New Insights into D-Aspartate Signaling in Testicular Activity †
Abstract
:1. Introduction
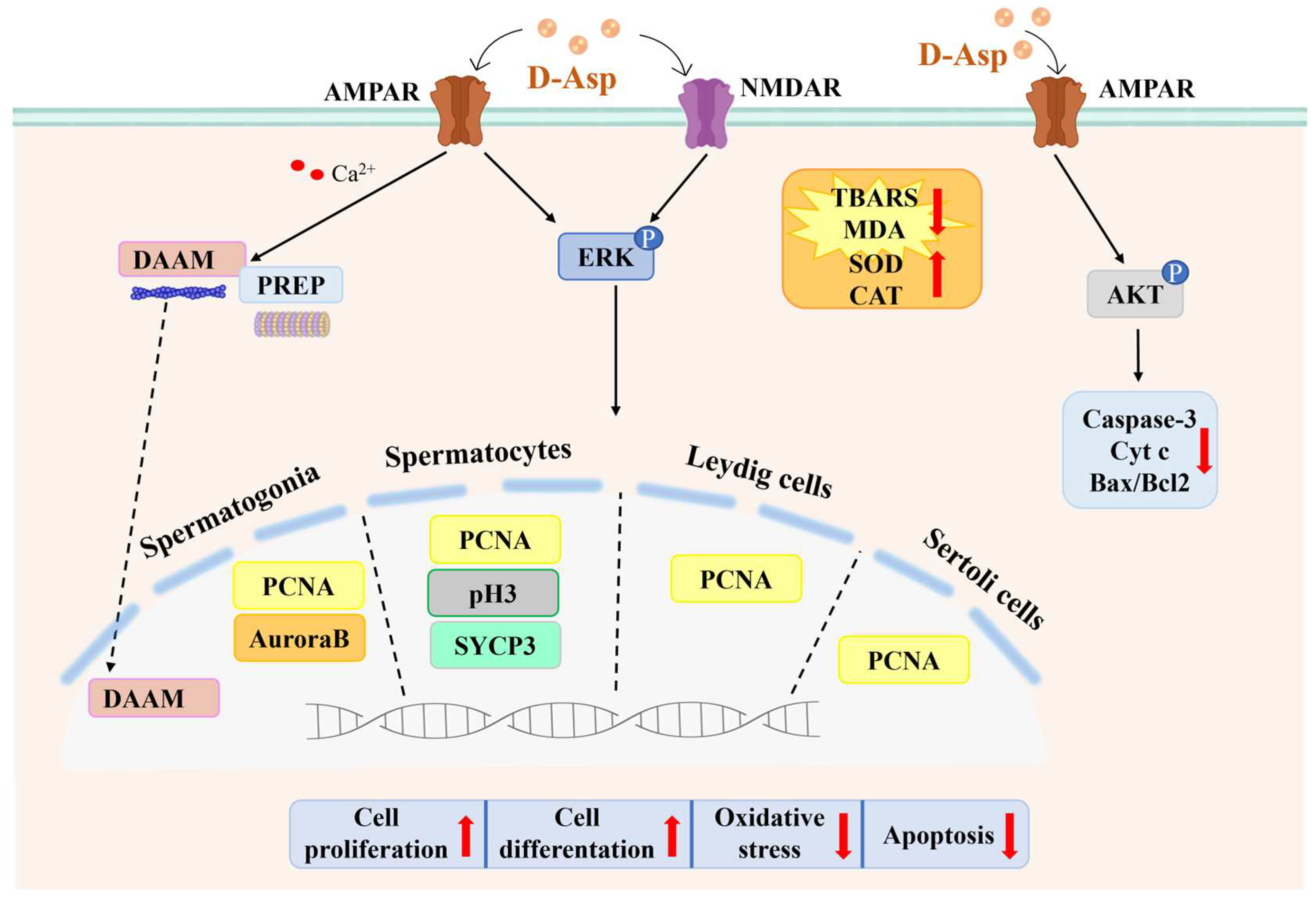
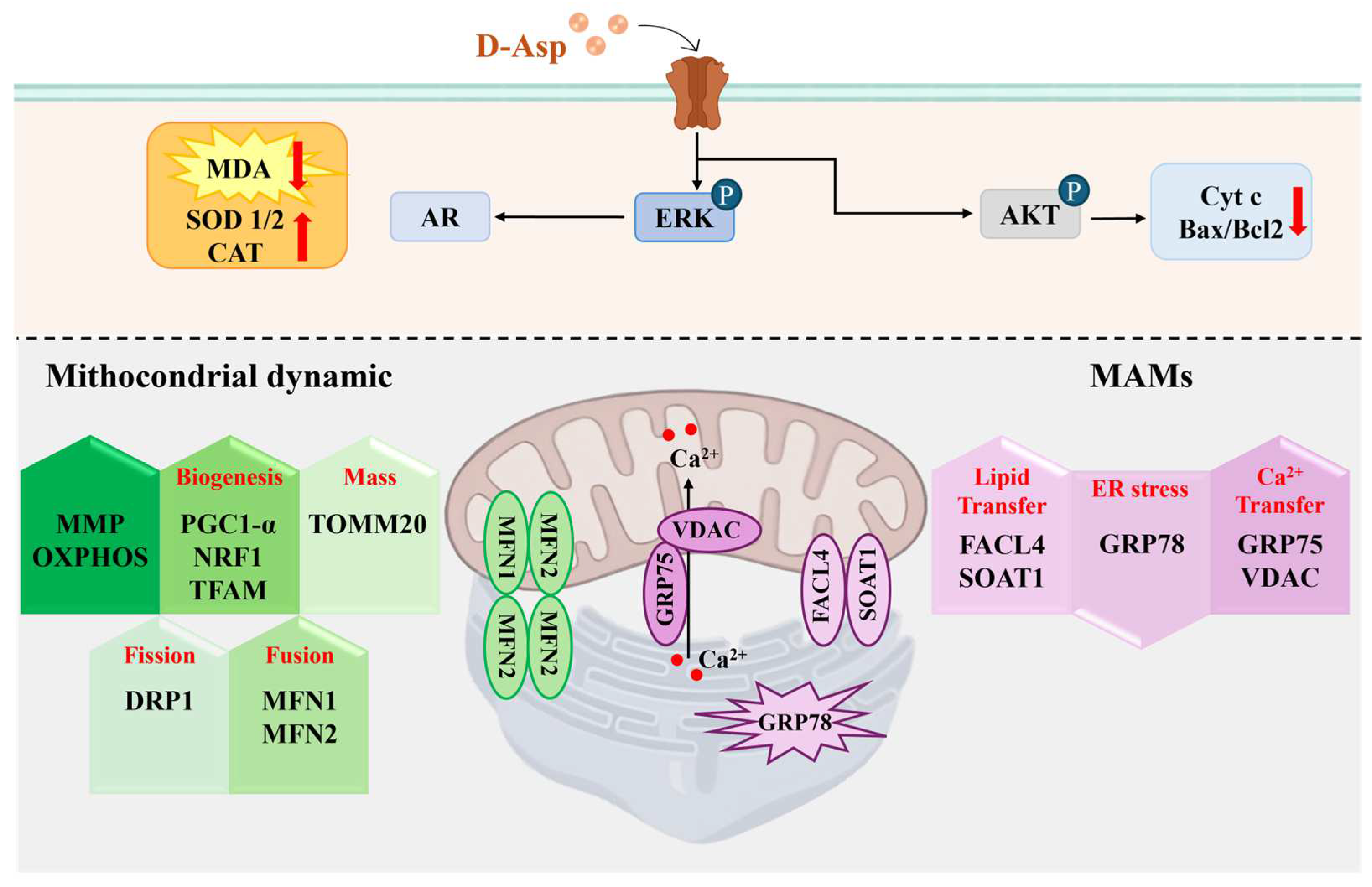
2. D-Asp Regulates the Functions of Leydig Cells via the cAMP/ERK1/2 Signaling Pathway
3. D-Asp Activates Spermatogenesis via the GluR-ERK1/2 Signaling Pathway
D-Asp Is Involved in the Metabolic Shift Occurring during Meiosis
4. D-Asp Modulates Oxidative Stress in the Testis
5. D-Asp Inhibits Apoptosis via the AMPAR/AKT Signaling Pathway
6. D-Asp Improves the Capacity of Sertoli Cells to Sustain Spermatogenesis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- D’Aniello, A. D-Aspartic acid: An endogenous amino acid with an important neuroendocrine role. Brain Res. Rev. 2007, 53, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Hamase, K. Sensitive two-dimensional determination of small amounts of d-amino acids in mammals and the study on their functions. Chem. Pharm. Bull. 2007, 55, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Hamase, K.; Morikawa, A.; Etoh, S.; Tojo, Y.; Miyoshi, Y.; Zaitsu, K. Analysis of small amounts of d-amino acids and the study of their physiological functions in mammals. Anal. Sci. 2009, 25, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, M.M.; Santillo, A.; Chieffi Baccari, G. Current knowledge of d-aspartate in glandular tissues. Amino Acids 2014, 46, 1805–1818. [Google Scholar] [CrossRef]
- Karakawa, S.; Shimbo, K.; Yamada, N.; Mizukoshi, T.; Miyano, H.; Mita, M.; Lindner, W.; Hamase, K. Simultaneous analysis of d-alanine, d-aspartic acid, and d-serine using chiral high-performance liquid chromatography-tandem mass spectrometry and its application to the rat plasma and tissues. J. Pharm. Biomed. Anal. 2015, 115, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Chieffi Baccari, G.; Falvo, S.; Santillo, A.; Di Giacomo Russo, F.; Di Fiore, M.M. D-amino acids in mammalian endocrine tissues. Amino Acids 2020, 52, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Monteforte, R.; Santillo, A.; Di Giovanni, M.; D’Aniello, A.; Di Maro, A.; Chieffi Baccari, G. d-Aspartate affects secretory activity in rat Harderian gland: Molecular mechanism and functional significance. Amino Acids 2009, 37, 653–664. [Google Scholar] [CrossRef]
- Di Giovanni, M.; Topo, E.; Santillo, A.; D’Aniello, A.; Chieffi Baccari, G. d-Aspartate binding sites in rat Harderian gland. Amino Acids 2010, 38, 229–235. [Google Scholar] [CrossRef]
- D’Aniello, S.; Somorjai, I.; Garcia-Fernàndez, J.; Topo, E.; D’Aniello, A. D-Aspartic acid is a novel endogenous neurotransmitter. FASEB J. 2011, 25, 1014–1027. [Google Scholar] [CrossRef]
- Errico, F.; Napolitano, F.; Nisticò, R.; Usiello, A. New insights on the role of free d-aspartate in the mammalian brain. Amino Acids 2012, 43, 1861–1871. [Google Scholar] [CrossRef]
- Ota, N.; Shi, T.; Sweedler, J.V. d-Aspartate acts as a signaling molecule in nervous and neuroendocrine systems. Amino Acids 2012, 43, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Bastings, J.J.A.J.; van Eijk, H.M.; Olde Damink, S.W.; Rensen, S.S. d-amino Acids in Health and Disease: A Focus on Cancer. Nutrients 2019, 11, 2205. [Google Scholar] [CrossRef]
- Wolosker, H.; D’Aniello, A.; Snyder, S.H. d-Aspartate disposition in neuronal and endocrine tissues: Ontogeny, biosynthesis and release. Neuroscience 2000, 100, 183–189. [Google Scholar] [CrossRef]
- Topo, E.; Soricelli, A.; D’Aniello, A.; Ronsini, S.; D’Aniello, G. The role and molecular mechanism of d-aspartic acid in the release and synthesis of LH and testosterone in humans and rats. Reprod. Biol. Endocrinol. 2009, 7, 120. [Google Scholar] [CrossRef]
- Topo, E.; Fisher, G.; Sorricelli, A.; Errico, F.; Usiello, A.; D’Aniello, A. Thyroid hormones and d-aspartic acid, d-aspartate oxidase, d-aspartate racemase, H2O2, and ROS in rats and mice. Chem. Biodivers. 2010, 7, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Raucci, F.; Assisi, L.; D’Aniello, S.; Spinelli, P.; Botte, V.; Di Fiore, M.M. Testicular endocrine activity is upregulated by D-aspartic acid in the green frog, Rana esculenta. J. Endocrinol. 2004, 182, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, M.M.; Santillo, A.; Falvo, S.; Longobardi, S.; Chieffi, G. Molecular mechanisms elicited by D-aspartate in Leydig cells and spermatogonia. Int. J. Mol. Sci. 2016, 17, 1127. [Google Scholar] [CrossRef]
- Di Fiore, M.M.; Boni, R.; Santillo, A.; Falvo, S.; Gallo, A.; Esposito, S.; Chieffi Baccari, G. d-aspartic acid in vertebrate reproduction: Animal models and experimental designs. Biomolecules 2019, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Falvo, S.; Di Fiore, M.M.; Burrone, L.; Chieffi Baccari, G.; Longobardi, S.; Santillo, A. Androgen and oestrogen modulation by d-aspartate in rat epididymis. Reprod. Fertil. Dev. 2016, 28, 1865–1872. [Google Scholar] [CrossRef]
- Usiello, A.; Di Fiore, M.M.; De Rosa, A.; Falvo, S.; Errico, F.; Santillo, A.; Nuzzo, T.; Chieffi, G. New Evidence on the Role of D-Aspartate Metabolism in Regulating Brain and Endocrine System Physiology: From Preclinical Observations to Clinical Applications. Int. J. Mol. Sci. 2020, 21, 8718. [Google Scholar] [CrossRef]
- D’Aniello, A.; Di Cosmo, A.; Di Cristo, C.; Annunziato, L.; Petrucelli, L.; Fisher, G. Involvement of D-aspartic acid in the synthesis of testosterone in rat testes. Life Sci. 1996, 59, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Homma, H.; Lee, J.A.; Fukushima, T.; Santa, T.; Tashiro, K.; Iwatsubo, T.; Imai, K. Localization of D-aspartic acid in elongate spermatids in rat testis. Arch. Biochem. Biophys. 1998, 351, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Santillo, A.; Falvo, S.; Venditti, M.; Di Maio, A.; Chieffi Baccari, G.; Errico, F.; Usiello, A.; Minucci, S.; Di Fiore, M.M. D-Aspartate Depletion Perturbs Steroidogenesis and Spermatogenesis in Mice. Biomolecules 2023, 13, 621. [Google Scholar] [CrossRef]
- Di Giovanni, M.; Burrone, L.; Chieffi Baccari, G.; Topo, E.; Santillo, A. Distribution of free d-aspartic acid and d-aspartate oxidase in frog Rana esculenta tissues. J. Exp. Zool. A Ecol. Genet. Physiol. 2010, 313, 137–143. [Google Scholar] [CrossRef]
- D’Aniello, A.; Di Fiore, M.M.; D’Aniello, G.; Colin, F.E.; Lewis, G.; Setchell, B.P. Secretion of D-aspartic acid by the rat testis and its role in endocrinology of the testis and spermatogenesis. FEBS Lett. 1998, 436, 23–27. [Google Scholar] [CrossRef]
- D’Aniello, G.; Ronsini, S.; Guida, F.; Spinelli, P.; D’Aniello, A. Occurrence of D-aspartic acid in human seminal plasma and spermatozoa: Possible role in reproduction. Fertil. Steril. 2005, 84, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Santillo, A.; Falvo, S.; Chieffi, P.; Di Fiore, M.M.; Senese, R.; Chieffi Baccari, G. D-Aspartate Induces Proliferative Pathways in Spermatogonial GC-1 Cells. J. Cell. Physiol. 2016, 231, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Santillo, A.; Falvo, S.; Di Fiore, M.M.; Chieffi Baccari, G.; Minucci, S. D-Aspartate upregulates DAAM1 protein levels in the rat testis and induces its localization in spermatogonia nucleus. Biomolecules 2020, 10, 677. [Google Scholar] [CrossRef]
- Falvo, S.; Santillo, A.; Chieffi Baccari, G.; Cioffi, F.; Di Fiore, M.M. D-aspartate and N-methyl-D-aspartate promote proliferative activity in mouse spermatocyte GC-2 cells. Reprod. Biol. 2022, 22, 100601. [Google Scholar] [CrossRef] [PubMed]
- Falvo, S.; Grillo, G.; Latino, D.; Chieffi Baccari, G.; Di Fiore, M.M.; Venditti, M.; Petito, G.; Santillo, A. Potential role of mitochondria and endoplasmic reticulum in the response elicited by D-Aspartate in TM4 Sertoli cells. Front. Cell Dev. Biol. 2024, 12, 1438231. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Oka, T. Free d-aspartate and d-serine in the mammalian brain and periphery. Prog. Neurobiol. 1997, 52, 325–353. [Google Scholar] [CrossRef]
- Modirshanechi, G.; Eslampour, M.A.; Abdolmaleki, Z. Agonist and antagonist NMDA receptor effect on cell fate during germ cell differentiation and regulate apoptotic process in 3D organ culture. Andrologia 2020, 52, e13764. [Google Scholar] [CrossRef] [PubMed]
- Noghani, A.E.; Asadpour, R.; Saberivand, A.; Mazaheri, Z.; Rodriguez-Wallberg, K.A.; Hamidian, G. Differentiation of neonate mouse spermatogonia on two-dimensional and three-dimensional culture systems supplemented with d-Serine and Dizocilpine (MK-801). Theriogenology 2022, 191, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.H.; Youngblood, G.L. Regulation of expression of steroidogenic enzymes in Leydig cells. Biol. Reprod. 1995, 52, 217–225. [Google Scholar] [CrossRef]
- Almog, T.; Naor, Z. Mitogen activated protein kinases (MAPKs) as regulators of spermatogenesis and spermatozoa functions. Mol. Cell. Endocrinol. 2008, 282, 39–44. [Google Scholar] [CrossRef]
- Du, Y.; Chi, X.; Wang, Y.; Cai, X.; Zeng, W.; Huo, Y.; Zhang, M.; Wang, Z.; Guo, Z.; Qiu, J.; et al. Advancements in the ERK1/2 Signaling Pathway Affecting Male Reproduction. Front. Biosci. 2024, 29, 23. [Google Scholar] [CrossRef] [PubMed]
- Raucci, F.; D’Aniello, A.; Di Fiore, M.M. Stimulation of androgen production by d-aspartate through the enhancement of StAR, P450scc and 3β-HSD mRNA levels in vivo rat testis and in culture of immature rat Leydig cells. Steroids 2014, 84, 103–110. [Google Scholar] [CrossRef]
- Venditti, M.; Santillo, A.; Latino, D.; Ben Rhouma, M.; Romano, M.Z.; Haddadi, A.; Di Fiore, M.M.; Minucci, S.; Messaoudi, I.; Chieffi Baccari, G. Evidence of the protective role of D-Aspartate in counteracting/preventing cadmium-induced oxidative stress in the rat testis. Ecotoxicol. Environ. Saf. 2023, 9, 115067. [Google Scholar] [CrossRef]
- Latino, D.; Venditti, M.; Falvo, S.; Grillo, G.; Santillo, A.; Messaoudi, I.; Ben Rhouma, M.; Minucci, S.; Chieffi Baccari, G.; Di Fiore, M.M. Steroidogenesis Upregulation through Mitochondria-Associated Endoplasmic Reticulum Membranes and Mitochondrial Dynamics in Rat Testes: The Role of D-Aspartate. Cells 2024, 13, 523. [Google Scholar] [CrossRef]
- Nagata, Y.; Homma, H.; Lee, J.-A.; Imai, K. D-Aspartate stimulation of testosterone synthesis in rat Leydig cells. FEBS Lett. 1999, 444, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, A.; De Toni, L.; Ferigo, M.; Rocca, M.S.; Speltra, E.; Ferlin, A.; Foresta, C. d-Aspartic acid stimulates steroidogenesis through the delay of LH receptor internalization in a mammalian Leydig cell line. J. Endocrinol. Investig. 2015, 39, 207–213. [Google Scholar] [CrossRef]
- Ge, X.Y.; Shao, L.L.; Gao, X.L.; He, R.X. Extracellular Signalregulated Kinase 1/2 Signaling Regulates Cell Invasion:a Review. Acta Acad. Med. Sin. 2023, 45, 155–160. [Google Scholar]
- Pogrmic-Majkic, K.; Fa, S.; Samardzija, D.; Hrubik, J.; Kaisarevic, S.; Andric, N. Atrazine activates multiple signaling pathways enhancing the rapid hCG-induced androgenesis in rat Leydig cells. Toxicology 2016, 368–369, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ge, F.; Li, X.; Ni, C.; Wu, K.; Zheng, W.; Chen, Y.; Lian, Q.; Ge, R.S. Propofol Inhibits Androgen Production in Rat Immature Leydig Cells. Front. Pharmacol. 2019, 10, 760. [Google Scholar] [CrossRef]
- Li, L.; Mu, X.; Ye, L.; Ze, Y.; Hong, F. Suppression of testosterone production by nanoparticulate TiO2 is associated with ERK1/2-PKA-PKC signaling pathways in rat primary cultured Leydig cells. Int. J. Nanomed. 2018, 13, 5909–5924. [Google Scholar] [CrossRef]
- Latino, D.; Falvo, S.; Santillo, A.; Chieffi Baccari, G.; Venditti, M.; Grillo, G.; Di Fiore, M.M. Regulative Mechanisms Induced by D-Asp into TM3 Leydig Cells. manuscript in preparation.
- Gill, S.S.; Mueller, R.W.; McGuire, P.F.; Pulido, O.M. Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicol. Pathol. 2000, 28, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Pulido, O.M. Glutamate receptors in peripheral tissues: Current knowledge, future research, and implications for toxicology. Toxicol. Pathol. 2001, 29, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Storto, M.; Sallese, M.; Salvatore, L.; Poulet, R.; Condorelli, D.F.; Dell’Albani, P.; Marcello, M.F.; Romeo, R.; Piomboni, P.; Barone, N.; et al. Expression of metabotropic glutamate receptors in the rat and human testis. J. Endocrinol. 2001, 170, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Yang, N.; Ma, Y.H.; Jiang, J.; Zhang, J.F.; Fei, J.; Guo, L.H. Identification of glutamate transporters and receptors in mouse testis. Acta Pharmacol. Sin. 2004, 25, 366–371. [Google Scholar]
- Takarada, T.; Hinoi, E.; Balcar, V.J.; Taniura, H.; Yoneda, Y. Possible expression of functional glutamate transporters in the rat testis. J. Endocrinol. 2004, 181, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, M.; Chruścicka, B.; Lech, T.; Burnat, G.; Pilc, A. Expression of group III metabotropic glutamate receptors in the reproductive system of male mice. Reprod. Fertil. Dev. 2016, 28, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Doherty, F.C.; Sladek, C.D. NMDA receptor subunit expression in the supraoptic nucleus of adult rats: Dominance of NR2B and NR2D. Brain Res. 2011, 1388, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Mao, L.; Tang, Q.; Samdani, S.; Liu, Z.; Wang, J.Q. A novel Ca2+-independent signaling pathway to extracellular signal-regulated protein kinase by coactivation of NMDA receptors and metabotropic glutamate receptor 5 in neurons. J. Neurosci. 2004, 24, 10846–10857. [Google Scholar] [CrossRef]
- Santillo, A.; Falvo, S.; Chieffi, P.; Burrone, L.; Chieffi Baccari, G.; Longobardi, S.; Di Fiore, M.M. d-Aspartate affects NMDA receptorextracellular signal-regulated kinase pathway and upregulates androgen receptor expression in the rat testis. Theriogenology 2014, 81, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Santillo, A.; Venditti, M.; Minucci, S.; Chieffi Baccari, G.; Falvo, S.; Rosati, L.; Di Fiore, M.M. d-Asp upregulates PREP and GluA2/3 expressions and induces p-ERK1/2 and p-Akt in rat testis. Reproduction 2019, 158, 357–367. [Google Scholar] [CrossRef]
- Santillo, A.; Falvo, S.; Di Fiore, M.M.; Di Giacomo Russo, F.; Chieffi, P.; Usiello, A.; Pinelli, C.; Chieffi Baccari, G. AMPA receptor expression in mouse testis and spermatogonial GC-1 cells: A study on its regulation by excitatory amino acids. J. Cell. Biochem. 2019, 120, 11044–11055. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.S.; Yi, J.; Kong, S.B.; Ding, J.C.; Zhao, Y.N.; Tian, Y.P.; Feng, G.S.; Li, C.J.; Liu, W.; et al. The role of tyrosine phosphatase Shp2 in spermatogonial differentiation and spermatocyte meiosis. Asian J. Androl. 2020, 22, 79–87. [Google Scholar] [CrossRef]
- Venditti, M.; Arcaniolo, D.; De Sio, M.; Minucci, S. Preliminary Investigation on the Involvement of Cytoskeleton-Related Proteins, DAAM1 and PREP, in Human Testicular Disorders. Int. J. Mol. Sci. 2021, 22, 8094. [Google Scholar] [CrossRef]
- Venditti, M.; Minucci, S. Subcellular Localization of Prolyl Endopeptidase During the First Wave of Rat Spermatogenesis and in Rat and Human Sperm. J. Histochem. Cytochem. 2019, 67, 229–243. [Google Scholar] [CrossRef]
- Dotolo, R.; Kim, J.D.; Pariante, P.; Minucci, S.; Diano, S. Prolyl Endopeptidase (PREP) is Associated With Male Reproductive Functions and Gamete Physiology in Mice. J. Cell. Physiol. 2016, 231, 551–557. [Google Scholar] [CrossRef]
- Szeltner, Z.; Polgár, L. Structure, function and biological relevance of prolyl oligopeptidase. Curr. Protein Pept. Sci. 2008, 9, 96–107. [Google Scholar] [PubMed]
- Chen, W.; Zhang, Z.; Chang, C.; Yang, Z.; Wang, P.; Fu, H.; Wei, X.; Chen, E.; Tan, S.; Huang, W.; et al. A bioenergetic shift is required for spermatogonial differentiation. Cell Discov. 2020, 6, 56. [Google Scholar] [CrossRef]
- Morimoto, H.; Yamamoto, T.; Miyazaki, T.; Ogonuki, N.; Ogura, A.; Tanaka, T.; Kanatsu-Shinohara, M.; Yabe-Nishimura, C.; Zhang, H.; Pommier, Y.; et al. An interplay of NOX1-derived ROS and oxygen determines the spermatogonial stem cell self-renewal efficiency under hypoxia. Genes Dev. 2021, 35, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Ramalho-Santos, J.; Varum, S.; Amaral, S.; Mota, P.C.; Sousa, A.P.; Amaral, A. Mitochondrial functionality in reproduction: From gonads and gametes to embryos and embryonic stem cells. Hum. Reprod. Update 2009, 15, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Almansa-Ordonez, A.; Bellido, R.; Vassena, R.; Barragan, M.; Zambelli, F. Oxidative Stress in Reproduction: A Mitochondrial Perspective. Biology 2020, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Pang, M.G. Mitochondrial Functionality in Male Fertility: From Spermatogenesis to Fertilization. Antioxidants 2021, 10, 98. [Google Scholar] [CrossRef]
- Varuzhanyan, G.; Rojansky, R.; Sweredoski, M.J.; Graham, R.L.J.; Hess, S.; Ladinsky, M.S.; Chan, D.C. Mitochondrial fusion is required for spermatogonial differentiation and meiosis. Elife 2019, 8, e51601. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, H.; Kanastu-Shinohara, M.; Ogonuki, N.; Kamimura, S.; Ogura, A.; Yabe-Nishimura, C.; Mori, Y.; Morimoto, T.; Watanabe, S.; Otsu, K.; et al. ROS amplification drives mouse spermatogonial stem cell self-renewal. Life Sci. Alliance 2019, 2, e201900374. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhu, T.; Chen, L.; Nishioka, T.; Tsuji, T.; Xiao, Z.X.; Chen, C.Y. Differential sensitization of different prostate cancer cells to apoptosis. Genes Cancer 2010, 1, 836–846. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, G.; Ronsini, S.; Notari, T.; Grieco, N.; Infante, V.; D’Angelo, N.; Mascia, F.; Di Fiore, M.M.; Fisher, G.; D’Aniello, A. D-Aspartate, a Key Element for the Improvement of Sperm Quality. Adv. Sex. Med. 2012, 2, 47–53. [Google Scholar] [CrossRef]
- Giacone, F.; Condorelli, R.A.; Mongioì, L.M.; Bullara, V.; La Vignera, S.; Calogero, A.E. In vitro effects of zinc, D-aspartic acid, and coenzyme-Q10 on sperm function. Endocrine 2017, 56, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Brunet, A.; Greenberg, M.E. Cellular survival: A play in three Akts. Genes Dev. 1999, 13, 2905–2927. [Google Scholar] [CrossRef]
- Kandel, E.S.; Hay, N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp. Cell Res. 1999, 253, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Burrone, L.; Di Giovanni, M.; Di Fiore, M.M.; Chieffi Baccari, G.; Santillo, A. Effects of d-aspartate treatment on d-aspartate oxidase, superoxide dismutase, and caspase 3 activities in frog (Rana esculenta) tissues. Chem. Biodivers. 2010, 7, 1459–1466. [Google Scholar] [CrossRef]
- Figueiredo, A.F.A.; Wnuk, N.T.; Tavares, A.O.; Miranda, J.R.; Hess, R.A.; de França, L.R.; Costa, G.M.J. Prepubertal PTU treatment in rat increases Sertoli cell number and sperm production. Reproduction 2019, 158, 199–209. [Google Scholar] [CrossRef]
- Galardo, M.N.; Regueira, M.; Riera, M.F.; Pellizzari, E.H.; Cigorraga, S.B.; Meroni, S.B. Lactate regulates rat male germ cell function through reactive oxygen species. PLoS ONE 2014, 9, e88024. [Google Scholar] [CrossRef]
- O’Donnell, L.; Smith, L.B.; Rebourcet, D. Sertoli cells as key drivers of testis function. Semin. Cell Dev. Biol. 2022, 121, 2–9. [Google Scholar] [CrossRef]
- Mather, J.P. Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol. Reprod. 1980, 23, 243–252. [Google Scholar]
- Luan, Y.; Luan, Y.; Yuan, R.X.; Feng, Q.; Chen, X.; Yang, Y. Structure and Function of Mitochondria-Associated Endoplasmic Reticulum Membranes (MAMs) and Their Role in Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 4578809. [Google Scholar] [CrossRef]
- Simmen, T.; Aslan, J.E.; Blagoveshchenskaya, A.D.; Thomas, L.; Wan, L.; Xiang, Y.; Feliciangeli, S.F.; Hung, C.H.; Crump, C.M.; Thomas, G. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 2005, 24, 717–729. [Google Scholar] [CrossRef]
- Ma, L.; Hai, S.; Wang, C.; Chen, C.; Rahman, S.U.; Zhao, C.; Bazai, M.A.; Feng, S.; Wang, X. Zearalenone induces mitochondria-associated endoplasmic reticulum membranes dysfunction in piglet Sertoli cells based on endoplasmic reticulum stress. Ecotoxicol. Environ. Saf. 2023, 254, 114710. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falvo, S.; Santillo, A.; Di Fiore, M.M.; Venditti, M.; Grillo, G.; Latino, D.; Baccari, I.; Petito, G.; Chieffi Baccari, G. New Insights into D-Aspartate Signaling in Testicular Activity. Cells 2024, 13, 1400. https://doi.org/10.3390/cells13161400
Falvo S, Santillo A, Di Fiore MM, Venditti M, Grillo G, Latino D, Baccari I, Petito G, Chieffi Baccari G. New Insights into D-Aspartate Signaling in Testicular Activity. Cells. 2024; 13(16):1400. https://doi.org/10.3390/cells13161400
Chicago/Turabian StyleFalvo, Sara, Alessandra Santillo, Maria Maddalena Di Fiore, Massimo Venditti, Giulia Grillo, Debora Latino, Isabella Baccari, Giuseppe Petito, and Gabriella Chieffi Baccari. 2024. "New Insights into D-Aspartate Signaling in Testicular Activity" Cells 13, no. 16: 1400. https://doi.org/10.3390/cells13161400
APA StyleFalvo, S., Santillo, A., Di Fiore, M. M., Venditti, M., Grillo, G., Latino, D., Baccari, I., Petito, G., & Chieffi Baccari, G. (2024). New Insights into D-Aspartate Signaling in Testicular Activity. Cells, 13(16), 1400. https://doi.org/10.3390/cells13161400







