Cigarette Smoke-Induced Epithelial-to-Mesenchymal Transition: Insights into Cellular Mechanisms and Signaling Pathways
Abstract
1. Introduction
2. Cigarette Smoke (CS) and Human Bronchial Epithelial Cells
2.1. Toxic Components of Cigarette Smoke
2.2. Human Bronchial Epithelial Cells
3. Evidence of Cigarette Smoke-Induced EMT
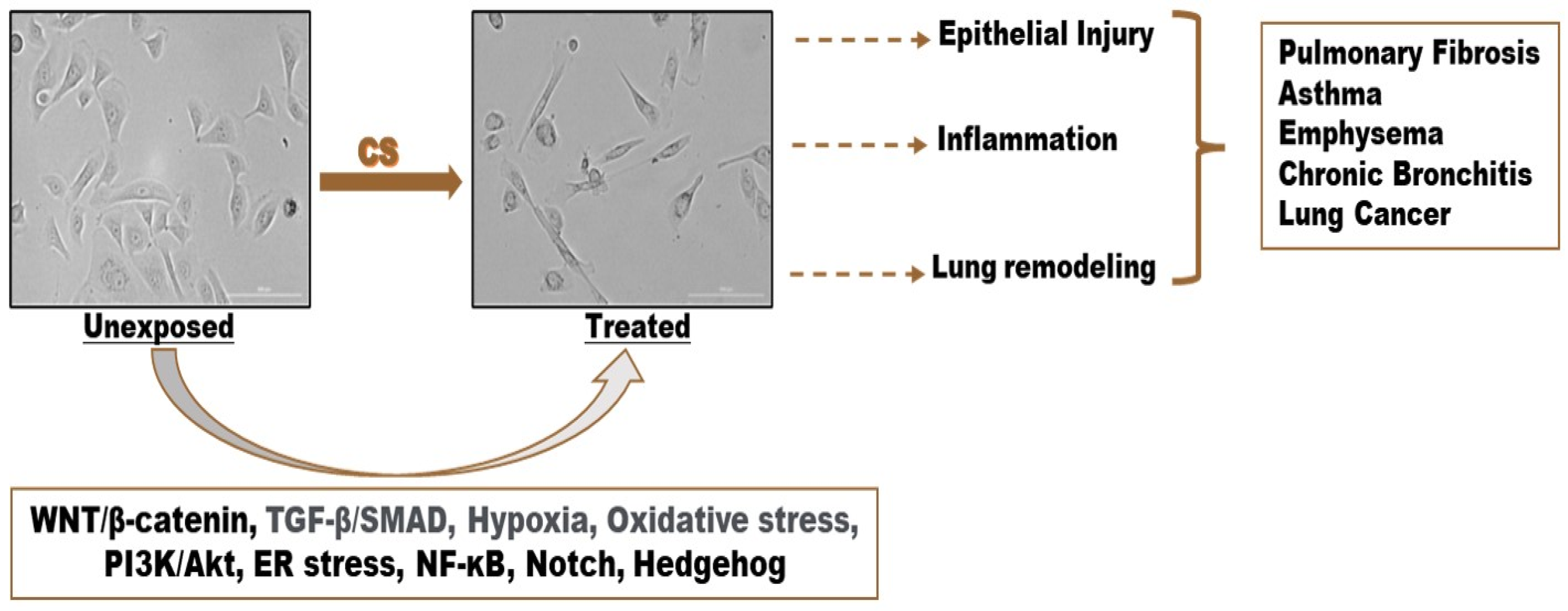
3.1. Cigarette Smoke (CS)-Induced Epithelial-to-Mesenchymal Transition (EMT)
3.2. Structural Evidence
3.3. Functional Evidence
4. Molecular Mechanisms of CS-Induced EMT
4.1. WNT/β-Catenin Signaling Pathway
4.2. TGF-β/SMAD Signaling Pathway
4.3. Hypoxia Signaling Pathway
4.4. Oxidative Stress Signaling Pathway
4.5. PI3K/Akt Pathway
4.6. Endoplasmic Reticulum (ER) Stress
4.7. NF-KB Signaling Pathway
4.8. Notch Signaling Pathway
4.9. Hedgehog Signaling Pathway
4.10. Macrophages and CS
5. Strengths and Limitations
6. Conclusions
7. Methodology
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balogh, E.P.; Dresler, C.; Fleury, M.E.; Gritz, E.R.; Kean, T.J.; Myers, M.L.; Nass, S.J.; Nevidjon, B.; Toll, B.A.; Warren, G.W.; et al. Reducing tobacco-related cancer incidence and mortality: Summary of an institute of medicine workshop. Oncologist 2014, 19, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- CDC. What Are the Risk Factors for Lung Cancer? Available online: https://www.cdc.gov/lung-cancer/risk-factors/index.html (accessed on 12 June 2023).
- Aredo, J.V.; Luo, S.J.; Gardner, R.M.; Sanyal, N.; Choi, E.; Hickey, T.P.; Riley, T.L.; Huang, W.Y.; Kurian, A.W.; Leung, A.N.; et al. Tobacco Smoking and Risk of Second Primary Lung Cancer. J. Thorac. Oncol. 2021, 16, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Phua, Z.J.; MacInnis, R.J.; Jayasekara, H. Cigarette smoking and risk of second primary cancer: A systematic review and meta-analysis. Cancer Epidemiol. 2022, 78, 102160. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Tsuruta, M.; Okabayashi, K.; Ishida, T.; Shigeta, K.; Seishima, R.; Ikebata, A.; Koishikawa, K.; Hasegawa, H.; Shimoda, M. The impact of smoking on pulmonary metastasis in colorectal cancer. OncoTargets Ther. 2020, 13, 9623–9629. [Google Scholar] [CrossRef]
- Jiang, Y.-J.; Chao, C.-C.; Chang, A.-C.; Chen, P.-C.; Cheng, F.-J.; Liu, J.-F.; Liu, P.-I.; Huang, C.-L.; Guo, J.-H.; Huang, W.-C. Cigarette smoke-promoted increases in osteopontin expression attract mesenchymal stem cell recruitment and facilitate lung cancer metastasis. J. Adv. Res. 2022, 41, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y. Smoking and respiratory diseases. Nihon Rinsho. Jpn. J. Clin. Med. 2013, 71, 416–420. [Google Scholar]
- Behr, J.; Nowak, D. Tobacco smoke and respiratory disease. World 2002, 58, 1–20. [Google Scholar]
- Lugade, A.A.; Bogner, P.N.; Thatcher, T.H.; Sime, P.J.; Phipps, R.P.; Thanavala, Y. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J. Immunol. 2014, 192, 5226–5235. [Google Scholar] [CrossRef]
- Alavinezhad, A.; Boskabady, M.H. The prevalence of asthma and related symptoms in Middle East countries. Clin. Respir J. 2018, 12, 865–877. [Google Scholar] [CrossRef]
- Anthonisen, N.R.; Connett, J.E.; Murray, R.P. Smoking and lung function of Lung Health Study participants after 11 years. Am. J. Respir. Crit. Care Med. 2002, 166, 675–679. [Google Scholar] [CrossRef]
- Mistry, A.; Tyagi, R.; Kagathara, J.; Vaidya, L.; Dholakiya, U.; Shah, C. Comparative study of pulmonary function tests in smokers and non-smokers. GCSMC J. Med. Sci 2014, 3, 22–27. [Google Scholar]
- Karia, R.M. Comparative study of peak expiratory flow rate and maximum voluntary ventilation between smokers and non-smokers. Natl. J. Med. Res. 2012, 2, 191–193. [Google Scholar]
- Papi, A.; Casoni, G.; Caramori, G.; Guzzinati, I.; Boschetto, P.; Ravenna, F.; Calia, N.; Petruzzelli, S.; Corbetta, L.; Cavallesco, G. COPD increases the risk of squamous histological subtype in smokers who develop non-small cell lung carcinoma. Thorax 2004, 59, 679–681. [Google Scholar] [CrossRef]
- Szalontai, K.; Gémes, N.; Furák, J.; Varga, T.; Neuperger, P.; Balog, J.Á.; Puskás, L.G.; Szebeni, G.J. Chronic obstructive pulmonary disease: Epidemiology, biomarkers, and paving the way to lung cancer. J. Clin. Med. 2021, 10, 2889. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Gakidou, E.; Lopez, A.D. Evolution of the global smoking epidemic over the past half century: Strengthening the evidence base for policy action. Tob. Control 2022, 31, 129–137. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Trends in Prevalence of Tobacco Smoking 2000–2025; World Health Organization: Geneva, Switzerland, 2018. Available online: https://www.who.int/publications/i/item/who-global-report-on-trends-in-prevalence-of-tobacco-use-2000-2025-third-edition (accessed on 15 June 2023).
- Faux, S.P.; Tai, T.; Thorne, D.; Xu, Y.; Breheny, D.; Gaca, M. The role of oxidative stress in the biological responses of lung epithelial cells to cigarette smoke. Biomarkers 2009, 14, 90–96. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Ju, Y.S.; Haase, K.; Van Loo, P.; Martincorena, I.; Nik-Zainal, S.; Totoki, Y.; Fujimoto, A.; Nakagawa, H.; Shibata, T.; et al. Mutational signatures associated with tobacco smoking in human cancer. Science 2016, 354, 618–622. [Google Scholar] [CrossRef]
- Sohal, S.S.; Walters, E.H. Epithelial mesenchymal transition (EMT) in small airways of COPD patients. Thorax 2013, 68, 783–784. [Google Scholar] [CrossRef]
- Hou, W.; Hu, S.; Li, C.; Ma, H.; Wang, Q.; Meng, G.; Guo, T.; Zhang, J. Cigarette smoke induced lung barrier dysfunction, EMT, and tissue remodeling: A possible link between COPD and lung cancer. BioMed Res. Int. 2019, 2019, 2025636. [Google Scholar] [CrossRef]
- Milara, J.; Peiró, T.; Serrano, A.; Cortijo, J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax 2013, 68, 410–420. [Google Scholar] [CrossRef]
- Gohy, S.T.; Hupin, C.; Fregimilicka, C.; Detry, B.R.; Bouzin, C.; Chevronay, H.G.; Lecocq, M.; Weynand, B.; Ladjemi, M.Z.; Pierreux, C.E. Imprinting of the COPD airway epithelium for dedifferentiation and mesenchymal transition. Eur. Respir. J. 2015, 45, 1258–1272. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.Q.; Sohal, S.S.; Shukla, S.D.; Ward, C.; Hardikar, A.; Noor, W.D.; Muller, H.K.; Knight, D.A.; Walters, E.H. Epithelial mesenchymal transition in smokers: Large versus small airways and relation to airflow obstruction. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Sohal, S.S.; Reid, D.; Soltani, A.; Ward, C.; Weston, S.; Muller, H.K.; Wood-Baker, R.; Walters, E.H. Reticular basement membrane fragmentation and potential epithelial mesenchymal transition is exaggerated in the airways of smokers with chronic obstructive pulmonary disease. Respirology 2010, 15, 930–938. [Google Scholar] [CrossRef]
- Lan, L.; Luo, Y.; Cui, D.; Shi, B.; Deng, W.; Huo, L.; Chen, H.; Zhang, G.; Deng, L. Epithelial-Mesenchymal Transition Triggers Cancer Stem Cell Generation in Human Thyroid Cancer Cells. Int. J. Oncol. 2013, 43, 113–120. [Google Scholar] [CrossRef]
- Akunuru, S.; Zhai, Q.; Zheng, Y.W. Non-Small Cell Lung Cancer Stem/Progenitor Cells Are Enriched in Multiple Distinct Phenotypic Subpopulations and Exhibit Plasticity. Cell Death Dis. 2012, 3, e352. [Google Scholar] [CrossRef]
- Wei, X.; Dou, X.; Bai, J.; Luo, X.Q.; Qiu, S.; Xi, D.; Huang, W.H.; Du, C.; Man, K.; Zhang, G. ERα Inhibits Epithelial-Mesenchymal Transition by Suppressing Bmi1 in Breast Cancer. Oncotarget 2015, 6, 21704–21717. [Google Scholar] [CrossRef]
- Wang, R.; Li, S.; Hou, Q.; Zhang, B.; Chu, H.; Hou, Y.; Ni, C.; Sun, L.; Ran, Y.; Zheng, H. Propofol Inhibits Colon Cancer Cell Stemness and Epithelial-Mesenchymal Transition by Regulating SIRT1, WNT/Β-Catenin and PI3K/AKT/mTOR Signaling Pathways. Discov. Oncol. 2023, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, B.P. Activation of Β-Catenin and Akt Pathways by Twist Are Critical for the Maintenance of EMT Associated Cancer Stem Cell-Like Characters. BMC Cancer 2011, 11, 49. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, L.; Zhang, T.; Wu, W.; Liu, J.; Wang, X.; Yang, W.; Geng, H.; Sun, X.; Qian, W.; et al. Curcumin negatively regulates cigarette smoke-induced renal cell carcinoma epithelial–mesenchymal transition through the ERK5/AP-1 pathway. Oncotargets Ther. 2020, 13, 9689–9700. [Google Scholar] [CrossRef]
- Geng, H.; Zhao, L.; Liang, Z.; Zhang, Z.; Xie, D.; Bi, L.; Wang, Y.; Zhang, T.; Cheng, L.; Yu, D.; et al. ERK5 Positively Regulates Cigarette Smoke-Induced Urocystic Epithelial-Mesenchymal Transition in SV-40 Immortalized Human Urothelial Cells. Oncol. Rep. 2015, 34, 1581–1588. [Google Scholar] [CrossRef]
- Bijani, M.; Abedi, S.; Karimi, S.; Tehranineshat, B. Major Challenges and Barriers in Clinical Decision-Making as Perceived by Emergency Medical Services Personnel: A Qualitative Content Analysis. BMC Emerg. Med. 2021, 21, 11. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, T.; Tang, L.; Min, J.; Yu, D. Curcumin Reversed Cigarette Smoke Extract-Induced Epithelial-to-Mesenchymal Transition of Human Bladder Cancer Cell UMUC3 Through Suppression of ERK1/2 Pathway. Res. Sq. 2022; preprint. [Google Scholar] [CrossRef]
- Su, X.; Wu, W.; Zhu, Z.; Lin, X.; Zeng, Y. The effects of epithelial-mesenchymal transitions in COPD induced by cigarette smoke: An update. Respir. Res. 2022, 23, 225. [Google Scholar] [CrossRef]
- Vu, T.; Jin, L.; Datta, P.K. Effect of Cigarette Smoking on Epithelial to Mesenchymal Transition (EMT) in Lung Cancer. J. Clin. Med. 2016, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hecht, S.S. Carcinogenic components of tobacco and tobacco smoke: A 2022 update. Food Chem. Toxicol. 2022, 165, 113179. [Google Scholar] [CrossRef]
- Rodgman, A.; Perfetti, T.A. The Chemical Components of Tobacco and Tobacco Smoke, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9780429146831. [Google Scholar]
- Boo, H.-J.; Min, H.-Y.; Hwang, S.J.; Lee, H.-J.; Lee, J.-W.; Oh, S.-R.; Park, C.-S.; Park, J.-S.; Lee, Y.M.; Lee, H.-Y. The tobacco-specific carcinogen NNK induces pulmonary tumorigenesis via nAChR/Src/STAT3-mediated activation of the renin-angiotensin system and IGF-1R signaling. Exp. Mol. Med. 2023, 55, 1131–1144. [Google Scholar] [CrossRef]
- Hudlikar, R.R.; Sargsyan, D.; Cheng, D.; Kuo, H.-C.D.; Wu, R.; Su, X.; Kong, A.-N. Tobacco carcinogen 4-[methyl (nitroso) amino]-1-(3-pyridinyl)-1-butanone (NNK) drives metabolic rewiring and epigenetic reprograming in A/J mice lung cancer model and prevention with diallyl sulphide (DAS). Carcinogenesis 2022, 43, 140–149. [Google Scholar] [CrossRef]
- Doukas, S.G.; Vageli, D.P.; Lazopoulos, G.; Spandidos, D.A.; Sasaki, C.T.; Tsatsakis, A. The effect of NNK, a tobacco smoke carcinogen, on the miRNA and mismatch DNA repair expression profiles in lung and head and neck squamous cancer cells. Cells 2020, 9, 1031. [Google Scholar] [CrossRef] [PubMed]
- Food, U.; Administration, D. Harmful and potentially harmful constituents in tobacco products and tobacco smoke; established list. Fed. Regist. 2012, 77, 20034–20037. [Google Scholar]
- International Agency for Research on Cancer. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/monographs-available/ (accessed on 15 June 2023).
- Hecht, S.S. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 1999, 91, 1194–1210. [Google Scholar] [CrossRef]
- World Health Organization. WHO Study Group on Tobacco Product Regulation: Report on the Scientific Basis of Tobacco Product Regulation: Seventh Report of a WHO Study Group. Available online: https://apps.who.int/iris/handle/10665/329445 (accessed on 15 June 2023).
- Kia’i, N.; Bajaj, T. Histology, Respiratory Epithelium. 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541061/ (accessed on 15 June 2023).
- Tulen, C.B.M.; Duistermaat, E.; Cremers, J.; Klerx, W.N.M.; Fokkens, P.H.B.; Weibolt, N.; Kloosterboer, N.; Dentener, M.A.; Gremmer, E.R.; Jessen, P.J.J.; et al. Smoking-Associated Exposure of Human Primary Bronchial Epithelial Cells to Aldehydes: Impact on Molecular Mechanisms Controlling Mitochondrial Content and Function. Cells 2022, 11, 3481. [Google Scholar] [CrossRef]
- Ramirez, R.D.; Sheridan, S.; Girard, L.; Sato, M.; Kim, Y.; Pollack, J.; Peyton, M.; Zou, Y.; Kurie, J.M.; DiMaio, J.M. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004, 64, 9027–9034. [Google Scholar] [CrossRef]
- Walters, M.S.; Gomi, K.; Ashbridge, B.; Moore, M.A.; Arbelaez, V.; Heldrich, J.; Ding, B.-S.; Rafii, S.; Staudt, M.R.; Crystal, R.G. Generation of a human airway epithelium derived basal cell line with multipotent differentiation capacity. Respir. Res. 2013, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.-R.; Jang, J.; Park, S.-M.; Ryu, S.M.; Cho, S.-J.; Yang, S.-R. Cigarette Smoke-Induced Respiratory Response: Insights into Cellular Processes and Biomarkers. Antioxidants 2023, 12, 1210. [Google Scholar] [CrossRef]
- Bukhari, B.; Naveed, M.; Ahmed, N.; Makhdoom, S.; Jabeen, K.; Asif, M.F.; Batool, H.; Yean, C. A Comparison Between Organic and Inorganic Nanoparticles: Prime Nanoparticles for Tumor Curation. Nano 2021, 16, 2130011. [Google Scholar] [CrossRef]
- Murray, L.A.; Dunmore, R.; Camelo, A.; Da Silva, C.A.; Gustavsson, M.J.; Habiel, D.M.; Hackett, T.L.; Hogaboam, C.M.; Sleeman, M.A.; Knight, D.A. Acute cigarette smoke exposure activates apoptotic and inflammatory programs but a second stimulus is required to induce epithelial to mesenchymal transition in COPD epithelium. Respir. Res. 2017, 18, 82. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Wang, Z.; Yang, Y.; Li, M.; Yuan, D.; Zhang, X.; Li, Y. CD147 Promoted Epithelial Mesenchymal Transition in Airway Epithelial Cells Induced by Cigarette Smoke via Oxidative Stress Signaling Pathway. Copd 2020, 17, 269–279. [Google Scholar] [CrossRef]
- Eurlings, I.M.; Reynaert, N.L.; van den Beucken, T.; Gosker, H.R.; de Theije, C.C.; Verhamme, F.M.; Bracke, K.R.; Wouters, E.F.; Dentener, M.A. Cigarette smoke extract induces a phenotypic shift in epithelial cells; involvement of HIF1α in mesenchymal transition. PLoS ONE 2014, 9, e107757. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic, E.; Jiricny, J.; Menigatti, M.; Rehrauer, H.; Han, W. Chronic exposure to cigarette smoke condensate in vitro induces epithelial to mesenchymal transition-like changes in human bronchial epithelial cells, BEAS-2B. Toxicol. Vitr. 2011, 25, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, C.; Sun, L.; Li, D.; Hong, X. Cigarette smoke condensate could promote human bronchial epithelial BEAS-2B cell migration through shifting neprilysin traffickingJ. Cancer Res. Ther. 2018, 14, S680–S687. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Zou, Y.; Zhao, Z.; Li, B.; Ran, P. Nicotine-induced epithelial-mesenchymal transition via Wnt/β-catenin signaling in human airway epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2013, 304, L199–L209. [Google Scholar] [CrossRef]
- Wu, Y.; Niu, Y.; Leng, J.; Xu, J.; Chen, H.; Li, H.; Wang, L.; Hu, J.; Xia, D.; Wu, Y. Benzo (a) pyrene regulated A549 cell migration, invasion and epithelial-mesenchymal transition by up-regulating long non-coding RNA linc00673. Toxicol. Lett. 2020, 320, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Piadé, J.-J.; Wajrock, S.; Jaccard, G.; Janeke, G. Formation of mainstream cigarette smoke constituents prioritized by the World Health Organization–yield patterns observed in market surveys, clustering and inverse correlations. Food Chem. Toxicol. 2013, 55, 329–347. [Google Scholar] [CrossRef]
- Jenkins, R.A.; Tomkins, B.; Guerin, M.R. The Chemistry of Environmental Tobacco Smoke: Composition and Measurement, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9780429182945. [Google Scholar]
- Kim, Y.H.; An, Y.J.; Jo, S.; Lee, S.H.; Lee, S.J.; Choi, S.J.; Lee, K. Comparison of volatile organic compounds between cigarette smoke condensate (CSC) and extract (CSE) samples. Environ. Health Toxicol. 2018, 33, e2018012. [Google Scholar] [CrossRef] [PubMed]
- Heijink, I.H.; de Bruin, H.G.; Dennebos, R.; Jonker, M.R.; Noordhoek, J.A.; Brandsma, C.A.; van den Berge, M.; Postma, D.S. Cigarette smoke-induced epithelial expression of WNT-5B: Implications for COPD. Eur. Respir. J. 2016, 48, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Lu, L.; Xia, H.; Xiang, Q.; Sun, J.; Xue, J.; Xiao, T.; Cheng, C.; Liu, Q.; Shi, A. Circ0061052 regulation of FoxC1/Snail pathway via miR-515-5p is involved in the epithelial-mesenchymal transition of epithelial cells during cigarette smoke-induced airway remodeling. Sci. Total Environ. 2020, 746, 141181. [Google Scholar] [CrossRef]
- Mao, Y.; Feng, H. Vitamin D3 alleviates cigarette smoke extract-mediated epithelial-mesenchymal transition and fibrogenesis by upregulating CC16 expression in bronchial epithelial cells. Exp. Med. 2022, 23, 357. [Google Scholar] [CrossRef]
- Liang, Z.; Xie, W.; Wu, R.; Geng, H.; Zhao, L.; Xie, C.; Li, X.; Huang, C.; Zhu, J.; Zhu, M.; et al. ERK5 negatively regulates tobacco smoke-induced pulmonary epithelial-mesenchymal transition. Oncotarget 2015, 6, 19605–19618. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Zhang, Y.; Zhang, Y.; Xiao, W. Activation of uPAR is required for cigarette smoke extract-induced epithelial-mesenchymal transition in lung epithelial cells. Oncol. Res. 2013, 21, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Polk, W.W. FH535 potentiation of cigarette smoke condensate cytotoxicity is associated with changes in β-catenin and EGR-1 signaling. Int. J. Toxicol. 2012, 31, 380–389. [Google Scholar] [CrossRef]
- Mathyssen, C.; Serré, J.; Sacreas, A.; Everaerts, S.; Maes, K.; Verleden, S.; Verlinden, L.; Verstuyf, A.; Pilette, C.; Gayan-Ramirez, G. Vitamin D modulates the response of bronchial epithelial cells exposed to cigarette smoke extract. Nutrients 2019, 11, 2138. [Google Scholar] [CrossRef] [PubMed]
- Bersaas, A.; Arnoldussen, Y.J.; Sjøberg, M.; Haugen, A.; Mollerup, S. Epithelial-mesenchymal transition and FOXA genes during tobacco smoke carcinogen induced transformation of human bronchial epithelial cells. Toxicol. Vitr. 2016, 35, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Killian, J.K.; Yang, M.; Walker, R.L.; Hong, J.A.; Zhang, M.; Davis, S.; Zhang, Y.; Hussain, M.; Xi, S.; et al. Epigenomic alterations and gene expression profiles in respiratory epithelia exposed to cigarette smoke condensate. Oncogene 2010, 29, 3650–3664. [Google Scholar] [CrossRef]
- Wang, J.; Yu, X.F.; Ouyang, N.; Zhao, S.; Yao, H.; Guan, X.; Tong, J.; Chen, T.; Li, J.X. MicroRNA and mRNA Interaction Network Regulates the Malignant Transformation of Human Bronchial Epithelial Cells Induced by Cigarette Smoke. Front. Oncol. 2019, 9, 1029. [Google Scholar] [CrossRef]
- van der Toorn, M.; Sewer, A.; Marescotti, D.; Johne, S.; Baumer, K.; Bornand, D.; Dulize, R.; Merg, C.; Corciulo, M.; Scotti, E.; et al. The biological effects of long-term exposure of human bronchial epithelial cells to total particulate matter from a candidate modified-risk tobacco product. Toxicol. Vitr. 2018, 50, 95–108. [Google Scholar] [CrossRef]
- Thapa, R.; Moglad, E.; Goyal, A.; Bhat, A.A.; Almalki, W.H.; Kazmi, I.; Alzarea, S.I.; Ali, H.; Oliver, B.G.; MacLoughlin, R. Deciphering NF-κB pathways in smoking-related lung carcinogenesis. EXCLI J. 2024, 23, 991–1017. [Google Scholar]
- Liang, X.; He, X.; Li, Y.; Wang, J.; Wu, D.; Yuan, X.; Wang, X.; Li, G. Lyn regulates epithelial-mesenchymal transition in CS-exposed model through Smad2/3 signaling. Respir. Res. 2019, 20, 201. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Xu, W.; Han, F.; Gu, W.; Song, L.; Ye, W.; Liu, Q.; Guo, X. Ginsenoside Rg1 Attenuates Cigarette Smoke-Induced Pulmonary Epithelial-Mesenchymal Transition via Inhibition of the TGF-β1/Smad Pathway. Biomed. Res. Int. 2017, 2017, 7171404. [Google Scholar] [CrossRef]
- Su, X.; Chen, J.; Lin, X.; Chen, X.; Zhu, Z.; Wu, W.; Lin, H.; Wang, J.; Ye, X.; Zeng, Y. FERMT3 mediates cigarette smoke-induced epithelial–mesenchymal transition through Wnt/β-catenin signaling. Respir. Res. 2021, 22, 286. [Google Scholar] [CrossRef]
- Vaz, M.; Hwang, S.Y.; Kagiampakis, I.; Phallen, J.; Patil, A.; O’Hagan, H.M.; Murphy, L.; Zahnow, C.A.; Gabrielson, E.; Velculescu, V.E.; et al. Chronic Cigarette Smoke-Induced Epigenomic Changes Precede Sensitization of Bronchial Epithelial Cells to Single-Step Transformation by KRAS Mutations. Cancer Cell 2017, 32, 360–376.e6. [Google Scholar] [CrossRef]
- Lin, F.; Liao, C.; Sun, Y.; Zhang, J.; Lu, W.; Bai, Y.; Liao, Y.; Li, M.; Ni, X.; Hou, Y. Hydrogen sulfide inhibits cigarette smoke-induced endoplasmic reticulum stress and apoptosis in bronchial epithelial cells. Front. Pharmacol. 2017, 8, 675. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Liao, C.; Zhang, J.; Sun, Y.; Lu, W.; Bai, Y.; Liao, Y.; Li, M.; Qi, Y.; Chen, Y. Hydrogen Sulfide Inhibits Bronchial Epithelial Cell Epithelial Mesenchymal Transition Through Regulating Endoplasm Reticulum Stress. Front. Mol. Biosci. 2022, 9, 828766. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Liu, J.; Yong, J.; Li, X.; Ji, W.; Zhao, Z.; Wang, X.; Xiao, C.; Wu, S.; Liu, H.; et al. Strategies for understanding the role of cellular heterogeneity in the pathogenesis of lung cancer: A cell model for chronic exposure to cigarette smoke extract. BMC Pulm. Med. 2022, 22, 333. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, C.; He, B.; Du, X.; Liu, J.; Xia, H.; Wang, P.; Wu, M.; Wu, H.; Liu, Q. Cigarette smoking, by accelerating the cell cycle, promotes the progression of non-small cell lung cancer through an HIF-1α-METTL3-m6A/CDK2AP2 axis. J. Hazard. Mater. 2023, 455, 131556. [Google Scholar] [CrossRef]
- Anzalone, G.; Arcoleo, G.; Bucchieri, F.; Montalbano, A.M.; Marchese, R.; Albano, G.D.; Di Sano, C.; Moscato, M.; Gagliardo, R.; Ricciardolo, F.L.M.; et al. Cigarette smoke affects the onco-suppressor DAB2IP expression in bronchial epithelial cells of COPD patients. Sci. Rep. 2019, 9, 15682. [Google Scholar] [CrossRef]
- Liu, C.; Lu, J.; Yuan, T.; Xie, L.; Zhang, L. EPC-exosomal miR-26a-5p improves airway remodeling in COPD by inhibiting ferroptosis of bronchial epithelial cells via PTGS2/PGE2 signaling pathway. Sci. Rep. 2023, 13, 6126. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, Y.; Lu, B.; Huang, Y.; Xu, S.; Xie, J.; Liu, M.; Che, D.; Ma, L.; Tao, J.; et al. Inositol Alleviates Pulmonary Fibrosis by Promoting Autophagy via Inhibiting the HIF-1α-SLUG Axis in Acute Respiratory Distress Syndrome. Oxid. Med. Cell. Longev. 2022, 2022, 1030238. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Tan, J.-Y.; Meng, X.; Xie, H.; Wang, R. Lysyl Oxidase Promotes Epithelial-to-Mesenchymal Transition During Paraquat-Induced Pulmonary Fibrosis. Mol. Biosyst. 2016, 12, 499–507. [Google Scholar] [CrossRef]
- Wadhwa, R.; Kalra, R.S.; Chaudhary, A.; Yu, Y.; Li, L.; Kaul, S. CARF regulates EMT through Wnt/b-catenin signaling: Its clinical relevance & potential as a therapeutic cancer target. Ann. Oncol. 2018, 29, vii52. [Google Scholar]
- Yang, S.; Liu, Y.; Li, M.Y.; Ng, C.S.H.; Yang, S.L.; Wang, S.; Zou, C.; Dong, Y.; Du, J.; Long, X.; et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol. Cancer 2017, 16, 124. [Google Scholar] [CrossRef]
- Jiang, Y.G.; Luo, Y.; He, D.L.; Li, X.; Zhang, L.L.; Peng, T.; Li, M.C.; Lin, Y.H. Role of Wnt/beta-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. Int. J. Urol. 2007, 14, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal. Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Heijink, I.H.; de Bruin, H.G.; van den Berge, M.; Bennink, L.J.; Brandenburg, S.M.; Gosens, R.; van Oosterhout, A.J.; Postma, D.S. Role of aberrant WNT signalling in the airway epithelial response to cigarette smoke in chronic obstructive pulmonary disease. Thorax 2013, 68, 709–716. [Google Scholar] [CrossRef]
- Malyla, V.; Paudel, K.R.; De Rubis, G.; Hansbro, N.G.; Hansbro, P.M.; Dua, K. Cigarette smoking induces lung cancer tumorigenesis via upregulation of the WNT/β-catenin signaling pathway. Life Sci. 2023, 326, 121787. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Pan, H.Y.; Wang, D.X.; Deng, Z.T.; Ye, X.L. TGF-beta1 induces human bronchial epithelial cell-to-mesenchymal transition in vitro. Lung 2009, 187, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Doerner, A.M.; Zuraw, B.L. TGF-beta1 induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells is enhanced by IL-1beta but not abrogated by corticosteroids. Respir. Res. 2009, 10, 100. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef]
- Mahmood, M.Q.; Reid, D.; Ward, C.; Muller, H.K.; Knight, D.A.; Sohal, S.S.; Walters, E.H. Transforming growth factor (TGF) β(1) and Smad signalling pathways: A likely key to EMT-associated COPD pathogenesis. Respirology 2017, 22, 133–140. [Google Scholar] [CrossRef]
- Jiang, B.; Guan, Y.; Shen, H.J.; Zhang, L.H.; Jiang, J.X.; Dong, X.W.; Shen, H.H.; Xie, Q.M. Akt/PKB signaling regulates cigarette smoke-induced pulmonary epithelial-mesenchymal transition. Lung Cancer 2018, 122, 44–53. [Google Scholar] [CrossRef]
- Zuo, H.; Trombetta-Lima, M.; Heijink, I.H.; van der Veen, C.; Hesse, L.; Faber, K.N.; Poppinga, W.J.; Maarsingh, H.; Nikolaev, V.O.; Schmidt, A.M. A-Kinase Anchoring Proteins Diminish TGF-β(1)/Cigarette Smoke-Induced Epithelial-to-Mesenchymal Transition. Cells 2020, 9, 356. [Google Scholar] [CrossRef]
- Guan, R.; Wang, J.; Cai, Z.; Li, Z.; Wang, L.; Li, Y.; Xu, J.; Li, D.; Yao, H.; Liu, W.; et al. Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway. Redox. Biol. 2020, 28, 101356. [Google Scholar] [CrossRef]
- Sarker, A.H.; Chatterjee, A.; Williams, M.; Lin, S.; Havel, C.; Jacoblill, P., 3rd; Boldogh, I.; Hazra, T.K.; Talbot, P.; Hang, B. NEIL2 protects against oxidative DNA damage induced by sidestream smoke in human cells. PLoS ONE 2014, 9, e90261. [Google Scholar] [CrossRef]
- Akil, A.; Gutiérrez-García, A.K.; Guenter, R.; Rose, J.B.; Beck, A.W.; Chen, H.; Ren, B. Notch Signaling in Vascular Endothelial Cells, Angiogenesis, and Tumor Progression: An Update and Prospective. Front. Cell Dev. Biol. 2021, 9, 642352. [Google Scholar] [CrossRef] [PubMed]
- Larue, L.; Bellacosa, A. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3’ kinase/AKT pathways. Oncogene 2005, 24, 7443–7454. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.J.; Sun, Y.H.; Zhang, S.J.; Jiang, J.X.; Dong, X.W.; Jia, Y.L.; Shen, J.; Guan, Y.; Zhang, L.H.; Li, F.F.; et al. Cigarette smoke-induced alveolar epithelial-mesenchymal transition is mediated by Rac1 activation. Biochim. Biophys. Acta. 2014, 1840, 1838–1849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Adiseshaiah, P.; Kalvakolanu, D.V.; Reddy, S.P. A Phosphatidylinositol 3-kinase-regulated Akt-independent signaling promotes cigarette smoke-induced FRA-1 expression. J. Biol. Chem. 2006, 281, 10174–10181. [Google Scholar] [CrossRef]
- Somborac-Bačura, A.; van der Toorn, M.; Franciosi, L.; Slebos, D.J.; Žanić-Grubišić, T.; Bischoff, R.; van Oosterhout, A.J. Cigarette smoke induces endoplasmic reticulum stress response and proteasomal dysfunction in human alveolar epithelial cells. Exp. Physiol. 2013, 98, 316–325. [Google Scholar] [CrossRef]
- Song, M.; Peng, H.; Guo, W.; Luo, M.; Duan, W.; Chen, P.; Zhou, Y. Cigarette smoke extract promotes human lung myofibroblast differentiation by the induction of endoplasmic reticulum stress. Respiration 2019, 98, 347–356. [Google Scholar] [CrossRef]
- Jorgensen, E.; Stinson, A.; Shan, L.; Yang, J.; Gietl, D.; Albino, A.P. Cigarette smoke induces endoplasmic reticulum stress and the unfolded protein response in normal and malignant human lung cells. BMC Cancer 2008, 8, 229. [Google Scholar] [CrossRef]
- Ahn, K.S.; Aggarwal, B.B. Transcription factor NF-kappaB: A sensor for smoke and stress signals. Ann. N. Y. Acad. Sci. 2005, 1056, 218–233. [Google Scholar] [CrossRef]
- Anto, R.J.; Mukhopadhyay, A.; Shishodia, S.; Gairola, C.G.; Aggarwal, B.B. Cigarette smoke condensate activates nuclear transcription factor-kappaB through phosphorylation and degradation of IkappaB(alpha): Correlation with induction of cyclooxygenase-2. Carcinogenesis 2002, 23, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Togo, S.; Al-Mugotir, M.; Kim, H.; Fang, Q.; Kobayashi, T.; Wang, X.; Mao, L.; Bitterman, P.; Rennard, S. NF-kappaB mediates the survival of human bronchial epithelial cells exposed to cigarette smoke extract. Respir. Res. 2008, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Allison, D.F.; Baranova, N.N.; Wamsley, J.J.; Katz, A.J.; Bekiranov, S.; Jones, D.R.; Mayo, M.W. NF-κB regulates mesenchymal transition for the induction of non-small cell lung cancer initiating cells. PLoS ONE 2013, 8, e68597. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Y.; Li, Y.; Xu, W.; Luo, F.; Wang, B.; Pang, Y.; Xiang, Q.; Zhou, J.; Wang, X.; et al. NF-κB-mediated inflammation leading to EMT via miR-200c is involved in cell transformation induced by cigarette smoke extract. Toxicol. Sci. 2013, 135, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, J.; Chen, Y.; Zhang, G.; Jiang, P.; Hong, L.; Shen, Y.; Wang, X.; Gong, X. Cigarette Smoke Enhances Initiation and Progression of Lung Cancer by Mutating Notch1/2 and Dysregulating Downstream Signaling Molecules. Oncotarget 2017, 8, 115128–115139. [Google Scholar] [CrossRef][Green Version]
- Salama, R. Lung Cancer Stem Cells: Current Progress and Future Perspectives. J. Stem Cell Res. Ther. 2013, 7, 2. [Google Scholar] [CrossRef]
- Espinoza, I.; Pochampally, R.; Xing, F.; Watabe, K.; Miele, L. Notch Signaling: Targeting Cancer Stem Cells and Epithelial-to-Mesenchymal Transition. Oncotargets Ther. 2013, 6, 1249–1259. [Google Scholar] [CrossRef]
- Venkatesh, V.; Nataraj, R.; Thangaraj, G.; Muthusamy, K.; Gnanasekaran, A.; Kaginelli, S.; Gobianand, K.; Kallappa, C.G.; Basalingappa, K.M. Targeting Notch Signalling Pathway of Cancer Stem Cells. Stem Cell Investig. 2018, 5, 5. [Google Scholar] [CrossRef]
- Weiss, G.J. Targeting the Hedgehog and Notch Signaling Pathways. J. Thorac. Oncol. 2011, 6, S1820–S1821. [Google Scholar] [CrossRef]
- Qu, H.; Liu, L.; Zhe, L.; Qin, H.; Liao, Z.; Xia, P.; Yang, Y.; Li, B.; Gao, F.; Cai, J. Blocking TBK1 Alleviated Radiation-Induced Pulmonary Fibrosis and Epithelial-Mesenchymal Transition Through Akt-Erk Inactivation. Exp. Mol. Med. 2019, 51, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Li, Y.; Yang, X.; Wu, Y.; Li, L.; Liu, X. Hsa_circ_0044226 Knockdown Attenuates Progression of Pulmonary Fibrosis by Inhibiting CDC27. Aging 2020, 12, 14808–14818. [Google Scholar] [CrossRef]
- Shan, B.; Yao, T.P.; Nguyen, H.T.; Zhuo, Y.; Levy, D.R.; Klingsberg, R.C.; Tao, H.; Palmer, M.L.; Holder, K.N.; Lasky, J.A. Requirement of HDAC6 for Transforming Growth Factor-Β1-Induced Epithelial-Mesenchymal Transition. J. Biol. Chem. 2008, 283, 21065–21073. [Google Scholar] [CrossRef] [PubMed]
- Bocci, F.; Gearhart-Serna, L.; Boareto, M.; Ribeiro, M.P.; Ben-Jacob, E.; Devi, G.R.; Levine, H.; Onuchic, J.N.; Jolly, M.K. Toward Understanding Cancer Stem Cell Heterogeneity in the Tumor Microenvironment. Proc. Natl. Acad. Sci. USA 2018, 116, 148–157. [Google Scholar] [CrossRef]
- Zhu, W.; Han, L.; Wu, Y.; Tong, L.; He, L.; Wang, Q.; Yan, Y.; Pan, T.; Shen, J.; Song, Y.; et al. Keratin 15 protects against cigarette smoke-induced epithelial mesenchymal transformation by MMP-9. Respir. Res. 2023, 24, 297. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Xue, J.; Xu, H.; Lin, M.; Shi, M.; Sun, Q.; Xiao, T.; Dai, X.; Wu, L.; Li, J.; et al. Andrographolide antagonizes the cigarette smoke-induced epithelial-mesenchymal transition and pulmonary dysfunction through anti-inflammatory inhibiting HOTAIR. Toxicology 2019, 422, 84–94. [Google Scholar] [CrossRef]

| Type of Cancer | Smoking Status | Prognostic Findings | Impact on Survival | Study |
|---|---|---|---|---|
| Multiple cancers | Current/former smokers | Higher incidence of second primary cancers in smokers. | Significantly reduced overall survival rates. | [6] |
| Bladder cancer | Current/former smokers | Higher recurrence rates and more aggressive tumor phenotypes in smokers. | Lower disease-free survival and overall survival in smokers. | [6] |
| Esophageal cancer | Current smokers | Smoking-related esophageal cancer has a higher likelihood of recurrence post-treatment. | Reduced survival and higher recurrence rates post-esophagectomy. | [10] |
| Lung cancer | Current smokers | Increased risk of second primary lung cancer among smokers. | Reduced overall survival and progression-free survival. | [5] |
| Non-small cell lung cancer (NSCLC) | Current smokers | Higher incidence of squamous cell carcinoma subtype among smokers. | Lower survival rates in smokers compared to nonsmokers. | [3] |
| Colorectal cancer | Current/former smokers | Increased pulmonary metastasis in smokers. | Increased mortality due to liver and lung metastasis. | [7] |
| Study | Type of Cell Line | Rate of CS Exposure | Downregulated Epithelial Marker | Upregulated Mesenchymal Marker |
|---|---|---|---|---|
| [123] | Primary HBE | 1.5% CSE for 48 h | E-cadherin | Vimentin, MMP-9 |
| [85] | Primary HBEC | 5% CSE for 24 h | E-cadherin ZO-1 | Vimentin |
| [81] | Immortalized 16HBE14o- | 40 μmol/L nicotine treated for 72 h | E-cadherin | α-SMA |
| [100] | Immortalized 16HBE | 3% CSE for 48 h | E-cadherin | E-cadherin fibronectin, α-SMA, Collagen 1, Collagen 3 |
| [66] | Immortalized 16-HBE | 5, 10 and 20% CSE for 24 h | E-cadherin | N-cadherin, α-SMA, Slug, FN1, Collagen IV |
| [124] | Immortalized HBE | 0.5, 1, and 2% CSE for 24 h | E-cadherin | N-cadherin, Vimentin α -SMA |
| [65] | Immortalized HBE | 1, 2, 4% CSE for 48 h | E-cadherin | N-cadherin, Vimentin, α-SMA, Snail, FoxC1 |
| [55] | Primary HBE | 10% CSE for 24 and 48 h | E-cadherin ZO-1 | α-SMA, Vimentin |
| [98] | Immortalized 16HBE | 2.5% CSE for 48 h | E-cadherin | α-SMA, Vimentin, MMP-9, MMP-2 |
| [76] | Immortalized 16HBE | 2.5, 5% CSE for 72 h, and 5% CSE for 6 h, 12 h, 24 h, 48 h, 72 h | E-cadherin | α-SMA, Vimentin |
| [77] | Immortalized BEAS-2B | 10% CSE for 48 h | E-cadherin | α-SMA |
| [67] | Primary NHBE Normal human bronchial epithelial cells | 2 and 4% CSE for 7 days | E-cadherin ZO-1 | N-cadherin, Vimentin |
| [68] | Immortalized BEAS-2B | 5% CSE for 5 days | E-cadherin | Vimentin, N-cadherin, α-SMA |
| [56] | Immortalized BEAS-2B | 1% CSE every 24 h for 48 h | E-cadherin ZO-1 keratin 18 | Vimentin, PAI-1, Fibronectin, Snail |
| [59] | Primary HBEC | 6 × 10−6 mol/L nicotine for 24 h | E-cadherin | α-SMA, Vimentin, Collagen I, MMP-9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqithami, S.M.; Machwe, A.; Orren, D.K. Cigarette Smoke-Induced Epithelial-to-Mesenchymal Transition: Insights into Cellular Mechanisms and Signaling Pathways. Cells 2024, 13, 1453. https://doi.org/10.3390/cells13171453
Alqithami SM, Machwe A, Orren DK. Cigarette Smoke-Induced Epithelial-to-Mesenchymal Transition: Insights into Cellular Mechanisms and Signaling Pathways. Cells. 2024; 13(17):1453. https://doi.org/10.3390/cells13171453
Chicago/Turabian StyleAlqithami, Sarah Mohammed, Amrita Machwe, and David K. Orren. 2024. "Cigarette Smoke-Induced Epithelial-to-Mesenchymal Transition: Insights into Cellular Mechanisms and Signaling Pathways" Cells 13, no. 17: 1453. https://doi.org/10.3390/cells13171453
APA StyleAlqithami, S. M., Machwe, A., & Orren, D. K. (2024). Cigarette Smoke-Induced Epithelial-to-Mesenchymal Transition: Insights into Cellular Mechanisms and Signaling Pathways. Cells, 13(17), 1453. https://doi.org/10.3390/cells13171453






