Structural and Functional Dysregulation of the Brain Endothelium in HIV Infection and Substance Abuse
Abstract
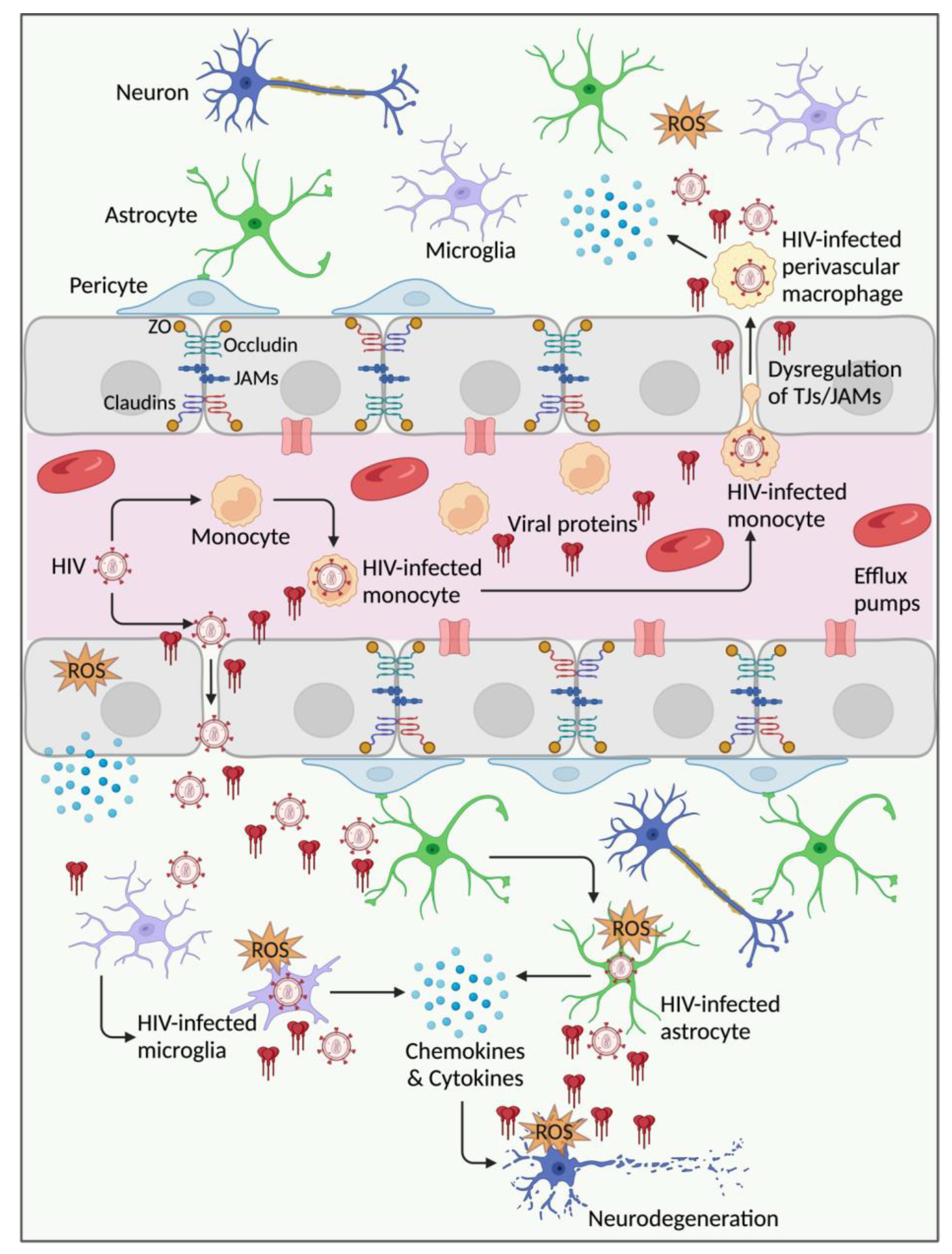
:1. Introduction
2. HIV Infection, BBB Injury, and Dysfunction
2.1. Evidence from Human Studies
2.2. Evidence from Animal Studies
2.3. Evidence from In Vitro Studies
3. Factors and Mechanisms Involved in HIV-Mediated Endothelial Injury and BBB Dysfunction
3.1. HIV and Infected Leukocytes
3.2. HIV Proteins
3.2.1. HIV gp120
3.2.2. HIV Tat
3.2.3. HIV Nef, Viral Protein R (Vpr), and p17
4. Endothelial Injury and BBB Dysfunction in HIV Infection and Substance Abuse
4.1. Cocaine
4.2. Meth
4.3. Alcohol
4.4. Tobacco
4.5. Opioids
4.6. Cannabinoids
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDS | Acquired immunodeficiency syndrome |
| ALCAM | Activated leukocyte cell adhesion molecule |
| AJs | Adherent junctions |
| AKT | Protein kinase B |
| AP-1 | Activator protein 1 |
| ART | Antiretroviral therapy |
| BBB | Blood–brain barrier |
| CB1R | Cannabinoid-type 1 receptor |
| CB2R | Cannabinoid-type 2 receptor |
| CCL2: | Chemokine ligand 2 |
| CCL5 | Chemokine ligand 5 |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| ECs | Endothelial cells |
| Gp120 | Glycoprotein 120 |
| HAND | HIV-associated neurocognitive disorder |
| HBMECs | Human brain microvascular endothelial cells |
| HIV | Human immunodeficiency virus |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IL | Interleukin |
| JAM | Junctional adhesion molecule |
| MAPK | Mitogen-activated protein kinase |
| MMPs | Matrix metalloproteinases |
| MOR | Morphine |
| Nef | Negative factor |
| NF-κB | Nuclear factor kappa B |
| QAIb | CSF/serum albumin quotient |
| PLWH | People living with HIV |
| PI3K | Phosphatidyl inositol 3 kinase |
| PPARα/γ | Peroxisome proliferator-activated receptor alpha/gamma |
| ROS | Reactive oxygen species |
| RTV/r | Ritonavir |
| SQV | Saquinavir |
| STAT | Signal transducer and activator of transcription |
| Tat | Trans-activator of transcription |
| Tg | Transgenic |
| TEER | Transendothelial electrical resistance |
| TJs | Tight junctions |
| TNF-α | Tumor necrosis factor-alpha |
| SIV | Simian immunodeficiency virus |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| Vpr | Viral protein R |
| ZO | Zonula occludens |
References
- Alahmari, A. Blood-Brain Barrier Overview: Structural and Functional Correlation. Neural Plast. 2021, 2021, 6564585. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Segarra, M.; Aburto, M.R.; Acker-Palmer, A. Blood–Brain Barrier Dynamics to Maintain Brain Homeostasis. Trends Neurosci. 2021, 44, 393–405. [Google Scholar] [CrossRef]
- Borrajo, A.; Spuch, C.; Penedo, M.A.; Olivares, J.M.; Agís-Balboa, R.C. Important role of microglia in HIV-1 associated neurocognitive disorders and the molecular pathways implicated in its pathogenesis. Ann. Med. 2021, 53, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, S.D.; Alldred, M.J.; Gunnam, S.M.; Schiroli, C.; Lee, S.H.; Morgello, S.; Fischer, T. Expression profiling suggests microglial impairment in human immunodeficiency virus neuropathogenesis. Ann. Neurol. 2018, 83, 406–417. [Google Scholar] [CrossRef]
- Mattson, M.P.; Haughey, N.J.; Nath, A. Cell death in HIV dementia. Cell Death Differ. 2005, 12, 893–904. [Google Scholar] [CrossRef]
- Kaul, M.; Zheng, J.; Okamoto, S.; Gendelman, H.E.; Lipton, S.A. HIV-1 infection and AIDS: Consequences for the central nervous system. Cell Death Differ. 2005, 12, 878–892. [Google Scholar] [CrossRef] [PubMed]
- Saylor, D.; Dickens, A.M.; Sacktor, N.; Haughey, N.; Slusher, B.; Pletnikov, M.; Mankowski, J.L.; Brown, A.; Volsky, D.J.; McArthur, J.C. HIV-associated neurocognitive disorder—Pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016, 12, 234–248. [Google Scholar] [CrossRef]
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef]
- Bandera, A.; Taramasso, L.; Bozzi, G.; Muscatello, A.; Robinson, J.A.; Burdo, T.H.; Gori, A. HIV-Associated Neurocognitive Impairment in the Modern ART Era: Are We Close to Discovering Reliable Biomarkers in the Setting of Virological Suppression? Front. Aging Neurosci. 2019, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.B.; Ances, B.M. HIV-associated neurocognitive disorder. Lancet Infect. Dis. 2013, 13, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, S.; Ances, B.; Cinque, P.; Dravid, A.; Dreyer, A.J.; Gisslén, M.; Joska, J.A.; Kwasa, J.; Meyer, A.-C.; Mpongo, N.; et al. Cognitive impairment in people living with HIV: Consensus recommendations for a new approach. Nat. Rev. Neurol. 2023, 19, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Sacktor, N.; McDermott, M.P.; Marder, K.; Schifitto, G.; Selnes, O.A.; McArthur, J.C.; Stern, Y.; Albert, S.; Palumbo, D.; Kieburtz, K.; et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J. NeuroVirol. 2002, 8, 136–142. [Google Scholar] [CrossRef]
- Marcus, J.L.; Leyden, W.A.; Alexeeff, S.E.; Anderson, A.N.; Hechter, R.C.; Hu, H.; Lam, J.O.; Towner, W.J.; Yuan, Q.; Horberg, M.A.; et al. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults With and Without HIV Infection, 2000-2016. JAMA Netw. Open 2020, 3, e207954. [Google Scholar] [CrossRef]
- Trickey, A.; Sabin, C.A.; Burkholder, G.; Crane, H.; d‘Arminio Monforte, A.; Egger, M.; Gill, M.J.; Grabar, S.; Guest, J.L.; Jarrin, I.; et al. Life expectancy after 2015 of adults with HIV on long-term antiretroviral therapy in Europe and North America: A collaborative analysis of cohort studies. Lancet HIV 2023, 10, e295–e307. [Google Scholar] [CrossRef]
- Anesten, B.; Zetterberg, H.; Nilsson, S.; Brew, B.J.; Fuchs, D.; Price, R.W.; Gisslén, M.; Yilmaz, A. Effect of antiretroviral treatment on blood-brain barrier integrity in HIV-1 infection. BMC Neurol. 2021, 21, 494. [Google Scholar] [CrossRef]
- Calcagno, A.; Alberione, M.C.; Romito, A.; Imperiale, D.; Ghisetti, V.; Audagnotto, S.; Lipani, F.; Raviolo, S.; Di Perri, G.; Bonora, S. Prevalence and predictors of blood-brain barrier damage in the HAART era. J. NeuroVirol. 2014, 20, 521–525. [Google Scholar] [CrossRef]
- Caligaris, G.; Trunfio, M.; Ghisetti, V.; Cusato, J.; Nigra, M.; Atzori, C.; Imperiale, D.; Bonora, S.; Di Perri, G.; Calcagno, A. Blood–Brain Barrier Impairment in Patients Living with HIV: Predictors and Associated Biomarkers. Diagnostics 2021, 11, 867. [Google Scholar] [CrossRef]
- Avison, M.J.; Nath, A.; Greene-Avison, R.; Schmitt, F.A.; Greenberg, R.N.; Berger, J.R. Neuroimaging correlates of HIV-associated BBB compromise. J. Neuroimmunol. 2004, 157, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Rahimy, E.; Li, F.-Y.; Hagberg, L.; Fuchs, D.; Robertson, K.; Meyerhoff, D.J.; Zetterberg, H.; Price, R.W.; Gisslén, M.; Spudich, S. Blood-Brain Barrier Disruption Is Initiated During Primary HIV Infection and Not Rapidly Altered by Antiretroviral Therapy. J. Infect. Dis. 2017, 215, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Chaganti, J.; Marripudi, K.; Staub, L.P.; Rae, C.D.; Gates, T.M.; Moffat, K.J.; Brew, B.J. Imaging correlates of the blood–brain barrier disruption in HIV-associated neurocognitive disorder and therapeutic implications. AIDS 2019, 33, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, A.; Atzori, C.; Romito, A.; Vai, D.; Audagnotto, S.; Stella, M.L.; Montrucchio, C.; Imperiale, D.; Di Perri, G.; Bonora, S. Blood brain barrier impairment is associated with cerebrospinal fluid markers of neuronal damage in HIV-positive patients. J. NeuroVirol. 2016, 22, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.L.; Wang, H.; Zhang, Z.; Millien, G.; Tyagi, M.; Hongpaisan, J. HIV Promotes Neurocognitive Impairment by Damaging the Hippocampal Microvessels. Mol. Neurobiol. 2022, 59, 4966–4986. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Yang, B.; Gendelman, H.E.; Persidsky, Y.; Kanmogne, G.D. STAT1 signaling modulates HIV-1–induced inflammatory responses and leukocyte transmigration across the blood-brain barrier. Blood 2008, 111, 2062–2072. [Google Scholar] [CrossRef]
- Dallasta, L.M.; Pisarov, L.A.; Esplen, J.E.; Werley, J.V.; Moses, A.V.; Nelson, J.A.; Achim, C.L. Blood-Brain Barrier Tight Junction Disruption in Human Immunodeficiency Virus-1 Encephalitis. Am. J. Pathol. 1999, 155, 1915–1927. [Google Scholar] [CrossRef]
- Bai, F.; Bono, V.; Borghi, L.; Bonazza, F.; Falcinella, C.; Vitaletti, V.; Miraglia, F.; Trunfio, M.; Calcagno, A.; Cusato, J.; et al. Association between tight junction proteins and cognitive performance in untreated Persons living with HIV (PLWH). AIDS 2024, 38, 1292–1303. [Google Scholar] [CrossRef]
- Petito, C.K.; Cash, K.S. Blood-brain barrier abnormalities in acquired immunodeficiency syndrome: Immunohistochemical localization of serum proteins in postmortem brain. Ann. Neurol. 1992, 32, 658–666. [Google Scholar] [CrossRef] [PubMed]
- MacLean, A.G.; Belenchia, G.E.; Bieniemy, D.N.; Moroney-Rasmussen, T.A.; Lackner, A.A. Simian immunodeficiency virus disrupts extended lengths of the blood–brain barrier. J. Med. Primatol. 2005, 34, 237–242. [Google Scholar] [CrossRef]
- Luabeya, M.K.; Dallasta, L.M.; Achim, C.L.; Pauza, C.D.; Hamilton, R.L. Blood–brain barrier disruption in simian immunodeficiency virus encephalitis. Neuropathol. Appl. Neurobiol. 2000, 26, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Orandle Marlene, S.; MacLean Andrew, G.; Sasseville Vito, G.; Alvarez, X.; Lackner Andrew, A. Enhanced Expression of Proinflammatory Cytokines in the Central Nervous System Is Associated with Neuroinvasion by Simian Immunodeficiency Virus and the Development of Encephalitis. J. Virol. 2002, 76, 5797–5802. [Google Scholar] [CrossRef] [PubMed]
- Clay Candice, C.; Rodrigues Denise, S.; Ho Yan, S.; Fallert Beth, A.; Janatpour, K.; Reinhart Todd, A.; Esser, U. Neuroinvasion of Fluorescein-Positive Monocytes in Acute Simian Immunodeficiency Virus Infection. J. Virol. 2007, 81, 12040–12048. [Google Scholar] [CrossRef] [PubMed]
- Sasseville, V.G.; Lane, J.H.; Walsh, D.; Ringler, D.J.; Lackner, A.A. VCAM-1 expression and leukocyte trafficking to the CNS occur early in infection with pathogenic isolates of SIV. J. Med. Primatol. 1995, 24, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Sasseville, V.G.; Smith, M.M.; Mackay, C.R.; Pauley, D.R.; Mansfield, K.G.; Ringler, D.J.; Lackner, A.A. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am. J. Pathol. 1996, 149, 1459. [Google Scholar]
- Bhargavan, B.; Woollard, S.M.; McMillan, J.E.; Kanmogne, G.D. CCR5 antagonist reduces HIV-induced amyloidogenesis, tau pathology, neurodegeneration, and blood-brain barrier alterations in HIV-infected hu-PBL-NSG mice. Mol. Neurodegener. 2021, 16, 78. [Google Scholar] [CrossRef]
- Cioni, C.; Annunziata, P. Circulating gp120 alters the blood–brain barrier permeability in HIV-1 gp120 transgenic mice. Neurosci. Lett. 2002, 330, 299–301. [Google Scholar] [CrossRef]
- Toneatto, S.; Finco, O.; van der Putten, H.; Abrignani, S.; Annunziata, P. Evidence of blood-brain barrier alteration and activation in HIV-1 gp120 transgenic mice. AIDS 1999, 13, 2343–2348. [Google Scholar] [CrossRef]
- Louboutin, J.-P.; Agrawal, L.; Reyes, B.A.S.; Van Bockstaele, E.J.; Strayer, D.S. HIV-1 gp120-Induced Injury to the Blood-Brain Barrier: Role of Metalloproteinases 2 and 9 and Relationship to Oxidative Stress. J. Neuropathol. Exp. Neurol. 2010, 69, 801–816. [Google Scholar] [CrossRef]
- Louboutin, J.-P.; Reyes, B.A.S.; Agrawal, L.; Van Bockstaele, E.J.; Strayer, D.S. HIV-1 gp120 upregulates matrix metalloproteinases and their inhibitors in a rat model of HIV encephalopathy. Eur. J. Neurosci. 2011, 34, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Niu, F.; Hu, G.; Guo, M.-L.; Sil, S.; Buch, S. HIV Tat-mediated induction of autophagy regulates the disruption of ZO-1 in brain endothelial cells. Tissue Barriers 2020, 8, 1748983. [Google Scholar] [CrossRef]
- Leibrand, C.R.; Paris, J.J.; Ghandour, M.S.; Knapp, P.E.; Kim, W.-K.; Hauser, K.F.; McRae, M. HIV-1 Tat disrupts blood-brain barrier integrity and increases phagocytic perivascular macrophages and microglia in the dorsal striatum of transgenic mice. Neurosci. Lett. 2017, 640, 136–143. [Google Scholar] [CrossRef]
- Sporer, B.; Koedel, U.; Paul, R.; Kohleisen, B.; Erfle, V.; Fontana, A.; Pfister, H.W. Human immunodeficiency virus type-1 Nef protein induces blood-brain barrier disruption in the rat: Role of matrix metalloproteinase-9. J. Neuroimmunol. 2000, 102, 125–130. [Google Scholar] [CrossRef]
- Rivera, J.; Isidro, R.A.; Loucil-Alicea, R.Y.; Cruz, M.L.; Appleyard, C.B.; Isidro, A.A.; Chompre, G.; Colon-Rivera, K.; Noel, R.J., Jr. Infusion of HIV-1 Nef-expressing astrocytes into the rat hippocampus induces enteropathy and interstitial pneumonitis and increases blood–brain-barrier permeability. PLoS ONE 2019, 14, e0225760. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.B.; Singh, M.V.; Gorantla, S.; Poluektova, L.Y.; Maggirwar, S.B. Smoothened Agonist Reduces Human Immunodeficiency Virus Type-1-Induced Blood-Brain Barrier Breakdown in Humanized Mice. Sci. Rep. 2016, 6, 26876. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Duan, F.; Morsey, B.; Persidsky, Y.; Kanmogne, G.D. HIV-1 Activates Proinflammatory and Interferon-Inducible Genes in Human Brain Microvascular Endothelial Cells: Putative Mechanisms of Blood—Brain Barrier Dysfunction. J. Cereb. Blood Flow Metab. 2008, 28, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Singh, S.; Bressani, R.; Kanmogne, G.D. Cross-talk between STAT1 and PI3K/AKT signaling in HIV-1-induced blood–brain barrier dysfunction: Role of CCR5 and implications for viral neuropathogenesis. J. Neurosci. Res. 2010, 88, 3090–3101. [Google Scholar] [CrossRef] [PubMed]
- Kanmogne, G.D.; Kennedy, R.C.; Grammas, P. HIV-1 gp120 Proteins and gp160 Peptides Are Toxic to Brain Endothelial Cells and Neurons: Possible Pathway for HIV Entry into the Brain and HIV-Associated Dementia. J. Neuropathol. Exp. Neurol. 2002, 61, 992–1000. [Google Scholar] [CrossRef]
- Kanmogne, G.D.; Primeaux, C.; Grammas, P. HIV-1 gp120 Proteins Alter Tight Junction Protein Expression and Brain Endothelial Cell Permeability: Implications for the Pathogenesis of HIV-Associated Dementia. J. Neuropathol. Exp. Neurol. 2005, 64, 498–505. [Google Scholar] [CrossRef]
- Kanmogne, G.D.; Schall, K.; Leibhart, J.; Knipe, B.; Gendelman, H.E.; Persidsky, Y. HIV-1 gp120 Compromises Blood–Brain Barrier Integrity and Enhance Monocyte Migration across Blood–Brain Barrier: Implication for Viral Neuropathogenesis. J. Cereb. Blood Flow Metab. 2007, 27, 123–134. [Google Scholar] [CrossRef]
- Yang, B.; Akhter, S.; Chaudhuri, A.; Kanmogne, G.D. HIV-1 gp120 induces cytokine expression, leukocyte adhesion, and transmigration across the blood–brain barrier: Modulatory effects of STAT1 signaling. Microvasc. Res. 2009, 77, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Avraham, H.K.; Jiang, S.; Lee, T.-H.; Prakash, O.; Avraham, S. HIV-1 Tat-Mediated Effects on Focal Adhesion Assembly and Permeability in Brain Microvascular Endothelial Cells1. J. Immunol. 2004, 173, 6228–6233. [Google Scholar] [CrossRef]
- Bhargavan, B.; Kanmogne, G.D. Differential Mechanisms of Inflammation and Endothelial Dysfunction by HIV-1 Subtype-B and Recombinant CRF02_AG Tat Proteins on Human Brain Microvascular Endothelial Cells: Implications for Viral Neuropathogenesis. Mol. Neurobiol. 2018, 55, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- András, I.E.; Pu, H.; Deli, M.A.; Nath, A.; Hennig, B.; Toborek, M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J. Neurosci. Res. 2003, 74, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, W.; Jiang, W.; Wu, X.; Ye, B.; Zhou, X. HIV-1 Tat Regulates Occludin and Aβ Transfer Receptor Expression in Brain Endothelial Cells via Rho/ROCK Signaling Pathway. Oxidative Med. Cell. Longev. 2016, 2016, 4196572. [Google Scholar] [CrossRef]
- Xu, R.; Feng, X.; Xie, X.; Zhang, J.; Wu, D.; Xu, L. HIV-1 Tat protein increases the permeability of brain endothelial cells by both inhibiting occludin expression and cleaving occludin via matrix metalloproteinase-9. Brain Res. 2012, 1436, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Pu, H.; Tian, J.; Andras, I.E.; Lee, Y.W.; Hennig, B.; Toborek, M. HIV-Tat protein induces P-glycoprotein expression in brain microvascular endothelial cells. J. Neurochem. 2005, 93, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Hennig, B.; Toborek, M. Intact Lipid Rafts Regulate HIV-1 Tat Protein-Induced Activation of the Rho Signaling and Upregulation of P-Glycoprotein in Brain Endothelial Cells. J. Cereb. Blood Flow Metab. 2010, 30, 522–533. [Google Scholar] [CrossRef]
- Acheampong Edward, A.; Parveen, Z.; Muthoga Lois, W.; Kalayeh, M.; Mukhtar, M.; Pomerantz Roger, J. Human Immunodeficiency Virus Type 1 Nef Potently Induces Apoptosis in Primary Human Brain Microvascular Endothelial Cells via the Activation of Caspases. J. Virol. 2005, 79, 4257–4269. [Google Scholar] [CrossRef] [PubMed]
- Raymond, A.D.; Diaz, P.; Chevelon, S.; Agudelo, M.; Yndart-Arias, A.; Ding, H.; Kaushik, A.; Jayant, R.D.; Nikkhah-Moshaie, R.; Roy, U.; et al. Microglia-derived HIV Nef+ exosome impairment of the blood–brain barrier is treatable by nanomedicine-based delivery of Nef peptides. J. NeuroVirol. 2016, 22, 129–139. [Google Scholar] [CrossRef]
- Dohgu, S.; Ryerse, J.S.; Robinson, S.M.; Banks, W.A. Human Immunodeficiency Virus-1 Uses the Mannose-6-Phosphate Receptor to Cross the Blood-Brain Barrier. PLoS ONE 2012, 7, e39565. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, J.; Goldstein, H. Human Immunodeficiency Virus Type 1 Infection Increases the In Vivo Capacity of Peripheral Monocytes To Cross the Blood-Brain Barrier into the Brain and the In Vivo Sensitivity of the Blood-Brain Barrier to Disruption by Lipopolysaccharide. J. Virol. 2008, 82, 7591–7600. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.W.; Eugenin, E.A.; Calderon, T.M.; Berman, J.W. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J. Leukoc. Biol. 2012, 91, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Eugenin, E.A.; Osiecki, K.; Lopez, L.; Goldstein, H.; Calderon, T.M.; Berman, J.W. CCL2/Monocyte Chemoattractant Protein-1 Mediates Enhanced Transmigration of Human Immunodeficiency Virus (HIV)-Infected Leukocytes across the Blood–Brain Barrier: A Potential Mechanism of HIV–CNS Invasion and NeuroAIDS. J. Neurosci. 2006, 26, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Nottet, H.S.L.M. Interactions between macrophages and brain microvascular endothelial cells: Role in pathogenesis of HIV-1 infection and blood-brain barrier function. J. Neurovirol. 1999, 5, 659–669. [Google Scholar] [CrossRef]
- Persidsky, Y.; Zheng, J.; Miller, D.; Gendelman, H.E. Mononuclear phagocytes mediate blood-brain barrier compromise and neuronal injury during HIV-1-associated dementia. J. Leukoc. Biol. 2000, 68, 413–422. [Google Scholar] [CrossRef]
- Chandra, P.K.; Rutkai, I.; Kim, H.; Braun, S.E.; Abdel-Mageed, A.B.; Mondal, D.; Busija, D.W. Latent HIV-Exosomes Induce Mitochondrial Hyperfusion Due to Loss of Phosphorylated Dynamin-Related Protein 1 in Brain Endothelium. Mol. Neurobiol. 2021, 58, 2974–2989. [Google Scholar] [CrossRef]
- Persidsky, Y.; Heilman, D.; Haorah, J.; Zelivyanskaya, M.; Persidsky, R.; Weber, G.A.; Shimokawa, H.; Kaibuchi, K.; Ikezu, T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE). Blood 2006, 107, 4770–4780. [Google Scholar] [CrossRef] [PubMed]
- Ricardo-Dukelow, M.; Kadiu, I.; Rozek, W.; Schlautman, J.; Persidsky, Y.; Ciborowski, P.; Kanmogne, G.D.; Gendelman, H.E. HIV-1 infected monocyte-derived macrophages affect the human brain microvascular endothelial cell proteome: New insights into blood-brain barrier dysfunction for HIV-1-associated dementia. J. Neuroimmunol. 2007, 185, 37–46. [Google Scholar] [CrossRef]
- McRae, M. HIV and viral protein effects on the blood brain barrier. Tissue Barriers 2016, 4, e1143543. [Google Scholar] [CrossRef]
- Langford, D.; Grigorian, A.; Hurford, R.; Adame, A.; Ellis, R.J.; Hansen, L.; Masliah, E. Altered P-Glycoprotein Expression in AIDS Patients with HIV Encephalitis. J. Neuropathol. Exp. Neurol. 2004, 63, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Turchan, J.; Pocernich, C.B.; Gairola, C.; Chauhan, A.; Schifitto, G.; Butterfield, D.A.; Buch, S.; Narayan, O.; Sinai, A.; Geiger, J.; et al. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology 2003, 60, 307–314. [Google Scholar] [CrossRef]
- Huang, W.; András, I.E.; Rha, G.B.; Hennig, B.; Toborek, M. PPARα and PPARγ protect against HIV-1-induced MMP-9 overexpression via caveolae-associated ERK and Akt signaling. FASEB J. 2011, 25, 3979–3988. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Eum, S.Y.; András, I.E.; Hennig, B.; Toborek, M. PPARα and PPARγ attenuate HIV-induced dysrégulation of tight junction proteins by modulations of matrix metalloproteinase and proteasome activities. FASEB J. 2009, 23, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Rha, G.B.; Han, M.-J.; Eum, S.Y.; András, I.E.; Zhong, Y.; Hennig, B.; Toborek, M. PPARα and PPARγ effectively protect against HIV-induced inflammatory responses in brain endothelial cells. J. Neurochem. 2008, 107, 497–509. [Google Scholar] [CrossRef]
- Ramirez, S.H.; Heilman, D.; Morsey, B.; Potula, R.; Haorah, J.; Persidsky, Y. Activation of Peroxisome Proliferator-Activated Receptor γ (PPARγ) Suppresses Rho GTPases in Human Brain Microvascular Endothelial Cells and Inhibits Adhesion and Transendothelial Migration of HIV-1 Infected Monocytes1. J. Immunol. 2008, 180, 1854–1865. [Google Scholar] [CrossRef]
- Khan, N.A.; Di Cello, F.; Stins, M.; Kim, K.S. Gp120-mediated cytotoxicity of human brain microvascular endothelial cells is dependent on p38 mitogen-activated protein kinase activation. J. NeuroVirol. 2007, 13, 242–251. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Brennan, J.M.; Vallance, K.L. Adsorptive Endocytosis of HIV-1gp120 by Blood–Brain Barrier Is Enhanced by Lipopolysaccharide. Exp. Neurol. 1999, 156, 165–171. [Google Scholar] [CrossRef]
- Lu, T.-S.; Avraham, H.K.; Seng, S.; Tachado, S.D.; Koziel, H.; Makriyannis, A.; Avraham, S. Cannabinoids Inhibit HIV-1 Gp120-Mediated Insults in Brain Microvascular Endothelial Cells1. J. Immunol. 2008, 181, 6406–6416. [Google Scholar] [CrossRef]
- Ramirez, S.H.; Buzhdygan, T.P.; Hale, J.F.; Cheng, L.; Li, G.; Hoover-Hankerson, B.; Razmpour, R.; Sriram, U.; Su, L.; Potula, R.; et al. Extracellular Microvesicles Released From Brain Endothelial Cells are Detected in Animal Models Of HIV-1 Signifying Unresolved Inflammation. J. Neuroimmune Pharmacol. 2021, 16, 785–795. [Google Scholar] [CrossRef]
- Banerjee, A.; Zhang, X.; Manda, K.R.; Banks, W.A.; Ercal, N. HIV proteins (gp120 and Tat) and methamphetamine in oxidative stress-induced damage in the brain: Potential role of the thiol antioxidant N-acetylcysteine amide. Free Radic. Biol. Med. 2010, 48, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Price, T.O.; Ercal, N.; Nakaoke, R.; Banks, W.A. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005, 1045, 57–63. [Google Scholar] [CrossRef]
- Price, T.O.; Uras, F.; Banks, W.A.; Ercal, N. A novel antioxidant N-acetylcysteine amide prevents gp120- and Tat-induced oxidative stress in brain endothelial cells. Exp. Neurol. 2006, 201, 193–202. [Google Scholar] [CrossRef]
- Ma, R.; Yang, L.; Niu, F.; Buch, S. HIV Tat-Mediated Induction of Human Brain Microvascular Endothelial Cell Apoptosis Involves Endoplasmic Reticulum Stress and Mitochondrial Dysfunction. Mol. Neurobiol. 2016, 53, 132–142. [Google Scholar] [CrossRef] [PubMed]
- ZIDOVETZKI, R.; WANG, J.-L.; CHEN, P.; JEYASEELAN, R.; HOFMAN, F. Human Immunodeficiency Virus Tat Protein Induces Interleukin 6 mRNA Expression in Human Brain Endothelial Cells via Protein Kinase C- and cAMP-Dependent Protein Kinase Pathways. AIDS Res. Hum. Retroviruses 1998, 14, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.M.; Nath, A.; Major, E.O.; Berman, J.W. HIV-1 Tat Induces Monocyte Chemoattractant Protein-1-Mediated Monocyte Transmigration Across a Model of the Human Blood-Brain Barrier and Up-Regulates CCR5 Expression on Human Monocytes. J. Immunol. 1999, 163, 2953–2959. [Google Scholar] [CrossRef] [PubMed]
- Hofman, F.M.; Dohadwala, M.M.; Wright, A.D.; Hinton, D.R.; Walker, S.M. Exogenous tat protein activates central nervous system-derived endothelial cells. J. Neuroimmunol. 1994, 54, 19–28. [Google Scholar] [CrossRef]
- Huang, W.; Mo, X.; Wu, X.; Luo, W.; Chen, Y. Rosiglitazone suppresses HIV-1 Tat-induced vascular inflammation via Akt signaling. Mol. Cell. Biochem. 2015, 407, 173–179. [Google Scholar] [CrossRef]
- Mishra, R.; Singh, S.K. HIV-1 Tat C phosphorylates VE-cadherin complex and increases human brain microvascular endothelial cell permeability. BMC Neurosci. 2014, 15, 80. [Google Scholar] [CrossRef]
- Mishra, R.; Singh, S.K. HIV-1 Tat C Modulates Expression of miRNA-101 to Suppress VE-Cadherin in Human Brain Microvascular Endothelial Cells. J. Neurosci. 2013, 33, 5992–6000. [Google Scholar] [CrossRef]
- Toborek, M.; Lee, Y.W.; Pu, H.; Malecki, A.; Flora, G.; Garrido, R.; Hennig, B.; Bauer, H.-C.; Nath, A. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. J. Neurochem. 2003, 84, 169–179. [Google Scholar] [CrossRef] [PubMed]
- András, I.E.; Pu, H.; Tian, J.; Deli, M.A.; Nath, A.; Hennig, B.; Toborek, M. Signaling Mechanisms of HIV-1 Tat-Induced Alterations of Claudin-5 Expression in Brain Endothelial Cells. J. Cereb. Blood Flow Metab. 2005, 25, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Di Cello, F.; Nath, A.; Kim, K.S. Human immunodeficiency virus type 1 Tat-mediated cytotoxicity of human brain microvascular endothelial cells. J. NeuroVirol. 2003, 9, 584–593. [Google Scholar] [CrossRef]
- Kim, T.-A.; Avraham, H.K.; Koh, Y.-H.; Jiang, S.; Park, I.-W.; Avraham, S. HIV-1 Tat-Mediated Apoptosis in Human Brain Microvascular Endothelial Cells1. J. Immunol. 2003, 170, 2629–2637. [Google Scholar] [CrossRef]
- Jiang, W.; Huang, W.; Chen, Y.; Zou, M.; Peng, D.; Chen, D. HIV-1 Transactivator Protein Induces ZO-1 and Neprilysin Dysfunction in Brain Endothelial Cells via the Ras Signaling Pathway. Oxidative Med. Cell. Longev. 2017, 2017, 3160360. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, E.; Mukhtar, M.; Parveen, Z.; Ngoubilly, N.; Ahmad, N.; Patel, C.; Pomerantz, R.J. Ethanol Strongly Potentiates Apoptosis Induced by HIV-1 Proteins in Primary Human Brain Microvascular Endothelial Cells. Virology 2002, 304, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Caccuri, F.; Neves, V.; Gano, L.; Correia, J.D.G.; Oliveira, M.C.; Mazzuca, P.; Caruso, A.; Castanho, M. The HIV-1 Matrix Protein p17 Does Cross the Blood-Brain Barrier. J. Virol. 2022, 96, e01200-21. [Google Scholar] [CrossRef]
- Chibanda, D.; Benjamin, L.; Weiss, H.A.; Abas, M. Mental, neurological, and substance use disorders in people living with HIV/AIDS in low- and middle-income countries. J. Acquir. Immune Defic. Syndr. 2014, 67 (Suppl. 1), S54–S67. [Google Scholar] [CrossRef]
- Chilunda, V.; Calderon, T.M.; Martinez-Aguado, P.; Berman, J.W. The impact of substance abuse on HIV-mediated neuropathogenesis in the current ART era. Brain Res. 2019, 1724, 146426. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, N.K.; Peng, F.; Bokhari, S.; Callen, S.; Shin, S.-H.; Zhu, X.; Kim, K.-J.; Buch, S.J. Cocaine-mediated Alteration in Tight Junction Protein Expression and Modulation of CCL2/CCR2 Axis Across the Blood-Brain Barrier: Implications for HIV-Dementia. J. Neuroimmune Pharmacol. 2008, 3, 52–56. [Google Scholar] [CrossRef]
- Yao, H.; Kim, K.; Duan, M.; Hayashi, T.; Guo, M.; Morgello, S.; Prat, A.; Wang, J.; Su, T.-P.; Buch, S. Cocaine Hijacks σ1 Receptor to Initiate Induction of Activated Leukocyte Cell Adhesion Molecule: Implication for Increased Monocyte Adhesion and Migration in the CNS. J. Neurosci. 2011, 31, 5942–5955. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.; Saiyed, Z.M.; Napuri, J.; Samikkannu, T.; Reddy, P.V.B.; Agudelo, M.; Khatavkar, P.; Saxena, S.K.; Nair, M.P.N. Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific Tat protein and cocaine in blood-brain barrier dysfunction: Implications for HIV-1-associated neurocognitive disorder. J. NeuroVirol. 2010, 16, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Fiala, M.; Eshleman, A.J.; Cashman, J.; Lin, J.; Lossinsky, A.S.; Suarez, V.; Yang, W.; Zhang, J.; Popik, W.; Singer, E.; et al. Cocaine increases human immunodeficiency virus type 1 neuroinvasion through remodeling brain microvascular endothelial cells. J. NeuroVirol. 2005, 11, 281–291. [Google Scholar] [CrossRef]
- Fiala, M.; Gan, X.H.; Zhang, L.; House, S.D.; Newton, T.; Graves, M.C.; Shapshak, P.; Stins, M.; Kim, K.S.; Witte, M.; et al. Cocaine Enhances Monocyte Migration Across the Blood-Brain Barrier. In Drugs of Abuse, Immunomodulation, and Aids; Friedman, H., Madden, J.J., Klein, T.W., Eds.; Springer: Boston, MA, USA, 1998; pp. 199–205. [Google Scholar]
- Gan, X.; Zhang, L.; Berger, O.; Stins, M.F.; Way, D.; Taub, D.D.; Chang, S.L.; Kim, K.S.; House, S.D.; Weinand, M.; et al. Cocaine Enhances Brain Endothelial Adhesion Molecules and Leukocyte Migration. Clin. Immunol. 1999, 91, 68–76. [Google Scholar] [CrossRef]
- Ezeomah, C.; Fongsaran, C.; Persons, A.L.; Napier, T.C.; Cisneros, I.E. Cocaine Self-Administration Influences Central Nervous System Immune Responses in Male HIV-1 Transgenic Rats. Cells 2022, 11, 2405. [Google Scholar] [CrossRef]
- Fattakhov, N.; Torices, S.; Stangis, M.; Park, M.; Toborek, M. Synergistic Impairment of the Neurovascular Unit by HIV-1 Infection and Methamphetamine Use: Implications for HIV-1-Associated Neurocognitive Disorders. Viruses 2021, 13, 1883. [Google Scholar] [CrossRef]
- Mediouni, S.; Marcondes, M.C.; Miller, C.; McLaughlin, J.P.; Valente, S.T. The cross-talk of HIV-1 Tat and methamphetamine in HIV-associated neurocognitive disorders. Front. Microbiol. 2015, 6, 1164. [Google Scholar] [CrossRef] [PubMed]
- Northrop, N.A.; Yamamoto, B.K. Methamphetamine effects on blood-brain barrier structure and function. Front. Neurosci. 2015, 9, 69. [Google Scholar] [CrossRef]
- Patel, S.; Leibrand, C.R.; Palasuberniam, P.; Couraud, P.-O.; Weksler, B.; Jahr, F.M.; McClay, J.L.; Hauser, K.F.; McRae, M. Effects of HIV-1 Tat and Methamphetamine on Blood-Brain Barrier Integrity and Function In Vitro. Antimicrob. Agents Chemother. 2017, 61, e01307. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, R.; Wang, S.; Zhang, D.; Leung, C.-K.; Yang, G.; Li, Y.; Liu, L.; Xu, Y.; Lin, S.; et al. Methamphetamine and HIV-Tat Protein Synergistically Induce Oxidative Stress and Blood-Brain Barrier Damage via Transient Receptor Potential Melastatin 2 Channel. Front. Pharmacol. 2021, 12, 619436. [Google Scholar] [CrossRef]
- Zhang, X.; Banerjee, A.; Banks, W.A.; Ercal, N. N-Acetylcysteine amide protects against methamphetamine-induced oxidative stress and neurotoxicity in immortalized human brain endothelial cells. Brain Res. 2009, 1275, 87–95. [Google Scholar] [CrossRef]
- Mahajan, S.D.; Aalinkeel, R.; Sykes, D.E.; Reynolds, J.L.; Bindukumar, B.; Adal, A.; Qi, M.; Toh, J.; Xu, G.; Prasad, P.N.; et al. Methamphetamine alters blood brain barrier permeability via the modulation of tight junction expression: Implication for HIV-1 neuropathogenesis in the context of drug abuse. Brain Res. 2008, 1203, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Ohene-Nyako, M.; Persons, A.L.; Napier, T.C. Hippocampal blood–brain barrier of methamphetamine self-administering HIV-1 transgenic rats. Eur. J. Neurosci. 2021, 53, 416–429. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; He, Y.; Wang, W.; Leung, C.-K.; Zhang, D.; Zhang, R.; Wang, S.; Li, Y.; Liu, L.; et al. The protective effect of gastrodin against the synergistic effect of HIV-Tat protein and METH on the blood–brain barrier via glucose transporter 1 and glucose transporter 3. Toxicol. Res. 2021, 10, 91–101. [Google Scholar] [CrossRef]
- Mack, M.L.; Huang, W.; Chang, S.L. Involvement of TRPM7 in Alcohol-Induced Damage of the Blood–Brain Barrier in the Presence of HIV Viral Proteins. Int. J. Mol. Sci. 2023, 24, 1910. [Google Scholar] [CrossRef] [PubMed]
- Agas, A.; Garcia, R.; Kalluru, J.; Leiser, B.; Haorah, J. Synergistic effects of alcohol and HIV TAT protein on macrophage migration and neurotoxicity. J. Neuroimmunol. 2022, 368, 577869. [Google Scholar] [CrossRef]
- Shiu, C.; Barbier, E.; Cello, F.D.; Choi, H.J.; Stins, M. HIV-1 gp120 as Well as Alcohol Affect Blood–Brain Barrier Permeability and Stress Fiber Formation: Involvement of Reactive Oxygen Species. Alcohol. Clin. Exp. Res. 2007, 31, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Manda, V.K.; Mittapalli, R.K.; Geldenhuys, W.J.; Lockman, P.R. Chronic exposure to nicotine and saquinavir decreases endothelial Notch-4 expression and disrupts blood-brain barrier integrity. J. Neurochem. 2010, 115, 515–525. [Google Scholar] [CrossRef]
- Manda, V.K.; Mittapalli, R.K.; Bohn, K.A.; Adkins, C.E.; Lockman, P.R. Nicotine and cotinine increases the brain penetration of saquinavir in rat. J. Neurochem. 2010, 115, 1495–1507. [Google Scholar] [CrossRef]
- Bhalerao, A.; Cucullo, L. HIV-1 gp120 and tobacco smoke synergistically disrupt the integrity of the blood-brain barrier. Eur. J. Cell Biol. 2022, 101, 151271. [Google Scholar] [CrossRef]
- Murphy, A.; Barbaro, J.; Martinez-Aguado, P.; Chilunda, V.; Jaureguiberry-Bravo, M.; Berman, J.W. The Effects of Opioids on HIV Neuropathogenesis. Front. Immunol. 2019, 10, 2445. [Google Scholar] [CrossRef] [PubMed]
- Buch, S.; Periyasamy, P.; Thangaraj, A.; Sil, S.; Chivero, E.T.; Tripathi, A. Opioid-Mediated HIV-1 Immunopathogenesis. J. Neuroimmune Pharmacol. 2020, 15, 628–642. [Google Scholar] [CrossRef] [PubMed]
- Marino, J.; Maubert, M.E.; Lawrence, J.M.; Wigdahl, B.; Nonnemacher, M.R. Chronic Low Dose Morphine Does Not Alter Two In Vitro BBB Models. Brain Sci. 2022, 12, 888. [Google Scholar] [CrossRef]
- Dutta, R.; Roy, S. Chronic morphine and HIV-1 Tat promote differential central nervous system trafficking of CD3+ and Ly6C+ immune cells in a murine Streptococcus pneumoniae infection model. J. Neuroinflamm. 2015, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Rademeyer, K.M.; Nass, S.R.; Jones, A.M.; Ohene-Nyako, M.; Hauser, K.F.; McRae, M. Fentanyl dysregulates neuroinflammation and disrupts blood-brain barrier integrity in HIV-1 Tat transgenic mice. J. NeuroVirol. 2024, 30, 1–21. [Google Scholar] [CrossRef]
- Leibrand, C.R.; Paris, J.J.; Jones, A.M.; Masuda, Q.N.; Halquist, M.S.; Kim, W.-K.; Knapp, P.E.; Kashuba, A.D.M.; Hauser, K.F.; McRae, M. HIV-1 Tat and opioids act independently to limit antiretroviral brain concentrations and reduce blood–brain barrier integrity. J. NeuroVirol. 2019, 25, 560–577. [Google Scholar] [CrossRef]
- Leibrand, C.R.; Paris, J.J.; Jones, A.M.; Ohene-Nyako, M.; Rademeyer, K.M.; Nass, S.R.; Kim, W.-K.; Knapp, P.E.; Hauser, K.F.; McRae, M. Independent actions by HIV-1 Tat and morphine to increase recruitment of monocyte-derived macrophages into the brain in a region-specific manner. Neurosci. Lett. 2022, 788, 136852. [Google Scholar] [CrossRef]
- Jaureguiberry-Bravo, M.; Lopez, L.; Berman, J.W. Frontline Science: Buprenorphine decreases CCL2-mediated migration of CD14+CD16+ monocytes. J. Leukoc. Biol. 2018, 104, 1049–1059. [Google Scholar] [CrossRef]
- Mahajan, S.D.; Aalinkeel, R.; Sykes, D.E.; Reynolds, J.L.; Bindukumar, B.; Fernandez, S.F.; Chawda, R.; Shanahan, T.C.; Schwartz, S.A. Tight Junction Regulation by Morphine and HIV-1 Tat Modulates Blood–Brain Barrier Permeability. J. Clin. Immunol. 2008, 28, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.H.; Ismaiel, O.A.; Mylott, W.R., Jr.; Yuan, M.; McClay, J.L.; Paris, J.J.; Hauser, K.F.; McRae, M. Cell-type specific differences in antiretroviral penetration and the effects of HIV-1 Tat and morphine among primary human brain endothelial cells, astrocytes, pericytes, and microglia. Neurosci. Lett. 2019, 712, 134475. [Google Scholar] [CrossRef] [PubMed]
- Hind, W.H.; England, T.J.; O’Sullivan, S.E. Cannabidiol protects an in vitro model of the blood-brain barrier from oxygen-glucose deprivation via PPARgamma and 5-HT1A receptors. Br. J. Pharmacol. 2016, 173, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.L.; England, T.J.; O’Sullivan, S.E. Protective Effects of Cannabidivarin and Cannabigerol on Cells of the Blood-Brain Barrier Under Ischemic Conditions. Cannabis Cannabinoid Res. 2021, 6, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Panikashvili, D.; Shein, N.a.A.; Mechoulam, R.; Trembovler, V.; Kohen, R.; Alexandrovich, A.; Shohami, E. The endocannabinoid 2-AG protects the blood–brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol. Dis. 2006, 22, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Starr, A.; Jordan-Sciutto, K.L.; Mironets, E. Confound, Cause, or Cure: The Effect of Cannabinoids on HIV-Associated Neurological Sequelae. Viruses 2021, 13, 1242. [Google Scholar] [CrossRef] [PubMed]
- Persidsky, Y.; Fan, S.; Dykstra, H.; Reichenbach, N.L.; Rom, S.; Ramirez, S.H. Activation of Cannabinoid Type Two Receptors (CB2) Diminish Inflammatory Responses in Macrophages and Brain Endothelium. J. Neuroimmune Pharmacol. 2015, 10, 302–308. [Google Scholar] [CrossRef]
- Rom, S.; Zuluaga-Ramirez, V.; Dykstra, H.; Reichenbach, N.L.; Pacher, P.; Persidsky, Y. Selective activation of cannabinoid receptor 2 in leukocytes suppresses their engagement of the brain endothelium and protects the blood-brain barrier. Am. J. Pathol. 2013, 183, 1548–1558. [Google Scholar] [CrossRef]
- Ramirez, S.H.; Hasko, J.; Skuba, A.; Fan, S.; Dykstra, H.; McCormick, R.; Reichenbach, N.; Krizbai, I.; Mahadevan, A.; Zhang, M.; et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J. Neurosci. 2012, 32, 4004–4016. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Cai, S.; Li, R.; Cao, G. Cannabinoid receptor 2 agonist attenuates blood-brain barrier damage in a rat model of intracerebral hemorrhage by activating the Rac1 pathway. Int. J. Mol. Med. 2018, 42, 2914–2922. [Google Scholar] [CrossRef]
- Fujii, M.; Sherchan, P.; Krafft, P.R.; Rolland, W.B.; Soejima, Y.; Zhang, J.H. Cannabinoid type 2 receptor stimulation attenuates brain edema by reducing cerebral leukocyte infiltration following subarachnoid hemorrhage in rats. J. Neurol. Sci. 2014, 342, 101–106. [Google Scholar] [CrossRef]
- Bullock, T.A.; Galpayage Dona, K.N.U.; Hale, J.F.; Morales, P.; Jagerovic, N.; Andrews, A.M.; Ramirez, S.H. Activation of CB2R by synthetic CB2R agonist, PM289, improves brain endothelial barrier properties, decreases inflammatory response and enhances endothelial repair. NeuroImmune Pharm. Ther. 2023, 2, 387–400. [Google Scholar] [CrossRef]
- Amenta, P.S.; Jallo, J.I.; Tuma, R.F.; Elliott, M.B. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J. Neurosci. Res. 2012, 90, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Peterson, S.N.; Li, Y.; Schrier, R.; Iudicello, J.; Letendre, S.; Morgan, E.; Tang, B.; Grant, I.; Cherner, M. Recent cannabis use in HIV is associated with reduced inflammatory markers in CSF and blood. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e809. [Google Scholar] [CrossRef]
- Kim, H.J.; Shin, A.H.; Thayer, S.A. Activation of cannabinoid type 2 receptors inhibits HIV-1 envelope glycoprotein gp120-induced synapse loss. Mol. Pharmacol. 2011, 80, 357–366. [Google Scholar] [CrossRef]
- Ellis, R.J.; Peterson, S.; Cherner, M.; Morgan, E.; Schrier, R.; Tang, B.; Hoenigl, M.; Letendre, S.; Iudicello, J. Beneficial Effects of Cannabis on Blood–Brain Barrier Function in Human Immunodeficiency Virus. Clin. Infect. Dis. 2020, 73, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.W.-M.; Campbell, L.M.; Sun-Suslow, N.; Hong, S.; Umlauf, A.; Ellis, R.J.; Iudicello, J.E.; Letendre, S.; Marcotte, T.D.; Heaton, R.K.; et al. Daily Cannabis Use is Associated With Lower CNS Inflammation in People With HIV. J. Int. Neuropsychol. Soc. 2021, 27, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.W.; Sundermann, E.; Helm, J.; Paolillo, E.W.; Hong, S.; Ellis, R.J.; Letendre, S.; Marcotte, T.D.; Heaton, R.K.; Morgan, E.E.; et al. A longitudinal study of cannabis use and risk for cognitive and functional decline among older adults with HIV. AIDS Behav. 2023, 27, 3401–3413. [Google Scholar] [CrossRef]
- Swinton, M.K.; Sundermann, E.E.; Pedersen, L.; Nguyen, J.D.; Grelotti, D.J.; Taffe, M.A.; Iudicello, J.E.; Fields, J.A. Alterations in Brain Cannabinoid Receptor Levels Are Associated with HIV-Associated Neurocognitive Disorders in the ART Era: Implications for Therapeutic Strategies Targeting the Endocannabinoid System. Viruses 2021, 13, 1742. [Google Scholar] [CrossRef]
- Cosenza-Nashat, M.A.; Bauman, A.; Zhao, M.L.; Morgello, S.; Suh, H.S.; Lee, S.C. Cannabinoid receptor expression in HIV encephalitis and HIV-associated neuropathologic comorbidities. Neuropathol. Appl. Neurobiol. 2011, 37, 464–483. [Google Scholar] [CrossRef]
- Jones, L.D.; Jackson, J.W.; Maggirwar, S.B. Modeling HIV-1 Induced Neuroinflammation in Mice: Role of Platelets in Mediating Blood-Brain Barrier Dysfunction. PLoS ONE 2016, 11, e0151702. [Google Scholar] [CrossRef]
- Haorah, J.; Knipe, B.; Gorantla, S.; Zheng, J.; Persidsky, Y. Alcohol-induced blood–brain barrier dysfunction is mediated via inositol 1,4,5-triphosphate receptor (IP3R)-gated intracellular calcium release. J. Neurochem. 2007, 100, 324–336. [Google Scholar] [CrossRef]
- Muneer, P.M.A.; Alikunju, S.; Szlachetka, A.M.; Haorah, J. The Mechanisms of Cerebral Vascular Dysfunction and Neuroinflammation by MMP-Mediated Degradation of VEGFR-2 in Alcohol Ingestion. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.; Pu, H.; Lee, Y.W.; Ravikumar, R.; Nath, A.; Hennig, B.; Toborek, M. Proinflammatory synergism of ethanol and HIV-1 Tat protein in brain tissue. Exp. Neurol. 2005, 191, 2–12. [Google Scholar] [CrossRef] [PubMed]
| Effects on the BBB Structure and Function | Mechanisms | |
|---|---|---|
| HIV | Downregulation of claudin-5, occludin, ZO-1, and ZO-2, [30,36,45,62,150]. Increased BBB permeability, and infiltration of leukocytes into the brain [36,45,62,150]. | Altered expression of ICAM-1 and VCAM-1 [34,150]. Increased levels of C-C chemokines, IFN-γ, CCL5, MIP-1α, MIP-1β, MCP-3, and IP-10 [35]. |
| HIV gp120 | Decreased expression of occludin and ZO-1 [81]. Increased BBB permeability [38,81]. | Increased ICAM-1, VCAM-1, substance-P, TIMP-1, TIMP-2, MMP-2, and MMP-9 [38,40]. Increased ROS, MDA and protein carbonyl; decreased GSH and GPx activity [40,81]. |
| HIV Tat | Decreased expression of claudin-5, and ZO-1 [41,54]. Disrupted BBB integrity; increased recruitment of activated, phagocytic, and perivascular macrophages into the CNS [42]. | Upregulation of ICAM-1, VCAM-1, and AKT activation [88]; autophagy [41]. |
| HIV Nef | Downregulation of claudin-5 [44]. Induced BBB disruption and increased BBB permeability [43,44]. | Upregulated MMP-9 [43] and IL-1β [44]. |
| Cocaine and HIV/viral proteins | Cocaine downregulated ZO-1 [101]. Cocaine and HIV-1 increased monocyte adhesion and transmigration [101]. | Cocaine induced PDGF-β [101]. Cocaine and HIV-1 increased ALCAM [101] and cytokines [106]. |
| Meth and HIV/viral proteins | Meth and HIV or Tat induced downregulation of ZO-1, occludin, claudin-5, and JAM-A [111,114,115]. Meth and HIV or Tat increased BBB permeability [111,114]. | Meth and HIV increased MMP-9 [114]. Meth and Tat downregulated GLUT-1 and GLUT-3 [115]; increased NF-κB [114]; induced ROS, reduced CAT, GPx, and SOD activity; and increased MDA [111]. |
| Alcohol and HIV/viral proteins | Alcohol altered occludin, claudin-5, and ZO-1, and phosphorylated ZO-1 [151,152]. Alcohol increased BBB permeability and transmigration of immune cells into the brain [151,152]. | Alcohol activated MMP-3 and MMP-9 [152]. Alcohol and Tat downregulated TRPM7 [116]; increased ICAM-1, IL-1β, MCP-1, CREB, and NF-κB DNA-binding activity; activated ERK1/2 [153]. |
| Tobacco and gp120/antiretrovirals | Nicotine and SQV or SQV/r decreased ZO-1 and increased BBB permeability [119]. | Nicotine and SQV or SQV/r decreased Notch-4 and increased ROS [119]. |
| MOR and Tat | MOR or fentanyl and Tat decreased ZO-1, claudin-5, and increased P-gp [126,128]. MOR or fentanyl and Tat increased BBB disruption and permeability [126,128]. | Fentanyl and Tat dysregulated inflammatory cytokines [126]. |
| Effects on the BBB Structure and Function | Mechanisms | |
|---|---|---|
| HIV | Downregulation of ZO-1, ZO-2, claudin-1, claudin-5, occludin, and JAM-A [46,64,74]; phosphorylation of occludin and claudin-5 [68]; reduced TEER [68]. Increased BBB permeability, leukocyte adhesion and transmigration [26,46,47,64,68,74]. | Increased MMP-2 and MMP-9 [64,73,74]; altered ICAM-1, and E-selectin [26,75]; increased IL-6, IL-8, IL-1β, TNF-α, MCP-1, and IFN-inducible genes; activation of SP-1, AP-1, NF-κB, STAT-1, and STAT-3 [26,46,47,73,75]. Increased ROS and redox proteins (peroxiredoxin, SOD); reduced p-eNOS, p-DRP1, and mitochondrial membrane potential [67,69,73]. Activation of Rac1, RhoA GTPases, Rho-kinase, PI3K, PDK1, ERK1/2, and AKT [47,68,73,76]. |
| HIV gp120 | Decreased expression of ZO-1, ZO-2, and occludin [49]. Increased BBB permeability, monocyte adhesion, and transendothelial migration [37,49,51]. | Increased IL-6, IL-8, ROS, and MDA; decreased activity of GSH, GPx, GR, and CAT [51,82,83]. Activation of STAT-1, p38 MAPK, MEK, and PI3K pathways [51,77]; increased caspase-3 and cytotoxicity [77]. |
| HIV Tat | Decreased expression of claudin-1, claudin-5, ZO-1, ZO-2, occludin, and TEER; increased P-gp, disruption and phosphorylation of VE-cadherin and β-catenin [41,53,54,55,56,57,58,89,90,92,95]. Increased BBB permeability, leukocyte adhesion and transendothelial migration [52,56,86,87,89,90]. | Increased E-selectin, ICAM-1 and VCAM-1 [87,88]; MMP-9 [56]; IL-6, PAI-1, MCP-1, and AP-1 [53,85,86,87,91]. Decreased GSH and increased ROS, ER stress, mitochondrial dysfunction, activated UPR, upregulated NOX-2 and NOX-4 [45,89,91,95]. Induced apoptosis, autophagy, and cytotoxicity [41,45,93,95]. Upregulated VEGFR-2 and activated redox-regulated pathways [92,93]. Increased NF-κB binding activity and activation of NF-κB, ERK1/2, IRAK-1/4, MKK JNK, AP-1, PI3K, AKT, FAK, RhoA, ROCK, Ras, PKA, PKC, and PYK2 pathways [52,53,55,56,58,85,87,89,92,93,95]. |
| HIV Nef | Decreased ZO-1, TEER, and altered BBB permeability [60]. | Increased IL-12, IL-8, IL-6, CCL5, and IL-17A [60]. Induced PARP cleavage, upregulated Fas/FasL, activation of caspases and mitochondrial apoptotic pathways [59]. |
| Cocaine and HIV/viral proteins | Cocaine, or cocaine and HIV-1 or Tat, decreased ZO-1, JAM-2, and TEER, and increased stress fiber formation [100,102]. Cocaine or cocaine and HIV-1 or Tat increased BBB permeability, monocyte adhesion and transendothelial migration [101,102,104,105]. | Cocaine induced activation of ERK1/2, p38 MAPK, JNK, and PI3K/AKT pathways [101]. Cocaine or cocaine and HIV-1 upregulated ICAM-1, VCAM-1, PECAM-1, ELAM-1, and ALCAM [101,104,105]. Cocaine or cocaine and Tat increased inflammatory cytokines, MCP-1 and its receptor in monocytes; increased secretion of TNF-α and IL-6, and activation of NF-κB [100,101,105]. |
| Meth and HIV/viral proteins | Meth, or Meth and gp120 or Tat, decreased ZO-1, JAM-A, occludin, claudin-5, claudin-3, JAM-2, and TEER; and impaired P-gp function [110,111,112,113,115]. Meth or Meth and gp120 or Tat induced BBB disruption, increased BBB permeability and leukocyte transmigration [111,113]. | Meth and Tat reduced GLUT-1 and GLUT-3 [115]. Meth or Meth and Tat increased ROS and MDA, reduced CAT, GPx, and SOD activity [111,112]; decreased viability and induced apoptosis in ECs [111,112,115]. Meth and gp120 activate Rho-A GTPase [113]. |
| Alcohol and HIV/viral proteins | Alcohol, alcohol plus gp120 or Tat, decreased claudin-3, claudin-5, occludin, JAM-1, ZO-1, and induced stress fiber formation [116,117,118]. Alcohol and gp120 or Tat increased BBB permeability and macrophage transmigration [117,118]. | Alcohol and gp120 or Tat downregulated TRPM7 [116], increased ECs ROS and NO [117,118]. Ethanol and Nef, Tat, gp120, or Vpr increased ECs LDH, TNF-α, and apoptosis [96]. |
| Tobacco and gp120/antiretrovirals | Nicotine and SQV or SQV/r decreased ZO-1 [119]. Tobacco smoke extracts and gp120 decreased occludin, ZO-1, and TEER [121]. Tobacco and SQV or gp120, or tobacco smoke extracts and gp120, increased BBB permeability [119,121]. | Nicotine and SQV or SQV/r increased ROS and downregulated Notch-4 [119]. Tobacco smoke extracts and gp120 increased ROS, NF-κB, disrupted mitochondrial function, decreased NRF-2 and EC viability [121]. |
| MOR and Tat | MOR and/or Tat decreased ZO-1, JAM-2, occludin, TEER, and increased P-gp [130]. MOR and/or Tat increased BBB permeability and leukocytes’ transendothelial migration [130]. | MOR and/or Tat activated MLCK, increased pro-inflammatory cytokines, and intracellular calcium release [130]. |
| Interaction | Effects on the BBB | |
|---|---|---|
| Cocaine + HIV | Additive | Upregulation of ALCAM [101] |
| Cocaine + Tat | Synergistic | Decreased ZO-1, JAM-2, and TEER, increased BBB permeability, and monocyte transmigration across BBB [102]. |
| Meth + Tat | Synergistic | Decreased ZO-1, occludin, and JAM-A [110,111,115]. Decreased CAT, SOD, and GPx, induced oxidative stress, and increased TRPM2 [111]. Decreased P-gp [110]; decreased claudin-5, GLUT-1, GLUT-3, and cell viability [115]. |
| Meth + gp120 | Synergistic | Decreased ZO-1, JAM-2, claudin-3, claudin-5, and TEER; increased leukocytes’ transmigration across the BBB [113]. |
| Meth + gp120 or Tat | Synergistic | Decreased ZO-1, occludin, glutathione, and GPx, increased ROS, protein carbonyls, lipid peroxidation [81]. |
| Ethanol + gp120 | Synergistic | Decreased claudin-3, occludin, ZO-1, JAM-2, and TRPM7 [116]. |
| Ethanol + Tat | Synergistic | Increased macrophage migration across BBB [117]. |
| Nicotine + SQR/r | Additive | Decreased Notch-4, and ZO-1, increased ROS, and BBB disruption [119]. |
| Tobacco smoke extracts + gp120 | Synergistic | Decreased ZO-1, occludin, and NRF-2; increased ROS, NF-κB, and BBB permeability; decreased TEER, and cell viability [121]. |
| Morphine + Tat | Synergistic | Reduced TEER; increased JAM-2, P-gp, and migration of non-infected and HIV-1 infected leukocytes across the BBB [130]. |
| Fentanyl + Tat | Additive | Decreased claudin-5 [126]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annadurai, N.; Kanmogne, G.D. Structural and Functional Dysregulation of the Brain Endothelium in HIV Infection and Substance Abuse. Cells 2024, 13, 1415. https://doi.org/10.3390/cells13171415
Annadurai N, Kanmogne GD. Structural and Functional Dysregulation of the Brain Endothelium in HIV Infection and Substance Abuse. Cells. 2024; 13(17):1415. https://doi.org/10.3390/cells13171415
Chicago/Turabian StyleAnnadurai, Narendran, and Georgette D. Kanmogne. 2024. "Structural and Functional Dysregulation of the Brain Endothelium in HIV Infection and Substance Abuse" Cells 13, no. 17: 1415. https://doi.org/10.3390/cells13171415




