Anti-Allergic and Anti-Inflammatory Signaling Mechanisms of Natural Compounds/Extracts in In Vitro System of RBL-2H3 Cell: A Systematic Review
Abstract
:1. Introduction
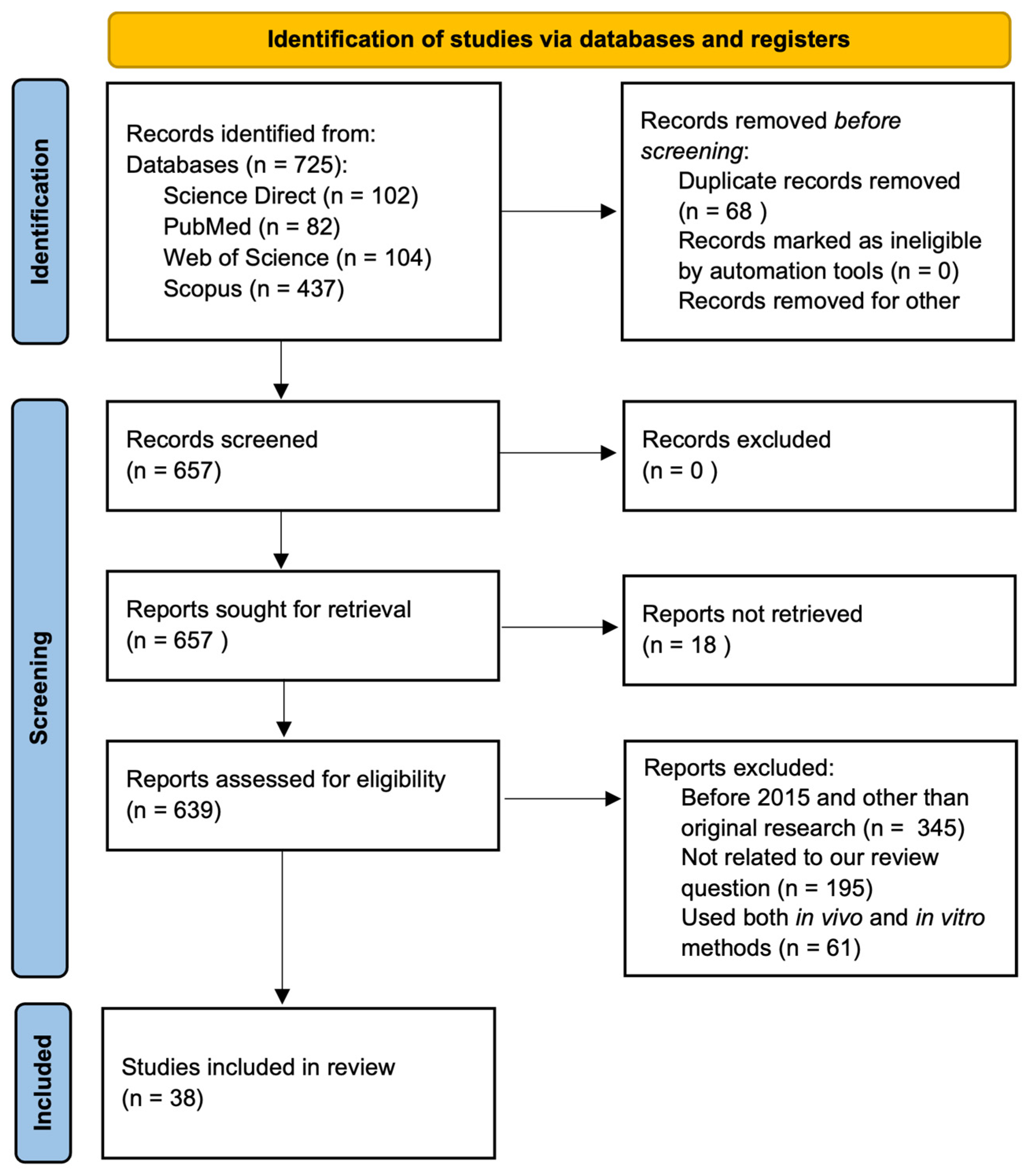
2. Materials and Methods
3. Results
3.1. Study Characteristics, Risk of Bias, and Reporting Quality
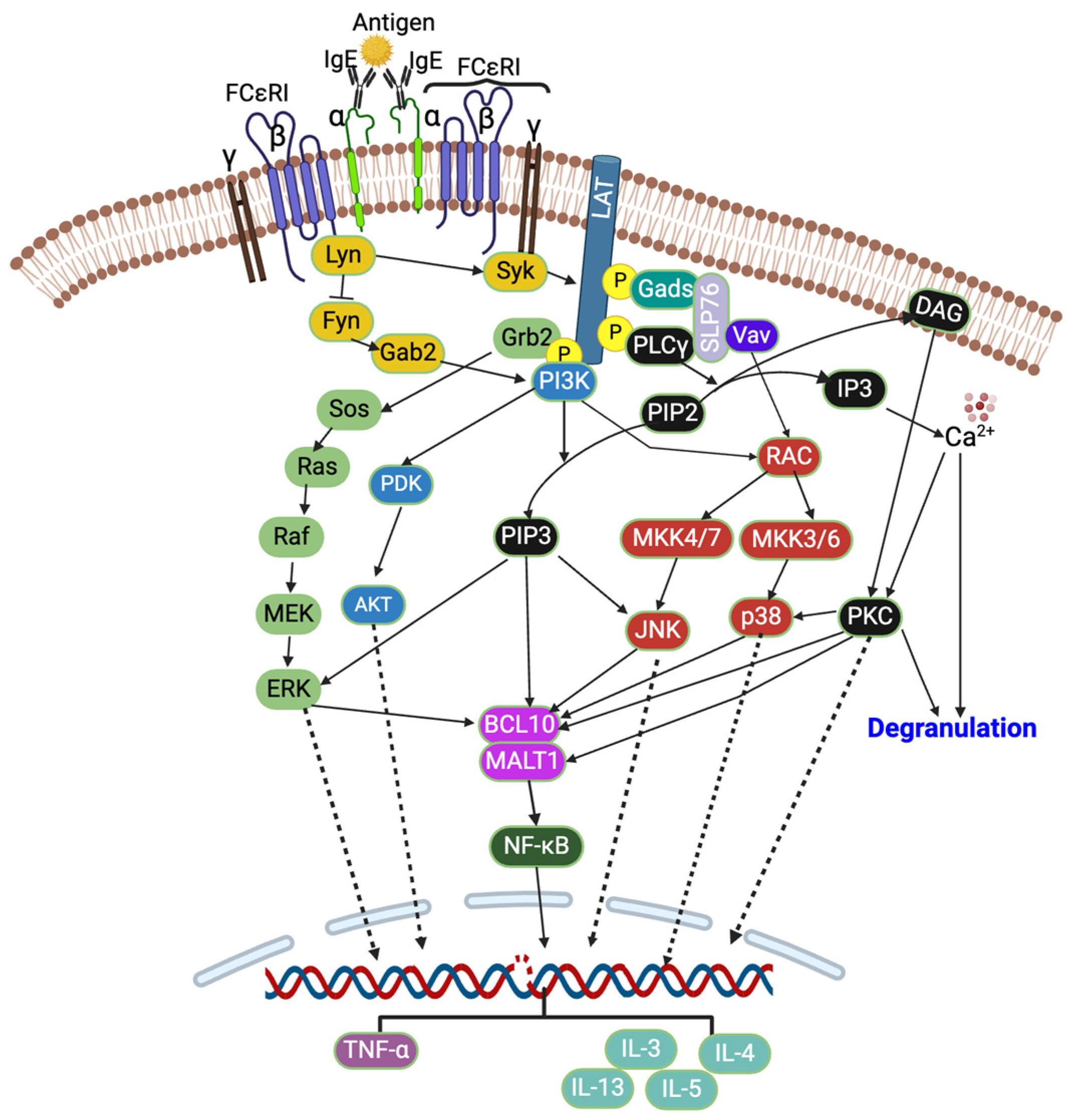
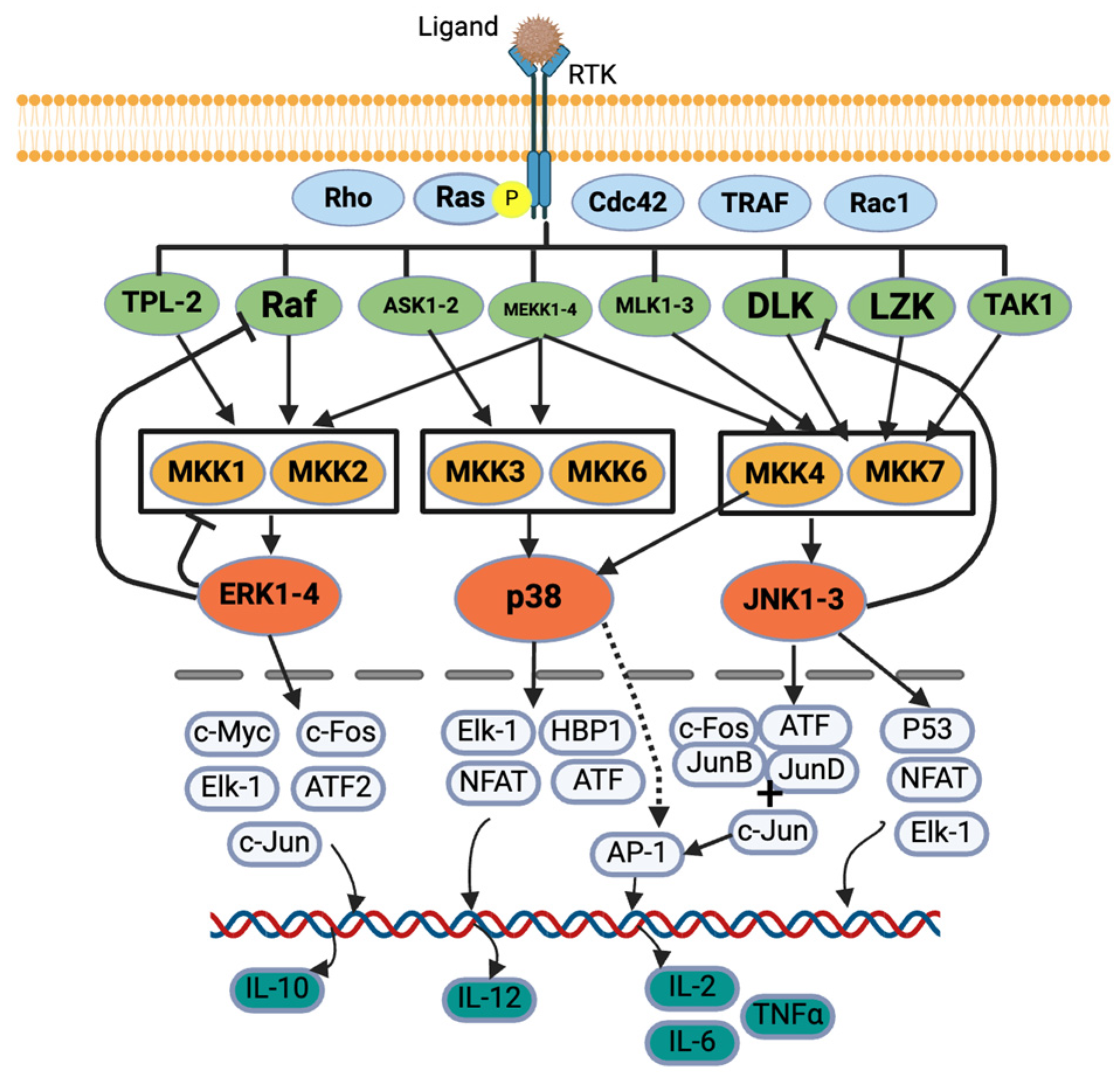
3.2. Signaling Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badger-Emeka, L.I.; Emeka, P.M.; Thirugnanasambantham, K.; Ibrahim, H.I.M. Anti-Allergic Potential of Cinnamaldehyde via the Inhibitory Effect of Histidine Decarboxylase (HDC) Producing Klebsiella pneumonia. Molecules 2020, 25, 5580. [Google Scholar] [CrossRef]
- Barbosa, M.; Lopes, G.; Valentão, P.; Ferreres, F.; Gil-Izquierdo, Á; Pereira, D.M.; Andrade, P.B. Edible seaweeds’ phlorotannins in allergy: A natural multi-target approach. Food Chem. 2018, 265, 233. [Google Scholar] [CrossRef]
- Chang, Y.; Hsu, W.; Pan, T. Monascus Secondary Metabolites Monascin and Ankaflavin Inhibit Activation of RBL-2H3 Cells. J. Agric. Food Chem. 2015, 63, 192. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kato, T.; Azuma, T.; Kikuzaki, H.; Abe, K. Anti-allergenic activity of polymethoxyflavones from Kaempferia parviflora. J. Funct. Foods 2015, 13, 100. [Google Scholar] [CrossRef]
- Lorenz, P.; Heinrich, M.; Garcia-Käufer, M.; Grunewald, F.; Messerschmidt, S.; Herrick, A.; Gruber, K.; Beckmann, C.; Knoedler, M.; Huber, R.; et al. Constituents from oak bark (Quercus robur L.) inhibit degranulation and allergic mediator release from basophils and mast cells in vitro. J. Ethnopharmacol. 2016, 194, 642. [Google Scholar] [CrossRef] [PubMed]
- Mwakalukwa, R.; Ashour, A.; Amen, Y.; Niwa, Y.; Tamrakar, S.; Miyamoto, T.; Shimizu, K. Anti-allergic activity of polyphenolic compounds isolated from olive mill wastes. J. Funct. Foods 2019, 58, 207. [Google Scholar] [CrossRef]
- Shankar, A.; Mcalees, J.W.; Lewkowich, I.P. Modulation of IL-4/IL-13 cytokine signaling in the context of allergic disease. J. Allergy Clin. Immunol. 2022, 150, 266. [Google Scholar] [CrossRef]
- Lee, E.; Yu, M.; Garcia, C.V.; Jhee, K.; Yang, S. Inhibitory effect of Zizania latifolia chloroform fraction on allergy-related mediator production in RBL-2H3 cells. Food Sci. Biotechnol. 2017, 26, 481. [Google Scholar] [CrossRef]
- Liu, Y.; Shu, Z.; Li, Y.; Chen, H.; Liu, H.; Yang, X.; Liu, G.; Liu, Q. Deep-sea-derived viridicatol relieves allergic response by suppressing MAPK and JAK-STAT signalling pathways of RBL-2H3 cells. Food Agric. Immunol. 2023, 34, 2207791. [Google Scholar] [CrossRef]
- Ma, J.; Tong, P.; Chen, Y.; Wang, Y.; Ren, H.; Gao, Z.; Yue, T.; Long, F. The inhibition of pectin oligosaccharides on degranulation of RBL-2H3 cells from apple pectin with high hydrostatic pressure assisted enzyme treatment. Food Chem. 2022, 371, 131097. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Wang, T.; Zhao, Y.; Shao, Y.; Sun, Y.; Zhang, Y.; Liu, Z. Network pharmacology-based analysis of the mechanism of Saposhnikovia divaricata for the treatment of type I allergy. Pharm. Biol. 2022, 60, 1224. [Google Scholar] [CrossRef]
- Yan, Z.; Feng, X.; Li, X.; Gao, Z.; Wang, Z.; Ren, G.; Long, F. Sea Buckthorn Flavonoid Extracted by High Hydrostatic Pressure Inhibited IgE-Stimulated Mast Cell Activation through the Mitogen-Activated Protein Kinase Signaling Pathway. Foods 2024, 13, 560. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Inoue, M.; Yoshioka, H.; Kitakaze, T.; Furuyashiki, T.; Abe, N.; Ashida, H. Enzymatically synthesized glycogen inhibited degranulation and inflammatory responses through stimulation of intestine. J. Clin. Biochem. Nutr. 2020, 67, 67–73. [Google Scholar] [CrossRef]
- Li, Y.; Leung, P.S.C.; Gershwin, M.E.; Song, J. New Mechanistic Advances in FcεRI-Mast Cell–Mediated Allergic Signaling. Clin. Rev. Allerg. Immunol. 2022, 63, 431. [Google Scholar] [CrossRef]
- Dera, A.; Rajagopalan, P.; Ahmed, I.; Alfhili, M.; Alsughayyir, J.; Chandramoorthy, H.C. Thymoquinone attenuates IgE-mediated allergic response via pi3k-Akt-NFκB pathway and upregulation of the Nrf2-HO1 axis. J. Food Biochem. 2020, 44, e13216. [Google Scholar] [CrossRef]
- Park, C.; Min, S.; Yu, H.; Kim, K.; Kim, S.; Lee, H.; Kim, J.; Park, Y. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. IJMS 2020, 21, 4620. [Google Scholar] [CrossRef]
- Yoo, G.; Lee, K.; Lee, D. Inhibitory effects of 2-oxo-2H-chromen-4-yl 4-methylbenzenesulfonate on allergic inflammatory responses in rat basophilic leukemia cells. Int. Immunopharmacol. 2017, 48, 196. [Google Scholar] [CrossRef]
- Vo, T.S.; Le, T.T.; Kim, S.; Ngo, D. The role of myricetin fromRhodomyrtus tomentosa(Aiton) Hassk fruits on downregulation of FcεRI-mediated mast cell activation. J. Food Biochem. 2020, 44, e13143. [Google Scholar] [CrossRef]
- Hagenlocher, Y.; Kießling, K.; Schäffer, M.; Bischoff, S.C.; Lorentz, A. Cinnamaldehyde is the main mediator of cinnamon extract in mast cell inhibition. Eur. J. Nutr. 2015, 54, 1297. [Google Scholar] [CrossRef]
- Jiao, W.; Cheng, B.; Shi, G.; Chen, G.; Gu, B.; Zhou, Y.; Hong, L.; Yang, F.; Liu, Z.; Qiu, S.; et al. Dysivillosins A–D, Unusual Anti-allergic Meroterpenoids from the Marine Sponge Dysidea villosa. Sci. Rep. 2017, 7, 8947. [Google Scholar] [CrossRef]
- Kawai, J.; Higuchi, Y.; Hirota, M.; Hirasawa, N.; Mori, K. Ergosterol and its derivatives from Grifola frondosa inhibit antigen-induced degranulation of RBL-2H3 cells by suppressing the aggregation of high affinity IgE receptors. Biosci. Biotechnol. Biochem. 2018, 82, 1803. [Google Scholar] [CrossRef]
- Kong, Z.L.; Sudirman, S.; Lin, H.J.; Chen, W.N. In vitro anti-inflammatory effects of curcumin on mast cell-mediated allergic responses via inhibiting FceRI protein expression and protein kinase C delta translocation. Cytotechnology 2020, 72, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhu, S.; Tamura, T.; Kadowaki, M.; Wang, Z.; Yoshimatsu, K.; Komatsu, K. Chemical constituents with anti-allergic activity from the root of Edulis Superba, a horticultural cultivar of Paeonia lactiflora. J. Nat. Med. 2016, 70, 234. [Google Scholar] [CrossRef]
- Athari, S.S. Targeting cell signaling in allergic asthma. Sig. Transduct Target Ther. 2019, 4, 45. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Jhee, K.; Yang, S. Tricin Isolated from Enzyme-Treated Zizania latifolia Extract Inhibits IgE-Mediated Allergic Reactions in RBL-2H3 Cells by Targeting the Lyn/Syk Pathway. Molecules 2020, 25, 2084. [Google Scholar] [CrossRef]
- Min, S.; Park, C.; Yu, H.; Park, Y. Anti-Inflammatory and Anti-Allergic Effects of Saponarin and Its Impact on Signaling Pathways of RAW 264.7, RBL-2H3, and HaCaT Cells. IJMS 2021, 22, 8431. [Google Scholar] [CrossRef]
- Lim, S.; Oh, S.; Nguyen, Q.T.N.; Kim, M.; Zheng, S.; Fang, M.; Yi, T. Rosa davurica Inhibited Allergic Mediators by Regulating Calcium and Histamine Signaling Pathways. Plants 2023, 12, 1572. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Shim, K.; Jeoung, D. The Crosstalk between FcεRI and Sphingosine Signaling in Allergic Inflammation. IJMS 2022, 23, 3892. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Soares-Silva, M.; Diniz, F.F.; Gomes, G.N.; Bahia, D. The Mitogen-Activated Protein Kinase (MAPK) Pathway: Role in Immune Evasion by Trypanosomatids. Front. Microbiol. 2016, 7, 183. [Google Scholar] [CrossRef]
- Yuan, J.; Dong, X.; Yap, J.; Hu, J. The MAPK and AMPK signalings: Interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020, 13, 113. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, M.; Zhang, Y.; Xu, Z. JNK pathway: Diseases and therapeutic potential. Acta Pharmacol. Sin. 2007, 28, 601. [Google Scholar] [CrossRef]
- Davis, R.J. Signal Transduction by the JNK Group Review of MAP Kinases. Cell 2000, 103, 239–252. [Google Scholar]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar]
- Zeke, A.; Misheva, M.; Reményi, A.; Bogoyevitch, M.A. JNK Signaling: Regulation and Functions Based on Complex Protein-Protein Partnerships. Microbiol. Mol. Biol. Rev. 2016, 80, 793. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Sig. Transduct Target Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Schulze-Osthoff, K.; Ferrari, D.; Riehemann, K.; Wesselborg, S. Regulation of NF-KB Activation by MAP Kinase Cascades. Immunobiology 1997, 198, 35–49. [Google Scholar]
- Wang, Q.; Gao, H.; Yuan, R.; Han, S.; Li, X.; Tang, M.; Dong, B.; Li, J.; Zhao, L.; Feng, J.; et al. Procyanidin A2, a polyphenolic compound, exerts anti-inflammatory and anti-oxidative activity in lipopolysaccharide-stimulated RAW264.7 cells. PLoS ONE 2020, 15, e0237017. [Google Scholar] [CrossRef]
- Chu, H.; Tang, Q.; Huang, H.; Hao, W.; Wei, X. Grape-seed proanthocyanidins inhibit the lipopolysaccharide-induced inflammatory mediator expression in RAW264.7 macrophages by suppressing MAPK and NF-κb signal pathways. Environ. Toxicol. Pharmacol. 2015, 41, 159. [Google Scholar] [CrossRef]
- Dasiman, R.; Md Nor, N.; Eshak, Z.; Syairah Mohd Mutalip, S.; Suwandi, R.; Bidin, H. A Review of Procyanidin: Updates on Current Bioactivities and Potential Health Benefits. Biointerface Res. Appl. Chem. 2021, 12, 5918. [Google Scholar] [CrossRef]
- Bischoff, S.C. Role of mast cells in allergic and non-allergic immune responses: Comparison of human and murine data. Nat. Rev. Immunol. 2007, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Elieh-Ali-Komi, D.; Metz, M.; Siebenhaar, F.; Maurer, M. Understanding human mast cells: Lesson from therapies for allergic and non-allergic diseases. Nat. Rev. Immunol. 2021, 22, 294. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Wilson, T.M.; Metcalfe, D.D. The mast cell and allergic diseases: Role in pathogenesis and implications for therapy. Clin. Exp. Allergy 2007, 38, 4. [Google Scholar] [CrossRef]
- Metz, M.; Kolkhir, P.; Altrichter, S.; Siebenhaar, F.; Levi-schaffer, F.; Youngblood, B.A.; Church, M.K.; Maurer, M. Mast cell silencing: A novel therapeutic approach for urticaria and other mast cell-mediated diseases. Allergy 2023, 79, 37. [Google Scholar] [CrossRef]
- Korinek, M.; Chen, K.; Jiang, Y.; El-Shazly, M.; Stocker, J.; Chou, C.; Hwang, T.; Wu, Y.; Chen, B.; Chang, F. Anti-allergic potential of Typhonium blumei: Inhibition of degranulation via suppression of PI3K/PLCγ2 phosphorylation and calcium influx. Phytomedicine 2016, 23, 1706. [Google Scholar] [CrossRef]
- Wagner, A.; Alam, S.B.; Kulka, M. The effects of age, origin, and biological sex on rodent mast cell (BMMC and MC/9) and basophil (RBL-2H3) phenotype and function. Cell. Immunol. 2023, 391–392, 104751. [Google Scholar] [CrossRef]
- Passante, E.; Frankish, N. The RBL-2H3 cell line: Its provenance and suitability as a model for the mast cell. Inflamm. Res. 2009, 58, 737. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Mckenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021. [Google Scholar] [CrossRef]
- Faggion, C.M. Guidelines for Reporting Pre-clinical In Vitro Studies on Dental Materials. J. Evid. Based Dent. Pract. 2012, 12, 182. [Google Scholar] [CrossRef]
- Lam, T.; Tran, N.N.; Pham, L.D.; Lai, N.V.; Dang, B.N.; Truong, N.N.; Nguyen-Vo, S.; Hoang, T.; Mai, T.T.; Tran, T. Flavonoids as dual-target inhibitors against α-glucosidase and α-amylase: A systematic review of in vitro studies. Nat. Prod. Bioprospect. 2024, 14, 4. [Google Scholar] [CrossRef]
- Bansode, R.R.; Plundrich, N.J.; Randolph, P.D.; Lila, M.A.; Williams, L.L. Peanut flour aggregation with polyphenolic extracts derived from peanut skin inhibits IgE binding capacity and attenuates RBL-2H3 cells degranulation via MAPK signaling pathway. Food Chem. 2018, 263, 307. [Google Scholar] [CrossRef]
- Dippenaar, C.; Shimbo, H.; Okon, K.; Miller, N.; Joubert, E.; Yoshida, T.; De Beer, D. Anti-Allergic and Antioxidant Potential of Polyphenol-Enriched Fractions from Cyclopia subternata (Honeybush) Produced by a Scalable Process. Separations 2022, 9, 278. [Google Scholar] [CrossRef]
- Gaihre, Y.R.; Iwamoto, A.; Oogai, S.; Hamajima, H.; Tsuge, K.; Nagata, Y.; Yanagita, T. Perilla pomace obtained from four different varieties have different levels and types of polyphenols and anti-allergic activity. Cytotechnology 2022, 74, 341. [Google Scholar] [CrossRef]
- Hamauzu, Y.; Kawahara, T. Anti-degranulation and bile acid-binding activity of extracts from fruits and agro-industrial by-products. Food Biosci. 2021, 43, 101244. [Google Scholar] [CrossRef]
- Liu, M.; Lu, J.; Chen, Y.; Shi, X.; Li, Y.; Yang, S.; Yu, J.; Guan, S. Sodium Sulfite-Induced Mast Cell Pyroptosis and Degranulation. J. Agric. Food Chem. 2021, 69, 7755. [Google Scholar] [CrossRef]
- Lv, L.; Ahmed, I.; Qu, X.; Ju, G.; Yang, N.; Guo, Y.; Li, Z. Effect of the structure and potential allergenicity of glycated tropomyosin, the shrimp allergen. Int. J. Food Sci. Technol. 2022, 57, 1782. [Google Scholar] [CrossRef]
- Matsui, T.; Ito, C.; Masubuchi, S.; Itoigawa, M. Licarin A is a candidate compound for the treatment of immediate hypersensitivity via inhibition of rat mast cell line RBL-2H3 cells. J. Pharm. Pharmacol. 2015, 67, 1723. [Google Scholar] [CrossRef]
- Matsui, T.; Ito, C.; Itoigawa, M.; Shibata, T. Three phlorotannins from Sargassum carpophyllum are effective against the secretion of allergic mediators from antigen-stimulated rat basophilic leukemia cells. Food Chem. 2022, 377, 131992. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Wei, J.; Li, X.; Jin, Y.; Shi, X. Inhibitory activity of narirutin on RBL-2H3 cells degranulation. Immunopharmacol. Immunotoxicol. 2020, 43, 68. [Google Scholar] [CrossRef]
- Vo, T.S.; Kim, S.; Ryu, B.; Ngo, D.H.; Yoon, N.; Bach, L.G.; Hang, N.; Ngo, D.H. The Suppressive Activity of Fucofuroeckol-A Derived from Brown Algal Ecklonia stolonifera Okamura on UVB-Induced Mast Cell Degranulation. Mar. Drugs 2018, 16, 1. [Google Scholar] [CrossRef]
- Zeng, J.; Hao, J.; Yang, Z.; Ma, C.; Gao, L.; Chen, Y.; Li, G.; Li, J. Anti-Allergic Effect of Dietary Polyphenols Curcumin and Epigallocatechin Gallate via Anti-Degranulation in IgE/Antigen-Stimulated Mast Cell Model: A Lipidomics Perspective. Metabolites 2023, 13, 628. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Li, X.; Sun, Y.; Shao, Y.; Zhang, Y.; Liu, Z. Network pharmacology-based analysis and experimental in vitro validation on the mechanism of Paeonia lactiflora Pall. in the treatment for type I allergy. BMC Complement Med. Ther. 2022, 22, 199. [Google Scholar] [CrossRef]
- Hanieh, H.; Hairul Islam, V.I.; Saravanan, S.; Chellappandian, M.; Ragul, K.; Durga, A.; Venugopal, K.; Senthilkumar, V.; Senthilkumar, P.; Thirugnanasambantham, K. Pinocembrin, a novel histidine decarboxylase inhibitor with anti-allergic potential in in vitro. Eur. J. Pharmacol. 2017, 814, 178. [Google Scholar] [CrossRef]
- Passante, E.; Frankish, N. Deficiencies in elements involved in TLR4-receptor signalling in RBL-2H3 cells. Inflamm. Res. 2009, 59, 185. [Google Scholar] [CrossRef]
- Falcone, F.H.; Wan, D.; Barwary, N.; Sagi-eisenberg, R. RBL cells as models for in vitro studies of mast cells and basophils. Immunol. Rev. 2018, 282, 47. [Google Scholar] [CrossRef] [PubMed]
- Passante, E. Mast Cells and Basophil Cell Lines: A Compendium. Basophils and Mast Cells: Methods and Protocols, 2nd ed.; Gibbs, B.F., Falcone, F.H., Eds.; Humana Press: New York, NY, USA, 2023; pp. 127–144. [Google Scholar]


| Ref. | Objectives | Methods: Treatment; Control | Methods: Dose (Duration) | Method: Sensitization, Treatment, Stimulation Sequence | Methods: Assays | Main Findings | Findings: Signaling Pathways in RBL-2H3 Cells |
|---|---|---|---|---|---|---|---|
| Bansode et al., 2018 [51] | Anti-allergic activity of PSP-enriched PN protein aggregate | PSP-PN aggregates; peanut flour w/o ionomycin (control) | Anti-DNP-IgE @ 1 µg/mL (overnight); PN: PSP aggregate (0–40% PSP: PN flour ratio, w/w), the protein exposure level @ 100 μg/mL; DNP-BSA @ 1 μg/mL + ionomycin @ 1 μM (3 h) | IgE > Tmt > DNP-BSA+ Ionomycin | WB, ELISA, histamine assay, β-hex assay | 30% PSP-PN ↓ β-hex (54.2%) and histamine (49.2%). 40% PSP-PN ↓ IgE binding by 19%. PSP-PN aggregates ↓ p44/42 MAPK, but ↑ p38 MAPK and SAPK/JNK | ↓ MAPK p44/42 |
| Barbosa et al., 2018 [2] | Anti-allergic property of phlorotannin-targeted extract of seaweed | Phlorotannin-targeted extracts; Quercetin (+ve) | Extract @ 125–500 μg/mL (30 min); A23187 @ 150 ng/mL (30 min); OR Anti-DNP IgE @ 50 ng/mL (16 h); DNP-BSA @ 50 ng/mL+ dry extract @ 125–500 μg/mL (1 h) | Tmt > A23187; IgE > DNP-BSA + Tmt | MTT reduction assay, CVS assay, HAase inhibition assay, β-hex assay | Extracts ↓ β-hex (79% to 31% ↓ at 500 μg/mL extract) and histamine (67 to 55% ↓ at 500 μg/mL extract) released in a dose-dependent manner | NA |
| Chang et al., 2015 [3] | Beneficial effect of the Monascin and ankaflvin | Monascin, ankaflavin, rosiglitazone, and GW9662 (PPARγ antagonist); Ionomycin/PMA (+ve), 15% FBS (MEM) (-ve) | Monascin, ankaflavin, rosiglitazone (each @ 40 μM) (24 h); PMA @ 50 nM PMA + ionomycin @ 500 nM (3 h). For β-hex, treated cells > ionomycin @ 10 μM (30 min) | Tmt > PMA + Ionomycin | CVS assay, WB, ELISA, β-hex assay | Forty micromolar monascin and ankaflavin ↓ PMA/ionomycin-induced mast cell degranulation and TNF-α secretion through ↓ PKC and MAPK family (ERK, JNK, and p38) | ↓ MAPK (ERK, JNK, and p38) and PKC |
| Dippenaar et al., 2022 [52] | Develop a protocol for Honeybush extract to yield fractions with higher anti-allergy potential | Hot water extract, four fractions on XAD 1180N; MEM (−ve), Wortmannin (W) (+ve) | Anti-DNP-IgE @ 500 ng/mL (24 h); extract @ 62.5–250 μg/mL or W @ 100 nM (30 min); DNP-hSA @ 5 ng/mL (1 h) | IgE > Tmt > DNP-hSA | β-hex assay, SARS assay, DPPH scavenging assay, ORAC assay, XOI assay | Fraction 1 ↓ the β-hex activity compared to extract | NA |
| Gaihre et al., 2022 [53] | Perilla pomace’s (i.e., Egoma) function in allergy | Egoma extracts (four cultivars); Tyrode buffer (control) | Anti-DNP-IgE @ 33.3 ng/mL (2 h); Tyrode’s buffer +/− extract (10 min); 30 μL (for β-hex) or 150 μL of DNP-HSA @ 200 μg/mL (1 h) | IgE > Tmt > DNP-HSA | CCK-8 assay, WB, HPLC, β-hex assay | Compared to the control, extracts from two Japanese cultivars ↓ β-hex, Syk, PLCγ2, and Akt proteins | ↓ Syk, PLCγ2, and Akt |
| Hamauzu et al., 2021 [54] | Anti-allergic effect of fruit extracts and agro-industrial by-products | Hot water and ethanol extracts of mature fruits, immature fruits, liquor residue, lima bean pod, and pomace | Extracts @ 100 μg/mL (24 h); anti-DNP IgE @ 1 μg/mL (2 h); DNP-hSA @ 10 ng/mL (30 min) | Tmt > IgE > DNP-hSA | β-hex assay, TPC assay, proanthocyanidin assay, HPLC, pectin content assay, bile acid-binding assay | Grape bunch stem ethanol extract had the highest ↓ (63.6%) on β-hex. No correlation between TPC and anti-degranulation property, +ve correlation between proanthocyanidin and degranulation. Higher proanthocyanidin content (100 mg GAE/g dry extract) adversely affected degranulation | NA |
| Jiao et al., 2017 [20] | The anti-allergic potential of dysivillosins A–D (form Marine sponge) | Dysivillosins A–D; ketotifen (+ve) | DNP-IgE @ 1 μg/mL (overnight); dysivillosins A–D @ 6 and 12 μM (30 min); DNP-BSA @ 1 μg/mL (1.5 h) | IgE > Tmt > DNP-BSA | MTT assay, WB, ELISA, β-hex assay | All compounds dose-dependently ↓ β-hex, IL-4, and LTB4. Dysivillonsin A was themost potent and reduced PLCγ1 and Syk in a dose-dependent manner | ↓ PLCγ1, Syk |
| Kawai et al., 2018 [21] | Anti-allergic effect of Grifola frondosa mushroom extract (GFE) | GFE; DNP-hSA (-ve), Tranilast (+ve) | Anti-DNP IgE @ 10 μL/mL (24 h); DMSO (0.5%) or ethanol (1%) dissolved extracts @ 10–50 μM (20 min); DNP-hSA @ 20 ng/mL (30 min) and β-hex and histamines measured. For mRNA detection, the anti-DNP IgE @ 10 μL/mL (24 h); ergosterol @ 50 ng/mL (20 min or for 0–10 min for phospho-tyrosine); DNP-hSA @ 20 ng/mL (2 h) or @ 50 ng/mL (0–10 min) for phospho-tyrosine | IgE > Tmt > DNP-hSA | β-hex assay, histamine release assay, RT-qPCR, WB, immunofluorescent microscopy | The active compounds ↓ β-hex, histamine. Ergosterol ↓ FcεRI aggregation, IL-4, TNF-α mRNA | ↓ FcεRI aggregation |
| Kobayashi et al., 2015 [4] | Cell degranulation property of PMFs from Kaempferia parviflora | PMFs; Wortmannin (control for IgE induced β-hex), A23187 and DTBHQ + PMA (control for A23187 induced β-hex) | Anti-DNP IgE @ 50 ng/mL (2 h); whole extracts @ 0–1000 μg/mL or extract components @ 0–100 μM or Wortmannin @ 25 μM (10 min); DNP-hSA @ 50 ng/mL (30 min) and β-hex measured. For A23187 induced degranulation, A23187 @ 10 μL of 250 μg/mL or 10 μL of 500 μM of DTBHQ+ 10 μL PMA (30 min). For PLCγ1, PLCγ2, Syk, FcεRI protein measurement, the sensitized cells were incubated with KP02 and KP10 @ 100 μM (1 h); DNP-hSA @ 50 ng/mL (1 min) | IgE > Tmt > DNP-hSA; A23187 or DTBHQ + PMA > Tmt> DNP-hSA | β-hex assay, WB | All the flavones ↓ β-hex. The KP02 and KP10 ↓ PLCγ1 and Syk. No effect on cytoplasmic FcεRI but ↓ membrane FcεRI | ↓ PLCγ1, Syk and membrane FcεRI |
| Lee et al., 2017 [8] | Mechanism of anti-allergic effect of the Zizania latifolia extract | Extracts or its fractions; IgE+ DNP-BSA (control) | Anti-DNP IgE @ 450 ng/mL (overnight); 20 μL of extract @ 10–100 μg/mL or the extract fractions @ 0–50 μg/mL (10 min) (or 30 min for COX-2); DNP-BSA @ 20 μL of 10 μg/mL (10 min) (or 4 h for COX-2) and β-hex was measured. For TNF-α, chloroform fraction @ 0–50 μg/mL; A23187 @ 1 μM+ 50 ng/mL PMA (4 h). For protein analysis, anti-DNP IgE @ 450 ng/mL (overnight); chloroform fraction 0–50 μg/mL; DNP-BSA @ 10 μg/mL (15 min) | IgE > Tmt > DNP-BSA; IgE > Tmt > A23187 + PMA | β-hex assay, WB, MTT assay, ELISA | Chloroform faction ↓ β-hex, TNF-α, COX-2, and MAPKs | ↓ MAPK family (p38, ERK, JNK) |
| Lee et al., 2020 [25] | Mechanism of tricin and enzyme-treated wild rice Zizania latifolia extract (ETZL) on cell degranulation | Tricin or ETZL; DNP-IgE and/or DNP-hSA (control) | Anti-DNP IgE @ 0.05 μg/mL (24 h); tricin @ 10–500 ng/mL or ETZL @ 10–500 μg/mL (1 h); DNP-hSA @ 0.1 μg/mL (4 h) (or 10 min for ERK, AKT, p38, JNK proteins) | IgE > Tmt > DNP-hSA | β-hex assay, WB, MTT assay, ELISA | Tricin and ETZL ↓ β-hex, TNF-α, IL-4, LTB4, LTC4, PGE2, cytosolic phospholipase A2, 5-lipoxygenase and COX-2, Akt, ERK, p38, JNK, PKC-δ, PLCγ1, Lyn, and Syk, but not much effect on Fyn | ↓ MAPK, PKC-δ, PLCγ1, Lyn, Syk, Akt |
| Li et al., 2022 [11] | Mechanism of Schischk (Sk) on type I allergy | Sk; KF (+ve), POG (model group) | Anti-DNP IgE @ 0.4 μg/mL (12 h); Sk @ 0.5–2 mg/mL or POG @ 10–80 μg/mL or KF @ 30 μM (12 h); DNP-BSA @ 0.4 μg/mL (1 h) | IgE > Tmt > DNP-BSA | β-hex assay, RT-qPCR | SD ↓ β-hex, proteins in Lyn/Syk, PI3/AKT and MAPK | ↓ Lyn/Syk, PI3/AKT and MAPK |
| Liu et al., 2021 [55] | Mechanism of sodium sulfite-induced pyroptosis and its effect on degranulation | Sodium sulfite; NAC or MCC950 (+ve) | Sodium sulfite @ 2–8 mM (30 min) or NAC @ 5 mM or MCC950 @ 10 μM (24 h) before treated with sodium sulfite @ 8 mM (30 min) | NAC orMCC950 > Sodium sulfite | β-hex assay, histamine assay, WB, MTT assay, ELISA | Sodium sulfite ↑ ROS, NLRP3, caspase-1, GSDMD-N, IL-1β, IL-18, cell membrane rupture, β-hex, histamine | ↑ NLRP3, caspase-1 |
| Liu et al., 2023 [9] | Viridicatol’s from Penicillium griseofulvum cell activation mechanism | Viridicatol; DNP-BSA (+ve) | Anti-DNP IgE @ 100 ng/mL (16 h); viridicatol @ 10 μg/mL (or @ 2.5–10 μg/mL for proteins JNK, ERK, p38, STAT6 study) (1 h); DNP-BSA @ 500 ng/mL (1 h) (or 15 min for protein measurement) | IgE > Tmt > DNP-BSA | RT-qPCR, RNA sequencing, WB | Viridicatol ↓ cell activation related genes Tnfα, Ccl2, Jun, Fos, Il4, Ccl7, Il13, and Socs1 and proteins JNK, ERK, P38, and STAT6 | ↓ JNK, ERK, P38, and STAT6 |
| Lv et al., 2022 [56] | Effect of ribose treated tropomyosin (from shrimp) (TM) on allergenicity | TM, glycated TM; Growth media (control) | Mouse sera @ 1:10 dilution (overnight); TM and 100 μL glycated TM @ 4000 mmol/l ribose (1 h) | Mouse Sera > Tmt | β-hex assay, ELISA | The glycated TM ↓ the histamine and β-hex | NA |
| Ma et al., 2022 [10] | Effect of POS on mast cell activation and degranulation | Oligosachharides; KF (+ve) | Anti-DNP IgE @ 0.5 μg/mL (overnight); galacturonic acid (GalA), Di-GalA, Tri-GalA or POS @ 75–300 μg/mL or KM (1 h); DNP-BSA @ 10 μg/mL or with PIPES @ 200 μL (30 min). For intracellular Ca2+ measurement, cells treated with inhibitors U73122, 2-APB, SKF96365 @ 19 μL (30 min); anti-DNP IgE; Tri-GalA @ 150 μg/mL; DNP-BSA | IgE > Tmt > DNP-BSA | β-hex assay, histamine release assay, ELISA, MTT assay | Tri-GalA and POS ↓ histamine, β-hex, IL-4, and Ca2+ influx | NA |
| Matsui et al., 2015 [57] | Anti-allergic effect of licarin A (a neolignan from plants) | Licarin; DNP-hSA (+ve) | Anti-DNP IgE @ 0.5 μg/mL (24 h); licarin A @ 0–20 μM (1 h) or only 20 μM (30 min) for Ca2+ measurement); DNP-hSA @ 0.2 μg/mL (30 min for histamine or (6 h) for PGD2 and COX-2 protein or (12 h) for TNF-α. For (p65, NF-κB, p38 MAPK, JNK, PKCα/β II proteins, licarin A @ 20 μM; DNP-HSA (0–30 min). For mRNA of COX-2 and TNF-α, sensitized and treated cell were stimulated with DNP-hSA (1–5 h) | IgE > Tmt > DNP-hSA | Spectro fluorometry, MTT assay, ELISA and WB, PGD2 assay | Licarin A ↓ TNF-α, PGD2, COX-2 (mRNA level), PKCα/β II and p38 MAPK proteins | ↓ PKCα/β II and p38 MAPK |
| Matsui et al., 2022 [58] | Anti-allergic activity of phlorotannins from brown seaweed | Phlorotannin; DNP-hSA (control) | Anti-DNP IgE @ 100 ng/mL (overnight); phlorotannins @ 0.2–300 μM (30 min); DNP-hSA @ 100 ng/mL (1 h) for β-hex or (8 h) for PGD2 and TNF-α or (1 h and 3 h) for mRNA of COX-2 and TNF-α or (10 h) for ROS | IgE > Tmt > DNP-hSA | β-hex assay, ELISA, MTT assay, PGD2 assay, WB | Phlorotannins ↓ β-hex, PGD2, TNF-α | NA |
| Mwakalukwa et al., 2019 [6] | Anti-allergic activity of olive mill waste (OMW) polyphenolic compounds | OMW extracts; DNP-BSA or A23187 (control) | Fractions of extracts such as new HDOA, luteolin, HOTy acetate @ 3.125–100 μg/mL or HOTy and 1-acetoxypin @ 5–250 μg/mL or DMSO @ 0.5 μL/well or quercetin (1 h); A23187 @ 5μM (1 h) or A23187 @10 μg/mL or DNP-BSA@ 100 ng/mL (180 sec) for Ca2+ measurement. For mRNAs of calcium channel proteins, TRPC1, STIM1, and Orai1 and ER membrane protein, IP3R, anti-DNP IgE sensitized cells were treated with compounds (16 h); DNP-BSA | Tmt> A23187;IgE > Tmt > DNP-BSA | RT-qPCR, β-hex assay, Calcium kit, MTT assay | Novel compound of OMW ↓ intracellular Ca2+ influx and calcium channel proteins | NA |
| Niu et al., 2020 [59] | Narirutin’s inhibition mechanism on degranulation | Narirutin; anti-DNP-IgE/DNP-BSA (control) | Anti-DNP IgE @ 0.5 μg/mL (overnight) or for Ca2+ (12 h); narirutin @ 0–200 μM (or 0–100 μM for histamine, Ca2+, IL-4 and TNF-α or 0–80 μM for mRNA of FcεRI α/β/γ) (2.5 h); DNP-BSA @ 0.5 μg/mL (1 h) | IgE > Tmt > DNP-BSA | CCK-8 assay, ELISA, WB, PCR, microscopy, β-hex assay, histamine assay | Narirutin ↓ Ca2+ influx via ↓ Syk, LAT, PLCγ1, and ↓ Ca2+ causes ↓ NF-κB. Narirutin also ↓ phosphorylated P38, ERK, JNK leading to ↓ of IL-4, TNF-α, histamine and β-hex | ↓ MAPK family (p38, ERK, JNK), Syk, LAT, PLCγ1, and NF-κB |
| Shi et al., 2016 [23] | The anti-allergic activity of the Edulis Superba root extract | Extract; baicalein (+ve) | Anti-DNP IgE @ 0.5 μg/mL (24 h); extract @ 50 μM (30 min); DNP-BSA @ 1 μg/mL (1 h) and β-hex was measured | IgE > Tmt > DNP-BSA | β-hex assay, CCK-8 assay | The extract (mudanpioside E and quercetin) ↓ β-hex | NA |
| Vo et al., 2018 [60] | Anti-allergic property of brown algae extract (fucofuroeckol-A) | Fucofuroeckol-A (F-A); no UVB (-ve) | F-A @ 0–50 μM (24 h); UVB exposure (1 h) for ROS and histamine or (2 h) for cytokines or (10 min) for Ca2+ | Tmt > UVB | ELISA, histamine assay, microscopy, MTT assay | F-A ↓ histamine, Ca2+, IL-1β, TNF-α, ROS | NA |
| Vo et al., 2020 [18] | Myricetin’s (from Aiton fruit) effect on mast cell activation | Myricetin; DNP-BSA (Control) | Myricetin @ 10–40 μM or only 40 μM for Ca2+ (24 h); anti-DNP-IgE @ 1μg/mL (10 min); DNP-BSA @ 1 μg/mL (1 h) or 10 min for Syk, PLCγ1, and NF-κB, or 24 h for IL-4, TNF-α. For DPPH and ABST assays, 100 μL of myricetin @ 2–16 μM were used | Tmt > IgE > DNP-BSA | MTT assay, WB, β-hex assay, ELISA, DPPH scavenging assay, ABTS scavenging assay | Myricetin ↓ β-hex and Ca2+, IL-4, TNF-α, Syk, PLCγ1, NF-κB, DPPH and ABTS+ radicals | ↓ Syk, PLCγ1, NF-κB |
| Yan et al., 2024 [12] | Anti-allergic property of sea buckthorn flavonoid (SBF) or its purified form (PSBF) | SBF or PSBF; KM (+ve) | Anti-DNP-IgE @ 0.5 μg/mL (overnight) or 12 h for Ca2+; SBF or PSBF @ 25–100 μg/mL or five flavonoids of SBF/PSBF @ 10–40 μg/mL (1 h); DNP-BSA @ 10 μg/mL (30 min). For protein analysis, the five compounds, and SBF and PSBF were used @ 100 and 40 μg/mL, respectively | IgE > Tmt > DNP-BSA | β-hex assay, histamine release assay, WB, MTT assay, ELISA | PSBF ↓ degranulation, IL-4, extracellular Ca2+ influx, SBF ↓ p38, and JNK expression | ↓ p38 and JNK expression |
| Zeng et al., 2023 [61] | Anti-allergic activity of curcumin and EGCG | Curcumin or EGCG; anti-DNP-IgE/DNP-BSA (+ve) | Anti-DNP-IgE @ 200 ng/mL (18 h); (curcumin @ 5–50 μM or 5–30μM for IL-4 and TNF-α or EGCG @ 100–650 μM or @ 100–500 μM for IL-4 and TNF-α)+ DNP-BSA @ 500 ng/mL (1 h or 3 h) or (3 h) for IL-4 and TNF-α | IgE > Tmt + DNP-BSA | β-hex assay, MTT assay, and ELISA | Both curcumin and EGCG ↓ β-hex, IL-4, and TNF-α | NA |
| Zhao et al., 2022 [62] | Mechanism of Paeonia lactiflora Pall (PLP) on anti-allergic effect | Paeoniflorin; KF (+ve) | Anti-DNP-IgE @ 0.2 μg/mL (12 h); Paeoniflorin @ 0.5–5 μg/mL or KF @ 25 μg/mL (1 h); DNP-BSA @ 0.4 μg/mL (30 min) | IgE > Tmt > DNP-BSA | WB, RT-qPCR | Paeoniflorin ↓ Lyn, Syk, Fyn, PLCγ, PI3K, Akt, p38, ERK, JNK, and p65 genes | ↓ Lyn, Syk, Fyn, PLCγ, PI3K, Akt, p38, ERK, JNK, and p65 |
| Ref. | Objectives | Methods: Cell Line | Methods: Treatment; Control | Methods: Dose (Duration) | Method: Sensitization, Treatment, Stimulation Sequence | Methods: Assays | Main Findings | Findings: Signaling Pathways in RBL-2H3 Cells |
|---|---|---|---|---|---|---|---|---|
| Badger-Emeka et al., 2020 [1] | Anti-allergic effect of cinnamaldehyde (CA) from cinnamon bark | RBL-2H3, Klebsiella pneumoniae (bacteria) | CA; DMSO (control),DNP-IgE (control) | For RBL-2H3: CA or DMSO @ 5–50 µM (10 min); DNP-IgE (24 h); DNP-BSA (24 h). For bacteria, the 25 µL of 5–50 µM CA dissolved in 0.1% DMSO was applied to bacterial culture and incubated at 37 °C (12 h), and inhibition zones for HDC activity were measured. | Tmt > IgE > DNP-BSA | MTT assay, ELISA, WB, RT-qPCR, histamine assay, and β-hex assay | CA ↓ MAPK p38/ERK pathway. CA ↓ all the measured parameters compared to the DNP-IgE treated RBL-2H3 cells. CA ↓ the HDC activities both in RBL-2H3 and bacterial cells | ↓ MAPK p38/ERK |
| Dera et al., 2020 [15] | Effect of thymoquinone from black caraway on allergy | RBL-2H3, RAW264.6, human neutrophil basophil | Thymoquinone (Tq); anti-DNP-IgE + DNP-BSA (control) | For RBL-2H3: Anti-DNP IgE @ 1 μg/mL (overnight); Tq @ 0–50 μM (30 min) or 1 h for degranulation and cytokines; DNP-BSA @ 0.025 μg/mL (4 h). | IgE > Tmt > DNP-BSA | BAT assay, MTT assay, neutrophil migration assay, neutrophil elastase assay, ELISA, WB | Tq dose-dependently ↓ of TNF-α and IL-4. Tq ↓ Akt, NF-κB phosphorylation, and ↑ nuclear Nrf2 and HO-1 proteins in activated RBL-2H3 cells | ↓ NF-κB and Akt |
| Hanieh et al., 2017 [63] | Pinocembrin’s effect on IgE-mediated response | RBL-2H3, Klebsiella pneumoniae (bacteria) | Pinocembrin; DMSO (+ve) | For RBL-2H3: DMSO or Pinocembrin @ 10–50 μmole/l (10 min); anti-DNP-IgE @ 1 μg/mL (4 h) for cell viability test or 24 h for β-hex, NO, WB and ELISA; DNP-BSA @ 100 μg/mL (24 h). For bacteria and microbial integrity tests, the sensitized and stimulated cells were stained with rhodamine123 @ 1 μg/mL. The bacteria were also used for a preliminary study of HDC activity. | Tmt > IgE > DNP-BSA | β-hex assay, WB, RT-qPCR, HDC activity and inhibitory activity assays, MTT assay, MMIT, NO assay | Pinocembrin ↓ HDC activity, histamine, damage of mitochondrial membrane, β-hex, TNF-α, IL-6, iNOS, PGE-2, and COX-2. Pinocembrin ↑ p38 MAPK through IkB pathway | ↑ p38 MAPK through IkB pathway |
| Hagenlocher et al., 2015 [19] | Cinnamaldehyde’s (CA) or Cinnamon extract (CE) effect on mast cell activation | RBL-2H3, human intestine mast cell (hiMC) | CA, CE; 0.1% DMSO (control for CA), 70% ethanol (control for CE) | For RBL-2H3 and hiMC cells: CA @ 5– 500 μM or CE @ 1 and 10 μL/mL or DMSO or 70% ethanol (18 h). (a) for IgE-dependent activation: DNP-IgE for RBL-2H3 or myeloma IgE for hiMC @ 0.1 μg/mL; DNP-BSA (RBL-2H3) @ 0.1 μg/mL or polyclonal anti-human IgE (hiMC) (5 min) or (10 min) for ERK and PLCγ1 or (90 min) for cytokine and mediator release or (6 h) for CXCL8. (b) For IgE-independent activation, cells were stimulated with ionomycin/PMA) @ 1 μM. | Tmt > IgE > DNP-BSA;Tmt > myeloma IgE > Anti-human IgE; Tmt > IgE > Ionomycin or PMA | Cell death detection kit, ELISA, β-hex assay, RT-PCR, WB | In RBL-2H3, CA ↓ the β-hex in dose-dependent manner to 10%. In hiMC, CA ↓ β-hex, LTC4, CXCL8, CCL2–4, ERK and PLCγ1 | NA |
| Kong et al., 2020 [22] | Curcumin’s effect on allergic inflammation | RBL-2H3 and huma pre-basophils (KU812) | Curcumin; no curcumin, no IgE, or no A23187(controls) | For RBL-2H3: anti-DNP IgE @ 500 ng/mL (24 h) or @ 1 μg/mL (overnight) for histamine or ROS, respectively; curcumin @ 1–30 μM; DNP-BSA @ 500 ng/mL (1 h) or (13 min) for ROS. For A23187 dependent activation: curcumin: A23187 @ 1μM or @ 2 μM for histamine or ROS, respectively (30 min) or ROS (13 min). For RT-PCR, KU812; for PKC, RBL-2H3; and for FcεRI protein, both cell types were used. | IgE > Tmt > DNP-BSA or A23187 | β-hex assay, WB, MTT assay, histamine release assay, ROS production assay, RT-PCR | Curcumin ↓ β-hex, histamine, ROS, FcεRI, IL-4, IL-13 production, and PKC-δ translocation in IgE or A23187 induced cells | ↓ FcεRI and PKC-δ |
| Korinek et al., 2016 [45] | Mechanism of the anti-allergic and anti-inflammatory effect of Typhonium blumei and T. roxburghii | RBL-2H3 and human neutrophil | Extracts (TBLE or TBE); dexamethasone (+ve) | For RBL-2H3: extracts (TBLE or TBE) @ 10–100 μg/mL or TBLE @ 1–100 μg/mL for mRNA study (20 h); (a) for IgE independent activation: A23187 @1 μM (1 h) or 2 μM (10 min) for proteins measurement) or (b) for IgE-dependent activation: anti-DNP IgE @ 5 μg/mL (2 h); DNP-BSA @ 100 ng/mL (1 h) or (10 min) for mRNA and protein study. Protein of ERK, JNK, p-38, Akt, PI3K and PLCγ2, and mRNA of IL-4 and MCP-1 were measured. | Tmt > IgE > A23187 or DNP-BSA | β-hex assay, WB, MTT assay, histamine release assay, flow cytometry, fluorescence microscopy, RT-qPCR, SAG assay | T. blumei’s non-polar fraction ↓ antigen-induced β-hex, histamine, and calcium influx (induced by antigen and A23187). No effect on FcεRI, IL-4, and MCP (mRNA) or MAPK, but ↓ PI3K/PLCγ2 | ↓ PI3K/PLCγ2 |
| Lim et al., 2023 [27] | Therapeutic potential of Rosa davurica leaf extract (RLE) against allergy | RBL-2H3 and RAW 264.7 | RLE; KF and tacrolimus (+ve control for RBL-2H3), dexamethasone (+ve control for RAW 264.7) | For RBL-2H3: anti-DNP IgE @ 50 ng/mL (24 h); RLE @ 10–100 μg/mL or KM @ 50 μM or tacrolimus @ 50 ng/mL (1 h); DNP-BSA @100 ng/mL (4 h). For RAW 264.7: RLE @ 10–100 μg/mL (1 h); LPS @ 1 μg/mL or dexamethasone @ 10 μM. In RAW 264.7, mRNA of inducible nitrogen oxygen synthase (iNOS), IL-1β, Il-6, TNF-α, COX-2, and NO were measured. | IgE > Tmt > DNP-BSA; Tmt > LPS | β-hex assay, histamine assay, WB, MTT assay, ELISA, calcium assay, RT-qPCR | In Raw 264.7, RLE ↓ NO, COX-2, iNOS, IL-1β, Il-6, TNF-α. In RBL-2H3, RLE ↓ β-hex, histamine, HDC, Ca2+ influx, Ca2+ pathways, and MAPK | ↓ MAPK (p38, JNK, ERK) |
| Lorenz et al., 2016 [5] | Effect of oak bark decoction (OBD) and tannin in degranulation | RBL-2H3 and human mast cell (HMC-1) | OBD tannin fractions; DMEM (-ve control), azelastine (+ve control) for RBL-2H3; DMEM and dexamethasone (+ve controls) for HMC-1 | For RBL-2H3: anti-DNP IgE @ 0.5 μg/mL (12–18 h); OBD tannin fractions A @ 0.017–0.17 mg/mL or fractions (B–D) @ 6–100 μg/mL (10 min); DNP-hSA @ 20 ng/mL DNP-HSA (30 min). For HMC-1: OBD fractions A–D at the same rate as for RBL-2H3 cells (30 min) or dexamethasone; PMA @ 40 nM and A23187 @ 1 μM (2.5 h) and IL-8, IL-6, and TNF-α were measured. | IgE > Tmt > DNP-hSA; Tmt > PMA + A23187 | WST-1 assay, β-hex assay, ELISA | The fractions ↓ β-hex (in RBL-2H3), IL-8, IL-6, and TNF-α in a dose-dependent manner | NA |
| Min et al., 2021 [26] | Anti-inflammatory, ani-allergic properties of saponarin from green barley leaves | RBL-2H3, RAW264.7, Human immortalized keratinocyte (HaCaT) cells | Saponarin; cyclosporine A (control for RBL-2H3), quercetin (control for RAW264.7) | For RAW264.7: LPS @ 1 μg/mL; saponarin @ 20–80 μM (or only 80 μM for ERK, JNK, p38, TNF-α, IL 6, IL-1β, iNOS, COX-2) or quercetin @ 15μM+ LPS @ 1 μg/mL (24 h) and NO was measured. For RBL-2H3: anti-DNP IgE @ 0.5 μg/mL (24 h); saponarin @ 5–40 μM for β-hex or only 40 μM for rest of all measurements or cyclosporine A @ 1 μg/mL (20 min); DNP-BSA @ 100 ng/mL (1 h). For HaCaT: 100 μM saponarin (24 h) or (1 h for ERK, JNK, p38) or (18 h for IL-33, IL-25, MDC, TARC, TSLP); 50 ng/mL of TNF-α and IFN-γ (24 h) (or 15 min for ERK, JNK, p38 or 6 h for IL-33, IL-25, MDC, TARC, TSLP) and STAT1 was measured. | LPS > Tmt;IgE > Tmt > DNP-BSA; Tmt > TNF-α and IFN-γ | β-hex assay, ELISA, MTT assay, RT-qPCR, WB | Saponarin (80 μM) ↓ TNF-α, IL-1β, iNOS, COX-2, ERK and p38 MAPK in RAW264.7 cells. Saponarin (40 μM) ↓ β-hex, Syk, PLCγ1, ERK, JNK, p38, TNF-α, IL-4, 5, 6, 13, COX-2, and FcεRI α/γ in RBL-2H3. Moreover, Saponarin (100 μM) ↓ IL-33, ERK, p38, STAT1, in HaCaT cells | ↓ FcεRI α/γ, PLCγ1 and MAPK family (ERK, JNK, p38) |
| Park et al., 2020 [16] | Anti-allergic effect of barley sprout extract (apigenin) on RBL-2H3, anti-inflammatory effect on RAW264.7 and AD potential on HaCaT cells | RBL-2H3, RAW264.7, Human epidermal keratinocyte (HaCaT) cells | Apigenin; cyclosporine A (control for RBL-2H3), quercetin (control for RAW264.7). | For RAW264.7: LPS @ 1 μg/mL and apigenin @ 20–100 μM (or only 100 μM or 15μM quercetin for cytokines and MAPK signaling proteins) (24 h) and NO was measured. For RBL-2H3: anti-DNP-IgE @ 0.5 μg/mL (24 h); apigenin @ 5–30 μM (or only 30 μM or cyclosporine A @ 1 μg/mL for mRNA of cytokines, FcεRI α, MAPK proteins) (20 min); DNP-BSA @ 100 ng/mL (1 h) and β-hex was measured. For HaCaT: apigenin @ 20 μM (24 h) and genes related to skin physical and chemical barrier function were measured. | Tmt + LPS; IgE > Tmt > DNP-BSA; Tmt | MTT assay, WB, ELISA, β-hex assay, RT-qPCR | In RAW264.7 cells, 100 μM apigenin ↓ NO, IL-1β, IL6, COX-2, iNOS, ERK, JNK. In RBL-2H3 cells, 30 μM apigenin ↓ Lyn, Syk, PLCγ1, ERK, JNK, FcεRI α, TNF-α, IL-4, -5, -6, and COX-2. In HaCaT cells, 20μM apigenin ↑ gene/protein of compounds related to chemical and physical barrier of skin | ↓ Lyn, Syk, PLCγ1, ERK, JNK, FcεRI α |
| Yoo et al., 2017 [17] | Coumarin derivative’s effect on mast cell degranulation | RBL-2H3, RAW264.7, MOLT-4 | Coumarin derivative 1 (C1); Loratadine (+ve control for RBL-2H3), LPS (+ve control for RAW264.7) | For RBL-2H3: C1 or loratadine (not for protein study) @ 0–25 μM (1 h); PMA @ 50 nM+ A23187 @ 1 μM (30 min) and β-hex, histamine, p38, ERK, JNK, MKK3, MEK1/2, and MKK4 were analyzed. For RAW264.7: C1 @ 0–25 μM (24 h) for NO production or (1 h for NO inhibition); LPS @ 1μg/mL (24 h). For MOLT-4: C1 0–25 μM (6 h) and mRNA of IL-4 and IFN-γ measured. | Tmt > PMA + A23187;Tmt > LPS; Tmt | β-hex assay, histamine release assay, WB, RT-qPCR, nitrite assay, MTT assay | C1 ↓ β-hex, histamine, and ERK with maximum effect at 25 μM | ↓ ERK |
| Yoshioka et al., 2020 [13] | Anti-allergic and anti-inflammatory effects of ESG | RBL-2H3, human epithelial cell lineage (Caco-2), BMMC | ESG; DMEM media (-ve control) | For RBL-2H3 or BMMC cells: Anti-DNP-IgE @ 1 μg/mL (16 h); ESG @ 100–1000 μg/mL (in co-culture system treated to Caco-2) (24 h); DNP-BSA @ 10 ng/mL (30 min) (in co-culture system, only RBL-2H3 and BMMC were sensitized and challenged). | IgE > Tmt > DNP-BSA | β-hex assay, WB, ELISA | ESG ↓ β-hex, TNF-α, IL-6, Lyn, Syk, PLCγ1/2, MAPK, and Akt in RBL-2H3 of co-culture system | ↓ Lyn, Syk, PLCγ1/2, MAPK and Akt |
| Ref. | Abstract | Introduction | Methods | Results | Discussion | Other Info. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2a | 2b | 3 | 4 | 10 | 11 | 12 | 13 | ||
| Background and Rationale | Objectives | Hypothesis | Intervention | Outcomes | Statistical Methods | Outcomes and Estimations | Limitations | Funding | ||
| Badger-Emeka et al., 2020 [1] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Bansode et al., 2018 [51] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Barbosa et al., 2018 [2] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Chang et al., 2015 [3] | Yes | Yes | Yes | No | Yes | No | Yes | Yes | No | Yes |
| Dera et al., 2020 [15] | Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | Yes |
| Dippenaar et al., 2022 [52] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Gaihre et al., 2022 [53] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Hamauzu et al., 2021 [54] | Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | Yes |
| Hanieh et al., 2017 [63] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Hagenlocher et al., 2015 [19] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| Jiao et al., 2017 [20] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Kawai et al., 2018 [21] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Kobayashi et al., 2015 [4] | Yes | Yes | Yes | No | No | No | Yes | Yes | No | Yes |
| Kong et al., 2020 [22] | Yes | Yes | Yes | No | No | No | Yes | No | No | Yes |
| Korinek et al., 2016 [45] | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes |
| Lee et al., 2017 [8] | Yes | Yes | Yes | No | No | No | Yes | Yes | No | Yes |
| Lee et al., 2020 [25] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Li et al., 2022 [11] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Lim et al., 2023 [27] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Liu et al., 2021 [55] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Liu et al., 2023 [9] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Lorenz et al., 2016 [5] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Lv et al., 2022 [56] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Ma et al., 2022 [10] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Matsui et al., 2015 [57] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Matsui et al., 2022 [58] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Min et al., 2021 [26] | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes |
| Mwakalukwa et al., 2019 [6] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Niu et al., 2020 [59] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Park et al., 2020 [16] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Shi et al., 2016 [23] | Yes | Yes | Yes | No | Yes | Yes | No | No | No | Yes |
| Vo et al., 2018 [60] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Vo et al., 2020 [18] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Yoo et al., 2017 [17] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Yan et al., 2024 [12] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Yoshioka et al., 2020 [13] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Zeng et al., 2023 [61] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Zhao et al., 2022 [62] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, T.S.; Bansode, R.R.; Williams, L.L. Anti-Allergic and Anti-Inflammatory Signaling Mechanisms of Natural Compounds/Extracts in In Vitro System of RBL-2H3 Cell: A Systematic Review. Cells 2024, 13, 1389. https://doi.org/10.3390/cells13161389
Rana TS, Bansode RR, Williams LL. Anti-Allergic and Anti-Inflammatory Signaling Mechanisms of Natural Compounds/Extracts in In Vitro System of RBL-2H3 Cell: A Systematic Review. Cells. 2024; 13(16):1389. https://doi.org/10.3390/cells13161389
Chicago/Turabian StyleRana, Tekan S., Rishipal R. Bansode, and Leonard L. Williams. 2024. "Anti-Allergic and Anti-Inflammatory Signaling Mechanisms of Natural Compounds/Extracts in In Vitro System of RBL-2H3 Cell: A Systematic Review" Cells 13, no. 16: 1389. https://doi.org/10.3390/cells13161389





