The Multifunctional Role of KCNE2: From Cardiac Arrhythmia to Multisystem Disorders
Abstract
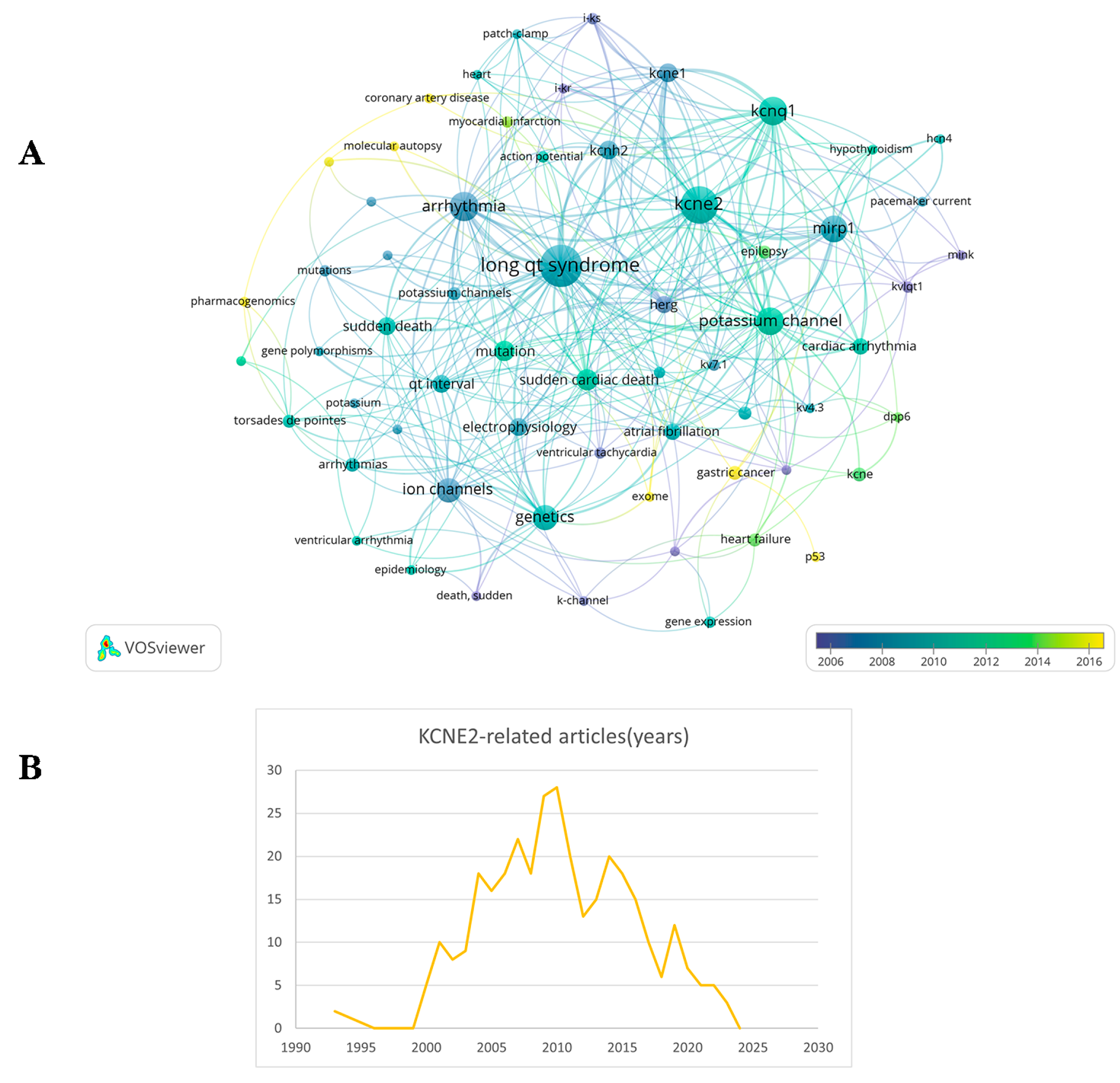
1. Introduction
2. Molecular Characterizations
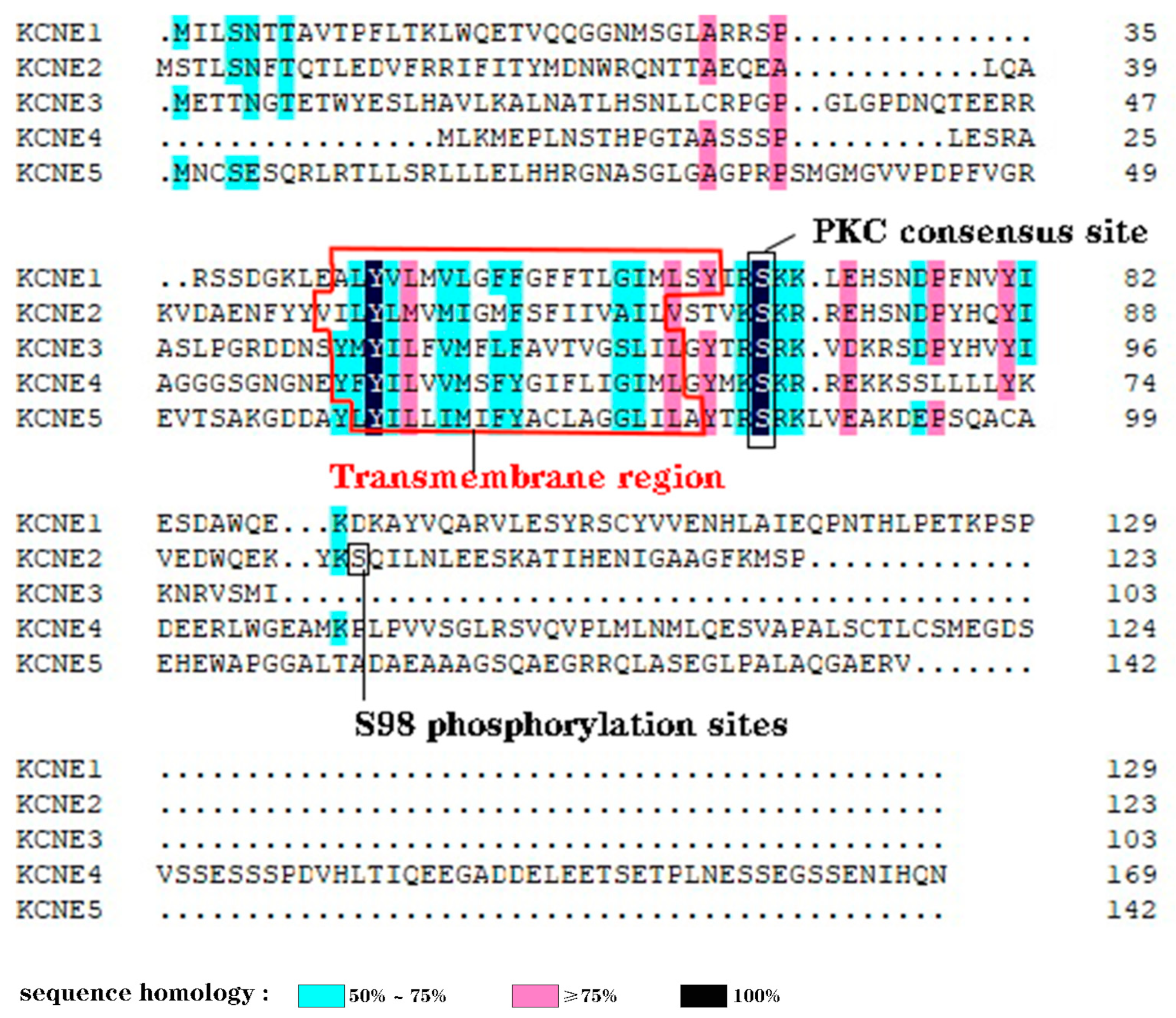
2.1. Structure
2.2. Tissue-Specific Distribution
2.3. Transcription Mechanisms
3. Pluripotent Biological Effects of KCNE2
3.1. KCNE2 Modulates the Ca2+ Channels
3.2. KCNE2 Modulates the HCN Channels
3.3. KCNE2 Modulates the HERG Channels
3.4. KCNE2 Modulate the Ito,fast Channel
3.5. KCNE2 Modulates the KCNQ1
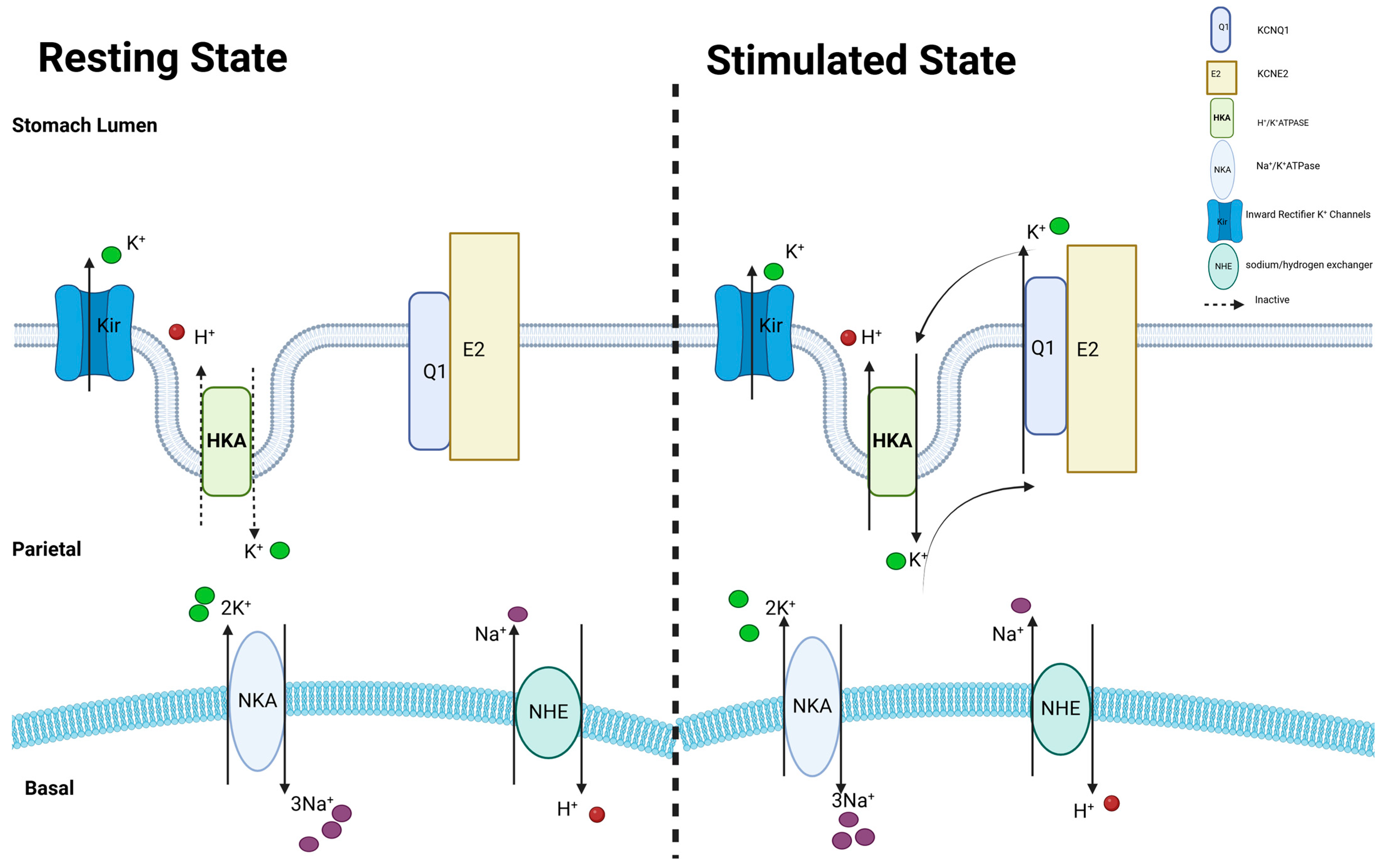
3.5.1. KCNQ1 in Stomach
3.5.2. KCNQ1 in Thyroid
3.5.3. KCNQ1 in Choroid Plexus Epithelium (CPe)
3.6. The Effect of KCNE2 on the Visual Pathway
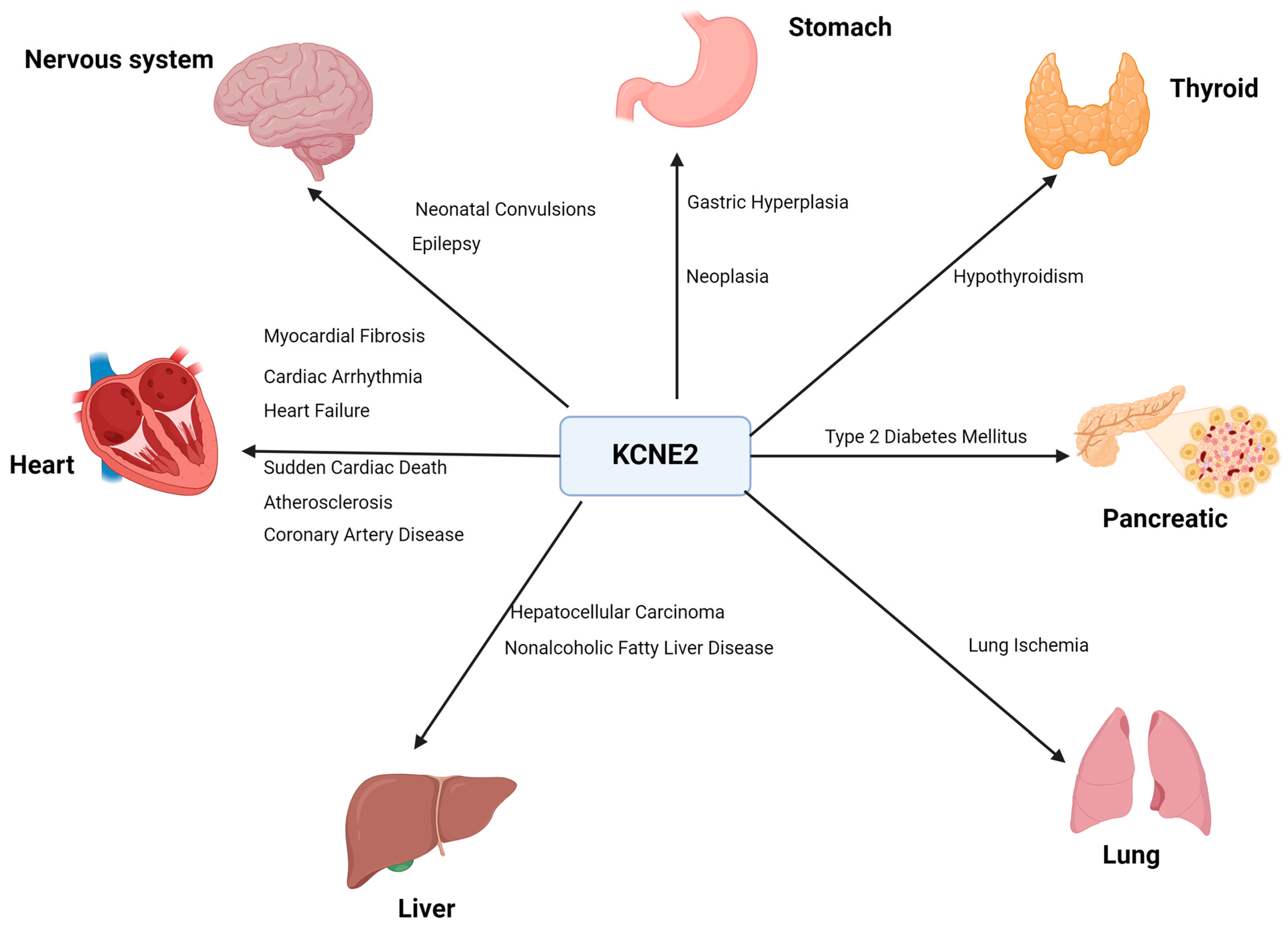
4. KCNE2 and Diseases
4.1. Cardiovascular Diseases
4.1.1. Cardiac Arrhythmia
4.1.2. Heart Failure
4.1.3. Sudden Cardiac Death
4.1.4. Atherosclerosis and Coronary Artery Disease
4.1.5. Myocardial Fibrosis
4.2. Neurological Diseases
4.3. Aging
4.4. Achlorhydia, Gastric Hyperplasia and Neoplasia
4.5. Hypothyroidism
4.6. Type 2 Diabetes Mellitus
4.7. Lung Ischemia and Reperfusion Injury
4.8. Non-Alcoholic Fatty Liver Disease (NAFLD)
4.9. Hepatocellular Carcinoma
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Benito-Bueno, A.; Socuellamos, P.G.; Merinero, Y.G.; Cercos, P.; Izquierdo, C.; Daniel-Mozo, M.; Marín-Olivero, I.; Perez-Lara, A.; Gonzalez-Vera, J.A.; Orte, A. Modulation of KV4. 3-KChIP2 Channels by IQM-266: Role of DPP6 and KCNE2. Int. J. Mol. Sci. 2022, 23, 9170. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, A.L.; Manderfield, L.J.; Vanoye, C.G.; Rogers, C.S.; Donahue, B.S.; Chang, P.A.; Drinkwater, D.C.; Murray, K.T.; George, A.L., Jr. Expression of multiple KCNE genes in human heart may enable variable modulation of I(Ks). J. Mol. Cell. Cardiol. 2005, 38, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Abbott, G.W. KCNE genetics and pharmacogenomics in cardiac arrhythmias: Much ado about nothing? Expert Rev. Clin. Pharmacol. 2013, 6, 49–60. [Google Scholar] [CrossRef][Green Version]
- Murai, T.; Kakizuka, A.; Takumi, T.; Ohkubo, H.; Nakanishi, S. Molecular cloning and sequence analysis of human genomic DNA encoding a novel membrane protein which exhibits a slowly activating potassium channel activity. Biochem. Biophys. Res. Commun. 1989, 161, 176–181. [Google Scholar] [CrossRef]
- Zou, X.; Wu, X.; Sampson, K.J.; Colecraft, H.M.; Larsson, H.P.; Kass, R.S. Pharmacological rescue of specific long QT variants of KCNQ1/KCNE1 channels. Front. Physiol. 2022, 13, 902224. [Google Scholar] [CrossRef]
- Duggal, P.; Vesely, M.R.; Wattanasirichaigoon, D.; Villafane, J.; Kaushik, V.; Beggs, A.H. Mutation of the gene for IsK associated with both Jervell and Lange-Nielsen and Romano-Ward forms of Long-QT syndrome. Circulation 1998, 97, 142–146. [Google Scholar] [CrossRef]
- Abbott, G.W.; Sesti, F.; Splawski, I.; Buck, M.E.; Lehmann, M.H.; Timothy, K.W.; Keating, M.T.; Goldstein, S.A. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell 1999, 97, 175–187. [Google Scholar] [CrossRef]
- Mazhari, R.; Greenstein, J.L.; Winslow, R.L.; Marbán, E.; Nuss, H.B. Molecular interactions between two long-QT syndrome gene products, HERG and KCNE2, rationalized by in vitro and in silico analysis. Circ. Res. 2001, 89, 33–38. [Google Scholar] [CrossRef]
- Deschênes, I.; Tomaselli, G.F. Modulation of Kv4. 3 current by accessory subunits. FEBS Lett. 2002, 528, 183–188. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, M.; Tseng, G.-N. minK-related peptide 1 associates with Kv4. 2 and modulates its gating function: Potential role as β subunit of cardiac transient outward channel? Circ. Res. 2001, 88, 1012–1019. [Google Scholar] [CrossRef]
- Sesti, F.; Tai, K.K.; Goldstein, S.A. MinK endows the I(Ks) potassium channel pore with sensitivity to internal tetraethylammonium. Biophys. J. 2000, 79, 1369–1378. [Google Scholar] [CrossRef]
- Pourrier, M.; Han, W.; Zicha, S.; Wang, Z.; Nattel, S. MiRP1-Kv3. 4 interaction: Possible role in Purkinje cell electrophysiology? Circulation 2002, 106, 47. [Google Scholar]
- Pourrier, M.; Zicha, S.; Ehrlich, J.; Han, W.; Nattel, S. Canine ventricular KCNE2 expression resides predominantly in Purkinje fibers. Circ. Res. 2003, 93, 189–191. [Google Scholar] [CrossRef]
- Hu, Z.; Kant, R.; Anand, M.; King, E.C.; Krogh-Madsen, T.; Christini, D.J.; Abbott, G.W. Kcne2 deletion creates a multisystem syndrome predisposing to sudden cardiac death. Circ. Cardiovasc. Genet. 2014, 7, 33–42. [Google Scholar] [CrossRef]
- Roepke, T.K.; King, E.C.; Reyna-Neyra, A.; Paroder, M.; Purtell, K.; Koba, W.; Fine, E.; Lerner, D.J.; Carrasco, N.; Abbott, G.W. Kcne2 deletion uncovers its crucial role in thyroid hormone biosynthesis. Nat. Med. 2009, 15, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Roepke, T.K.; Kontogeorgis, A.; Ovanez, C.; Xu, X.; Young, J.B.; Purtell, K.; Goldstein, P.A.; Christini, D.J.; Peters, N.S.; Akar, F.G.; et al. Targeted deletion of kcne2 impairs ventricular repolarization via disruption of I(K,slow1) and I(to,f). FASEB J. 2008, 22, 3648–3660. [Google Scholar] [CrossRef]
- Roepke, T.K.; Anantharam, A.; Kirchhoff, P.; Busque, S.M.; Young, J.B.; Geibel, J.P.; Lerner, D.J.; Abbott, G.W. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. J. Biol. Chem. 2006, 281, 23740–23747. [Google Scholar] [CrossRef]
- Lee, S.M.; Nguyen, D.; Hu, Z.; Abbott, G.W. Kcne2 deletion promotes atherosclerosis and diet-dependent sudden death. J. Mol. Cell. Cardiol. 2015, 87, 148–151. [Google Scholar] [CrossRef]
- Lee, S.M.; Baik, J.; Nguyen, D.; Nguyen, V.; Liu, S.; Hu, Z.; Abbott, G.W. Kcne2 deletion impairs insulin secretion and causes type 2 diabetes mellitus. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 2674–2685. [Google Scholar]
- Sesti, F.; Goldstein, S.A. Single-channel characteristics of wild-type IKs channels and channels formed with two minK mutants that cause long QT syndrome. J. Gen. Physiol. 1998, 112, 651–663. [Google Scholar] [CrossRef]
- McCrossan, Z.A.; Abbott, G.W. The MinK-related peptides. Neuropharmacology 2004, 47, 787–821. [Google Scholar] [CrossRef]
- Pongs, O.; Schwarz, J.R. Ancillary subunits associated with voltage-dependent K+ channels. Physiol. Rev. 2010, 90, 755–796. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Jiang, M.; Zankov, D.P.; Chowdhury, S.; Kasirajan, V.; Tseng, G.-N. KCNE2 protein is more abundant in ventricles than in atria and can accelerate hERG protein degradation in a phosphorylation-dependent manner. Am. J. Physiol.-Heart Circ. Physiol. 2012, 302, H910–H922. [Google Scholar] [CrossRef]
- Abbott, G.W. The KCNE2 K+ channel regulatory subunit: Ubiquitous influence, complex pathobiology. Gene 2015, 569, 162–172. [Google Scholar]
- Abbott, G.W. KCNE2 and the K+ channel: The tail wagging the dog. Channels 2012, 6, 1–10. [Google Scholar] [CrossRef]
- De Castro, M.P.; Aránega, A.; Franco, D. Protein distribution of Kcnq1, Kcnh2, and Kcne3 potassium channel subunits during mouse embryonic development. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2006, 288, 304–315. [Google Scholar] [CrossRef]
- Roepke, T.K.; Kanda, V.A.; Purtell, K.; King, E.C.; Lerner, D.J.; Abbott, G.W. KCNE2 forms potassium channels with KCNA3 and KCNQ1 in the choroid plexus epithelium. FASEB J. 2011, 25, 4264–4273. [Google Scholar] [CrossRef]
- Millar, I.D.; Bruce, J.I.; Brown, P.D. Ion channel diversity, channel expression and function in the choroid plexuses. Cerebrospinal Fluid Res. 2007, 4, 1–16. [Google Scholar] [CrossRef]
- De La Vieja, A.; Dohan, O.; Levy, O.; Carrasco, N. Molecular analysis of the sodium/iodide symporter: Impact on thyroid and extrathyroid pathophysiology. Physiol. Rev. 2000, 80, 1083–1105. [Google Scholar] [CrossRef]
- Lundquist, A.L.; Turner, C.L.; Ballester, L.Y.; George, A.L., Jr. Expression and transcriptional control of human KCNE genes. Genomics 2006, 87, 119–128. [Google Scholar] [CrossRef]
- ReferencesKundu, P.; Ciobotaru, A.; Foroughi, S.; Toro, L.; Stefani, E.; Eghbali, M. Hormonal regulation of cardiac KCNE2 gene expression. Mol. Cell. Endocrinol. 2008, 292, 50–62. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, M.; Tang, D.G.; Clemo, H.F.; Liu, J.; Holwitt, D.; Kasirajan, V.; Pond, A.L.; Wettwer, E.; Tseng, G.N. KCNE2 protein is expressed in ventricles of different species, and changes in its expression contribute to electrical remodeling in diseased hearts. Circulation 2004, 109, 1783–1788. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Liu, W.; Deng, J.; Wang, G.; Zhang, C.; Luo, X.; Yan, D.; Su, Q.; Liu, J. KCNE2 modulates cardiac L-type Ca2+ channel. J. Mol. Cell. Cardiol. 2014, 72, 208–218. [Google Scholar] [CrossRef]
- Yu, H.; Wu, J.; Potapova, I.; Wymore, R.T.; Holmes, B.; Zuckerman, J.; Pan, Z.; Wang, H.; Shi, W.; Robinson, R.B.; et al. MinK-related peptide 1: A β subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ. Res. 2001, 88, e84–e87. [Google Scholar] [CrossRef]
- Qu, J.; Kryukova, Y.; Potapova, I.A.; Doronin, S.V.; Larsen, M.; Krishnamurthy, G.; Cohen, I.S.; Robinson, R.B. MiRP1 modulates HCN2 channel expression and gating in cardiac myocytes. J. Biol. Chem. 2004, 279, 43497–43502. [Google Scholar] [CrossRef]
- Decher, N.; Bundis, F.; Vajna, R.; Steinmeyer, K. KCNE2 modulates current amplitudes and activation kinetics of HCN4: Influence of KCNE family members on HCN4 currents. Pflügers Arch. 2003, 446, 633–640. [Google Scholar] [CrossRef]
- Plotnikov, A.N.; Sosunov, E.A.; Qu, J.; Shlapakova, I.N.; Anyukhovsky, E.P.; Liu, L.; Janse, M.J.; Brink, P.R.; Cohen, I.S.; Robinson, R.B. Biological pacemaker implanted in canine left bundle branch provides ventricular escape rhythms that have physiologically acceptable rates. Circulation 2004, 109, 506–512. [Google Scholar] [CrossRef]
- Grosshans, B.L.; Ortiz, D.; Novick, P. Rabs and their effectors: Achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. USA 2006, 103, 11821–11827. [Google Scholar] [CrossRef]
- Abbott, G.W.; Goldstein, S.A. Potassium channel subunits encoded by the KCNE gene family: Physiology and pathophysiology of the MinK-related peptides (MiRPs). Mol. Interv. 2001, 1, 95–107. [Google Scholar]
- Sah, R.; Ramirez, R.J.; Oudit, G.Y.; Gidrewicz, D.; Trivieri, M.G.; Zobel, C.; Backx, P.H. Regulation of cardiac excitation-contraction coupling by action potential repolarization: Role of the transient outward potassium current (I(to)). J. Physiol. 2003, 546, 5–18. [Google Scholar] [CrossRef]
- Fedida, D.; Giles, W.R. Regional variations in action potentials and transient outward current in myocytes isolated from rabbit left ventricle. J. Physiol. 1991, 442, 191–209. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, D.; Gingell, R.L.; Moss, A.J.; Napolitano, C.; Priori, S.G.; Schwartz, P.J.; Kehoe, E.; Robinson, J.L.; Schulze-Bahr, E.; et al. Homozygous deletion in KVLQT1 associated with Jervell and Lange-Nielsen syndrome. Circulation 1999, 99, 1344–1347. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Nerbonne, J.M. Elimination of the transient outward current and action potential prolongation in mouse atrial myocytes expressing a dominant negative Kv4 alpha subunit. J. Physiol. 1999, 519 Pt 1, 11–21. [Google Scholar] [CrossRef]
- Bou-Abboud, E.; Nerbonne, J.M. Molecular correlates of the calcium-independent, depolarization-activated K+ currents in rat atrial myocytes. J. Physiol. 1999, 517 Pt 2, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Nerbonne, J.M. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J. Physiol. 2000, 525 Pt 2, 285–298. [Google Scholar] [PubMed]
- Wettwer, E.; Amos, G.J.; Posival, H.; Ravens, U. Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ. Res. 1994, 75, 473–482. [Google Scholar] [CrossRef]
- Liu, W.-J.; Deng, J.-X.; Wang, G.; Gao, K.-P.; Lin, Z.-X.; Liu, S.-Y.; Wang, Y.-H.; Liu, J. Manipulation of KCNE2 expression modulates action potential duration and I to and IK in rat and mouse ventricular myocytes. Am. J. Physiol.-Heart Circ. Physiol. 2015, 309, H1288–H1302. [Google Scholar] [CrossRef][Green Version]
- Heitzmann, D.; Grahammer, F.; von Hahn, T.; Schmitt-Gräff, A.; Romeo, E.; Nitschke, R.; Gerlach, U.; Lang, H.J.; Verrey, F.; Barhanin, J.; et al. Heteromeric KCNE2/KCNQ1 potassium channels in the luminal membrane of gastric parietal cells. J. Physiol. 2004, 561, 547–557. [Google Scholar] [CrossRef]
- Dedek, K.; Waldegger, S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflug. Arch. 2001, 442, 896–902. [Google Scholar] [CrossRef]
- Tinel, N.; Diochot, S.; Borsotto, M.; Lazdunski, M.; Barhanin, J. KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel. EMBO J. 2000, 19, 6326–6330. [Google Scholar] [CrossRef]
- Purtell, K.; Paroder-Belenitsky, M.; Reyna-Neyra, A.; Nicola, J.P.; Koba, W.; Fine, E.; Carrasco, N.; Abbott, G.W. The KCNQ1-KCNE2 K+ channel is required for adequate thyroid I−uptake. FASEB J. 2012, 26, 3252. [Google Scholar] [CrossRef]
- Wright, E.M. Transport processes in the formation of the cerebrospinal fluid. Rev. Physiol. Biochem. Pharmacol. 1978, 83, 3–34. [Google Scholar]
- Damkier, H.H.; Brown, P.D.; Praetorius, J. Epithelial pathways in choroid plexus electrolyte transport. Physiology 2010, 25, 239–249. [Google Scholar] [CrossRef]
- Kotera, T.; Brown, P.D. Evidence for two types of potassium current in rat choroid plexus epithelial cells. Pflug. Arch. 1994, 427, 317–324. [Google Scholar] [CrossRef]
- Grunnet, M.; Rasmussen, H.B.; Hay-Schmidt, A.; Rosenstierne, M.; Klaerke, D.A.; Olesen, S.P.; Jespersen, T. KCNE4 is an inhibitory subunit to Kv1.1 and Kv1.3 potassium channels. Biophys. J. 2003, 85, 1525–1537. [Google Scholar] [CrossRef]
- Solé, L.; Roura-Ferrer, M.; Pérez-Verdaguer, M.; Oliveras, A.; Calvo, M.; Fernández-Fernández, J.M.; Felipe, A. KCNE4 suppresses Kv1.3 currents by modulating trafficking, surface expression and channel gating. J. Cell Sci. 2009, 122, 3738–3748. [Google Scholar] [CrossRef]
- Van Hook, M.J.; Nawy, S.; Thoreson, W.B. Voltage- and calcium-gated ion channels of neurons in the vertebrate retina. Prog. Retin. Eye Res. 2019, 72, 100760. [Google Scholar] [CrossRef]
- Gayet-Primo, J.; Yaeger, D.B.; Khanjian, R.A.; Puthussery, T. Heteromeric K(V)2/K(V)8.2 Channels Mediate Delayed Rectifier Potassium Currents in Primate Photoreceptors. J. Neurosci. 2018, 38, 3414–3427. [Google Scholar] [CrossRef]
- Czirják, G.; Tóth, Z.E.; Enyedi, P. Characterization of the heteromeric potassium channel formed by kv2.1 and the retinal subunit kv8.2 in Xenopus oocytes. J. Neurophysiol. 2007, 98, 1213–1222. [Google Scholar] [CrossRef][Green Version]
- Lindner, M.; Gilhooley, M.J.; Palumaa, T.; Morton, A.J.; Hughes, S.; Hankins, M.W. Expression and Localization of Kcne2 in the Vertebrate Retina. Investig. Ophthalmol. Vis. Sci. 2020, 61, 33. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.E.; Splawski, I.; Timothy, K.W.; Vincen, G.M.; Green, E.D.; Keating, M.T. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 1995, 80, 795–803. [Google Scholar] [CrossRef]
- Sanguinetti, M.C.; Jiang, C.; Curran, M.E.; Keating, M.T. A mechanistic link between an inherited and an acquird cardiac arrthytmia: HERG encodes the IKr potassium channel. Cell 1995, 81, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, I.; Zareba, W.; Moss, A.J. Long QT Syndrome. Curr. Probl. Cardiol. 2008, 33, 629–694. [Google Scholar] [CrossRef]
- Purtell, K.; Roepke, T.K.; Abbott, G.W. Cardiac arrhythmia and thyroid dysfunction: A novel genetic link. Int. J. Biochem. Cell Biol. 2010, 42, 1767–1770. [Google Scholar] [CrossRef]
- Sesti, F.; Abbott, G.W.; Wei, J.; Murray, K.T.; Saksena, S.; Schwartz, P.J.; Priori, S.G.; Roden, D.M.; George, A.L., Jr.; Goldstein, S.A. A common polymorphism associated with antibiotic-induced cardiac arrhythmia. Proc. Natl. Acad. Sci. USA 2000, 97, 10613–10618. [Google Scholar] [CrossRef]
- Kapplinger, J.D.; Tester, D.J.; Salisbury, B.A.; Carr, J.L.; Harris-Kerr, C.; Pollevick, G.D.; Wilde, A.A.; Ackerman, M.J. Spectrum and prevalence of mutations from the first 2,500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm 2009, 6, 1297–1303. [Google Scholar] [CrossRef]
- Roberts, J.D.; Krahn, A.D.; Ackerman, M.J.; Rohatgi, R.K.; Moss, A.J.; Nazer, B.; Tadros, R.; Gerull, B.; Sanatani, S.; Wijeyeratne, Y.D.; et al. Loss-of-Function KCNE2 Variants: True Monogenic Culprits of Long-QT Syndrome or Proarrhythmic Variants Requiring Secondary Provocation? Circ. Arrhythm. Electrophysiol. 2017, 10, e005282. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xia, M.; Jin, Q.; Bendahhou, S.; Shi, J.; Chen, Y.; Liang, B.; Lin, J.; Liu, Y.; Liu, B.; et al. Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am. J. Hum. Genet. 2004, 75, 899–905. [Google Scholar] [CrossRef]
- Splawski, I.; Shen, J.; Timothy, K.W.; Lehmann, M.H.; Priori, S.; Robinson, J.L.; Moss, A.J.; Schwartz, P.J.; Towbin, J.A.; Vincent, G.M.; et al. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation 2000, 102, 1178–1185. [Google Scholar] [CrossRef]
- Isbrandt, D.; Friederich, P.; Solth, A.; Haverkamp, W.; Ebneth, A.; Borggrefe, M.; Funke, H.; Sauter, K.; Breithardt, G.; Pongs, O.; et al. Identification and functional characterization of a novel KCNE2 (MiRP1) mutation that alters HERG channel kinetics. J. Mol. Med. 2002, 80, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mahaut-Smith, M.P.; Huang, C.L.; Vandenberg, J.I. Mutant MiRP1 subunits modulate HERG K+ channel gating: A mechanism for pro-arrhythmia in long QT syndrome type 6. J. Physiol. 2003, 551, 253–262. [Google Scholar] [CrossRef]
- Wu, J.; Shimizu, W.; Ding, W.G.; Ohno, S.; Toyoda, F.; Itoh, H.; Zang, W.J.; Miyamoto, Y.; Kamakura, S.; Matsuura, H.; et al. KCNE2 modulation of Kv4.3 current and its potential role in fatal rhythm disorders. Heart Rhythm 2010, 7, 199–205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Lisewski, U.; Köhncke, C.; Schleussner, L.; Purfürst, B.; Lee, S.M.; De Silva, A.; Manville, R.W.; Abbott, G.W.; Roepke, T.K. Hypochlorhydria reduces mortality in heart failure caused by Kcne2 gene deletion. FASEB J. 2020, 34, 10699–10719. [Google Scholar] [CrossRef]
- Chang, P.-C.; Lin, S.-F.; Chu, Y.; Wo, H.-T.; Lee, H.-L.; Huang, Y.-C.; Wen, M.-S.; Chou, C.-C. LCZ696 therapy reduces ventricular tachyarrhythmia inducibility in a myocardial infarction-induced heart failure rat model. Cardiovasc. Ther. 2019, 2019, 6032631. [Google Scholar] [CrossRef]
- Nattel, S.; Maguy, A.; Le Bouter, S.; Yeh, Y.-H. Arrhythmogenic ion-channel remodeling in the heart: Heart failure, myocardial infarction, and atrial fibrillation. Physiol. Rev. 2007, 87, 425–456. [Google Scholar] [CrossRef] [PubMed]
- Caballero, R.; Delpón, E.; Valenzuela, C.; Longobardo, M.n.; Tamargo, J. Losartan and its metabolite E3174 modify cardiac delayed rectifier K+ currents. Circulation 2000, 101, 1199–1205. [Google Scholar] [CrossRef]
- Fishman, G.I.; Chugh, S.S.; DiMarco, J.P.; Albert, C.M.; Anderson, M.E.; Bonow, R.O.; Buxton, A.E.; Chen, P.-S.; Estes, M.; Jouven, X. Sudden cardiac death prediction and prevention: Report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation 2010, 122, 2335–2348. [Google Scholar] [CrossRef]
- George, A.L. Molecular and genetic basis of sudden cardiac death. J. Clin. Investig. 2013, 123, 75–83. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, J.; Zhou, L.; Tian, X.; Abbott, G.W. AKT and ERK 1/2 activation via remote ischemic preconditioning prevents Kcne2-dependent sudden cardiac death. Physiol. Rep. 2019, 7, e13957. [Google Scholar] [CrossRef]
- Watcharasit, P.; Bijur, G.N.; Song, L.; Zhu, J.; Chen, X.; Jope, R.S. Glycogen synthase kinase-3beta (GSK3beta) binds to and promotes the actions of p53. J. Biol. Chem. 2003, 278, 48872–48879. [Google Scholar] [CrossRef] [PubMed]
- Tamareille, S.; Mateus, V.; Ghaboura, N.; Jeanneteau, J.; Croué, A.; Henrion, D.; Furber, A.; Prunier, F. RISK and SAFE signaling pathway interactions in remote limb ischemic perconditioning in combination with local ischemic postconditioning. Basic Res. Cardiol. 2011, 106, 1329–1339. [Google Scholar] [CrossRef]
- Yang, S.; Abbott, G.W.; Gao, W.D.; Liu, J.; Luo, C.; Hu, Z. Involvement of glycogen synthase kinase-3β in liver ischemic conditioning induced cardioprotection against myocardial ischemia and reperfusion injury in rats. J. Appl. Physiol. 2017, 122, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Warsi, J.; Fezai, M.; Fores, M.; Elvira, B.; Lang, F. Up-regulation of voltage gated K+ channels Kv1. 3 and Kv1. 5 by protein kinase PKB/Akt. Cell. Physiol. Biochem. 2015, 37, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Teos, L.Y.; Zhao, A.; Alvin, Z.; Laurence, G.G.; Li, C.; Haddad, G.E. Basal and IGF-I-dependent regulation of potassium channels by MAP kinases and PI3-kinase during eccentric cardiac hypertrophy. Am. J. Physiol.-Heart Circ. Physiol. 2008, 295, H1834–H1845. [Google Scholar] [CrossRef]
- Cheng, W.; Zhu, Y.; Wang, H. The MAPK pathway is involved in the regulation of rapid pacing-induced ionic channel remodeling in rat atrial myocytes. Mol. Med. Rep. 2016, 13, 2677–2682. [Google Scholar] [CrossRef][Green Version]
- Fliegel, L. Regulation of myocardial Na+/H+ exchanger activity. Basic Res. Cardiol. 2001, 96, 301–305. [Google Scholar] [CrossRef]
- Kathiresan, S.; Voight, B.F.; Purcell, S.; Musunuru, K.; Ardissino, D.; Mannucci, P.M.; Anand, S.; Engert, J.C.; Samani, N.J.; Schunkert, H.; et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat. Genet. 2009, 41, 334–341. [Google Scholar] [CrossRef]
- Szpakowicz, A.; Kiliszek, M.; Pepiński, W.; Waszkiewicz, E.; Franaszczyk, M.; Skawrońska, M.; Dobrzycki, S.; Niemcunowicz-Janica, A.; Ploski, R.; Opolski, G.; et al. The rs9982601 polymorphism of the region between the SLC5A3/MRPS6 and KCNE2 genes associated with a prevalence of myocardial infarction and subsequent long-term mortality. Pol. Arch. Med. Wewn. 2015, 125, 240–248. [Google Scholar] [CrossRef]
- Sabater-Lleal, M.; Mälarstig, A.; Folkersen, L.; Soler Artigas, M.; Baldassarre, D.; Kavousi, M.; Almgren, P.; Veglia, F.; Brusselle, G.; Hofman, A.; et al. Common genetic determinants of lung function, subclinical atherosclerosis and risk of coronary artery disease. PLoS ONE 2014, 9, e104082. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Woodgett, J.; Maamari, M.; Force, T. Targeting GSK-3 family members in the heart: A very sharp double-edged sword. J. Mol. Cell. Cardiol. 2011, 51, 607–613. [Google Scholar] [CrossRef]
- Miura, T.; Miki, T. GSK-3beta, a therapeutic target for cardiomyocyte protection. Circ. J. 2009, 73, 1184–1192. [Google Scholar] [CrossRef]
- Tinel, N.; Diochot, S.; Lauritzen, I.; Barhanin, J.; Lazdunski, M.; Borsotto, M. M-type KCNQ2-KCNQ3 potassium channels are modulated by the KCNE2 subunit. FEBS Lett. 2000, 480, 137–141. [Google Scholar] [CrossRef] [PubMed]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef]
- Sohn, E.-J.; Kim, J.M.; Kang, S.-H.; Kwon, J.; An, H.J.; Sung, J.-S.; Cho, K.A.; Jang, I.-S.; Choi, J.-S. Restoring effects of natural anti-oxidant quercetin on cellular senescent human dermal fibroblasts. Am. J. Chin. Med. 2018, 46, 853–873. [Google Scholar] [CrossRef] [PubMed]
- Salsbury, G.; Cambridge, E.L.; McIntyre, Z.; Arends, M.J.; Karp, N.A.; Isherwood, C.; Shannon, C.; Hooks, Y.; Ramirez-Solis, R.; Adams, D.J. Disruption of the potassium channel regulatory subunit KCNE2 causes iron-deficient anemia. Exp. Hematol. 2014, 42, 1053–1058.e1051. [Google Scholar] [CrossRef]
- Chaker, L.; Razvi, S.; Bensenor, I.M.; Azizi, F.; Pearce, E.N.; Peeters, R.P. Hypothyroidism. Nat. Rev. Dis. Primers 2022, 8, 30. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E. Insulin Resistance: A Multifaceted Syndrome Responsible for NIDDM, Obesity, Hypertension, Dyslipidemia, and Atherosclerotic Cardiovascular Disease. Diabetes Care 1991, 14, 173–194. [Google Scholar] [CrossRef]
- Hu, Z.; Crump, S.M.; Zhang, P.; Abbott, G.W. Kcne2 deletion attenuates acute post-ischaemia/reperfusion myocardial infarction. Cardiovasc. Res. 2016, 110, 227–237. [Google Scholar] [CrossRef]
- Zhou, L.; Köhncke, C.; Hu, Z.; Roepke, T.K.; Abbott, G.W. The KCNE2 potassium channel β subunit is required for normal lung function and resilience to ischemia and reperfusion injury. FASEB J. 2019, 33, 9762. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M.; Diehl, A.M. Hepatic steatosis and type 2 diabetes mellitus. Curr. Diabetes Rep. 2002, 2, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Day, C.P. The genetics of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 645–655. [Google Scholar] [CrossRef]
- Lee, S.M.; Nguyen, D.; Anand, M.; Kant, R.; Köhncke, C.; Lisewski, U.; Roepke, T.K.; Hu, Z.; Abbott, G.W. Kcne2 deletion causes early-onset nonalcoholic fatty liver disease via iron deficiency anemia. Sci. Rep. 2016, 6, 23118. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ashtari, S.; Pourhoseingholi, M.A.; Sharifian, A.; Zali, M.R. Hepatocellular carcinoma in Asia: Prevention strategy and planning. World J. Hepatol. 2015, 7, 1708–1717. [Google Scholar] [CrossRef]
- Agarwal, R.; Narayan, J.; Bhattacharyya, A.; Saraswat, M.; Tomar, A.K. Gene expression profiling, pathway analysis and subtype classification reveal molecular heterogeneity in hepatocellular carcinoma and suggest subtype specific therapeutic targets. Cancer Genet. 2017, 216–217, 37–51. [Google Scholar] [CrossRef]
- Wei, H.; Wang, J.; Xu, Z.; Lu, Y.; Wu, X.; Zhuo, C.; Tan, C.; Tang, Q.; Pu, J. miR-584-5p regulates hepatocellular carcinoma cell migration and invasion through targeting KCNE2. Mol. Genet. Genom. Med. 2019, 7, e702. [Google Scholar] [CrossRef]

| Channel | Biological Effects | Affected Tissue | References |
|---|---|---|---|
| LTCC | KCNE2 caused a positive shift in the activation voltage and a negative shift in the voltage inactivation of LTCC, and KCNE2 slowed down the recovery from inactivation and accelerates the deactivation of LTCC. | Heart | [34] |
| HCN channel | KCNE2 fasted activation and deactivation kinetics of channel, and increased current amplitude | Heart | [36,37,38] |
| HERG channel | S98-phosphorylated KCNE2 accelerates the degradation of HERG protein and to inhibit the amplitude of HERG current | Heart | [23] |
| Ito,fast channel | KCNE2 knockdown increased gating kinetics of Ito,fast in both neonatal and adult cardiomyocytes. Overexpression of KCNE2 reduced the activation and inactivation of Ito,fast in neonatal cardiomyocytes, whereas no effect on the gating properties of Ito,fast in adult cardiomyocytes | Heart | [10,16,40,41,42,43,44,45,46,47,48] |
| KCNQ1-KCNE2 channel | KCNQ1-KCNE2 channel could be activated by low extracellular pH and acts as a channel for potassium cycling in parietal cells. Furthermore, it is postulated to be essential in regulating the potassium flux, which in turn is crucial for the optimal production of thyroid hormones in thyrocytes and the production, secretion, and regulation of the CSF in CPe. | Stomach, thyroid and choroid plexus epithelium | [15,25,27,49,50,51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Zhuge, Y.; Tu, Y.; Liu, J.; Liu, W. The Multifunctional Role of KCNE2: From Cardiac Arrhythmia to Multisystem Disorders. Cells 2024, 13, 1409. https://doi.org/10.3390/cells13171409
Song M, Zhuge Y, Tu Y, Liu J, Liu W. The Multifunctional Role of KCNE2: From Cardiac Arrhythmia to Multisystem Disorders. Cells. 2024; 13(17):1409. https://doi.org/10.3390/cells13171409
Chicago/Turabian StyleSong, Ming, Yixin Zhuge, Yuqi Tu, Jie Liu, and Wenjuan Liu. 2024. "The Multifunctional Role of KCNE2: From Cardiac Arrhythmia to Multisystem Disorders" Cells 13, no. 17: 1409. https://doi.org/10.3390/cells13171409
APA StyleSong, M., Zhuge, Y., Tu, Y., Liu, J., & Liu, W. (2024). The Multifunctional Role of KCNE2: From Cardiac Arrhythmia to Multisystem Disorders. Cells, 13(17), 1409. https://doi.org/10.3390/cells13171409





