Essential Roles of PIEZO1 in Mammalian Cardiovascular System: From Development to Diseases
Abstract
1. Introduction
2. Structure and Mechanogating Mechanism
3. PIEZO1 in Cardiovascular Development
3.1. PIEZO1 in Embryos
3.2. PIEZO1 in Vascular Development
3.3. PIEZO1 in Cardiac Development
3.4. PIEZO1 in Lymphatic Valve and Arteriovenous Valve Development
4. PIEZO1 in Cardiovascular Homeostasis and Diseases
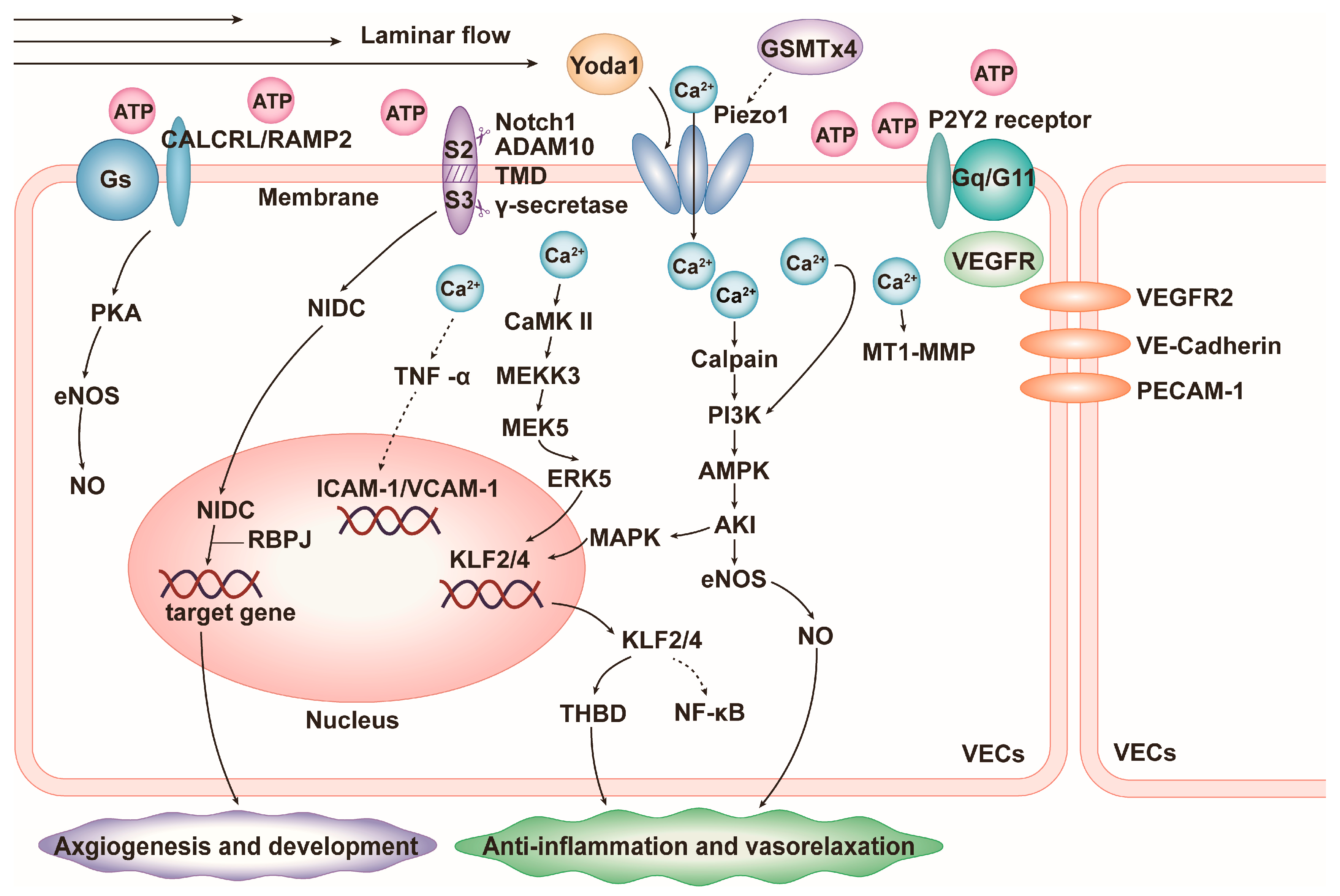
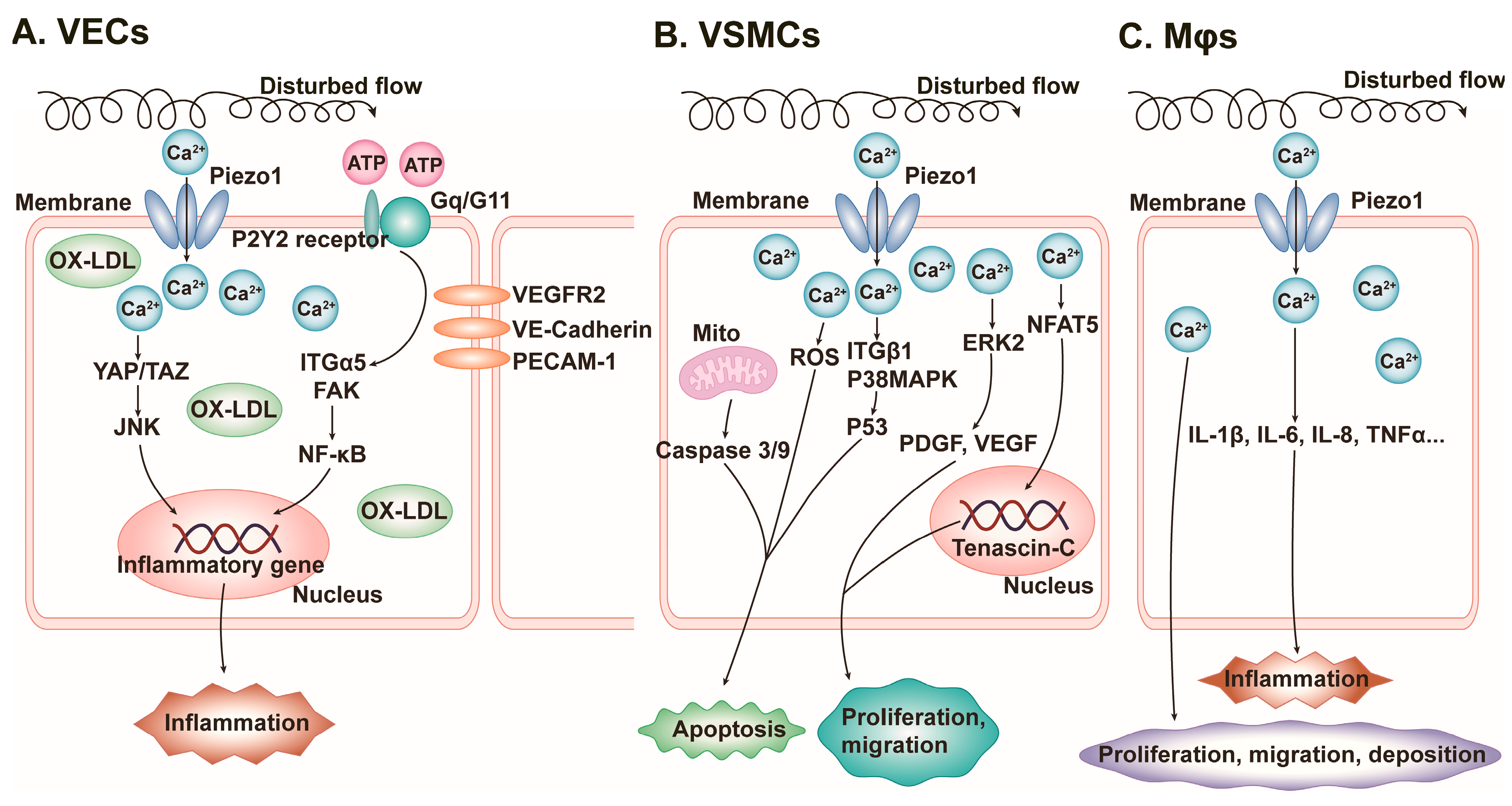
4.1. Vascular Inflammation
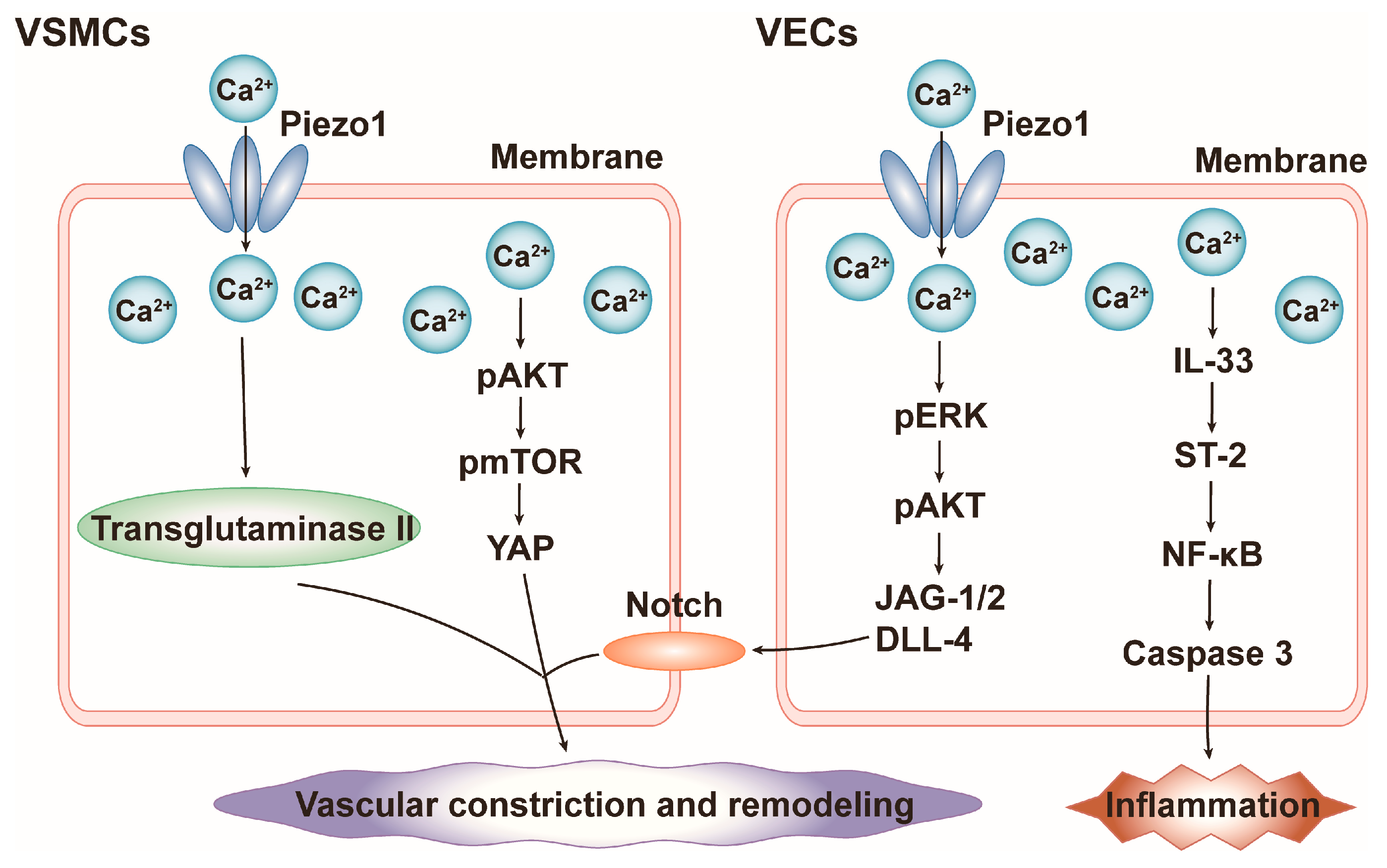
4.2. Blood Pressure
4.3. Cardiac Hypertrophy
4.4. Cardiac Fibrosis
4.5. Heart Failure (HF)
5. Potential Chemical Therapies Targeting PIEZO1
5.1. Agonists
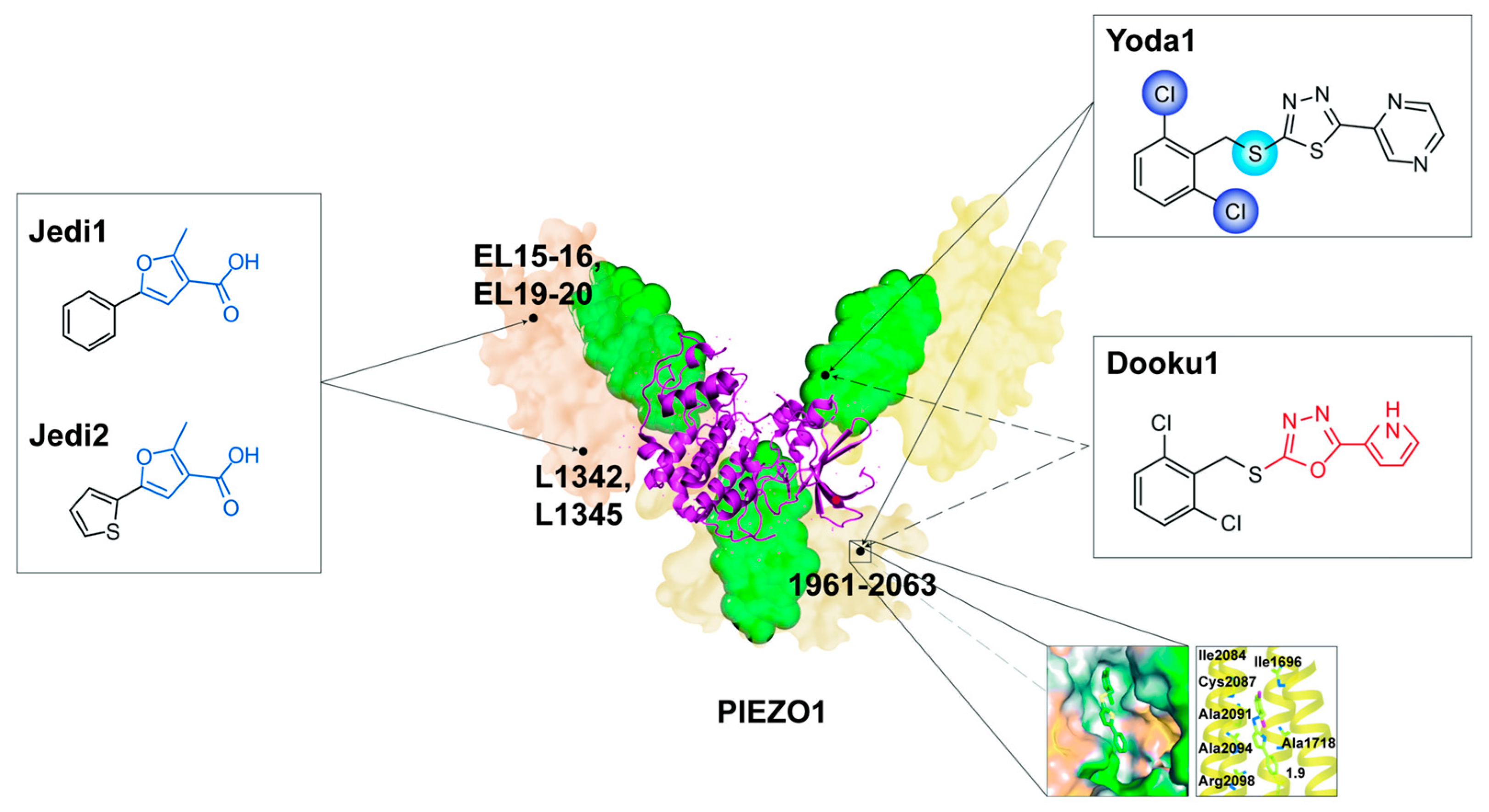
5.1.1. Yoda1
5.1.2. Jedi1 and Jedi2
5.2. Competitive Inhibitors
5.2.1. Dooku1
5.2.2. Tubeimoside I (TBMS1) and Salvianolic Acid B (SalB)
5.3. Non-Competitive Inhibitors
5.3.1. GsMTx-4
5.3.2. Ruthenium Red (RR), Amyloid β (Aβ), and Gadolinium (Gd3+)
5.4. Dietary Lipids
5.5. Others
6. Challenges and Prospects
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAA | abdominal aortic aneurysm | mPIEZO1 | mouse PIEZO1 |
| Aβ | amyloid β | MS | mechanosensitive |
| ADAM10 | Ca2+-dependent transmembrane abscission enzyme | MT1-MMP | membrane-type-1 matrix metalloproteinases |
| AF | atrial fibrillation | Møs | macrophages |
| AngII | angiotensin II | NFAT5 | nuclear factor of activated T-cells 5 |
| APD | action potential time duration | NF-κB | nuclear factor-κ-gene binding |
| ARB | angiotensin II receptor blocker | NPJc | nodose-petrosal-jugular ganglion complex |
| AS | atherosclerosis | OH | outer helix |
| CaM | calmodulin | OX-LDL | oxidized low-density lipoprotein |
| CaN | calcineurin | PAD | peripheral artery disease |
| CaMKII | calcium/calmodulin-dependent protein kinase II | PDGF | platelet-derived growth factor |
| CAVD | calcified aortic valvular disease | PH | pulmonary hypertension |
| CED | C-terminal extracellular domain | PIEZO1fl/fl | floxed PIEZO1 |
| CMs | cardiomyocytes | Pitx2 | paired like homeodomain 2 |
| COMP | cartilage oligomeric matrix protein | PKA | protein kinase A |
| CTD | C-terminal domain | PRP | platelet-rich plasma |
| DLL-4 | delta like-4 | RBPJ | recombination signal binding protein for immunoglobulin kappa J region |
| E | embryonic day | ROS | reactive oxygen species |
| ECM | extracellular matrix | RR | ruthenium red |
| ECs | endothelial cells | RyR2 | ryanodine receptor 2 |
| EL | extracellular loops | SalB | salvianolic acid B |
| ETS1 | ETS proto-oncogene 1 | SERCA2 | sarcoplasmic endoplasmic reticulum calcium ATPase 2 |
| FAK | focal adhesion kinase | SMPD3 | sphingomyelin phosphodiesterase 3 |
| FBs | fibroblasts | SNPs | single nucleotide polymorphisms |
| Gd3+ | gadolinium | SPT | single particle tracking |
| HAFBs | human atrial fibroblasts | ST2 | suppression of tumorigenicity 2 |
| HCFBs | human cardiac fibroblasts | TAZ | transcription coactivator with PDZ-binding motif |
| HDAC4 | histone deacetylase 4 | TBMS1 | tubeimoside I |
| HF | heart failure | TGF-β | transforming growth factor-β |
| HLI | hindlimb ischemia | THBD | thrombomodulin |
| hPIEZO1 | human PIEZO1 | TM | transmembrane |
| hUC-MSCs | human umbilical cord mesenchymal stem cells | TMEM150C | transmembrane protein 150C |
| ICAM-1 | intercellular cell adhesion molecule-1 | TNC | tenascin C |
| IH | inner helices | TNF-α | tumor necrosis factor-α |
| IL | interleukin | TRPM4 | transient receptor potential melastatin 4 |
| ITG | integrin | TRPV1 | transient receptor potential vanilloid subtype |
| JAG-1/2 | jagged1/2 | VCAM-1 | vascular cell adhesion molecule-1 |
| JNK | c-Jun N-terminal kinase | VE-cadherin | vascular endothelial cadherin |
| KLF | kruppel-like factor | VEGF | vascular endothelial growth factor |
| MCFBs | mouse cardiac fibroblasts | VEGFR2 | vascular endothelial growth factor receptor 2 |
| MEF2 | myocyte enhancer factor 2 | VECs | vascular endothelial cells |
| MI | myocardial infarction | VSMCs | vascular smooth muscle cells |
| Mito | mitochondrion | NO | nitric oxide |
| MLC2v | myosin light chain 2v | YAP | yes-associated protein |
References
- Majkut, S.; Dingal, P.D.P.; Discher, D.E. Stress Sensitivity and Mechanotransduction during Heart Development. Curr. Biol. 2014, 24, R495–R501. [Google Scholar] [CrossRef] [PubMed]
- Flournoy, J.; Ashkanani, S.; Chen, Y. Mechanical regulation of signal transduction in angiogenesis. Front. Cell Dev. Biol. 2022, 10, 933474. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.D.; Schwartz, M.A. Vascular Mechanobiology: Homeostasis, Adaptation, and Disease. Annu. Rev. Biomed. Eng. 2021, 23, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, Y.; Yanagisawa, H. The molecular mechanism of mechanotransduction in vascular homeostasis and disease. Clin. Sci. 2020, 134, 2399–2418. [Google Scholar] [CrossRef]
- Douguet, D.; Honoré, E. Mammalian Mechanoelectrical Transduction: Structure and Function of Force-Gated Ion Channels. Cell 2019, 179, 340–354. [Google Scholar] [CrossRef]
- Fancher, I.S. Cardiovascular mechanosensitive ion channels-Translating physical forces into physiological responses. Curr. Top. Membr. 2021, 87, 47–95. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Douguet, D.; Patel, A.; Xu, A.; Vanhoutte, P.M.; Honoré, E. Piezo Ion Channels in Cardiovascular Mechanobiology. Trends Pharmacol. Sci. 2019, 40, 956–970. [Google Scholar] [CrossRef]
- Beech, D.J.; Kalli, A.C. Force Sensing by Piezo Channels in Cardiovascular Health and Disease. Arter. Thromb. Vasc. Biol. 2019, 39, 2228–2239. [Google Scholar] [CrossRef]
- Zeng, W.-Z.; Marshall, K.L.; Min, S.; Daou, I.; Chapleau, M.W.; Abboud, F.M.; Liberles, S.D.; Patapoutian, A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 2018, 362, 464–467. [Google Scholar] [CrossRef]
- Szczot, M.; Nickolls, A.R.; Lam, R.M.; Chesler, A.T. The Form and Function of PIEZO2. Annu. Rev. Biochem. 2021, 90, 507–534. [Google Scholar] [CrossRef] [PubMed]
- Saotome, K.; Murthy, S.E.; Kefauver, J.M.; Whitwam, T.; Patapoutian, A.; Ward, A.B. Author Correction: Structure of the mechanically activated ion channel Piezo1. Nature 2018, 554, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhou, H.; Chi, S.; Wang, Y.; Wang, J.; Geng, J.; Wu, K.; Liu, W.; Zhang, T.; Dong, M.-Q.; et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature 2018, 554, 487–492. [Google Scholar] [CrossRef]
- Wang, Y.; Chi, S.; Guo, H.; Li, G.; Wang, L.; Zhao, Q.; Rao, Y.; Zu, L.; He, W.; Xiao, B. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat. Commun. 2018, 9, 113a–114a. [Google Scholar] [CrossRef]
- Bavi, N.; Richardson, J.; Heu, C.; Martinac, B.; Poole, K. PIEZO1-Mediated Currents Are Modulated by Substrate Mechanics. ACS Nano 2019, 13, 13545–13559. [Google Scholar] [CrossRef]
- Lewis, A.H.; Grandl, J. Stretch and poke stimulation for characterizing mechanically activated ion channels. Methods Enzym. 2021, 654, 225–253. [Google Scholar] [CrossRef]
- Ellefsen, K.L.; Holt, J.R.; Chang, A.C.; Nourse, J.L.; Arulmoli, J.; Mekhdjian, A.H.; Abuwarda, H.; Tombola, F.; Flanagan, L.A.; Dunn, A.R.; et al. Myosin-II mediated traction forces evoke localized Piezo1-dependent Ca2+ flickers. Commun. Biol. 2019, 2, 298. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Bae, C.; Gottlieb, P.A.; Sachs, F. Ionic Selectivity and Permeation Properties of Human PIEZO1 Channels. PLoS ONE 2015, 10, e0125503. [Google Scholar] [CrossRef]
- Martinac, B.; Adler, J.; Kung, C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature 1990, 348, 261–263. [Google Scholar] [CrossRef]
- Syeda, R.; Florendo, M.N.; Cox, C.D.; Kefauver, J.M.; Santos, J.S.; Martinac, B.; Patapoutian, A. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 2016, 17, 1739–1746. [Google Scholar] [CrossRef]
- Cox, C.D.; Bae, C.; Ziegler, L.; Hartley, S.; Nikolova-Krstevski, V.; Rohde, P.R.; Ng, C.-A.; Sachs, F.; Gottlieb, P.A.; Martinac, B. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat. Commun. 2016, 7, 10366. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Yang, X.; Zhou, G.; Wang, L.; Xiao, B. Tethering Piezo channels to the actin cytoskeleton for mechanogating via the cadherin-β-catenin mechanotransduction complex. Cell Rep. 2022, 38, 110342. [Google Scholar] [CrossRef]
- Ranade, S.S.; Qiu, Z.; Woo, S.-H.; Hur, S.S.; Murthy, S.E.; Cahalan, S.M.; Xu, J.; Mathur, J.; Bandell, M.; Coste, B.; et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 10347–10352. [Google Scholar] [CrossRef]
- Li, J.; Hou, B.; Tumova, S.; Muraki, K.; Bruns, A.; Ludlow, M.J.; Sedo, A.; Hyman, A.J.; McKeown, L.; Young, R.S.; et al. Piezo1 integration of vascular architecture with physiological force. Nature 2014, 515, 279–282. [Google Scholar] [CrossRef]
- del Mármol, J.I.; Touhara, K.K.; Croft, G.; MacKinnon, R. Piezo1 forms a slowly-inactivating mechanosensory channel in mouse embryonic stem cells. eLife 2018, 7, e33149. [Google Scholar] [CrossRef]
- Hyman, A.J.; Tumova, S.; Beech, D.J. Piezo1 Channels in Vascular Development and the Sensing of Shear Stress. Curr. Top Membr. 2017, 79, 37–57. [Google Scholar] [CrossRef]
- Kang, H.; Hong, Z.; Zhong, M.; Klomp, J.; Bayless, K.J.; Mehta, D.; Karginov, A.V.; Hu, G.; Malik, A.B. Piezo1 mediates angiogenesis through activation of MT1-MMP signaling. Am. J. Physiol. Physiol. 2019, 316, C92–C103. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, N.M.; Wang, Y.; Youn, J.Y.; Cai, H. Endothelial cell calpain as a critical modulator of angiogenesis. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2017, 1863, 1326–1335. [Google Scholar] [CrossRef]
- Caolo, V.; Debant, M.; Endesh, N.; Futers, T.S.; Lichtenstein, L.; Bartoli, F.; Parsonage, G.; Jones, E.A.; Beech, D.J.; Molecular, C.F.; et al. Shear stress activates ADAM10 sheddase to regulate Notch1 via the Piezo1 force sensor in endothelial cells. eLife 2020, 9, 50684. [Google Scholar] [CrossRef]
- Alabi, R.O.; Glomski, K.; Haxaire, C.; Weskamp, G.; Monette, S.; Blobel, C.P. ADAM10-Dependent Signaling Through Notch1 and Notch4 Controls Development of Organ-Specific Vascular Beds. Circ. Res. 2016, 119, 519–531. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, G.; Jiang, S.; Ning, Y.; Deng, B.; Pan, X.; Liu, S.; He, Y.; Zhang, L.; Wan, R.; et al. Mechanosensitive Piezo1 in endothelial cells promotes angiogenesis to support bone fracture repair. Cell Calcium 2021, 97, 102431. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Almalki, W.H.; Alzarea, S.I.; Kazmi, I.; Al-Abbasi, F.A.; Afzal, O.; Altamimi, A.S.A.; Singh, S.K.; Dua, K.; Gupta, G. A narrative review on the biology of piezo1 with platelet-rich plasma in cardiac cell regeneration. Chem. Interact. 2022, 363, 110011. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Xu, Z.; Lin, X.; Zhang, X.; Li, L.; Li, Y. Matrix stiffness regulates myocardial differentiation of human umbilical cord mesenchymal stem cells. Aging 2020, 13, 2231–2250. [Google Scholar] [CrossRef]
- Yao, M.; Tijore, A.; Cheng, D.; Li, J.V.; Hariharan, A.; Martinac, B.; Van Nhieu, G.T.; Cox, C.D.; Sheetz, M. Force- and cell state–dependent recruitment of Piezo1 drives focal adhesion dynamics and calcium entry. Sci. Adv. 2022, 8, eabo1461. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, D.; Yu, Z.; Schiatti, T.; Chan, A.Y.; Hill, A.P.; Peyronnet, R.; Feneley, M.P.; Cox, C.D.; Martinac, B. Functional coupling between Piezo1 and TRPM4 influences the electrical activity of HL-1 atrial myocytes. J. Physiol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Fotiou, E.; Martin-Almedina, S.; Simpson, M.A.; Lin, S.; Gordon, K.; Brice, G.; Atton, G.; Jeffery, I.; Rees, D.C.; Mignot, C.; et al. Author Correction: Novel mutations in PIEZO1 cause an autosomal recessive generalized lymphatic dysplasia with non-immune hydrops fetalis. Nat. Commun. 2015, 6, 8085. [Google Scholar] [CrossRef]
- Lukacs, V.; Mathur, J.; Mao, R.; Bayrak-Toydemir, P.; Procter, M.; Cahalan, S.M.; Kim, H.J.; Bandell, M.; Longo, N.; Day, R.W.; et al. Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat. Commun. 2015, 6, 8329. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, X.; Lin, Y.; Cheng, D.; Bavi, N.; Secker, G.A.; Li, J.V.; Janbandhu, V.; Sutton, D.L.; Scott, H.S.; et al. MyoD-family inhibitor proteins act as auxiliary subunits of Piezo channels. Science 2023, 381, 799–804. [Google Scholar] [CrossRef]
- Byrne, A.B.; Brouillard, P.; Sutton, D.L.; Kazenwadel, J.; Montazaribarforoushi, S.; Secker, G.A.; Oszmiana, A.; Babic, M.; Betterman, K.L.; Brautigan, P.J.; et al. Pathogenic variants in MDFIC cause recessive central conducting lymphatic anomaly with lymphedema. Sci. Transl. Med. 2022, 14, eabm4869. [Google Scholar] [CrossRef]
- Faucherre, A.; Maati, H.M.O.; Nasr, N.; Pinard, A.; Theron, A.; Odelin, G.; Desvignes, J.-P.; Salgado, D.; Collod-Béroud, G.; Avierinos, J.-F.; et al. Piezo1 is required for outflow tract and aortic valve development. J. Mol. Cell Cardiol. 2020, 143, 51–62. [Google Scholar] [CrossRef]
- Shadrina, A.S.; Sharapov, S.Z.; Shashkova, T.I.; Tsepilov, Y.A. Varicose veins of lower extremities: Insights from the first large-scale genetic study. PLoS Genet. 2019, 15, e1008110. [Google Scholar] [CrossRef]
- Fukaya, E.; Flores, A.M.; Lindholm, D.; Gustafsson, S.; Zanetti, D.; Ingelsson, E.; Leeper, N.J. Clinical and Genetic Determinants of Varicose Veins. Circulation 2018, 138, 2869–2880. [Google Scholar] [CrossRef]
- Smelser, D.T.; Haley, J.S.; Ryer, E.J.; Elmore, J.R.; Cook, A.M.; Carey, D.J. Association of varicose veins with rare protein-truncating variants in PIEZO1 identified by exome sequencing of a large clinical population. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 10, 382–389.e2. [Google Scholar] [CrossRef]
- Zhang, M.; Ding, X.; Zhang, Q.; Liu, J.; Zhang, Y.; Zhang, Y.; Tian, Z.; Li, W.; Zhu, W.; Kang, H.; et al. Exome sequencing of 112 trios identifies recessive genetic variants in brain arteriovenous malformations. J. NeuroIntervent. Surg. 2020, 13, 568–573. [Google Scholar] [CrossRef]
- Zheng, Q.; Zou, Y.; Teng, P.; Chen, Z.; Wu, Y.; Dai, X.; Li, X.; Hu, Z.; Wu, S.; Xu, Y.; et al. Mechanosensitive Channel PIEZO1 Senses Shear Force to Induce KLF2/4 Expression via CaMKII/MEKK3/ERK5 Axis in Endothelial Cells. Cells 2022, 11, 2191. [Google Scholar] [CrossRef]
- Davies, J.E.; Lopresto, D.; Apta, B.H.; Lin, Z.; Ma, W.; Harper, M.T. Using Yoda-1 to mimic laminar flow in vitro: A tool to simplify drug testing. Biochem. Pharmacol. 2019, 168, 473–480. [Google Scholar] [CrossRef]
- Shinge, S.A.U.; Zhang, D.; Din, A.U.; Yu, F.X.; Nie, Y.M. Emerging Piezo1 signaling in inflammation and atherosclerosis; a potential therapeutic target. Int. J. Biol. Sci. 2022, 18, 923–941. [Google Scholar] [CrossRef]
- Shinge, S.A.U.; Zhang, D.; Muluh, T.A.; Nie, Y.; Yu, F. Mechanosensitive Piezo1 Channel Evoked-Mechanical Signals in Atherosclerosis. J. Inflamm. Res. 2021, 14, 3621–3636. [Google Scholar] [CrossRef]
- Paz, N.G.D.; Frangos, J.A. Rapid flow-induced activation of Gαq/11 is independent of Piezo1 activation. Am. J. Physiol. Physiol. 2019, 316, C741–C752. [Google Scholar] [CrossRef]
- Albarrán-Juárez, J.; Iring, A.; Wang, S.; Joseph, S.; Grimm, M.; Strilic, B.; Wettschureck, N.; Althoff, T.F.; Offermanns, S. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J. Exp. Med. 2018, 215, 2655–2672. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, D.; Zhang, C.; Yang, W.; Li, C.; Gao, Z.; Pei, K.; Li, Y. Piezo1 mediates endothelial atherogenic inflammatory responses via regulation of YAP/TAZ activation. Hum. Cell 2021, 35, 51–62. [Google Scholar] [CrossRef]
- Wang, K.-C.; Yeh, Y.-T.; Nguyen, P.; Limqueco, E.; Lopez, J.; Thorossian, S.; Guan, K.-L.; Li, Y.-S.J.; Chien, S. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc. Natl. Acad. Sci. USA 2016, 113, 11525–11530. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Liu, S.; Li, C. Biomechanical signal communication in vascular smooth muscle cells. J. Cell Commun. Signal. 2020, 14, 357–376. [Google Scholar] [CrossRef]
- Scherer, C.; Pfisterer, L.; Wagner, A.H.; Hödebeck, M.; Cattaruzza, M.; Hecker, M.; Korff, T. Arterial Wall Stress Controls NFAT5 Activity in Vascular Smooth Muscle Cells. J. Am. Heart Assoc. 2014, 3, e000626. [Google Scholar] [CrossRef]
- Wernig, F.; Mayr, M.; Xu, Q. Mechanical Stretch-Induced Apoptosis in Smooth Muscle Cells Is Mediated by β 1 -Integrin Signaling Pathways. Hypertension 2003, 41, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Zang, G.; Li, N.; Sun, C.; Du, R. Agonist-induced Piezo1 activation promote mitochondrial-dependent apoptosis in vascular smooth muscle cells. BMC Cardiovasc. Disord. 2022, 22, 287. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Nemoto, E.; Yamada, S. Mechanical regulation of macrophage function—Cyclic tensile force inhibits NLRP3 inflammasome-dependent IL-1β secretion in murine macrophages. Inflamm. Regen. 2019, 39, 1–9. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, J.; Chen, W.; Li, W.; Chen, Z. Vascular Macrophages in Atherosclerosis. J. Immunol. Res. 2019, 2019, 4354786. [Google Scholar] [CrossRef]
- Xie, L.; Wang, X.; Ma, Y.; Ma, H.; Shen, J.; Chen, J.; Wang, Y.; Su, S.; Chen, K.; Xu, L.; et al. Piezo1 (Piezo-Type Mechanosensitive Ion Channel Component 1)-Mediated Mechanosensation in Macrophages Impairs Perfusion Recovery after Hindlimb Ischemia in Mice. Arter. Thromb. Vasc. Biol. 2023, 43, 504–518. [Google Scholar] [CrossRef]
- Baratchi, S.; Zaldivia, M.T.; Wallert, M.; Loseff-Silver, J.; Al-Aryahi, S.; Zamani, J.; Thurgood, P.; Salim, A.; Htun, N.M.; Stub, D.; et al. Transcatheter Aortic Valve Implantation Represents an Anti-Inflammatory Therapy Via Reduction of Shear Stress–Induced, Piezo-1–Mediated Monocyte Activation. Circulation 2020, 142, 1092–1105. [Google Scholar] [CrossRef]
- Qian, W.; Hadi, T.; Silvestro, M.; Ma, X.; Rivera, C.F.; Bajpai, A.; Li, R.; Zhang, Z.; Qu, H.; Tellaoui, R.S.; et al. Microskeletal stiffness promotes aortic aneurysm by sustaining pathological vascular smooth muscle cell mechanosensation via Piezo1. Nat. Commun. 2022, 13, 512. [Google Scholar] [CrossRef]
- Rode, B.; Shi, J.; Endesh, N.; Drinkhill, M.J.; Webster, P.J.; Lotteau, S.J.; Bailey, M.A.; Yuldasheva, N.Y.; Ludlow, M.J.; Cubbon, R.M.; et al. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat. Commun. 2017, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chennupati, R.; Kaur, H.; Iring, A.; Wettschureck, N.; Offermanns, S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J. Clin. Investig. 2016, 126, 4527–4536. [Google Scholar] [CrossRef] [PubMed]
- Iring, A.; Jin, Y.-J.; Albarrán-Juárez, J.; Siragusa, M.; Wang, S.; Dancs, P.T.; Nakayama, A.; Tonack, S.; Chen, M.; Künne, C.; et al. Shear stress–induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Investig. 2019, 129, 2775–2791. [Google Scholar] [CrossRef] [PubMed]
- Miroshnikova, Y.A.; Manet, S.; Li, X.; Wickström, S.A.; Faurobert, E.; Albiges-Rizo, C. Calcium signaling mediates a biphasic mechanoadaptive response of endothelial cells to cyclic mechanical stretch. Mol. Biol. Cell 2021, 32, 1724–1736. [Google Scholar] [CrossRef]
- Retailleau, K.; Duprat, F.; Arhatte, M.; Ranade, S.S.; Peyronnet, R.; Martins, J.R.; Jodar, M.; Moro, C.; Offermanns, S.; Feng, Y.; et al. Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. Cell Rep. 2015, 13, 1161–1171. [Google Scholar] [CrossRef]
- Liao, J.; Lu, W.; Chen, Y.; Duan, X.; Zhang, C.; Luo, X.; Lin, Z.; Chen, J.; Liu, S.; Yan, H.; et al. Upregulation of Piezo1 (Piezo Type Mechanosensitive Ion Channel Component 1) Enhances the Intracellular Free Calcium in Pulmonary Arterial Smooth Muscle Cells From Idiopathic Pulmonary Arterial Hypertension Patients. Hypertension 2021, 77, 1974–1989. [Google Scholar] [CrossRef]
- Chen, J.; Rodriguez, M.; Miao, J.; Liao, J.; Jain, P.P.; Zhao, M.; Zhao, T.; Babicheva, A.; Wang, Z.; Parmisano, S.; et al. Mechanosensitive channel Piezo1 is required for pulmonary artery smooth muscle cell proliferation. Am. J. Physiol. Cell Mol. Physiol. 2022, 322, L737–L760. [Google Scholar] [CrossRef]
- Chen, J.; Miao, J.; Zhou, D.; Liao, J.; Wang, Z.; Lin, Z.; Zhang, C.; Luo, X.; Li, Y.; Li, X.; et al. Upregulation of mechanosensitive channel Piezo1 involved in high shear stress-induced pulmonary hypertension. Thromb. Res. 2022, 218, 52–63. [Google Scholar] [CrossRef]
- Bertero, T.; Oldham, W.M.; Cottrill, K.A.; Pisano, S.; Vanderpool, R.R.; Yu, Q.; Zhao, J.; Tai, Y.; Tang, Y.; Zhang, Y.-Y.; et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Investig. 2016, 126, 3313–3335. [Google Scholar] [CrossRef]
- Hu, Y.; Chi, L.; Kuebler, W.M.; Goldenberg, N.M. Perivascular Inflammation in Pulmonary Arterial Hypertension. Cells 2020, 9, 2338. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Barbeau, S.; Baudrimont, I.; Vacher, P.; Freund-Michel, V.; Cardouat, G.; Berger, P.; Guibert, C.; Ducret, T.; Quignard, J.-F. Piezo1 Channel Activation Reverses Pulmonary Artery Vasoconstriction in an Early Rat Model of Pulmonary Hypertension: The Role of Ca2+ Influx and Akt-eNOS Pathway. Cells 2022, 11, 2349. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Babicheva, A.; Jain, P.P.; Rodriguez, M.; Ayon, R.J.; Ravellette, K.S.; Wu, L.; Balistrieri, F.; Tang, H.; et al. Endothelial upregulation of mechanosensitive channel Piezo1 in pulmonary hypertension. Am. J. Physiol. Physiol. 2021, 321, C1010–C1027. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Xu, M.; Wang, H.; Zhong, C.; Jiang, S.; Lichtenberger, F.-B.; Erdoğan, C.; Wang, H.; Bonk, J.S.; Lai, E.Y.; et al. Piezo1 Mediates Vasodilation Induced by Acute Hyperglycemia in Mouse Renal Arteries and Microvessels. Hypertension 2023, 80, 1598–1610. [Google Scholar] [CrossRef]
- Carson, P.E. Perils and Pitfalls With Associations in Heart Failure, Particularly in HF-pEF. JACC Asia 2023, 3, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Yin, K.; Wu, K.; Zhang, M.; Wang, S.; Cheng, H.; Zhou, Z.; Xiao, B. The mechanosensitive Piezo1 channel mediates heart mechano-chemo transduction. Nat. Commun. 2021, 12, 102a–103a. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, S.-A.; Li, W.; Ma, Y.; Shen, J.; Wang, Y.; Shen, Y.; Chen, J.; Ji, Y.; Xie, Y.; et al. Piezo1-Mediated Mechanotransduction Promotes Cardiac Hypertrophy by Impairing Calcium Homeostasis to Activate Calpain/Calcineurin Signaling. Hypertension 2021, 78, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-Y.; Gong, H.; Kesteven, S.; Guo, Y.; Wu, J.; Li, J.V.; Cheng, D.; Zhou, Z.; Iismaa, S.E.; Kaidonis, X.; et al. Piezo1 is the cardiac mechanosensor that initiates the cardiomyocyte hypertrophic response to pressure overload in adult mice. Nat. Cardiovasc. Res. 2022, 1, 577–591. [Google Scholar] [CrossRef]
- Stewart, L.; Turner, N.A. Channelling the Force to Reprogram the Matrix: Mechanosensitive Ion Channels in Cardiac Fibroblasts. Cells 2021, 10, 990. [Google Scholar] [CrossRef]
- Ploeg, M.C.; Munts, C.; Prinzen, F.W.; Turner, N.A.; van Bilsen, M.; van Nieuwenhoven, F.A. Piezo1 Mechanosensitive Ion Channel Mediates Stretch-Induced Nppb Expression in Adult Rat Cardiac Fibroblasts. Cells 2021, 10, 1745. [Google Scholar] [CrossRef]
- Braidotti, N.; Chen, S.N.; Long, C.S.; Cojoc, D.; Sbaizero, O. Piezo1 Channel as a Potential Target for Hindering Cardiac Fibrotic Remodeling. Int. J. Mol. Sci. 2022, 23, 8065. [Google Scholar] [CrossRef]
- Emig, R.; Knodt, W.; Krussig, M.J.; Zgierski-Johnston, C.M.; Gorka, O.; Groß, O.; Kohl, P.; Ravens, U.; Peyronnet, R. Piezo1 Channels Contribute to the Regulation of Human Atrial Fibroblast Mechanical Properties and Matrix Stiffness Sensing. Cells 2021, 10, 663. [Google Scholar] [CrossRef]
- Bartoli, F.; Evans, E.L.; Blythe, N.M.; Stewart, L.; Chuntharpursat-Bon, E.; Debant, M.; Musialowski, K.E.; Lichtenstein, L.; Parsonage, G.; Futers, T.S.; et al. Global PIEZO1 Gain-of-Function Mutation Causes Cardiac Hypertrophy and Fibrosis in Mice. Cells 2022, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Blythe, N.M.; Muraki, K.; Ludlow, M.J.; Stylianidis, V.; Gilbert, H.T.; Evans, E.L.; Cuthbertson, K.; Foster, R.; Swift, J.; Li, J.; et al. Mechanically activated Piezo1 channels of cardiac fibroblasts stimulate p38 mitogen-activated protein kinase activity and interleukin-6 secretion. J. Biol. Chem. 2019, 294, 17395–17408. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Zhang, X.; Fan, X. The Role of the Piezo1 Mechanosensitive Channel in Heart Failure. Curr. Issues Mol. Biol. 2023, 45, 5830–5848. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, L.; Chen, X.; Zhang, H.; Shi, Z. MiR-103a targeting Piezo1 is involved in acute myocardial infarction through regulating endothelium function. Cardiol. J. 2013, 23, 556–562. [Google Scholar] [CrossRef]
- Liang, J.; Huang, B.; Yuan, G.; Chen, Y.; Liang, F.; Zeng, H.; Zheng, S.; Cao, L.; Geng, D.; Zhou, S. Stretch-activated channel Piezo1 is up-regulated in failure heart and cardiomyocyte stimulated by AngII. Am. J. Transl. Res. 2017, 9, 2945–2955. [Google Scholar]
- Su, S.A.; Zhang, Y.; Li, W.; Xi, Y.; Lu, Y.; Shen, J.; Ma, Y.; Wang, Y.; Shen, Y.; Xie, L.; et al. Cardiac Piezo1 Exacerbates Lethal Ventricular Arrhythmogenesis by Linking Mechanical Stress with Ca2+ Handling after Myocardial Infarction. Research 2023, 6, 0165. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, Q.; Li, X.; Luo, G.-H.; Kuang, S.-J.; Luo, X.-S.; Li, Q.-Q.; Yang, H.; Liu, Y.; Deng, C.-Y.; et al. Piezo1 Participated in Decreased L-Type Calcium Current Induced by High Hydrostatic Pressure via. CaM/Src/Pitx2 Activation in Atrial Myocytes. Front. Cardiovasc. Med. 2022, 9, 842885. [Google Scholar] [CrossRef]
- Jakob, D.; Klesen, A.; Allegrini, B.; Darkow, E.; Aria, D.; Emig, R.; Chica, A.S.; Rog-Zielinska, E.A.; Guth, T.; Beyersdorf, F.; et al. Piezo1 and BKCa channels in human atrial fibroblasts: Interplay and remodelling in atrial fibrillation. J. Mol. Cell Cardiol. 2021, 158, 49–62. [Google Scholar] [CrossRef]
- Syeda, R.; Xu, J.; E Dubin, A.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H.; et al. Chemical activation of the mechanotransduction channel Piezo1. eLife 2015, 4, 07369. [Google Scholar] [CrossRef]
- Tang, H.; Zeng, R.; He, E.; Zhang, I.; Ding, C.; Zhang, A. Piezo-Type Mechanosensitive Ion Channel Component 1 (Piezo1): A Promising Therapeutic Target and Its Modulators. J. Med. Chem. 2022, 65, 6441–6453. [Google Scholar] [CrossRef]
- Botello-Smith, W.M.; Jiang, W.; Zhang, H.; Ozkan, A.D.; Lin, Y.-C.; Pham, C.N.; Lacroix, J.J.; Luo, Y. A mechanism for the activation of the mechanosensitive Piezo1 channel by the small molecule Yoda1. Nat. Commun. 2019, 10, 4503. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, J.J.; Botello-Smith, W.M.; Luo, Y. Probing the gating mechanism of the mechanosensitive channel Piezo1 with the small molecule Yoda1. Nat. Commun. 2018, 9, 2029. [Google Scholar] [CrossRef] [PubMed]
- Wijerathne, T.D.; Ozkan, A.D.; Lacroix, J.J. Yoda1’s energetic footprint on Piezo1 channels and its modulation by voltage and temperature. Proc. Natl. Acad. Sci. USA 2022, 119, e2202269119. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.L.; Cuthbertson, K.; Endesh, N.; Rode, B.; Blythe, N.M.; Hyman, A.J.; Hall, S.J.; Gaunt, H.J.; Ludlow, M.J.; Foster, R.; et al. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br. J. Pharmacol. 2018, 175, 1744–1759. [Google Scholar] [CrossRef]
- Liu, S.; Pan, X.; Cheng, W.; Deng, B.; He, Y.; Zhang, L.; Ning, Y.; Li, J. Tubeimoside I Antagonizes Yoda1-Evoked Piezo1 Channel Activation. Front. Pharmacol. 2020, 11, 768. [Google Scholar] [CrossRef]
- Pan, X.; Wan, R.; Wang, Y.; Liu, S.; He, Y.; Deng, B.; Luo, S.; Chen, Y.; Wen, L.; Hong, T.; et al. Inhibition of chemically and mechanically activated Piezo1 channels as a mechanism for ameliorating atherosclerosis with salvianolic acid B. Br. J. Pharmacol. 2022, 179, 3778–3814. [Google Scholar] [CrossRef]
- Yang, Y.; Pei, K.; Zhang, Q.; Wang, D.; Feng, H.; Du, Z.; Zhang, C.; Gao, Z.; Yang, W.; Wu, J.; et al. Salvianolic acid B ameliorates atherosclerosis via inhibiting YAP/TAZ/JNK signaling pathway in endothelial cells and pericytes. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2020, 1865, 158779. [Google Scholar] [CrossRef] [PubMed]
- Gnanasambandam, R.; Ghatak, C.; Yasmann, A.; Nishizawa, K.; Sachs, F.; Ladokhin, A.S.; Sukharev, S.I.; Suchyna, T.M. GsMTx4: Mechanism of Inhibiting Mechanosensitive Ion Channels. Biophys. J. 2017, 112, 31–45. [Google Scholar] [CrossRef]
- Bae, C.; Sachs, F.; Gottlieb, P.A. The Mechanosensitive Ion Channel Piezo1 Is Inhibited by the Peptide GsMTx4. Biochemistry 2011, 50, 6295–6300. [Google Scholar] [CrossRef]
- Copp, S.W.; Kim, J.S.; Ruiz-Velasco, V.; Kaufman, M.P. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J. Physiol. 2016, 594, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E.; et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef]
- Maneshi, M.M.; Ziegler, L.; Sachs, F.; Hua, S.Z.; Gottlieb, P.A. Enantiomeric Aβ peptides inhibit the fluid shear stress response of PIEZO1. Sci. Rep. 2018, 8, 14267. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.O.; Massey, A.E.; Mata-Daboin, A.D.; Sierra-Valdez, F.J.; Chauhan, S.C.; Cordero-Morales, J.F.; Vásquez, V. Dietary fatty acids fine-tune Piezo1 mechanical response. Nat. Commun. 2019, 10, 1200. [Google Scholar] [CrossRef]
- Ridone, P.; Pandzic, E.; Vassalli, M.; Cox, C.D.; Macmillan, A.; Gottlieb, P.A.; Martinac, B. Disruption of membrane cholesterol organization impairs the activity of PIEZO1 channel clusters. J. Gen. Physiol. 2020, 152, e201912515. [Google Scholar] [CrossRef]
- Buyan, A.; Cox, C.D.; Barnoud, J.; Li, J.; Chan, H.S.; Martinac, B.; Marrink, S.J.; Corry, B. Piezo1 Forms Specific, Functionally Important Interactions with Phosphoinositides and Cholesterol. Biophys. J. 2020, 119, 1683–1697. [Google Scholar] [CrossRef]
- Poole, K.; Herget, R.; Lapatsina, L.; Ngo, H.-D.; Lewin, G.R. Tuning Piezo ion channels to detect molecular-scale movements relevant for fine touch. Nat. Commun. 2014, 5, 3520. [Google Scholar] [CrossRef]
- Shi, J.; Hyman, A.J.; De Vecchis, D.; Chong, J.; Lichtenstein, L.; Futers, T.S.; Rouahi, M.; Salvayre, A.N.; Auge, N.; Kalli, A.C.; et al. Sphingomyelinase Disables Inactivation in Endogenous PIEZO1 Channels. Cell Rep. 2020, 33, 108225. [Google Scholar] [CrossRef]
- Borbiro, I.; Badheka, D.; Rohacs, T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci. Signal. 2015, 8, ra15. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.O.; Schneider, E.R.; Matson, J.D.; Gracheva, E.O.; Bagriantsev, S.N. TMEM150C/Tentonin3 Is a Regulator of Mechano-gated Ion Channels. Cell Rep. 2018, 23, 701–708. [Google Scholar] [CrossRef]
- Zhang, T.; Chi, S.; Jiang, F.; Zhao, Q.; Xiao, B. A protein interaction mechanism for suppressing the mechanosensitive Piezo channels. Nat. Commun. 2017, 8, 1797. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Z.; Wang, B.; Li, B.; Lv, H.; He, J.; Huang, Y.; Cui, Z.; Ma, Q.; Li, T.; et al. COMP (Cartilage Oligomeric Matrix Protein), a Novel PIEZO1 Regulator That Controls Blood Pressure. Hypertension 2022, 79, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Wang, J.; Xu, J.-P.; Wang, J.; Yang, X. Recent advances in CRISPR-based genome editing technology and its applications in cardiovascular research. Mil. Med. Res. 2023, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Stone, O.J.; Pablo, M.; Herron, J.C.; Nogueira, A.T.; Dagliyan, O.; Grimm, J.B.; Lavis, L.D.; Elston, T.C.; Hahn, K.M. Biosensors based on peptide exposure show single molecule conformations in live cells. Cell 2021, 184, 5670–5685. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Su, S.; Yu, S.; Zhang, Y.; Chen, K.; Xiang, M.; Ma, H. Essential Roles of PIEZO1 in Mammalian Cardiovascular System: From Development to Diseases. Cells 2024, 13, 1422. https://doi.org/10.3390/cells13171422
Jin C, Su S, Yu S, Zhang Y, Chen K, Xiang M, Ma H. Essential Roles of PIEZO1 in Mammalian Cardiovascular System: From Development to Diseases. Cells. 2024; 13(17):1422. https://doi.org/10.3390/cells13171422
Chicago/Turabian StyleJin, Chengjiang, Sheng’an Su, Shuo Yu, Yue Zhang, Kaijie Chen, Meixiang Xiang, and Hong Ma. 2024. "Essential Roles of PIEZO1 in Mammalian Cardiovascular System: From Development to Diseases" Cells 13, no. 17: 1422. https://doi.org/10.3390/cells13171422
APA StyleJin, C., Su, S., Yu, S., Zhang, Y., Chen, K., Xiang, M., & Ma, H. (2024). Essential Roles of PIEZO1 in Mammalian Cardiovascular System: From Development to Diseases. Cells, 13(17), 1422. https://doi.org/10.3390/cells13171422







