A.; Matute-Bello, G.; Borson, S.; Kohen, R.; Adams, S.G.; Fan, V.S. Symptom profiles and inflammatory markers in moderate to severe COPD. BMC Pulm. Med. 2016, 16, 173. [Google Scholar] [CrossRef]
- Mendy, A.; Forno, E.; Niyonsenga, T.; Gasana, J. Blood biomarkers as predictors of long-term mortality in COPD. Clin. Respir. J. 2018, 12, 1891–1899. [Google Scholar] [CrossRef]
- Dahl, M.; Vestbo, J.; Lange, P.; Bojesen, S.E.; Tybjærg-Hansen, A.; Nordestgaard, B.G. C-reactive Protein as a Predictor of Prognosis in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2007, 175, 250–255. [Google Scholar] [CrossRef]
- Kelly, E.; Owen, C.A.; Pinto-Plata, V.; Celli, B.R. The role of systemic inflammatory biomarkers to predict mortality in chronic obstructive pulmonary disease. Expert. Rev. Respir. Med. 2013, 7, 57–64. [Google Scholar] [CrossRef]
- Gea, J.; Sancho-Muñoz, A.; Chalela, R. Nutritional status and muscle dysfunction in chronic respiratory diseases: Stable phase versus acute exacerbations. J. Thorac. Dis. 2018, 10, S1332–S1354. [Google Scholar] [CrossRef]
- Marquis, K.; Debigaré, R.; Lacasse, Y.; LeBlanc, P.; Jobin, J.; Carrier, G.; Maltais, F. Midthigh Muscle Cross-Sectional Area Is a Better Predictor of Mortality than Body Mass Index in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2002, 166, 809–813. [Google Scholar] [CrossRef]
- Plante, E.; Vance, R. Selection of preschool language tests: A data-based approach. Language, Speech, and Hearing Services in Schools 1994, 25, 15–24. [Google Scholar] [CrossRef]
- Owusuaa, C.; Dijkland, S.A.; Nieboer, D.; van der Rijt, C.C.D.; van der Heide, A. Predictors of mortality in chronic obstructive pulmonary disease: A systematic review and meta-analysis. BMC Pulm. Med. 2022, 22, 125. [Google Scholar] [CrossRef]
- White, N.; Kupeli, N.; Vickerstaff, V.; Stone, P. How accurate is the ‘Surprise Question’ at identifying patients at the end of life? A systematic review and meta-analysis. BMC Med. 2017, 15, 139. [Google Scholar] [CrossRef]
- Aryal, S.; Diaz-Guzman, E.; Mannino, D.M. Influence of sex on chronic obstructive pulmonary disease risk and treatment out-comes. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 1145–1154. [Google Scholar]
- Ntritsos, G.; Franek, J.; Belbasis, L.; Christou, M.A.; Markozannes, G.; Altman, P.; Fogel, R.; Sayre, T.; Ntzani, E.E.; Evangelou, E. Gen-der-specific estimates of COPD prevalence: A systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 1507–1514. [Google Scholar] [CrossRef]
- Drummond, M.B.; Wise, R.A.; Hansel, N.N.; Putcha, N. Comorbidities and Chronic Obstructive Pulmonary Disease: Prevalence, Influence on Outcomes, and Management. Semin. Respir. Crit. Care Med. 2015, 36, 575–591. [Google Scholar] [CrossRef]
- Bai, S.; Ye, R.; Wang, C.; Sun, P.; Wang, D.; Yue, Y.; Wang, H.; Wu, S.; Yu, M.; Xi, S.; et al. Identification of Proteomic Signatures in Chronic Obstructive Pulmonary Disease Emphysematous Phenotype. Front. Mol. Biosci. 2021, 8, 650604. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Enríquez-Rodríguez, C.J.; Pascual-Guardia, S. Metabolomics in COPD. Arch. Bronconeumol. 2023, 59, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Casadevall, C.; Nebot, P.; Enríquez-Rodríguez, C.J.; Faner, R.; Cosio, B.G.; Haro, N.; Pascual-Guardia, S.; Peces-Barba, G.; Monsó, E.; et al. Aging and metabolic changes in COPD patients. Am. J. Respir. Crit. Care Med. 2004, 209, A4314. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).