Effect of Culture Temperature on 2-Methylisoborneol Production and Gene Expression in Two Strains of Pseudanabaena sp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of the Bacteria and Mic Gene
2.2. Culture Experiments
2.3. DNA and RNA Extraction
2.4. dPCR Setup for Mic Gene Quantification
| Primers /Probes | Target | Sequence (5′→3′) | Target Size (bp) | Ta (°C) | Reference |
|---|---|---|---|---|---|
| 27F | Universal bacteria | AGAGTTTGATYMTGGCTCAG | >1200 | 56 | Sung et al. [40] |
| 1492R | TACGGYTACCTTGTTACGACT | ||||
| CYAN 108F | Pseudanabaena- specific 16S rRNA | ACGGGTGAGTAACRCGTRA | 270 | 55 | Modified from Rinta-Kanto et al. [39] |
| CYAN 377R | CCATTGCGGAAAATTCCCC | ||||
| CYAN 328R | FAM-CTCAGTTCCAGTGTGACTGGTC-BHQ1 | ||||
| MIB3324F | Cyanobacterial MIB synthase | CATTACCGAGCGATTCAACGAGC | 726 | 52 | Suurnäkki et al. [33] |
| MIB4050R | CCGCAATCTGTAGCACCATGTTGA | ||||
| 3909F | Cyanobacterial MIB synthase (mic) | CACCAGATCTTTTCTTCGATC | 140 | 59 | Lee et al. [38] |
| 4028R | AATCTGTAGCACCATGTTGAC | ||||
| 3987P | FAM-TCCTTTCGGTTGCCA-BHQ1 | this study |
2.5. GC-MS Analysis of 2-MIB
2.6. Statistical Analysis
3. Results and Discussion
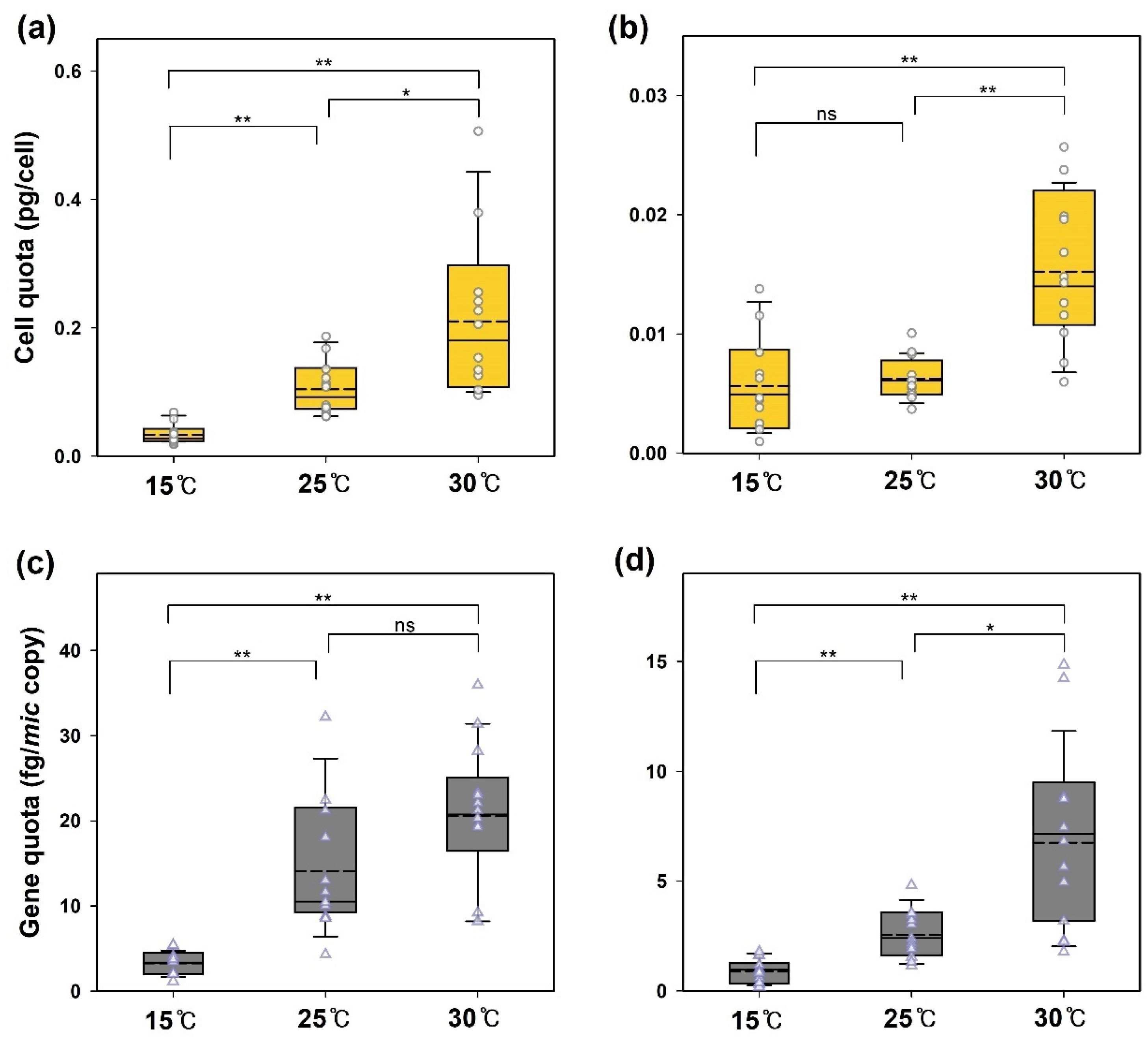
3.1. Cell Growth, 2-MIB Production, and Mic Gene Abundance
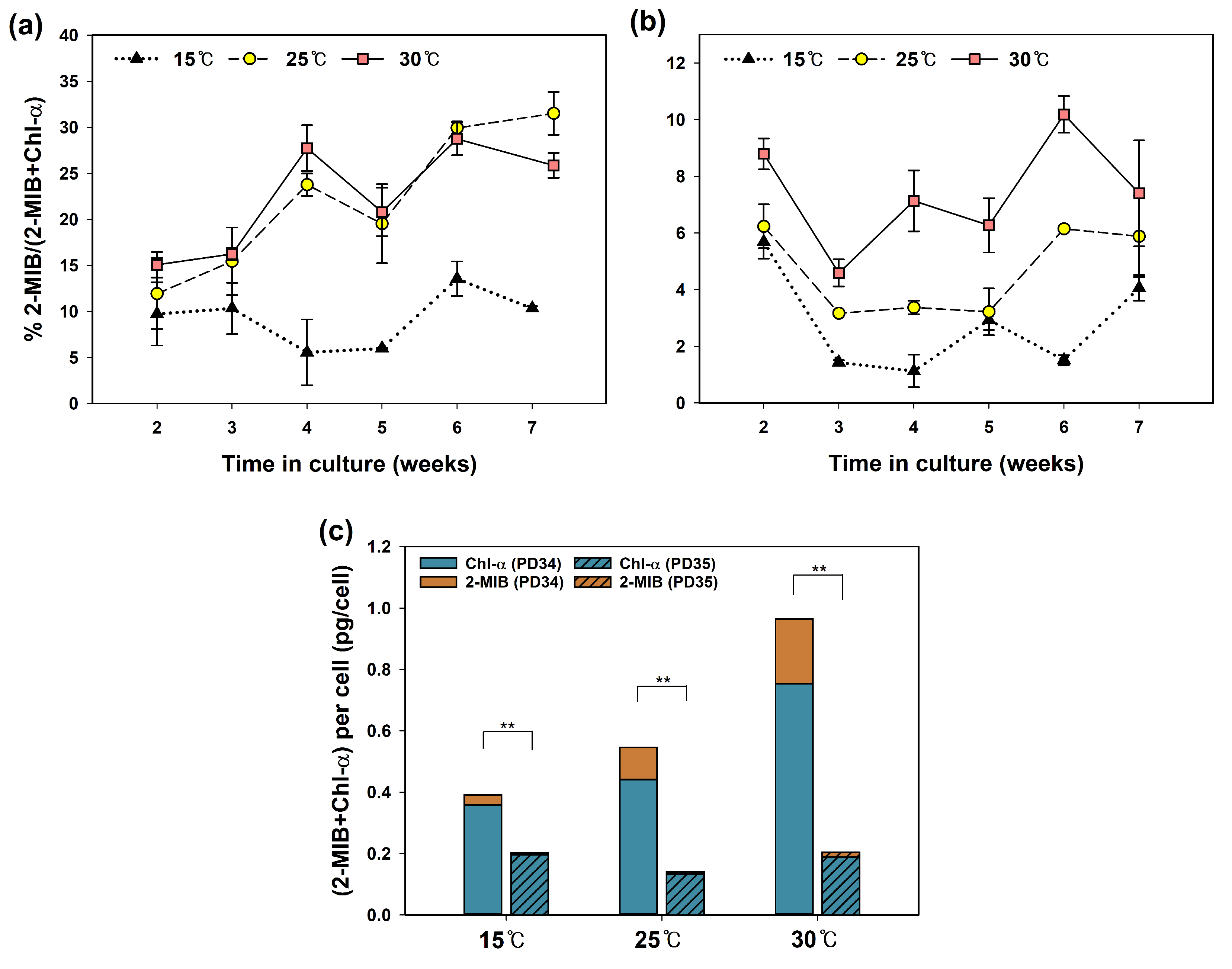
3.2. The Effect of Temperature on 2-MIB Production Yield
3.3. Temperature and Expression Level of the 2-MIB Synthase Gene
3.4. Relationship between Chl-a and 2-MIB Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Srinivasan, R.; Sorial, G.A. Treatment of taste and odor causing compounds 2-methyl isoborneol and geosmin in drinking water: A critical review. J. Environ. Sci. 2011, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Wang, M.; Ge, X.; Zhao, Q.; Sun, S.; Jia, R. Highly efficient removal of geosmin and 2-methylisoborneol by carboxylated multi-walled carbon nanotubes. Monatshefte Chem. Chem. Mon. 2014, 145, 747–754. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, L.; Li, L.; Song, L. 2-Methylisoborneol production characteristics of Pseudanabaena sp. FACHB 1277 isolated from Xionghe Reservoir, China. J. Appl. Phycol. 2016, 28, 3353–3362. [Google Scholar] [CrossRef]
- Pestana, C.J.; Lawton, L.A.; Kaloudis, T. Removal and/or Destruction of Cyanobacterial Taste and Odour Compounds by Conventional and Advanced Oxidation Processes. In Water Treatment for Purification from Cyanobacteria and Cyanotoxins; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2020; pp. 207–230. [Google Scholar]
- Manganelli, M.; Testai, E.; Tazart, Z.; Scardala, S.; Codd, G.A. Co-occurrence of taste and odor compounds and cyanotoxins in cyanobacterial blooms: Emerging risks to human health? Microorganisms 2023, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.B. Aquatic taste and odor: A primary signal of drinking-water integrity. J. Toxicol. Environ. Health A 2004, 67, 1779–1795. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Yu, M.; Go, J.; Kim, E.; Kim, H. Comparison between ozone and ferrate in oxidising geosmin and 2-MIB in water. Water Sci. Technol. 2007, 55, 117–125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berlt, M.M.G.; Schneider, R.D.C.D.S.; Machado, Ê.L.; Kist, L.T. Comparative assessment of the degradation of 2-methylisoborneol and geosmin in freshwater using advanced oxidation processes. Environ. Technol. 2021, 42, 3832–3839. [Google Scholar] [CrossRef]
- Watson, S.B.; Monis, P.; Baker, P.; Giglio, S. Biochemistry and genetics of taste-and odor-producing cyanobacteria. Harmful Algae 2016, 54, 112–127. [Google Scholar] [CrossRef]
- Anuar, N.S.S.; Kassim, A.A.; Utsumi, M.; Iwamoto, K.; Goto, M.; Shimizu, K.; Nor, A.O.; Zakaria, Z.; Sugiura, N.; Hara, H. Characterization of musty odor-producing actinomycetes from tropics and effects of temperature on the production of musty odor compounds. Microbes Environ. 2017, 32, 352–357. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Shao, J.; Wang, J.; Li, R. Genes associated with 2-methylisoborneol biosynthesis in cyanobacteria: Isolation, characterization, and expression in response to light. PLoS ONE 2011, 6, e18665. [Google Scholar] [CrossRef]
- Yamada, Y.; Kuzuyama, T.; Komatsu, M.; Shin-Ya, K.; Omura, S.; Cane, D.E.; Ikeda, H. Terpene synthases are widely distributed in bacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 857–862. [Google Scholar] [CrossRef]
- Havens, K.E.; Paerl, H.W. Climate Change at a Crossroad for Control of Harmful Algal Blooms; ACS Publications: Washington, DC, USA, 2015. [Google Scholar]
- Nowicka-Krawczyk, P.; Żelazna-Wieczorek, J.; Skrobek, I.; Ziułkiewicz, M.; Adamski, M.; Kaminski, A.; Żmudzki, P. Persistent cyanobacteria blooms in artificial water bodies—An effect of environmental conditions or the result of anthropogenic change. Int. J. Environ. Res. Public Health 2022, 19, 6990. [Google Scholar] [CrossRef]
- Lu, J.; Su, M.; Su, Y.; Wu, B.; Cao, T.; Fang, J.; Yu, J.; Zhang, H.; Yang, M. Driving forces for the growth of MIB-producing Planktothricoides raciborskii in a low-latitude reservoir. Water Res. 2022, 220, 118670. [Google Scholar] [CrossRef]
- Mohanty, B.; Majedi, S.M.; Pavagadhi, S.; Te, S.H.; Boo, C.Y.; Gin, K.Y.-H.; Swarup, S. Effects of light and temperature on the metabolic profiling of two habitat-dependent bloom-forming cyanobacteria. Metabolites 2022, 12, 406. [Google Scholar] [CrossRef]
- Izaguirre, G.; Taylor, W.D. A Pseudanabaena species from Castaic Lake, California, that produces 2-methylisoborneol. Water Res. 1998, 32, 1673–1677. [Google Scholar] [CrossRef]
- Lee, J.E.; Yu, M.N.; Yu, S.; Byeon, M. Occurrence and phylogenetic analysis of Pseudanabaena sp. producing 2-methylisoborneol in drinking water source of South Korea. Environ. Microbiol. Rep. 2022, 14, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Huang, Q.; Shen, X.; Wu, J.; Nan, J.; Li, J.; Lu, H.; Yang, C. Distribution, driving forces, and risk assessment of 2-MIB and its producer in a drinking water source-oriented shallow lake. Environ. Sci. Pollut. Res. 2023, 30, 71194–71208. [Google Scholar] [CrossRef]
- Wang, Z.; Li, R. Effects of light and temperature on the odor production of 2-methylisoborneol-producing Pseudanabaena sp. and geosmin-producing Anabaena ucrainica (cyanobacteria). Biochem. Syst. Ecol. 2015, 58, 219–226. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Q.; Miao, H.; Shimada, M.; Utsumi, M.; Lei, Z.; Zhang, Z.; Nishimura, O.; Asada, Y.; Fujimoto, N. Temperature affects growth, geosmin/2-methylisoborneol production, and gene expression in two cyanobacterial species. Environ. Sci. Pollut. Res. 2022, 29, 12017–12026. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Wan Omar, W.M.; Merican, F.M.M.S.; Azizan, A.A.; Foong, C.P.; Convey, P.; Najimuddin, N.; Smykla, J.; Alias, S.A. Identification and phenotypic plasticity of Pseudanabaena catenata from the Svalbard archipelago. Pol. Polar Res. 2017, 38, 445–458. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, J.; Wang, M.; Dong, W. Dominance and growth factors of Pseudanabaena sp. in drinking water source reservoirs, Southern China. Sustainability 2018, 10, 3936. [Google Scholar] [CrossRef]
- Shizuka, K.; Ikenaga, M.; Murase, J.; Nakayama, N.; Matsuya, N.; Kakino, W.; Taruya, H.; Maie, N. Diversity of 2-MIB-Producing cyanobacteria in Lake Ogawara: Microscopic and molecular ecological approaches. Aquac. Sci. 2020, 68, 9–23. [Google Scholar]
- Su, M.; Zhu, Y.; Andersen, T.; Wang, X.; Yu, Z.; Lu, J.; Song, Y.; Cao, T.; Yu, J.; Zhang, Y. Light-dominated selection shaping filamentous cyanobacterial assemblages drives odor problem in a drinking water reservoir. NPJ Clean Water 2022, 5, 37. [Google Scholar] [CrossRef]
- Kakimoto, M.; Ishikawa, T.; Miyagi, A.; Saito, K.; Miyazaki, M.; Asaeda, T.; Yamaguchi, M.; Uchimiya, H.; Kawai-Yamada, M. Culture temperature affects gene expression and metabolic pathways in the 2-methylisoborneol-producing cyanobacterium Pseudanabaena galeata. J. Plant Physiol. 2014, 171, 292–300. [Google Scholar] [CrossRef]
- Cao, T.; Fang, J.; Jia, Z.; Zhu, Y.; Su, M.; Zhang, Q.; Song, Y.; Yu, J.; Yang, M. Early warning of MIB episode based on gene abundance and expression in drinking water reservoirs. Water Res. 2023, 231, 119667. [Google Scholar] [CrossRef]
- Zimba, P.V.; Dionigi, C.P.; Millie, D.F. Evaluating the relationship between photopigment synthesis and 2-methylisoborneol accumulation in cyanobacteria. J. Phycol. 1999, 35, 1422–1429. [Google Scholar] [CrossRef]
- Shen, Q.; Shimizu, K.; Miao, H.; Tsukino, S.; Utsumi, M.; Lei, Z.; Zhang, Z.; Nishimura, O.; Asada, Y.; Fujimoto, N. Effects of elevated nitrogen on the growth and geosmin productivity of Dolichospermum smithii. Environ. Sci. Pollut. Res. 2021, 28, 177–184. [Google Scholar] [CrossRef]
- Watson, S.B. Cyanobacterial and eukaryotic algal odour compounds: Signals or by-products? A review of their biological activity. Phycologia 2003, 42, 332–350. [Google Scholar] [CrossRef]
- Watson, S.; Jüttner, F. Biological production of taste and odour compounds. In Taste and Odour in Source and Drinking Water: Causes, Controls, and Consequences; IWA Publishing: London, UK, 2019; pp. 63–112. [Google Scholar]
- Belcher, H.; Swale, E. Culturing Algae. A Guide for Schools and Colleges; Institute of Terrestrial Ecology: Cambridge, UK, 1982. [Google Scholar]
- Suurnäkki, S.; Gomez-Saez, G.V.; Rantala-Ylinen, A.; Jokela, J.; Fewer, D.P.; Sivonen, K. Identification of geosmin and 2-methylisoborneol in cyanobacteria and molecular detection methods for the producers of these compounds. Water Res. 2015, 68, 56–66. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Jeong Hwan, B.; Kim, H.N.; Kang, T.G.; Kim, B.-H.; Byeon, M.-S. Study of the cause of the generation of odor compounds (geosmin and 2-methylisoborneol) in the Han River system, the drinking water source, Republic of Korea. Water Supply 2023, 23, 1081–1093. [Google Scholar] [CrossRef]
- Lee, J.; Gil, K. Spatial optimization of operating microalgae bioreactor for nitrogen removal and electricity saving. Environ. Earth Sci. 2020, 79, 239. [Google Scholar] [CrossRef]
- Tan, L.L.; Loganathan, N.; Agarwalla, S.; Yang, C.; Yuan, W.; Zeng, J.; Wu, R.; Wang, W.; Duraiswamy, S. Current commercial dPCR platforms: Technology and market review. Crit. Rev. Biotechnol. 2023, 43, 433–464. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Park, R.; Yu, M.; Byeon, M.; Kang, T. qPCR-based monitoring of 2-Methylisoborneol/Geosmin-producing cyanobacteria in drinking water reservoirs in South Korea. Microorganisms 2023, 11, 2332. [Google Scholar] [CrossRef]
- Rinta-Kanto, J.; Ouellette, A.; Boyer, G.; Twiss, M.; Bridgeman, T.; Wilhelm, S. Quantification of toxic Microcystis spp. during the 2003 and 2004 blooms in western Lake Erie using quantitative real-time PCR. Environ. Sci. Technol. 2005, 39, 4198–4205. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y.; Hwang, Y.; Shin, M.H.; Park, M.S.; Lee, S.H.; Yong, D.; Lee, K. Utility of conventional culture and MALDI-TOF MS for identification of microbial communities in bronchoalveolar lavage fluid in comparison with the GS junior next generation sequencing system. Ann. Lab. Med. 2018, 38, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Hurlburt, B.; Lloyd, S.W.; Grimm, C.C. Comparison of analytical techniques for detection of geosmin and 2-methylisoborneol in aqueous samples. J. Chromatogr. Sci. 2009, 47, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.; Helmstetter, C.E. Chromosome replication and the division cycle of Escherichia coli Br. J. Mol. Biol. 1968, 31, 519–540. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, A.; Kaplan-Levy, R.N.; Welch, J.M.; Post, A.F. Massive multiplication of genome and ribosomes in dormant cells (akinetes) of Aphanizomenon ovalisporum (Cyanobacteria). ISME J. 2012, 6, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Jüttner, F.; Watson, S.B. Biochemical and ecological control of geosmin and 2-methylisoborneol in source waters. Appl. Environ. Microbiol. 2007, 73, 4395–4406. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-T.; Yen, H.-K.; Lin, T.-F. An alternative method to quantify 2-MIB producing cyanobacteria in drinking water reservoirs: Method development and field applications. Environ. Res. 2016, 151, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, L.; Cheng, S.; Chen, L.; Zhang, H.; Zhang, X. Production and release of 2-MIB in Pseudanabaena: Effects of growth phases on cell characteristics and 2-MIB yield. Ecotoxicol. Environ. Saf. 2024, 274, 116198. [Google Scholar] [CrossRef]
- Jia, Z.; Su, M.; Liu, T.; Guo, Q.; Wang, Q.; Burch, M.; Yu, J.; Yang, M. Light as a possible regulator of MIB-producing Planktothrix in source water reservoir, mechanism and in-situ verification. Harmful Algae 2019, 88, 101658. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, G.; Shao, J.; Tan, W.; Li, Y.; Li, R. Establishment and field applications of real-time PCR methods for the quantification of potential MIB-producing cyanobacteria in aquatic systems. J. Appl. Phycol. 2016, 28, 325–333. [Google Scholar] [CrossRef]
- Ren, X.; Sun, J.; Zhang, Q.; Zuo, Y.; Liu, J.; Liu, J.; Li, L.; Song, L. The Emergent Integrated Constructed Wetland-Reservoir (CW-R) Is Being Challenged by 2-Methylisoborneol Episode—A Case Study in Yanlonghu CW-R. Water 2022, 14, 2670. [Google Scholar] [CrossRef]
- Su, M.; Zhu, Y.; Jia, Z.; Liu, T.; Yu, J.; Burch, M.; Yang, M. Identification of MIB producers and odor risk assessment using routine data: A case study of an estuary drinking water reservoir. Water Res. 2021, 192, 116848. [Google Scholar] [CrossRef]
- Lu, K.-Y.; Chiu, Y.-T.; Burch, M.; Senoro, D.; Lin, T.-F. A molecular-based method to estimate the risk associated with cyanotoxins and odor compounds in drinking water sources. Water Res. 2019, 164, 114938. [Google Scholar] [CrossRef]
- Giglio, S.; Saint, C.P.; Monis, P.T. Expression of the geosmin synthase gene in the cyanobacterium Anabaena circinalis AWQC318 1. J. Phycol. 2011, 47, 1338–1343. [Google Scholar] [CrossRef]
- Blazewicz, S.J.; Barnard, R.L.; Daly, R.A.; Firestone, M.K. Evaluating rRNA as an indicator of microbial activity in environmental communities: Limitations and uses. ISME J. 2013, 7, 2061–2068. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta Biomembr. 2004, 1666, 142–157. [Google Scholar] [CrossRef]
- Avalos, M.; Garbeva, P.; Vader, L.; van Wezel, G.P.; Dickschat, J.S.; Ulanova, D. Biosynthesis, evolution and ecology of microbial terpenoids. Nat. Prod. Rep. 2022, 39, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.L.; da Silva, A.P.R.; de Magalhães, V.F. Use of the cell quota and chlorophyll content for normalization of cylindropermopsin produced by two Cylindrospermopsis raciborskii strains grown under different light intensities. Ecotoxicol. Environ. Contam. 2013, 8, 93–100. [Google Scholar] [CrossRef]
- Shi, R.; Li, G.; Zhou, L.; Liu, J.; Tan, Y. The increasing aluminum content affects the growth, cellular chlorophyll a and oxidation stress of cyanobacteria Synechocystis sp. WH7803. Oceanol. Hydrobiol. Stud. 2015, 44, 343–351. [Google Scholar] [CrossRef]
- Sigaud, T.C.S.; Aidar, E. Salinity and temperature effects on the growth and chlorophyll-α content of some planktonic aigae. Bol. Inst. Oceanogr. 1993, 41, 95–103. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.; Dai, X.; Sun, Y.; Chen, F. Effect of phosphorus and temperature on chlorophyll a contents and cell sizes of Scenedesmus obliquus and Microcystis aeruginosa. Limnology 2011, 12, 187–192. [Google Scholar] [CrossRef]
- Feng, Y.; Morgan, R.M.L.; Fraser, P.D.; Hellgardt, K.; Nixon, P.J. Crystal structure of geranylgeranyl pyrophosphate synthase (CrtE) involved in cyanobacterial terpenoid biosynthesis. Front. Plant Sci. 2020, 11, 589. [Google Scholar] [CrossRef]
- Lin, P.-C.; Pakrasi, H.B. Engineering cyanobacteria for production of terpenoids. Planta 2019, 249, 145–154. [Google Scholar] [CrossRef]
- Post, A.F.; de Wit, R.; Mur, L.R. Interactions between temperature and light intensity on growth and photosynthesis of the cyanobacterium Oscillatoria agardhii. J. Plankton Res. 1985, 7, 487–495. [Google Scholar] [CrossRef]
- Espinosa, C.; Abril, M.; Guasch, H.; Pou, N.; Proia, L.; Ricart, M.; Ordeix, M.; Llenas, L. Water flow and light availability influence on intracellular geosmin production in river biofilms. Front. Microbiol. 2020, 10, 3002. [Google Scholar] [CrossRef]
- Formighieri, C.; Melis, A. Sustainable heterologous production of terpene hydrocarbons in cyanobacteria. Photosynth. Res. 2016, 130, 123–135. [Google Scholar] [CrossRef]
- Lin, P.-C.; Saha, R.; Zhang, F.; Pakrasi, H.B. Metabolic engineering of the pentose phosphate pathway for enhanced limonene production in the cyanobacterium Synechocystis sp. PCC 6803. Sci. Rep. 2017, 7, 17503. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, R.; Yu, M.-N.; Park, J.-H.; Kang, T.; Lee, J.-E. Effect of Culture Temperature on 2-Methylisoborneol Production and Gene Expression in Two Strains of Pseudanabaena sp. Cells 2024, 13, 1386. https://doi.org/10.3390/cells13161386
Park R, Yu M-N, Park J-H, Kang T, Lee J-E. Effect of Culture Temperature on 2-Methylisoborneol Production and Gene Expression in Two Strains of Pseudanabaena sp. Cells. 2024; 13(16):1386. https://doi.org/10.3390/cells13161386
Chicago/Turabian StylePark, Rumi, Mi-Na Yu, Ji-Hyun Park, Taegu Kang, and Jung-Eun Lee. 2024. "Effect of Culture Temperature on 2-Methylisoborneol Production and Gene Expression in Two Strains of Pseudanabaena sp." Cells 13, no. 16: 1386. https://doi.org/10.3390/cells13161386
APA StylePark, R., Yu, M.-N., Park, J.-H., Kang, T., & Lee, J.-E. (2024). Effect of Culture Temperature on 2-Methylisoborneol Production and Gene Expression in Two Strains of Pseudanabaena sp. Cells, 13(16), 1386. https://doi.org/10.3390/cells13161386






