Expression of LOXL3, NES, and SNAI1 in Melanoma Genesis and Progression
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Procurement and Processing
2.2. Immunofluorescence
2.3. Data Acquisition
2.4. Semi-Quantification
2.5. Image Analysis of Area Percentage
2.6. Statistical Analysis of Area Percentage
2.7. RNA Isolation and Reverse Transcription
2.8. qPCR
2.9. Statistical Analysis of RT-qPCR
2.10. Transcriptomics
3. Results
3.1. H&E Staining of Dysplastic Nevus, Melanoma In Situ, and BRAF− and BRAF+ Melanoma
3.2. LOXL3 Expression
3.3. NES Expression
3.4. SNAI1 Expression
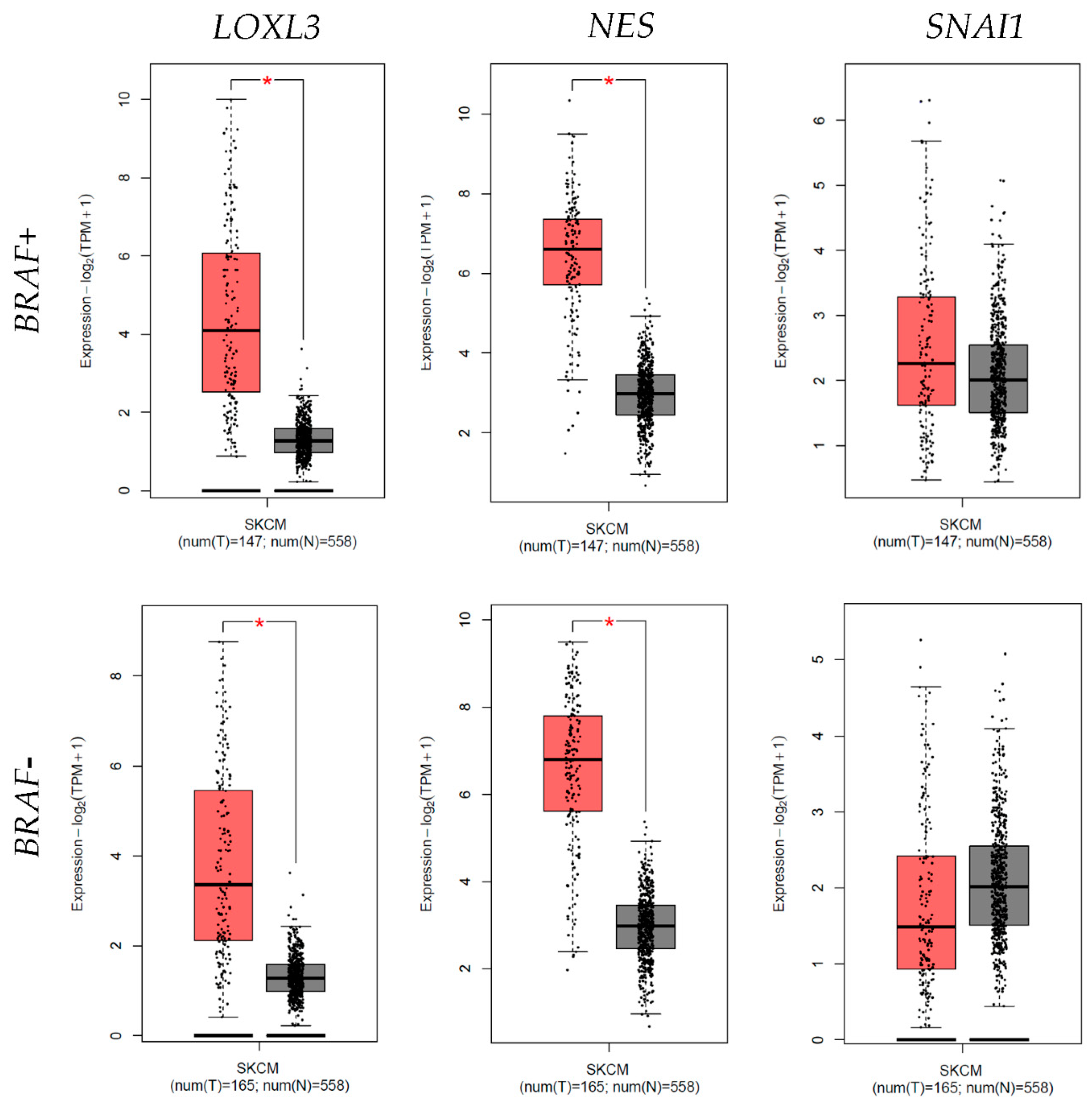
3.5. Differential Expression
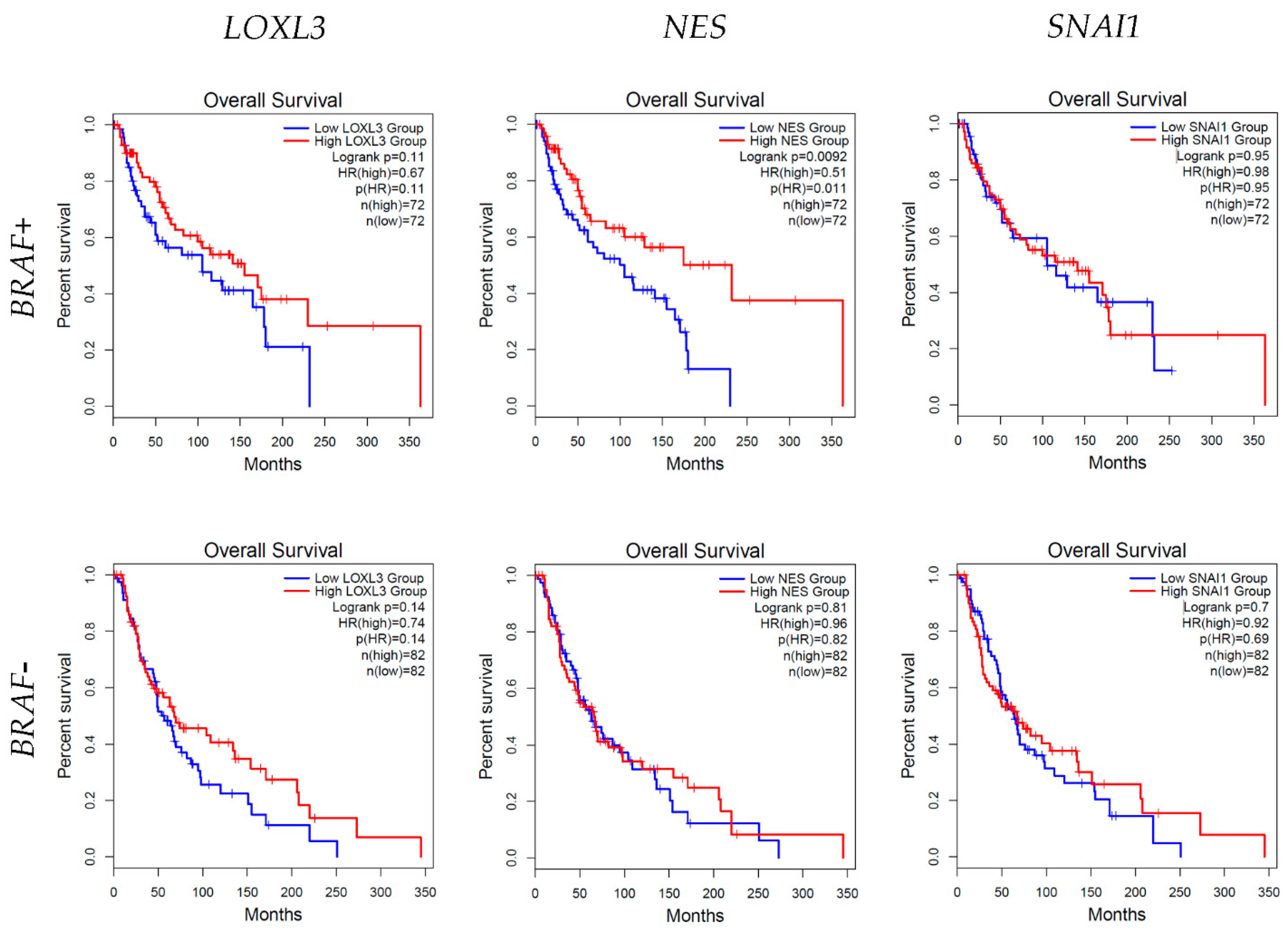
3.6. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heistein, J.B.; Acharya, U.; Mukkamalla, S.K.R. Malignant Melanoma. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Geller, A.C.; Clapp, R.W.; Sober, A.J.; Gonsalves, L.; Mueller, L.; Christiansen, C.L.; Shaikh, W.; Miller, D.R. Melanoma Epidemic: An Analysis of Six Decades of Data From the Connecticut Tumor Registry. J. Clin. Oncol. 2013, 31, 4172–4178. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur. J. Cancer 2022, 170, 236–255. [Google Scholar] [CrossRef]
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo 2014, 28, 1005–1011. [Google Scholar] [PubMed]
- Shain, A.H.; Yeh, I.; Kovalyshyn, I.; Sriharan, A.; Talevich, E.; Gagnon, A.; Dummer, R.; North, J.P.; Pincus, L.B.; Ruben, B.S.; et al. The Genetic Evolution of Melanoma from Precursor Lesions. N. Engl. J. Med. 2015, 373, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Paluncic, J.; Kovacevic, Z.; Jansson, P.J.; Kalinowski, D.; Merlot, A.M.; Huang, M.L.-H.; Lok, H.C.; Sahni, S.; Lane, D.J.; Richardson, D.R. Roads to melanoma: Key pathways and emerging players in melanoma progression and oncogenic signaling. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Elder, D.E.; Bastian, B.C.; Cree, I.A.; Massi, D.; Scolyer, R.A. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch. Pathol. Lab. Med. 2020, 144, 500–522. [Google Scholar] [CrossRef]
- Bastian, B.C. The Molecular Pathology of Melanoma: An Integrated Taxonomy of Melanocytic Neoplasia. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 239–271. [Google Scholar] [CrossRef]
- Ostrowski, S.M.; Fisher, D.E. Biology of Melanoma. Hematol. Oncol. Clin. N. Am. 2021, 35, 29–56. [Google Scholar] [CrossRef]
- Satyamoorthy, K.; Li, G.; Gerrero, M.R.; Brose, M.S.; Volpe, P.; Weber, B.L.; Van Belle, P.; E Elder, D.; Herlyn, M. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003, 63, 756–759. [Google Scholar]
- Jansen, P.; Cosgarea, I.; Murali, R.; Möller, I.; Sucker, A.; Franklin, C.; Paschen, A.; Zaremba, A.; Brinker, T.J.; Stoffels, I.; et al. Frequent Occurrence of NRAS and BRAF Mutations in Human Acral Naevi. Cancers 2019, 11, 546. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Cherepakhin, O.S.; Argenyi, Z.B.; Moshiri, A.S. Genomic and Transcriptomic Underpinnings of Melanoma Genesis, Progression, and Metastasis. Cancers 2021, 14, 123. [Google Scholar] [CrossRef]
- Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [CrossRef] [PubMed]
- Tsao, H.; Bevona, C.; Goggins, W.; Quinn, T. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: A population-based estimate. Arch. Dermatol. 2003, 139, 282–288. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. Snail: More than EMT. Cell Adhes. Migr. 2010, 4, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Cox, T.R.; Erler, J.T. The rationale for targeting the LOX family in cancer. Nat. Rev. Cancer 2012, 12, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Molnar, J.; Fong, K.; He, Q.; Hayashi, K.; Kim, Y.; Fong, S.; Fogelgren, B.; Szauter, K.M.; Mink, M.; Csiszar, K. Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2003, 1647, 220–224. [Google Scholar] [CrossRef]
- Trackman, P.C. Lysyl Oxidase Isoforms and Potential Therapeutic Opportunities for Fibrosis and Cancer. Expert Opin. Ther. Targets 2016, 20, 935–945. [Google Scholar] [CrossRef]
- Vázquez-Naharro, A.; Bustos-Tauler, J.; Floristán, A.; Yuste, L.; Oltra, S.S.; Vinyals, A.; Moreno-Bueno, G.; Fabra, À.; Portillo, F.; Cano, A.; et al. Loxl3 Promotes Melanoma Progression and Dissemination Influencing Cell Plasticity and Survival. Cancers 2022, 14, 1200. [Google Scholar] [CrossRef]
- Akiyama, M.; Matsuda, Y.; Ishiwata, T.; Naito, Z.; Kawana, S. Inhibition of the Stem Cell Marker Nestin Reduces Tumor Growth and Invasion of Malignant Melanoma. J. Investig. Dermatol. 2013, 133, 1384–1387. [Google Scholar] [CrossRef]
- Gomes, C.B.; Zechin, K.G.; Xu, S.; Stelini, R.F.; Nishimoto, I.N.; Zhan, Q.; Xu, T.; Qin, G.; Treister, N.S.; Murphy, G.F.; et al. TET2 Negatively Regulates Nestin Expression in Human Melanoma. Am. J. Pathol. 2016, 186, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Kuk, S.K.; Won, C.H.; Lee, W.J.; Shin, W.J.; Yoon, H.J.; Hong, S.D.; Hong, S.P.; Lee, J. Prognostic significance of nestin in primary malignant melanoma of the oral cavity. Melanoma Res. 2016, 26, 457–463. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, C.; Guan, W.; Chen, L.; Guo, K.; Yu, A.; Xie, K. Clinicopathological and prognostic significance of nestin expression in patients with breast cancer: A systematic review and meta-analysis. Cancer Cell Int. 2020, 20, 169. [Google Scholar] [CrossRef] [PubMed]
- A Flørenes, V.; Holm, R.; Myklebost, O.; Lendahl, U.; Fodstad, O. Expression of the neuroectodermal intermediate filament nestin in human melanomas. Cancer Res. 1994, 54, 354–356. [Google Scholar] [PubMed]
- Klein, W.M.; Wu, B.P.; Zhao, S.; Wu, H.; Klein-Szanto, A.J.P.; Tahan, S.R. Increased expression of stem cell markers in malignant melanoma. Mod. Pathol. 2007, 20, 102–107. [Google Scholar] [CrossRef]
- Dissanayake, S.K.; Wade, M.; Johnson, C.E.; O’Connell, M.P.; Leotlela, P.D.; French, A.D.; Shah, K.V.; Hewitt, K.J.; Rosenthal, D.T.; Indig, F.E.; et al. The Wnt5A/Protein Kinase C Pathway Mediates Motility in Melanoma Cells via the Inhibition of Metastasis Suppressors and Initiation of an Epithelial to Mesenchymal Transition. J. Biol. Chem. 2007, 282, 17259–17271. [Google Scholar] [CrossRef]
- Moody, S.E.; Perez, D.; Pan, T.-C.; Sarkisian, C.J.; Portocarrero, C.P.; Notorfrancesco, K.L.; Cardiff, R.D.; Chodosh, L.A. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 2005, 8, 197–209. [Google Scholar] [CrossRef]
- Sugimachi, K.; Tanaka, S.; Kameyama, T.; Taguchi, K.-I.; Aishima, S.-I.; Shimada, M.; Sugimachi, K.; Tsuneyoshi, M. Transcriptional repressor snail and progression of human hepatocellular carcinoma. Clin. Cancer Res. 2003, 9, 2657–2664. [Google Scholar]
- Yang, Z.; Zhang, X.; Gang, H.; Li, X.; Li, Z.; Wang, T.; Han, J.; Luo, T.; Wen, F.; Wu, X. Up-regulation of gastric cancer cell invasion by Twist is accompanied by N-cadherin and fibronectin expression. Biochem. Biophys. Res. Commun. 2007, 358, 925–930. [Google Scholar] [CrossRef]
- Arumi-Planas, M.; Rodriguez-Baena, F.J.; Cabello-Torres, F.; Gracia, F.; Lopez-Blau, C.; Nieto, M.A.; Sanchez-Laorden, B. Microenvironmental Snail1-induced immunosuppression promotes melanoma growth. Oncogene 2023, 42, 2659–2672. [Google Scholar] [CrossRef]
- Williams, J. The Declaration of Helsinki and public health. Bull. World Health Organ. 2008, 86, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Kelam, N.; Racetin, A.; Polović, M.; Benzon, B.; Ogorevc, M.; Vukojević, K.; Durdov, M.G.; Huljev, A.D.; Prusac, I.K.; Čarić, D.; et al. Aberrations in FGFR1, FGFR2, and RIP5 Expression in Human Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). Int. J. Mol. Sci. 2022, 23, 15537. [Google Scholar] [CrossRef] [PubMed]
- Lozić, M.; Filipović, N.; Jurić, M.; Kosović, I.; Benzon, B.; Šolić, I.; Kelam, N.; Racetin, A.; Watanabe, K.; Katsuyama, Y.; et al. Alteration of Cx37, Cx40, Cx43, Cx45, Panx1, and Renin Expression Patterns in Postnatal Kidneys of Dab1-/- (yotari) Mice. Int. J. Mol. Sci. 2021, 22, 1284. [Google Scholar] [CrossRef]
- Paštar, V.; Lozić, M.; Kelam, N.; Filipović, N.; Bernard, B.; Katsuyama, Y.; Vukojević, K. Connexin Expression Is Altered in Liver Development of Yotari (dab1 -/-) Mice. Int. J. Mol. Sci. 2021, 22, 10712. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Peinado, H.; Cruz, M.D.C.I.-D.L.; Olmeda, D.; Csiszar, K.; Fong, K.S.K.; Vega, S.; Nieto, M.A.; Cano, A.; Portillo, F. A molecular role for lysyl oxidase-like 2 enzyme in Snail regulation and tumor progression. EMBO J. 2005, 24, 3446–3458. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Hayashi, Y.; Sakaki, H.; Murakami, I. Evaluation of Clinical and Immunohistochemical Factors Relating to Melanoma Metastasis: Potential Roles of Nestin and Fascin in Melanoma. Diagnostics 2022, 12, 219. [Google Scholar] [CrossRef]
- Santamaría, P.G.; Floristán, A.; Fontanals-Cirera, B.; Vázquez-Naharro, A.; Santos, V.; Morales, S.; Yuste, L.; Peinado, H.; García-Gómez, A.; Portillo, F.; et al. Lysyl oxidase-like 3 is required for melanoma cell survival by maintaining genomic stability. Cell Death Differ. 2017, 25, 935–950. [Google Scholar] [CrossRef]
- Zhang, X.; Su, M.-W.; Cheng, Y.; Martinka, M.; Wang, G.; Huang, Y.; Li, L.; Zhou, Y. Immunohistochemistry analysis reveals lysyl oxidase-like 3 as a novel prognostic marker for primary melanoma. Melanoma Res. 2021, 31, 173–177. [Google Scholar] [CrossRef]
- Liu, S.; Kumar, S.M.; Martin, J.S.; Yang, R.; Xu, X. Snail1 Mediates Hypoxia-Induced Melanoma Progression. Am. J. Pathol. 2011, 179, 3020–3031. [Google Scholar] [CrossRef]
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; Del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor Snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Kaufhold, S.; Bonavida, B. Central role of Snail1 in the regulation of EMT and resistance in cancer: A target for therapeutic intervention. J. Exp. Clin. Cancer Res. 2014, 33, 014–0062. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Durand, S.; Dalle, S.; Caramel, J. EMT-Inducing Transcription Factors, Drivers of Melanoma Phenotype Switching, and Resistance to Treatment. Cancers 2020, 12, 2154. [Google Scholar] [CrossRef]
- Brychtova, S.; Fiuraskova, M.; Hlobilková, A.; Brychta, T.; Hirnak, J. Nestin expression in cutaneous melanomas and melanocytic nevi. J. Cutan. Pathol. 2006, 34, 370–375. [Google Scholar] [CrossRef]
- Akiyama, M.; Matsuda, Y.; Ishiwata, T.; Naito, Z.; Kawana, S. Nestin is highly expressed in advanced-stage melanomas and neurotized nevi. Oncol. Rep. 2013, 29, 1595–1599. [Google Scholar] [CrossRef]
- Piras, F.; Perra, M.T.; Murtas, D.; Minerba, L.; Floris, C.; Maxia, C.; Demurtas, P.; Ugalde, J.; Ribatti, D.; Sirigu, P. The stem cell marker nestin predicts poor prognosis in human melanoma. Oncol. Rep. 2009, 23, 17–24. [Google Scholar] [CrossRef]
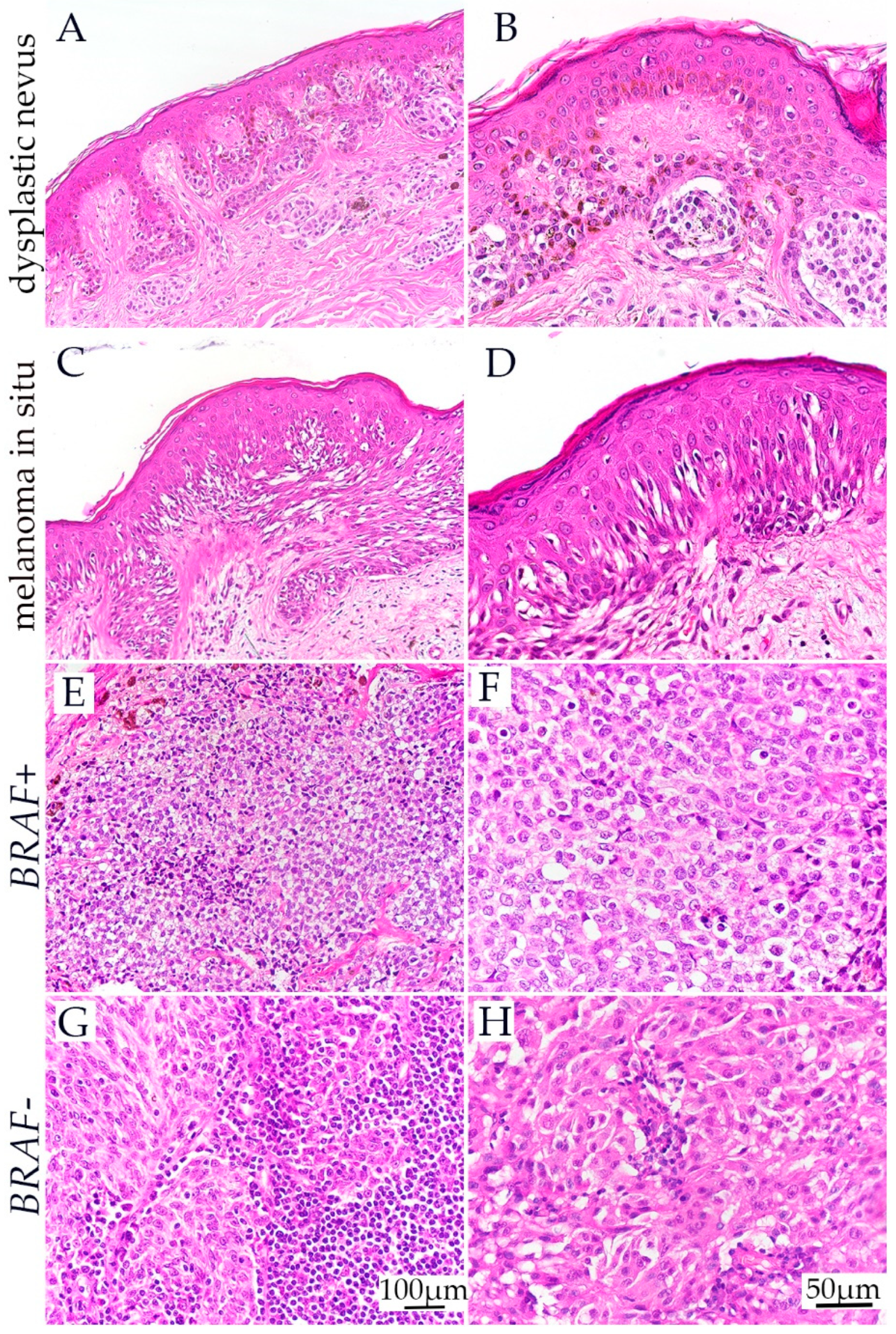
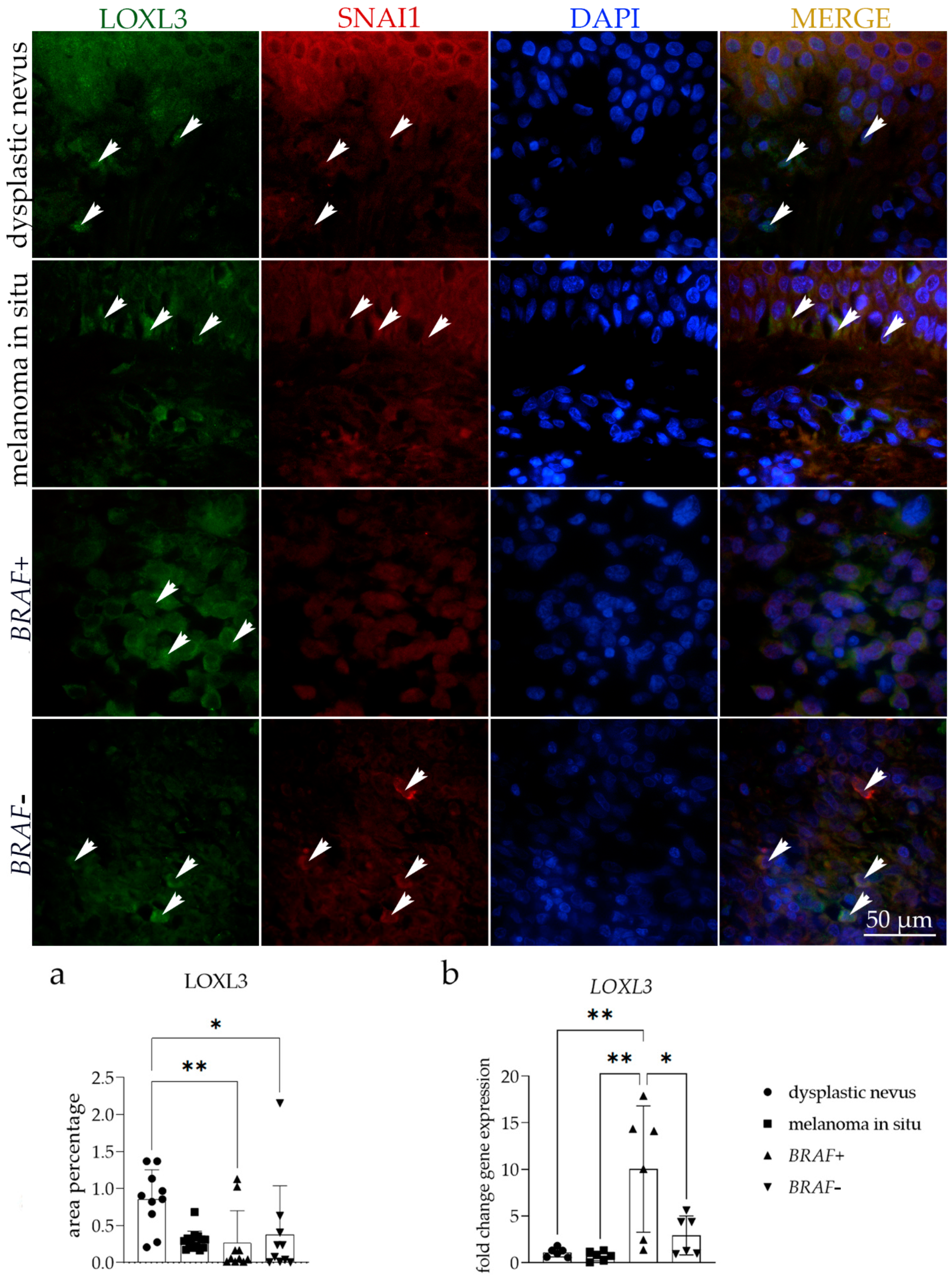
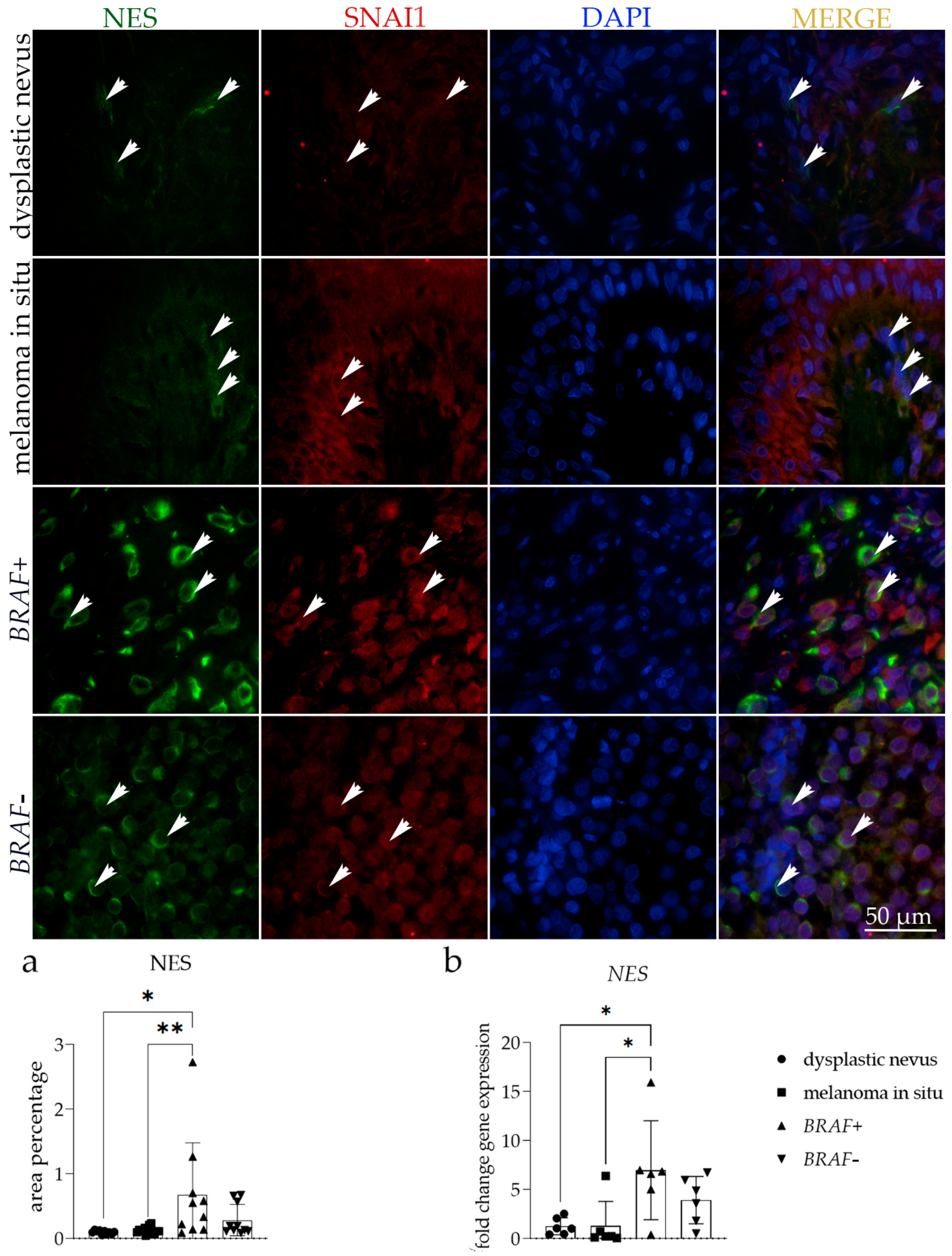

| Histology | No Patients | Age | Sex (Male/Female) |
|---|---|---|---|
| Dysplastic nevus | 10 | 36.9 ± 12.2 | 6/4 |
| Melanoma in situ | 15 | 55.9 ± 10.9 | 5/5 |
| BRAF+ melanoma | 10 | 60.8 ± 13.1 | 9/6 |
| BRAF− melanoma | 10 | 65.3 ± 15.9 | 7/3 |
| Antibodies | Catalog Number | Host | Dilution | Source | |
|---|---|---|---|---|---|
| Primary | Anti-LOXL3 | SAB4301652 | Rabbit | 1:100 | Merck KGaA, Darmstadt, Germany |
| Anti-SNAI1 antibody | ab53519 | Goat | 1:500 | Abcam, Cambridge, UK | |
| Anti-nestin antibody (SP103) | ab105389 | Rabbit | 1:100 | Abcam, Cambridge, UK | |
| Secondary | Rhodamine Red™-X (RRX) AffiniPure Anti-Goat IgG (H + L) | 705-295-003 | Donkey | 1:300 | Jackson Immuno Research Laboratories, Inc., (Baltimore, PA, USA) |
| Alexa Fluor®488 AffiniPure Anti- Rabbit lgG (H + L) | 711-545-152 | Donkey | 1:300 | Jackson Immuno Research Laboratories, Inc., (Baltimore, PA, USA) | |
| Structure | Antibodies | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOXL3 | NES | SNAI1 | ||||||||||
| Dysplastic Nevus | Melanoma In Situ | BRAF+ | BRAF− | Dysplastic Nevus | Melanoma In Situ | BRAF+ | BRAF− | Dysplastic Nevus | Melanoma In Situ | BRAF+ | BRAF− | |
| epithelium | + | + | ++ | ++ | + | + | +++ | ++ | + | + | ++ | ++ |
| lamina propria | +/− | +/− | + | + | +/− | +/− | + | + | +/− | +/− | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šitum Čeprnja, Z.; Kelam, N.; Ogorevc, M.; Racetin, A.; Vukoja, M.; Čeprnja, T.; Filipović, N.; Saraga-Babić, M.; Vukojević, K. Expression of LOXL3, NES, and SNAI1 in Melanoma Genesis and Progression. Cells 2024, 13, 1450. https://doi.org/10.3390/cells13171450
Šitum Čeprnja Z, Kelam N, Ogorevc M, Racetin A, Vukoja M, Čeprnja T, Filipović N, Saraga-Babić M, Vukojević K. Expression of LOXL3, NES, and SNAI1 in Melanoma Genesis and Progression. Cells. 2024; 13(17):1450. https://doi.org/10.3390/cells13171450
Chicago/Turabian StyleŠitum Čeprnja, Zdenka, Nela Kelam, Marin Ogorevc, Anita Racetin, Martina Vukoja, Toni Čeprnja, Natalija Filipović, Mirna Saraga-Babić, and Katarina Vukojević. 2024. "Expression of LOXL3, NES, and SNAI1 in Melanoma Genesis and Progression" Cells 13, no. 17: 1450. https://doi.org/10.3390/cells13171450
APA StyleŠitum Čeprnja, Z., Kelam, N., Ogorevc, M., Racetin, A., Vukoja, M., Čeprnja, T., Filipović, N., Saraga-Babić, M., & Vukojević, K. (2024). Expression of LOXL3, NES, and SNAI1 in Melanoma Genesis and Progression. Cells, 13(17), 1450. https://doi.org/10.3390/cells13171450









