In Vivo Tracking and 3D Mapping of Cell Death in Regeneration and Cancer Using Trypan Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Strains and Allele Deletion
2.2. Carbon Tetrachloride (CCl4) Administration
2.3. Ischemia-Reperfusion Injury
2.4. Thymus Atrophy and Toxic Injury
2.5. Plasmids
2.6. Hydrodynamic Tail Vein Injection (HDTVI)
2.7. Trypan Blue Levels in Serum and Toxicity Analysis
2.8. Pulse and Chase
2.9. Liver Sample Collection
2.10. Immunofluorescence
2.11. iDISCO+ Clearing of Mouse Liver
2.12. Light Sheet Fluorescence Microscopy
2.13. 3D Image Reconstruction and Analysis
3. Results
3.1. TBlue Marks Dead Cells during Liver Injury and Regeneration
3.2. TBlue Detects Apoptotic Cell Death
3.3. In Vivo “Pulse and Chase” Tracking of Dead Cells
3.4. In Vivo “Pulse and Chase” of Two Populations of Dead Cells
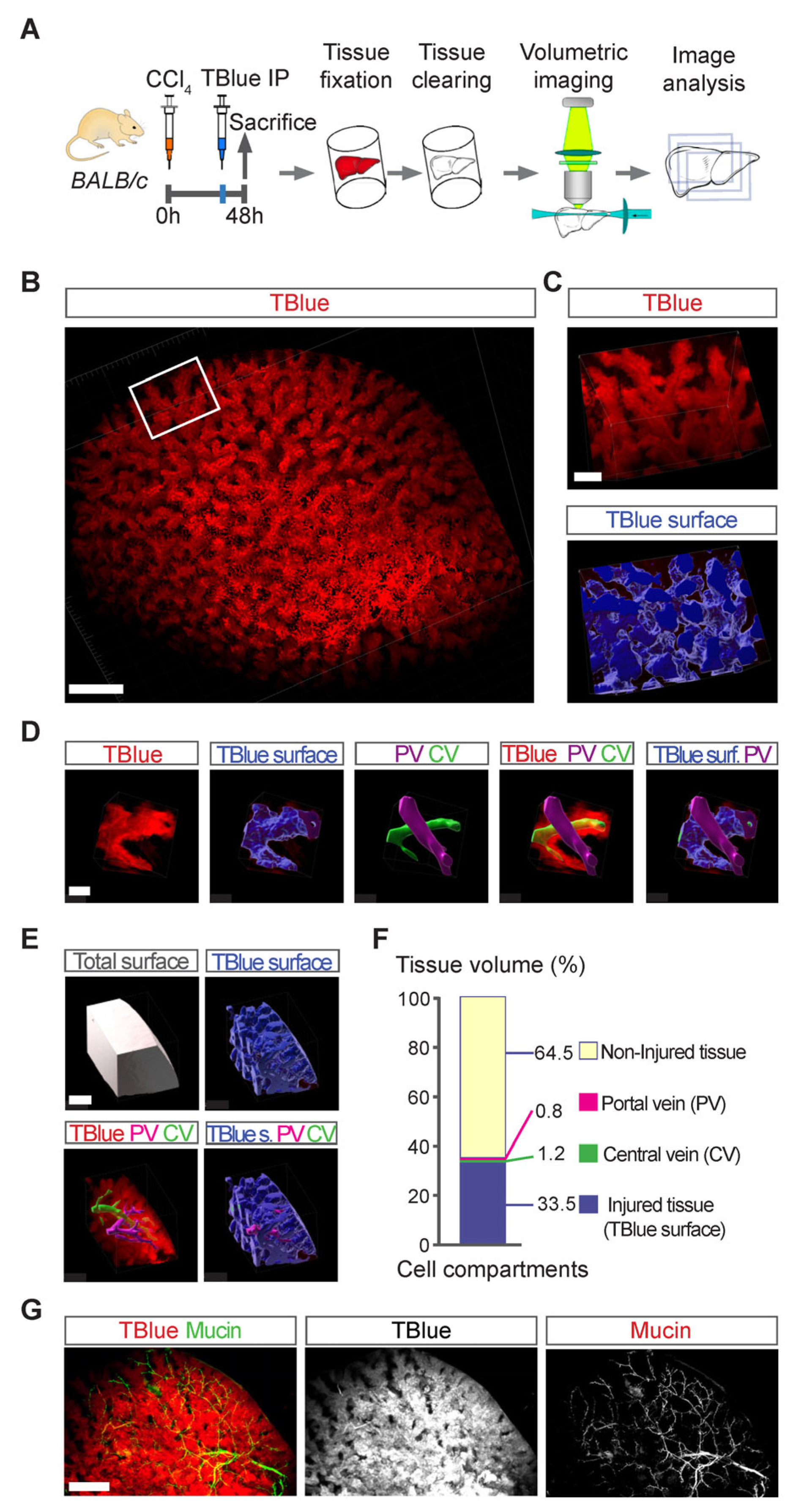
3.5. Three-Dimensional Mapping of Large Necrotic Areas in Whole Livers
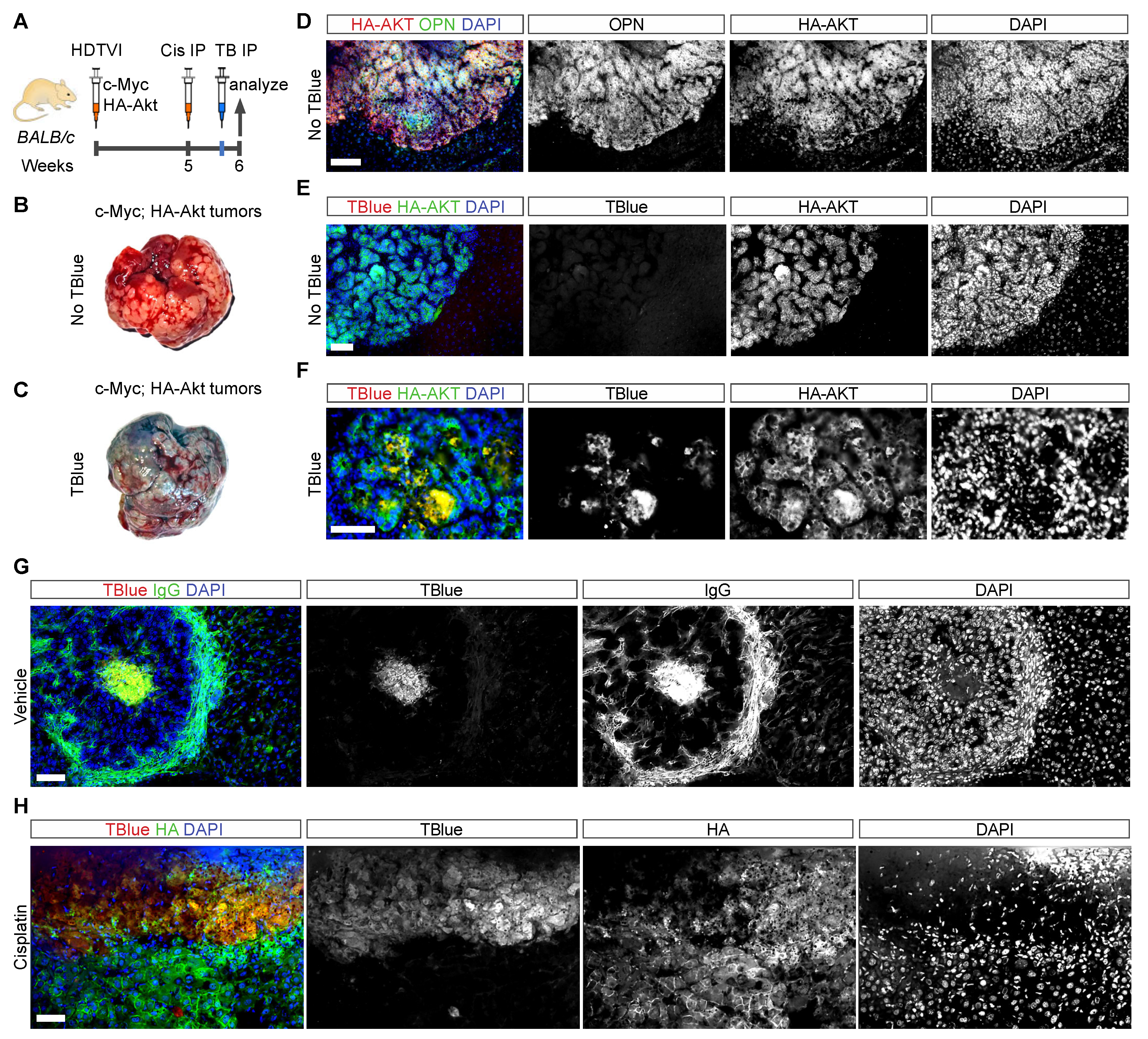
3.6. Detection of Cell Death in Liver Cancer Using TBlue Labelling
3.7. Detection of Cell Death in Other Organs Using TBlue Labeling
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.L.; Puhar, A.; Ngo-Camus, M.; Ramarao, N. Trypan blue dye enters viable cells incubated with the pore-forming toxin HlyII of Bacillus cereus. PLoS ONE 2011, 6, e22876. [Google Scholar] [CrossRef] [PubMed]
- Shilova, O.N.; Shilov, E.S.; Deyev, S.M. The effect of trypan blue treatment on autofluorescence of fixed cells. Cytometry A 2017, 91, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, F.; Tesei, A.; Arienti, C.; Bevilacqua, A. Cell Counting and Viability Assessment of 2D and 3D Cell Cultures: Expected Reliability of the Trypan Blue Assay. Biol. Proced. Online 2017, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Hammoudeh, S.M.; Hammoudeh, A.M.; Hamoudi, R. High-throughput quantification of the effect of DMSO on the viability of lung and breast cancer cells using an easy-to-use spectrophotometric trypan blue-based assay. Histochem. Cell Biol. 2019, 152, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Humeres, C.; González, V.; Gómez, M.T.; Montt, N.; Sanchez, G.; Chiong, M.; García, L. Insulin/NFκB protects against ischemia-induced necrotic cardiomyocyte death. Biochem. Biophys. Res. Commun. 2015, 467, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Lees, G.J. Trypan blue in vivo stains nigral dopaminergic neurons killed by 6-hydroxydopamine. Histochemistry 1989, 91, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.C.; Li, J.L.; Becker, D.; Weninger, W.; Angeli, V.; Ng, L.G. Inducing Ischemia-reperfusion Injury in the Mouse Ear Skin for Intravital Multiphoton Imaging of Immune Responses. J. Vis. Exp. 2016, 118, e54956. [Google Scholar] [CrossRef]
- Renier, N.; Adams, E.L.; Kirst, C.; Wu, Z.; Azevedo, R.; Kohl, J.; Autry, A.E.; Kadiri, L.; Venkataraju, K.U.; Zhou, Y.; et al. Mapping of Brain Activity by Automated Volume Analysis of Immediate Early Genes. Cell 2016, 165, 1789–1802. [Google Scholar] [CrossRef]
- Del Río, E.; Rojo, L.; Vilanova, E. Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 687–690. [Google Scholar]
- Verboven, E.; Moya, I.M.; Sansores-Garcia, L.; Xie, J.; Hillen, H.; Kowalczyk, W.; Vella, G.; Verhulst, S.; Castaldo, S.A.; Algueró-Nadal, A.; et al. Regeneration Defects in Yap and Taz Mutant Mouse Livers Are Caused by Bile Duct Disruption and Cholestasis. Gastroenterology 2021, 160, 847–862. [Google Scholar] [CrossRef]
- Hammad, S.; Hoehme, S.; Friebel, A.; von Recklinghausen, I.; Othman, A.; Begher-Tibbe, B.; Reif, R.; Godoy, P.; Johann, T.; Vartak, A.; et al. Protocols for staining of bile canalicular and sinusoidal networks of human, mouse and pig livers, three-dimensional reconstruction and quantification of tissue microarchitecture by image processing and analysis. Arch. Toxicol. 2014, 88, 1161–1183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Herweijer, H.; Zhang, G.; Subbotin, V.M.; Budker, V.; Williams, P.; Wolff, J.A. Time course of gene expression after plasmid DNA gene transfer to the liver. J. Gene Med. 2001, 3, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Watanabe, T.; Nagatomo, S.; Seki, T.; Niimi, S.; Ariga, T. Annexin A3-Expressing Cellular Phenotypes Emerge from Necrotic Lesion in the Pericentral Area in 2-Acetylaminofluoren/Carbon Tetrachloride-Treated Rat Livers. Biosci. Biotechnol. Biochem. 2007, 71, 3082–3089. [Google Scholar] [CrossRef] [PubMed]
- Renier, N.; Wu, Z.; Simon, D.J.; Yang, J.; Ariel, P.; Tessier-Lavigne, M. iDISCO: A simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 2014, 159, 896–910. [Google Scholar] [CrossRef]
- Moya, I.M.; Castaldo, S.A.; Van den Mooter, L.; Soheily, S.; Sansores-Garcia, L.; Jacobs, J.; Mannaerts, I.; Xie, J.; Verboven, E.; Hillen, H.; et al. Peritumoral activation of the Hippo pathway effectors YAP and TAZ suppresses liver cancer in mice. Science 2019, 366, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Song, W.H.; Kim, H.Y.; Lim, Y.S.; Hwang, S.Y.; Lee, C.; Lee, D.Y.; Moon, Y.; Song, Y.J.; Yoon, S. Fish Collagen Peptides Protect against Cisplatin-Induced Cytotoxicity and Oxidative Injury by Inhibiting MAPK Signaling Pathways in Mouse Thymic Epithelial Cells. Mar. Drugs 2022, 20, 232. [Google Scholar] [CrossRef] [PubMed]
- Lagou, M.K.; Anastasiadou, D.P.; Karagiannis, G.S. A Proposed Link Between Acute Thymic Involution and Late Adverse Effects of Chemotherapy. Front. Immunol. 2022, 13, 933547. [Google Scholar] [CrossRef]
- Howard, J.K.; Lord, G.M.; Matarese, G.; Vendetti, S.; Ghatei, M.A.; Ritter, M.A.; Lechler, R.I.; Bloom, S.R. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J. Clin. Investig. 1999, 104, 1051–1059. [Google Scholar] [CrossRef]
- Lotze, M.T.; Demarco, R.A. Dying dangerously: Necrotic cell death and chronic inflammation promote tumor growth. Discov. Med. 2004, 4, 448–456. [Google Scholar] [PubMed]
- Mazzolini, G.; Sowa, J.P.; Canbay, A. Cell death mechanisms in human chronic liver diseases: A far cry from clinical applicability. Clin. Sci. 2016, 130, 2121–2138. [Google Scholar] [CrossRef] [PubMed]
- Julien, O.; Wells, J.A. Caspases and their substrates. Cell Death Differ. 2017, 24, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of Apoptosis and Necrosis by Annexin V Binding, Propidium Iodide Uptake, and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087288. [Google Scholar] [CrossRef] [PubMed]
- Akekawatchai, C.; Roytrakul, S.; Phaonakrop, N.; Jaresitthikunchai, J.; Jitrapakdee, S. Proteomic Analysis of the Anoikis-Resistant Human Breast Cancer Cell Lines. Methods Mol. Biol. 2020, 2138, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Pedrera, L.; Espiritu, R.A.; Ros, U.; Weber, J.; Schmitt, A.; Stroh, J.; Hailfinger, S.; von Karstedt, S.; García-Sáez, A.J. Ferroptotic pores induce Ca2+ fluxes and ESCRT-III activation to modulate cell death kinetics. Cell Death Differ. 2021, 28, 1644–1657. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, G. Embryotoxische Dosis-Wirkungs-Beziehungen von Trypanblau bei Mäusen. Naunyn-Schmiedebergs Arch. Pharmakol. 1970, 267, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; O’Brien, M.A.; Moravec, R.A.; Kupcho, K.; Niles, A.L. Assay Guidance Manual; Markossian, S., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Didenko, V.V.; Ngo, H.; Baskin, D.S. Early necrotic DNA degradation: Presence of blunt-ended DNA breaks, 3′ and 5′ overhangs in apoptosis, but only 5′ overhangs in early necrosis. Am. J. Pathol. 2003, 162, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, A.R. The failure of glutamic acid to protect the rat embryo against the action of trypan blue. Teratology 1981, 23, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.M.; Daston, G.P.; Ebron, M.T.; Carver, B.; Stefanadis, J.G.; Grabowski, C.T. Studies on the mechanism of trypan blue teratogenicity in the rat developing in vivo and in vitro. Teratology 1985, 31, 389–399. [Google Scholar] [CrossRef]
- Tsaousis, K.T.; Kopsachilis, N.; Tsinopoulos, I.T.; Dimitrakos, S.A.; Kruse, F.E.; Welge-Luessen, U. Time-dependent morphological alterations and viability of cultured human trabecular cells after exposure to Trypan blue. Clin. Exp. Ophthalmol. 2013, 41, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Kwok, A.K.; Yeung, C.K.; Lai, T.Y.; Chan, K.P.; Pang, C.P. Effects of trypan blue on cell viability and gene expression in human retinal pigment epithelial cells. Br. J. Ophthalmol. 2004, 88, 1590–1594. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Procel, N.; Camacho, K.; Verboven, E.; Baroja, I.; Guerrero, P.A.; Hillen, H.; Estrella-García, C.; Vizcaíno-Rodríguez, N.; Sansores-Garcia, L.; Santamaría-Naranjo, A.; et al. In Vivo Tracking and 3D Mapping of Cell Death in Regeneration and Cancer Using Trypan Blue. Cells 2024, 13, 1379. https://doi.org/10.3390/cells13161379
Procel N, Camacho K, Verboven E, Baroja I, Guerrero PA, Hillen H, Estrella-García C, Vizcaíno-Rodríguez N, Sansores-Garcia L, Santamaría-Naranjo A, et al. In Vivo Tracking and 3D Mapping of Cell Death in Regeneration and Cancer Using Trypan Blue. Cells. 2024; 13(16):1379. https://doi.org/10.3390/cells13161379
Chicago/Turabian StyleProcel, Nicole, Karen Camacho, Elisabeth Verboven, Isabel Baroja, Priscila A. Guerrero, Hanne Hillen, Carlos Estrella-García, Nicole Vizcaíno-Rodríguez, Leticia Sansores-Garcia, Ana Santamaría-Naranjo, and et al. 2024. "In Vivo Tracking and 3D Mapping of Cell Death in Regeneration and Cancer Using Trypan Blue" Cells 13, no. 16: 1379. https://doi.org/10.3390/cells13161379
APA StyleProcel, N., Camacho, K., Verboven, E., Baroja, I., Guerrero, P. A., Hillen, H., Estrella-García, C., Vizcaíno-Rodríguez, N., Sansores-Garcia, L., Santamaría-Naranjo, A., Romero-Carvajal, A., Caicedo, A., Halder, G., & Moya, I. M. (2024). In Vivo Tracking and 3D Mapping of Cell Death in Regeneration and Cancer Using Trypan Blue. Cells, 13(16), 1379. https://doi.org/10.3390/cells13161379






