The Formation and Renewal of Photoreceptor Outer Segments
Abstract
:1. Introduction
2. Photoreceptor OS
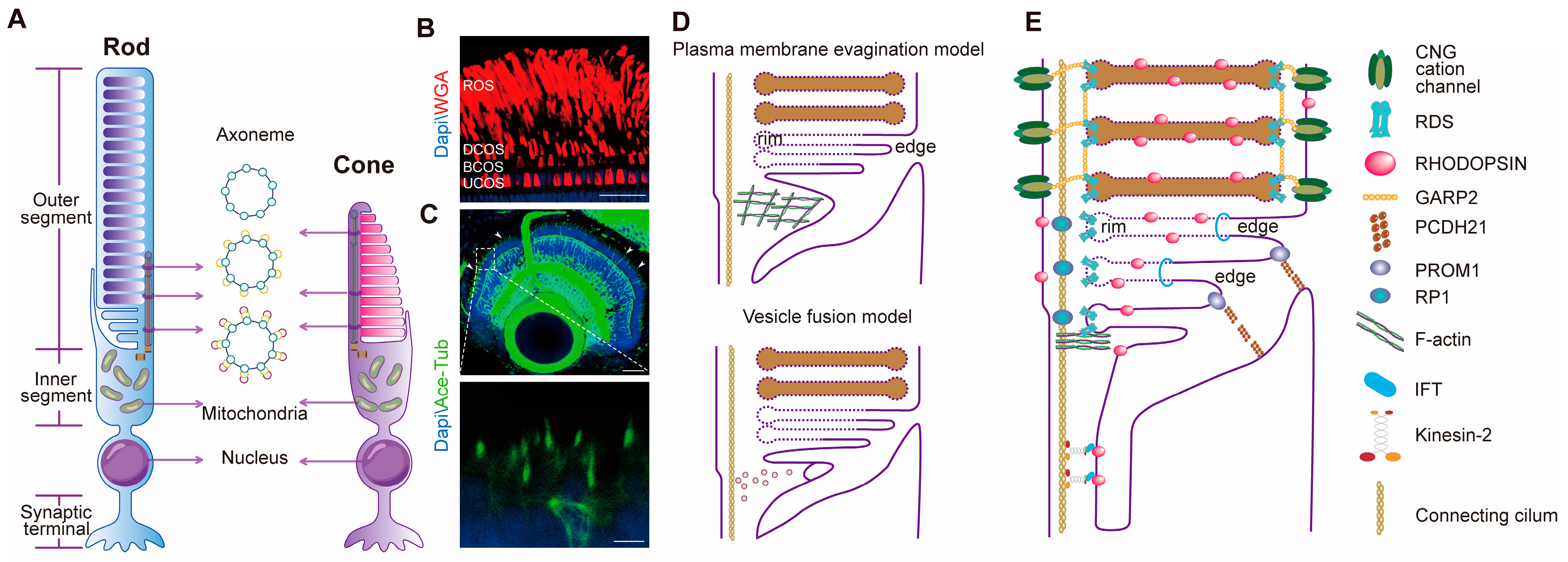
2.1. Structure and Differences between Rod and Cone OSs
2.2. Morphogenesis and Molecular Basis of Photoreceptor OSs
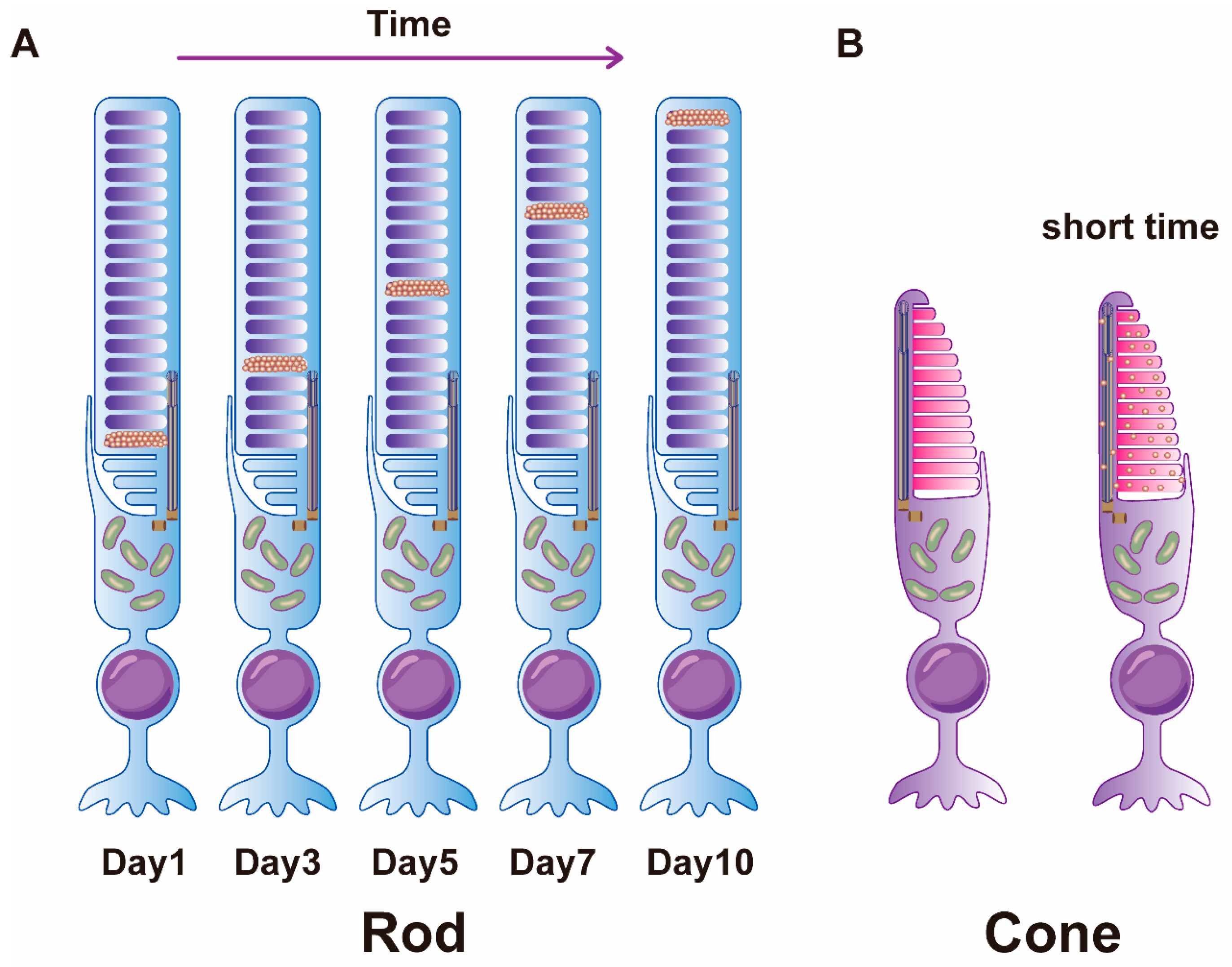
2.2.1. Morphogenesis of Photoreceptor OS Discs
2.2.2. Connecting Cilia
2.2.3. Essential Proteins Constitute the Structure of Photoreceptor OSs
3. Photoreceptor OS Renewal
3.1. The Discovery of OS Renewal
3.2. Regulation of OS Renewal by Light and Circadian Rhythms
3.3. Molecular Mechanisms of Photoreceptor OS Renewal
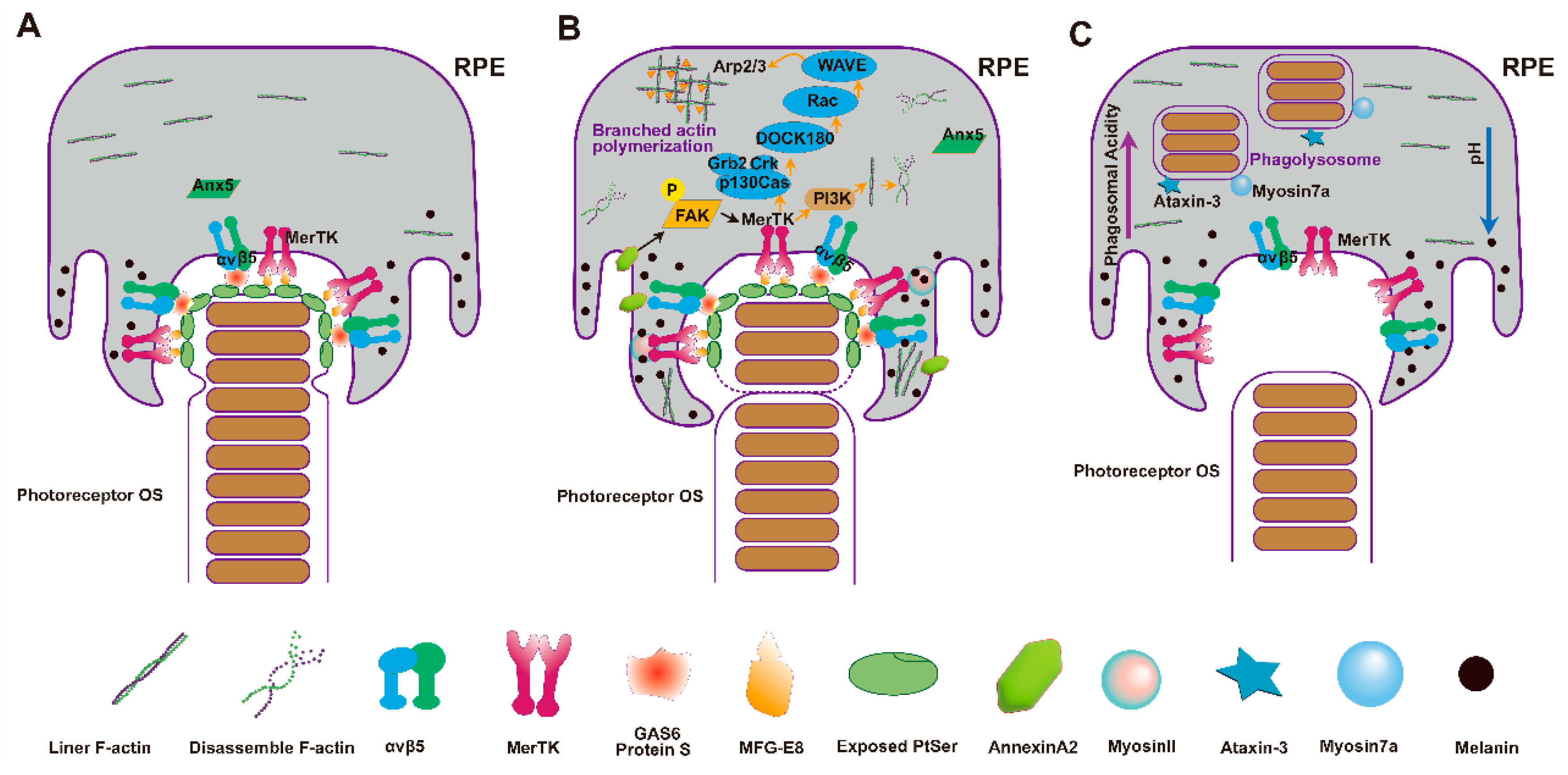
3.3.1. Recognition and Binding of RPE to OSs
3.3.2. Phagocytosis and Internalization
3.3.3. Degradation of Phagosomes in RPE Cells
3.3.4. Regulation of OS Renewal by Proteins in Photoreceptor Cells
3.4. Retinal Diseases Related with RPE Phagocytic Defects
4. Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Pearring, J.N.; Salinas, R.Y.; Baker, S.A.; Arshavsky, V.Y. Protein sorting, targeting and trafficking in photoreceptor cells. Prog. Retin. Eye Res. 2013, 36, 24–51. [Google Scholar] [CrossRef]
- Khanna, H. Photoreceptor Sensory Cilium: Traversing the Ciliary Gate. Cells 2015, 4, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Kevany, B.M.; Palczewski, K. Phagocytosis of retinal rod and cone photoreceptors. Physiology 2010, 25, 8–15. [Google Scholar] [CrossRef]
- Calvert, P.D.; Strissel, K.J.; Schiesser, W.E.; Pugh, E.N.; Arshavsky, V.Y. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006, 16, 560–568. [Google Scholar] [CrossRef]
- De Robertis, E. Some observations on the ultrastructure and morphogenesis of photoreceptors. J. Gen. Physiol. 1960, 43, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Roehlecke, C.; Schumann, U.; Ader, M.; Brunssen, C.; Bramke, S.; Morawietz, H.; Funk, R.H. Stress reaction in outer segments of photoreceptors after blue light irradiation. PLoS ONE 2013, 8, e71570. [Google Scholar] [CrossRef]
- Sung, C.H.; Chuang, J.Z. The cell biology of vision. J. Cell Biol. 2010, 190, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Young, R.W. The renewal of photoreceptor cell outer segments. J. Cell Biol. 1967, 33, 61–72. [Google Scholar] [CrossRef]
- Carter-Dawson, L.D.; LaVail, M.M. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J. Comp. Neurol. 1979, 188, 245–262. [Google Scholar] [CrossRef]
- Gilliam, J.C.; Chang, J.T.; Sandoval, I.M.; Zhang, Y.; Li, T.; Pittler, S.J.; Chiu, W.; Wensel, T.G. Three-Dimensional Architecture of the Rod Sensory Cilium and Its Disruption in Retinal Neurodegeneration. Cell 2012, 151, 1029–1041. [Google Scholar] [CrossRef]
- May-Simera, H.; Nagel-Wolfrum, K.; Wolfrum, U. Cilia—The sensory antennae in the eye. Prog. Retin. Eye Res. 2017, 60, 144–180. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.H.; Fisher, S.K.; Steinberg, R.H. Mammalian cones: Disc shedding, phagocytosis, and renewal. Investig. Ophthalmol. Vis. Sci. 1978, 17, 117–133. [Google Scholar]
- Cohen, A.I. The fine structure of the extrafoveal receptors of the Rhesus monkey. Exp. Eye Res. 1961, 1, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.I. Further studies on the question of the patency of saccules in outer segments of vertebrate photoreceptors. Vis. Res. 1970, 10, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, T.; Meschede, I.P.; Burden, J.J.; Bailly, M.; Seabra, M.C.; Futter, C.E. Rod disc renewal occurs by evagination of the ciliary plasma membrane that makes cadherin-based contacts with the inner segment. Proc. Natl. Acad. Sci. USA 2015, 112, 15922–15927. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.D.; Salinas, R.Y.; Arshavsky, V.Y. Discs of mammalian rod photoreceptors form through the membrane evagination mechanism. J. Cell Biol. 2015, 211, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Laties, A.M.; Bok, D.; Liebman, P. Procion yellow: A marker dye for outer segment disc patency and for rod renewal. Exp. Eye Res. 1976, 23, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, B.; Besharse, J.C. Light and temperature modulated staining of the rod outer segment distal tips with Lucifer yellow. Investig. Ophthalmol. Vis. Sci. 1985, 26, 628–635. [Google Scholar]
- Volland, S.; Hughes, L.C.; Kong, C.; Burgess, B.L.; Linberg, K.A.; Luna, G.; Zhou, Z.H.; Fisher, S.K.; Williams, D.S. Three-dimensional organization of nascent rod outer segment disk membranes. Proc. Natl. Acad. Sci. USA 2015, 112, 14870–14875. [Google Scholar] [CrossRef]
- Muresan, V.; Joshi, H.C.; Besharse, J.C. Gamma-tubulin in differentiated cell types: Localization in the vicinity of basal bodies in retinal photoreceptors and ciliated epithelia. J. Cell Sci. 1993, 104 Pt 4, 1229–1237. [Google Scholar] [CrossRef]
- Knabe, W.; Kuhn, H.J. Ciliogenesis in photoreceptor cells of the tree shrew retina. Anat. Embryol. 1997, 196, 123–131. [Google Scholar] [CrossRef]
- Fisch, C.; Dupuis-Williams, P. Ultrastructure of cilia and flagella—Back to the future! Biol. Cell 2011, 103, 249–270. [Google Scholar] [CrossRef]
- Sale, W.S.; Besharse, J.C.; Piperno, G. Distribution of acetylated alpha-tubulin in retina and in vitro-assembled microtubules. Cell Motil. Cytoskelet. 1988, 9, 243–253. [Google Scholar] [CrossRef]
- Bader, J.R.; Kusik, B.W.; Besharse, J.C. Analysis of KIF17 distal tip trafficking in zebrafish cone photoreceptors. Vis. Res. 2012, 75, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.F.; Moritz, O.L.; Williams, D.S. Molecular basis for photoreceptor outer segment architecture. Prog. Retin. Eye Res. 2016, 55, 52–81. [Google Scholar] [CrossRef]
- Chuang, J.-Z.; Zhao, Y.; Sung, C.-H. SARA-Regulated Vesicular Targeting Underlies Formation of the Light-Sensing Organelle in Mammalian Rods. Cell 2007, 130, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, R.H.; Fisher, S.K.; Anderson, D.H. Disc morphogenesis in vertebrate photoreceptors. J. Comp. Neurol. 1980, 190, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Kinney, M.S.; Fisher, S.K. The photoreceptors and pigment epithelium of the larval Xenopus retina: Morphogenesis and outer segment renewal. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1978, 201, 149–167. [Google Scholar] [CrossRef]
- Williams, D.S.; Linberg, K.A.; Vaughan, D.K.; Fariss, R.N.; Fisher, S.K. Disruption of microfilament organization and deregulation of disk membrane morphogenesis by cytochalasin D in rod and cone photoreceptors. J. Comp. Neurol. 1988, 272, 161–176. [Google Scholar] [CrossRef]
- Hale, I.L.; Fisher, S.K.; Matsumoto, B. The actin network in the ciliary stalk of photoreceptors functions in the generation of new outer segment discs. J. Comp. Neurol. 1996, 376, 128–142. [Google Scholar] [CrossRef]
- Spencer, W.J.; Ding, J.D.; Lewis, T.R.; Yu, C.; Phan, S.; Pearring, J.N.; Kim, K.Y.; Thor, A.; Mathew, R.; Kalnitsky, J.; et al. PRCD is essential for high-fidelity photoreceptor disc formation. Proc. Natl. Acad. Sci. USA 2019, 116, 13087–13096. [Google Scholar] [CrossRef] [PubMed]
- Spencer, W.J.; Lewis, T.R.; Pearring, J.N.; Arshavsky, V.Y. Photoreceptor Discs: Built Like Ectosomes. Trends Cell Biol. 2020, 30, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Kevany, B.M.; Tsybovsky, Y.; Campuzano, I.D.; Schnier, P.D.; Engel, A.; Palczewski, K. Structural and functional analysis of the native peripherin-ROM1 complex isolated from photoreceptor cells. J. Biol. Chem. 2013, 288, 36272–36284. [Google Scholar] [CrossRef] [PubMed]
- Milstein, M.L.; Cavanaugh, B.L.; Roussey, N.M.; Volland, S.; Williams, D.S.; Goldberg, A.F.X. Multistep peripherin-2/rds self-assembly drives membrane curvature for outer segment disk architecture and photoreceptor viability. Proc. Natl. Acad. Sci. USA 2020, 117, 4400–4410. [Google Scholar] [CrossRef] [PubMed]
- Bujakowska, K.M.; Liu, Q.; Pierce, E.A. Photoreceptor Cilia and Retinal Ciliopathies. Cold Spring Harb. Perspect. Biol. 2017, 9, a028274. [Google Scholar] [CrossRef]
- Cole, D.G.; Diener, D.R.; Himelblau, A.L.; Beech, P.L.; Fuster, J.C.; Rosenbaum, J.L. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 1998, 141, 993–1008. [Google Scholar] [CrossRef] [PubMed]
- Pazour, G.J.; Baker, S.A.; Deane, J.A.; Cole, D.G.; Dickert, B.L.; Rosenbaum, J.L.; Witman, G.B.; Besharse, J.C. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J. Cell Biol. 2002, 157, 103–113. [Google Scholar] [CrossRef]
- Tsujikawa, M.; Malicki, J. Intraflagellar Transport Genes Are Essential for Differentiation and Survival of Vertebrate Sensory Neurons. Neuron 2004, 42, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Krock, B.L.; Perkins, B.D. The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J. Cell Sci. 2008, 121, 1907–1915. [Google Scholar] [CrossRef]
- Jimeno, D.; Lillo, C.; Roberts, E.A.; Goldstein, L.S.B.; Williams, D.S. Kinesin-2 and photoreceptor cell death: Requirement of motor subunits. Exp. Eye Res. 2006, 82, 351–353. [Google Scholar] [CrossRef]
- Marszalek, J.R.; Liu, X.; Roberts, E.A.; Chui, D.; Marth, J.D.; Williams, D.S.; Goldstein, L.S.B. Genetic Evidence for Selective Transport of Opsin and Arrestin by Kinesin-II in Mammalian Photoreceptors. Cell 2000, 102, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Omori, Y.; Brodowska, K.; Kovach, P.; Malicki, J. Kinesin-2 family in vertebrate ciliogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 2388–2393. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, L.; Palczewski, K. The G protein-coupled receptor rhodopsin: A historical perspective. Methods Mol. Biol. 2015, 1271, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Liebman, P.A.; Entine, G. Visual pigments of frog and tadpole (Rana pipiens). Vis. Res. 1968, 8, 761–775. [Google Scholar] [CrossRef]
- Nathans, J. Rhodopsin: Structure, function, and genetics. Biochemistry 1992, 31, 4923–4931. [Google Scholar] [CrossRef]
- Humphries, M.M.; Rancourt, D.; Farrar, G.J.; Kenna, P.; Hazel, M.; Bush, R.A.; Sieving, P.A.; Sheils, D.M.; Creighton, P.; Erven, A.; et al. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat. Genet. 1997, 15, 216–219. [Google Scholar] [CrossRef]
- Malanson, K.M.; Lem, J. Rhodopsin-mediated retinitis pigmentosa. Prog. Mol. Biol. Transl. Sci. 2009, 88, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jin, N.; Chuang, J.Z.; Zhang, Z.; Zhong, X.; Zhang, Z.; Sung, C.H.; Ribelayga, C.P.; Fu, Y. Visual pigment-deficient cones survive and mediate visual signaling despite the lack of outer segments. Proc. Natl. Acad. Sci. USA 2022, 119, e2115138119. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.F. Role of peripherin/rds in vertebrate photoreceptor architecture and inherited retinal degenerations. Int. Rev. Cytol. 2006, 253, 131–175. [Google Scholar] [CrossRef]
- Khattree, N.; Ritter, L.M.; Goldberg, A.F. Membrane curvature generation by a C-terminal amphipathic helix in peripherin-2/rds, a tetraspanin required for photoreceptor sensory cilium morphogenesis. J. Cell Sci. 2013, 126, 4659–4670. [Google Scholar] [CrossRef]
- van Nie, R.; Iványi, D.; Démant, P. A new H-2-linked mutation, rds, causing retinal degeneration in the mouse. Tissue Antigens 1978, 12, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.K.; Jansen, H.G.; Sanyal, S. Development and degeneration of retina in rds mutant mice: Photoreceptor abnormalities in the heterozygotes. Exp. Eye Res. 1985, 41, 701–720. [Google Scholar] [CrossRef] [PubMed]
- Stuck, M.W.; Conley, S.M.; Naash, M.I. PRPH2/RDS and ROM-1: Historical context, current views and future considerations. Prog. Retin. Eye Res. 2016, 52, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Khan, M.; Rooijakkers, A.; Mulders, T.; Haer-Wigman, L.; Boon, C.J.F.; Klaver, C.C.W.; van den Born, L.I.; Hoyng, C.B.; Cremers, F.P.M.; et al. PRPH2 mutation update: In silico assessment of 245 reported and 7 novel variants in patients with retinal disease. Hum. Mutat. 2021, 42, 1521–1547. [Google Scholar] [CrossRef] [PubMed]
- Bascom, R.A.; Manara, S.; Collins, L.; Molday, R.S.; Kalnins, V.I.; McInnes, R.R. Cloning of the cDNA for a novel photoreceptor membrane protein (rom-1) identifies a disk rim protein family implicated in human retinopathies. Neuron 1992, 8, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, W.; Weng, J.; Travis, G.H. Analysis of the rds/peripherin.rom1 complex in transgenic photoreceptors that express a chimeric protein. J. Biol. Chem. 1999, 274, 29181–29187. [Google Scholar] [CrossRef] [PubMed]
- Loewen, C.J.; Molday, R.S. Disulfide-mediated oligomerization of Peripherin/Rds and Rom-1 in photoreceptor disk membranes. Implications for photoreceptor outer segment morphogenesis and degeneration. J. Biol. Chem. 2000, 275, 5370–5378. [Google Scholar] [CrossRef]
- Clarke, G.; Goldberg, A.F.; Vidgen, D.; Collins, L.; Ploder, L.; Schwarz, L.; Molday, L.L.; Rossant, J.; Szél, A.; Molday, R.S.; et al. Rom-1 is required for rod photoreceptor viability and the regulation of disk morphogenesis. Nat. Genet. 2000, 25, 67–73. [Google Scholar] [CrossRef]
- Michalakis, S.; Becirovic, E.; Biel, M. Retinal Cyclic Nucleotide-Gated Channels: From Pathophysiology to Therapy. Int. J. Mol. Sci. 2018, 19, 749. [Google Scholar] [CrossRef]
- Kaupp, U.B.; Seifert, R. Cyclic nucleotide-gated ion channels. Physiol. Rev. 2002, 82, 769–824. [Google Scholar] [CrossRef]
- Ritter, L.M.; Khattree, N.; Tam, B.; Moritz, O.L.; Schmitz, F.; Goldberg, A.F. In situ visualization of protein interactions in sensory neurons: Glutamic acid-rich proteins (GARPs) play differential roles for photoreceptor outer segment scaffolding. J. Neurosci. 2011, 31, 11231–11243. [Google Scholar] [CrossRef] [PubMed]
- Colville, C.A.; Molday, R.S. Primary structure and expression of the human beta-subunit and related proteins of the rod photoreceptor cGMP-gated channel. J. Biol. Chem. 1996, 271, 32968–32974. [Google Scholar] [CrossRef]
- Batra-Safferling, R.; Abarca-Heidemann, K.; Körschen, H.G.; Tziatzios, C.; Stoldt, M.; Budyak, I.; Willbold, D.; Schwalbe, H.; Klein-Seetharaman, J.; Kaupp, U.B. Glutamic Acid-rich Proteins of Rod Photoreceptors Are Natively Unfolded. J. Biol. Chem. 2006, 281, 1449–1460. [Google Scholar] [CrossRef]
- Röper, K.; Corbeil, D.; Huttner, W.B. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat. Cell Biol. 2000, 2, 582–592. [Google Scholar] [CrossRef]
- Han, Z.; Anderson, D.W.; Papermaster, D.S. Prominin-1 localizes to the open rims of outer segment lamellae in Xenopus laevis rod and cone photoreceptors. Investig. Opthalmology Vis. Sci. 2012, 53, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, Y.; Lillo, C.; Chien, J.; Yu, Z.; Michaelides, M.; Klein, M.; Howes, K.A.; Li, Y.; Kaminoh, Y.; et al. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J. Clin. Investig. 2008, 118, 2908–2916. [Google Scholar] [CrossRef]
- Lu, Z.; Hu, X.; Reilly, J.; Jia, D.; Liu, F.; Yu, S.; Liu, X.; Xie, S.; Qu, Z.; Qin, Y.; et al. Deletion of the transmembrane protein Prom1b in zebrafish disrupts outer-segment morphogenesis and causes photoreceptor degeneration. J. Biol. Chem. 2019, 294, 13953–13963. [Google Scholar] [CrossRef] [PubMed]
- Sotomayor, M.; Gaudet, R.; Corey, D.P. Sorting out a promiscuous superfamily: Towards cadherin connectomics. Trends Cell Biol. 2014, 24, 524–536. [Google Scholar] [CrossRef]
- Rattner, A.; Chen, J.; Nathans, J. Proteolytic shedding of the extracellular domain of photoreceptor cadherin. Implications for outer segment assembly. J. Biol. Chem. 2004, 279, 42202–42210. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, J.; Daiger, S.P.; Farber, D.B.; Heckenlively, J.R.; Smith, J.E.; Sullivan, L.S.; Zuo, J.; Milam, A.H.; Pierce, E.A. Identification and subcellular localization of the RP1 protein in human and mouse photoreceptors. Investig. Ophthalmol. Vis. Sci. 2002, 43, 22–32. [Google Scholar]
- Liu, Q.; Zuo, J.; Pierce, E.A. The retinitis pigmentosa 1 protein is a photoreceptor microtubule-associated protein. J. Neurosci. 2004, 24, 6427–6436. [Google Scholar] [CrossRef]
- Bowne, S.J.; Daiger, S.P.; Hims, M.M.; Sohocki, M.M.; Malone, K.A.; McKie, A.B.; Heckenlively, J.R.; Birch, D.G.; Inglehearn, C.F.; Bhattacharya, S.S.; et al. Mutations in the RP1 gene causing autosomal dominant retinitis pigmentosa. Hum. Mol. Genet. 1999, 8, 2121–2128. [Google Scholar] [CrossRef]
- Daiger, S.P.; Sullivan, L.S.; Gire, A.I.; Birch, D.G.; Heckenlively, J.R.; Bowne, S.J. Mutations in known genes account for 58% of autosomal dominant retinitis pigmentosa (adRP). Adv. Exp. Med. Biol. 2008, 613, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Sharkova, M.; Aparicio, G.; Mouzaaber, C.; Zolessi, F.R.; Hocking, J.C. Photoreceptor calyceal processes accompany the developing outer segment, adopting a stable length despite a dynamic core. J. Cell Sci. 2024, 137, jcs261721. [Google Scholar] [CrossRef]
- Nambiar, R.; McConnell, R.E.; Tyska, M.J. Myosin motor function: The ins and outs of actin-based membrane protrusions. Cell. Mol. Life Sci. 2010, 67, 1239–1254. [Google Scholar] [CrossRef] [PubMed]
- Schietroma, C.; Parain, K.; Estivalet, A.; Aghaie, A.; Boutet de Monvel, J.; Picaud, S.; Sahel, J.A.; Perron, M.; El-Amraoui, A.; Petit, C. Usher syndrome type 1-associated cadherins shape the photoreceptor outer segment. J. Cell Biol. 2017, 216, 1849–1864. [Google Scholar] [CrossRef]
- Hodel, C.; Niklaus, S.; Heidemann, M.; Klooster, J.; Kamermans, M.; Biehlmaier, O.; Gesemann, M.; Neuhauss, S.C. Myosin VIIA is a marker for the cone accessory outer segment in zebrafish. Anat. Rec. 2014, 297, 1777–1784. [Google Scholar] [CrossRef]
- Glover, G.; Mueller, K.P.; Söllner, C.; Neuhauss, S.C.; Nicolson, T. The Usher gene cadherin 23 is expressed in the zebrafish brain and a subset of retinal amacrine cells. Mol. Vis. 2012, 18, 2309–2322. [Google Scholar] [PubMed]
- Young, R.W.; Bok, D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J. Cell Biol. 1969, 42, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Young, R.W. The renewal of rod and cone outer segments in the rhesus monkey. J. Cell Biol. 1971, 49, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Young, R.W. Shedding of discs from rod outer segments in the rhesus monkey. J. Ultrastruct. Res. 1971, 34, 190–203. [Google Scholar] [CrossRef]
- Lakkaraju, A.; Umapathy, A.; Tan, L.X.; Daniele, L.; Philp, N.J.; Boesze-Battaglia, K.; Williams, D.S. The cell biology of the retinal pigment epithelium. Prog. Retin. Eye Res. 2020, 78, 100846. [Google Scholar] [CrossRef]
- Young, R.W. A difference between rods and cones in the renewal of outer segment protein. Investig. Ophthalmol. 1969, 8, 222–231. [Google Scholar]
- Young, R.W.; Droz, B. The renewal of protein in retinal rods and cones. J. Cell Biol. 1968, 39, 169–184. [Google Scholar] [CrossRef]
- Basinger, S.; Hoffman, R.; Matthes, M. Photoreceptor shedding is initiated by light in the frog retina. Science 1976, 194, 1074–1076. [Google Scholar] [CrossRef] [PubMed]
- LaVail, M.M. Rod outer segment disk shedding in rat retina: Relationship to cyclic lighting. Science 1976, 194, 1071–1074. [Google Scholar] [CrossRef]
- Vargas, J.A.; Finnemann, S.C. Probing Photoreceptor Outer Segment Phagocytosis by the RPE In Vivo: Models and Methodologies. Int. J. Mol. Sci. 2022, 23, 3661. [Google Scholar] [CrossRef]
- LaVail, M.M. Circadian nature of rod outer segment disc shedding in the rat. Investig. Ophthalmol. Vis. Sci. 1980, 19, 407–411. [Google Scholar]
- Cassone, V.M. Avian circadian organization: A chorus of clocks. Front. Neuroendocrinol. 2014, 35, 76–88. [Google Scholar] [CrossRef]
- Li, L. Circadian Vision in Zebrafish: From Molecule to Cell and from Neural Network to Behavior. J. Biol. Rhythm. 2019, 34, 451–462. [Google Scholar] [CrossRef]
- Abe, M.; Herzog, E.D.; Yamazaki, S.; Straume, M.; Tei, H.; Sakaki, Y.; Menaker, M.; Block, G.D. Circadian rhythms in isolated brain regions. J. Neurosci. 2002, 22, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Bhoi, J.D.; Goel, M.; Ribelayga, C.P.; Mangel, S.C. Circadian clock organization in the retina: From clock components to rod and cone pathways and visual function. Prog. Retin. Eye Res. 2023, 94, 101119. [Google Scholar] [CrossRef]
- Grace, M.S.; Chiba, A.; Menaker, M. Circadian control of photoreceptor outer segment membrane turnover in mice genetically incapable of melatonin synthesis. Vis. Neurosci. 1999, 16, 909–918. [Google Scholar] [CrossRef]
- DeVera, C.; Dixon, J.; Chrenek, M.A.; Baba, K.; Le, Y.Z.; Iuvone, P.M.; Tosini, G. The Circadian Clock in the Retinal Pigment Epithelium Controls the Diurnal Rhythm of Phagocytic Activity. Int. J. Mol. Sci. 2022, 23, 5302. [Google Scholar] [CrossRef]
- Milićević, N.; Hakkari, O.A.-H.; Bagchi, U.; Sandu, C.; Jongejan, A.; Moerland, P.D.; Ten Brink, J.B.; Hicks, D.; Bergen, A.A.; Felder-Schmittbuhl, M.-P. Core circadian clock genes Per1 and Per2 regulate the rhythm in photoreceptor outer segment phagocytosis. FASEB J. 2021, 35, e21722. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.L.; Fehilly, J.D.; Floss Jones, D.; Collery, R.; Kennedy, B.N. Regulation of the rhythmic diversity of daily photoreceptor outer segment phagocytosis in vivo. FASEB J. 2022, 36, e22556. [Google Scholar] [CrossRef]
- Fujimoto, T.; Parmryd, I. Interleaflet Coupling, Pinning, and Leaflet Asymmetry-Major Players in Plasma Membrane Nanodomain Formation. Front. Cell Dev. Biol. 2016, 4, 155. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Quinn, P.; Xue, D.; Kagan, V. Fat(al) attraction: Oxidized lipids act as “eat-me” signals. HFSP J. 2007, 1, 225–229. [Google Scholar] [CrossRef]
- Ruggiero, L.; Connor, M.P.; Chen, J.; Langen, R.; Finnemann, S.C. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5-/- or Mfge8-/- mouse retina. Proc. Natl. Acad. Sci. USA 2012, 109, 8145–8148. [Google Scholar] [CrossRef]
- Nandrot, E.F.; Kim, Y.; Brodie, S.E.; Huang, X.; Sheppard, D.; Finnemann, S.C. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J. Exp. Med. 2004, 200, 1539–1545. [Google Scholar] [CrossRef]
- Mazzoni, F.; Safa, H.; Finnemann, S.C. Understanding photoreceptor outer segment phagocytosis: Use and utility of RPE cells in culture. Exp. Eye Res. 2014, 126, 51–60. [Google Scholar] [CrossRef]
- Yu, C.; Muñoz, L.E.; Mallavarapu, M.; Herrmann, M.; Finnemann, S.C. Annexin A5 regulates surface αvβ5 integrin for retinal clearance phagocytosis. J. Cell Sci. 2019, 132, jcs232439. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Freeman, S.A. Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling. Front. Immunol. 2020, 11, 604205. [Google Scholar] [CrossRef]
- Lieffrig, S.A.; Gyimesi, G.; Mao, Y.; Finnemann, S.C. Clearance phagocytosis by the retinal pigment epithelial during photoreceptor outer segment renewal: Molecular mechanisms and relation to retinal inflammation. Immunol. Rev. 2023, 319, 81–99. [Google Scholar] [CrossRef]
- Finnemann, S.C. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003, 22, 4143–4154. [Google Scholar] [CrossRef] [PubMed]
- Ilić, D.; Furuta, Y.; Kanazawa, S.; Takeda, N.; Sobue, K.; Nakatsuji, N.; Nomura, S.; Fujimoto, J.; Okada, M.; Yamamoto, T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 1995, 377, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Maa, M.C.; Leu, T.H. Vanadate-dependent FAK activation is accomplished by the sustained FAK Tyr-576/577 phosphorylation. Biochem. Biophys. Res. Commun. 1998, 251, 344–349. [Google Scholar] [CrossRef]
- Sieg, D.J.; Hauck, C.R.; Schlaepfer, D.D. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 1999, 112 Pt 16, 2677–2691. [Google Scholar] [CrossRef]
- Prasad, D.; Rothlin, C.V.; Burrola, P.; Burstyn-Cohen, T.; Lu, Q.; Garcia de Frutos, P.; Lemke, G. TAM receptor function in the retinal pigment epithelium. Mol. Cell. Neurosci. 2006, 33, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Bok, D.; Hall, M.O. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J. Cell Biol. 1971, 49, 664–682. [Google Scholar] [CrossRef]
- D’Cruz, P.M.; Yasumura, D.; Weir, J.; Matthes, M.T.; Abderrahim, H.; LaVail, M.M.; Vollrath, D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000, 9, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.O.; Obin, M.S.; Heeb, M.J.; Burgess, B.L.; Abrams, T.A. Both protein S and Gas6 stimulate outer segment phagocytosis by cultured rat retinal pigment epithelial cells. Exp. Eye Res. 2005, 81, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Singh, S.; Georgescu, M.M.; Birge, R.B. A role for Mer tyrosine kinase in alphavbeta5 integrin-mediated phagocytosis of apoptotic cells. J. Cell Sci. 2005, 118, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Law, A.L.; Ling, Q.; Hajjar, K.A.; Futter, C.E.; Greenwood, J.; Adamson, P.; Wavre-Shapton, S.T.; Moss, S.E.; Hayes, M.J. Annexin A2 regulates phagocytosis of photoreceptor outer segments in the mouse retina. Mol. Biol. Cell 2009, 20, 3896–3904. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.A.; Johnson, M.T.; Beningo, K.; Post, P.; Mooseker, M.; Araki, N. A contractile activity that closes phagosomes in macrophages. J. Cell Sci. 1999, 112 Pt 3, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Strick, D.J.; Feng, W.; Vollrath, D. Mertk drives myosin II redistribution during retinal pigment epithelial phagocytosis. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Nuckels, R.J.; Ng, A.; Darland, T.; Gross, J.M. The vacuolar-ATPase complex regulates retinoblast proliferation and survival, photoreceptor morphogenesis, and pigmentation in the zebrafish eye. Investig. Ophthalmol. Vis. Sci. 2009, 50, 893–905. [Google Scholar] [CrossRef]
- Wavre-Shapton, S.T.; Meschede, I.P.; Seabra, M.C.; Futter, C.E. Phagosome maturation during endosome interaction revealed by partial rhodopsin processing in retinal pigment epithelium. J. Cell Sci. 2014, 127, 3852–3861. [Google Scholar] [CrossRef]
- Deguchi, J.; Yamamoto, A.; Yoshimori, T.; Sugasawa, K.; Moriyama, Y.; Futai, M.; Suzuki, T.; Kato, K.; Uyama, M.; Tashiro, Y. Acidification of phagosomes and degradation of rod outer segments in rat retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1994, 35, 568–579. [Google Scholar]
- Zaidi, N.; Maurer, A.; Nieke, S.; Kalbacher, H. Cathepsin D: A cellular roadmap. Biochem. Biophys. Res. Commun. 2008, 376, 5–9. [Google Scholar] [CrossRef]
- Gibbs, D.; Kitamoto, J.; Williams, D.S. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proc. Natl. Acad. Sci. USA 2003, 100, 6481–6486. [Google Scholar] [CrossRef]
- Yu, B.; Egbejimi, A.; Dharmat, R.; Xu, P.; Zhao, Z.; Long, B.; Miao, H.; Chen, R.; Wensel, T.G.; Cai, J.; et al. Phagocytosed photoreceptor outer segments activate mTORC1 in the retinal pigment epithelium. Sci. Signal. 2018, 11, eaag3315. [Google Scholar] [CrossRef]
- Toulis, V.; García-Monclús, S.; de la Peña-Ramírez, C.; Arenas-Galnares, R.; Abril, J.F.; Todi, S.V.; Khan, N.; Garanto, A.; Costa, M.D.C.; Marfany, G. The Deubiquitinating Enzyme Ataxin-3 Regulates Ciliogenesis and Phagocytosis in the Retina. Cell Rep. 2020, 33, 108360. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Chuang, J.Z.; Sung, C.H. Light regulates the ciliary protein transport and outer segment disc renewal of mammalian photoreceptors. Dev. Cell 2015, 32, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.; Chen, N.; Andreev, A.; Chen, J.; Kefalov, V.J.; Chen, J. Light regulation of rhodopsin distribution during outer segment renewal in murine rod photoreceptors. Curr. Biol. 2024, 34, 1492–1505.e1496. [Google Scholar] [CrossRef] [PubMed]
- Insinna, C.; Pathak, N.; Perkins, B.; Drummond, I.; Besharse, J.C. The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev. Biol. 2008, 316, 160–170. [Google Scholar] [CrossRef]
- Lewis, T.R.; Kundinger, S.R.; Link, B.A.; Insinna, C.; Besharse, J.C. Kif17 phosphorylation regulates photoreceptor outer segment turnover. BMC Cell Biol. 2018, 19, 25. [Google Scholar] [CrossRef]
- Moran, A.L.; Fehilly, J.D.; Blacque, O.; Kennedy, B.N. Gene therapy for RAB28: What can we learn from zebrafish? Vis. Res. 2023, 210, 108270. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.P.; Moran, A.L.; Matallanas, D.; McManus, G.J.; Blacque, O.E.; Kennedy, B.N. Genetic Deletion of Zebrafish Rab28 Causes Defective Outer Segment Shedding, but Not Retinal Degeneration. Front. Cell Dev. Biol. 2020, 8, 136. [Google Scholar] [CrossRef]
- Moran, A.L.; Carter, S.P.; Kaylor, J.J.; Jiang, Z.; Broekman, S.; Dillon, E.T.; Gómez Sánchez, A.; Minhas, S.K.; van Wijk, E.; Radu, R.A.; et al. Dawn and dusk peaks of outer segment phagocytosis, and visual cycle function require Rab28. FASEB J. 2022, 36, e22309. [Google Scholar] [CrossRef]
- Ying, G.; Boldt, K.; Ueffing, M.; Gerstner, C.D.; Frederick, J.M.; Baehr, W. The small GTPase RAB28 is required for phagocytosis of cone outer segments by the murine retinal pigmented epithelium. J. Biol. Chem. 2018, 293, 17546–17558. [Google Scholar] [CrossRef] [PubMed]
- Audo, I.; Mohand-Said, S.; Boulanger-Scemama, E.; Zanlonghi, X.; Condroyer, C.; Démontant, V.; Boyard, F.; Antonio, A.; Méjécase, C.; El Shamieh, S.; et al. MERTK mutation update in inherited retinal diseases. Hum. Mutat. 2018, 39, 887–913. [Google Scholar] [CrossRef] [PubMed]
- Gal, A.; Li, Y.; Thompson, D.A.; Weir, J.; Orth, U.; Jacobson, S.G.; Apfelstedt-Sylla, E.; Vollrath, D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet. 2000, 26, 270–271. [Google Scholar] [CrossRef] [PubMed]
- Tuson, M.; Marfany, G.; Gonzàlez-Duarte, R. Mutation of CERKL, a novel human ceramide kinase gene, causes autosomal recessive retinitis pigmentosa (RP26). Am. J. Hum. Genet. 2004, 74, 128–138. [Google Scholar] [CrossRef]
- Aleman, T.S.; Soumittra, N.; Cideciyan, A.V.; Sumaroka, A.M.; Ramprasad, V.L.; Herrera, W.; Windsor, E.A.; Schwartz, S.B.; Russell, R.C.; Roman, A.J.; et al. CERKL mutations cause an autosomal recessive cone-rod dystrophy with inner retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5944–5954. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, C.; Biswas, L.; Hu, X.; Liu, F.; Reilly, J.; Liu, X.; Liu, Y.; Huang, Y.; Lu, Z.; et al. CERKL gene knockout disturbs photoreceptor outer segment phagocytosis and causes rod-cone dystrophy in zebrafish. Hum. Mol. Genet. 2017, 26, 2335–2345. [Google Scholar] [CrossRef]
- Roosing, S.; Rohrschneider, K.; Beryozkin, A.; Sharon, D.; Weisschuh, N.; Staller, J.; Kohl, S.; Zelinger, L.; Peters, T.A.; Neveling, K.; et al. Mutations in RAB28, Encoding a Farnesylated Small GTPase, Are Associated with Autosomal-Recessive Cone-Rod Dystrophy. Am. J. Hum. Genet. 2013, 93, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Wensel, T.G.; Potter, V.L.; Moye, A.; Zhang, Z.; Robichaux, M.A. Structure and dynamics of photoreceptor sensory cilia. Pflug. Arch. Eur. J. Physiol. 2021, 473, 1517–1537. [Google Scholar] [CrossRef] [PubMed]
- Wensel, T.G.; Zhang, Z.; Anastassov, I.A.; Gilliam, J.C.; He, F.; Schmid, M.F.; Robichaux, M.A. Structural and molecular bases of rod photoreceptor morphogenesis and disease. Prog. Retin. Eye Res. 2016, 55, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Moye, A.R.; Robichaux, M.A.; Wensel, T. Expansion Microscopy of Mouse Photoreceptor Cilia. Adv. Exp. Med. Biol. 2023, 1415, 395–402. [Google Scholar] [CrossRef]
- Zang, J.; Neuhauss, S.C.F. Biochemistry and physiology of zebrafish photoreceptors. Pflügers Arch. Eur. J. Physiol. 2021, 473, 1569–1585. [Google Scholar] [CrossRef] [PubMed]
- Renninger, S.L.; Schonthaler, H.B.; Neuhauss, S.C.; Dahm, R. Investigating the genetics of visual processing, function and behaviour in zebrafish. Neurogenetics 2011, 12, 97–116. [Google Scholar] [CrossRef]
- Gordon, K.; Del Medico, A.; Sander, I.; Kumar, A.; Hamad, B. Gene therapies in ophthalmic disease. Nat. Rev. Drug Discov. 2019, 18, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, N.G.; Abboud, E.B.; Nowilaty, S.R.; Alkuraya, H.; Alhommadi, A.; Cai, H.; Hou, R.; Deng, W.-T.; Boye, S.L.; Almaghamsi, A.; et al. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: Results of a phase I trial. Hum. Genet. 2016, 135, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Amador, C.; Shah, R.; Ghiam, S.; Kramerov, A.A.; Ljubimov, A.V. Gene Therapy in the Anterior Eye Segment. Curr. Gene Ther. 2022, 22, 104–131. [Google Scholar] [CrossRef] [PubMed]
- Drag, S.; Dotiwala, F.; Upadhyay, A.K. Gene Therapy for Retinal Degenerative Diseases: Progress, Challenges, and Future Directions. Investig. Ophthalmol. Vis. Sci. 2023, 64, 39. [Google Scholar] [CrossRef]
- Kruczek, K.; Swaroop, A. Chapter Four—Patient stem cell-derived in vitro disease models for developing novel therapies of retinal ciliopathies. In Current Topics in Developmental Biology; Iomini, C., Sun, Y., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 155, pp. 127–163. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhao, C.; Kang, Y. The Formation and Renewal of Photoreceptor Outer Segments. Cells 2024, 13, 1357. https://doi.org/10.3390/cells13161357
Xu J, Zhao C, Kang Y. The Formation and Renewal of Photoreceptor Outer Segments. Cells. 2024; 13(16):1357. https://doi.org/10.3390/cells13161357
Chicago/Turabian StyleXu, Jingjin, Chengtian Zhao, and Yunsi Kang. 2024. "The Formation and Renewal of Photoreceptor Outer Segments" Cells 13, no. 16: 1357. https://doi.org/10.3390/cells13161357
APA StyleXu, J., Zhao, C., & Kang, Y. (2024). The Formation and Renewal of Photoreceptor Outer Segments. Cells, 13(16), 1357. https://doi.org/10.3390/cells13161357




